Characterization of the Gut Microbiota of Mackerel Icefish, Champsocephalus gunnari
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.3. Bioinformatics
2.4. Statistical Analysis
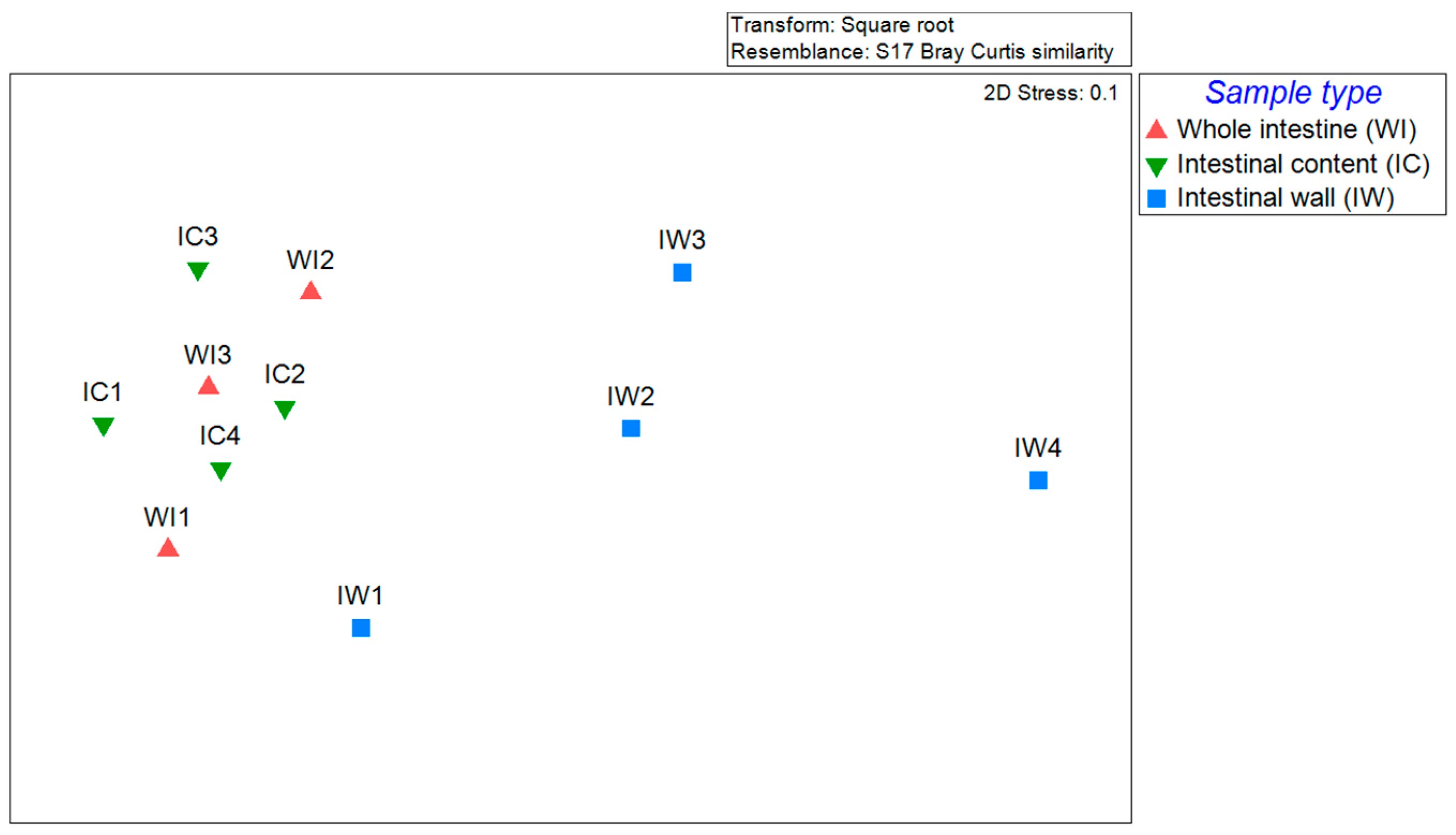
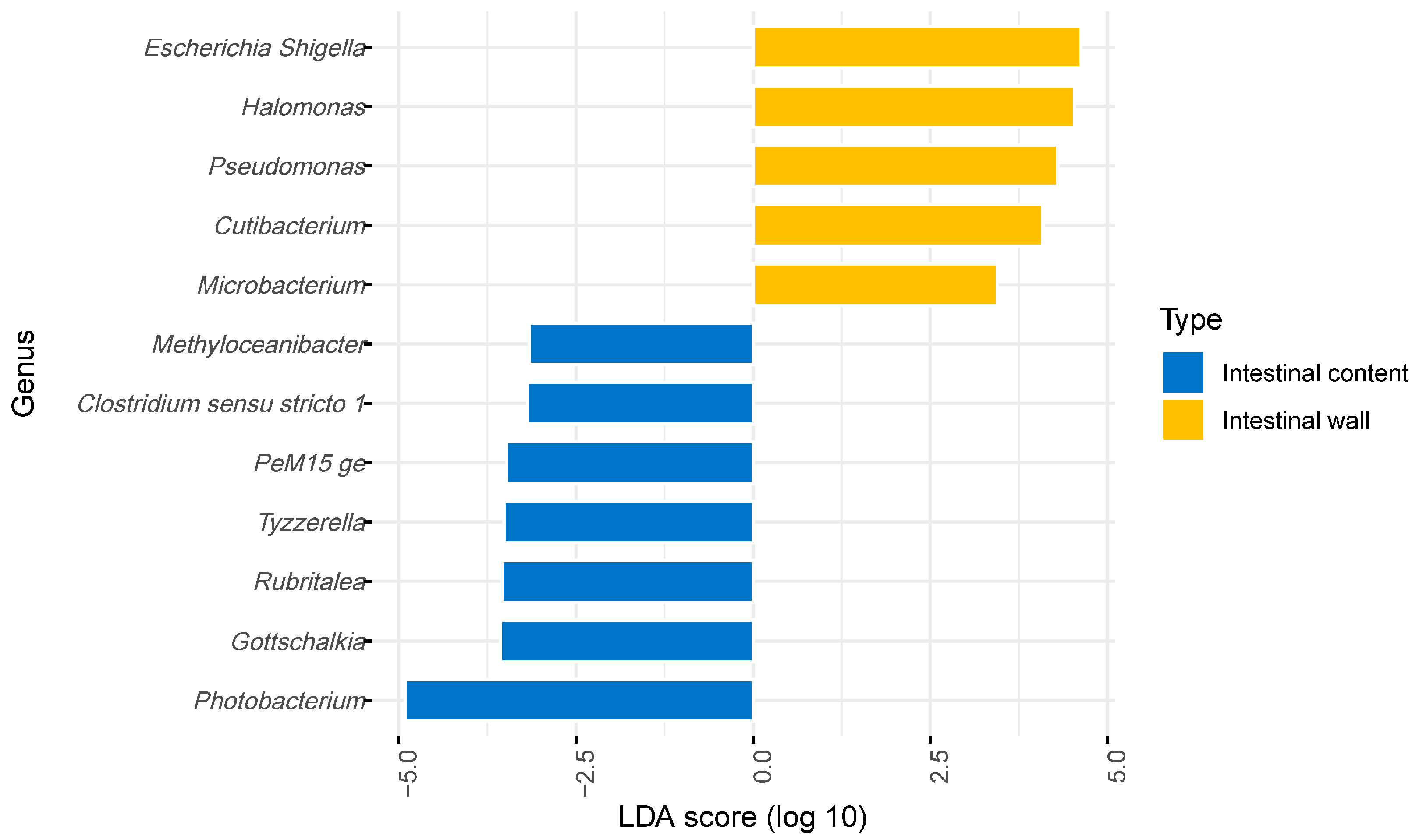
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youngblut, N.D.; Reischer, G.H.; Walters, W.; Schuster, N.; Walzer, C.; Stalder, G.; Ley, R.E.; Farnleitner, A.H. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat. Commun. 2019, 10, 2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.P.; Frank, K.M. Salmonella, shigella, and yersinia. Clin. Lab. Med. 2015, 35, 225–246. [Google Scholar] [CrossRef] [Green Version]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Senghor, B.; Sokhna, C.; Ruimy, R.; Lagier, J.-C. Gut microbiota diversity according to dietary habits and geographical provenance. Hum. Microbiome J. 2018, 7–8, 1–9. [Google Scholar] [CrossRef]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef] [Green Version]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The gut microbiota of marine fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; McGinnity, P.; Dionne, M.; Letourneau, J.; Thonier, F.; Carvalho, G.R.; Creer, S.; Derome, N. The biogeography of the atlantic salmon (Salmo salar) gut microbiome. ISME J. 2016, 10, 1280–1284. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, C.; Burgess, C.M.; Cotter, P.D.; Cabrera-Rubio, R.; Whyte, P.; Smyth, C.; Bolton, D.J. Diversity and composition of the gut microbiota of Atlantic salmon (Salmo salar) farmed in Irish waters. J. Appl. Microbiol. 2019, 127, 648–657. [Google Scholar] [CrossRef]
- Dvergedal, H.; Sandve, S.R.; Angell, I.L.; Klemetsdal, G.; Rudi, K. Association of gut microbiota with metabolism in juvenile Atlantic salmon. Microbiome 2020, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Huyben, D.; Roehe, B.K.; Bekaert, M.; Ruyter, B.; Glencross, B. Dietary Lipid:Protein Ratio and n-3 Long-Chain Polyunsaturated fatty acids alters the gut microbiome of atlantic salmon under hypoxic and normoxic conditions. Front. Microbiol. 2020, 11, 589898. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, D.; Rasmussen, J.A.; Carøe, C.; Sveier, H.; Nordøy, K.; Gilbert, M.T.P.; Limborg, M.T. Salmon gut microbiota correlates with disease infection status: Potential for monitoring health in farmed animals. Anim. Microbiome 2021, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jaramillo-Torres, A.; Li, Y.; Kortner, T.M.; Gajardo, K.; Brevik, Ø.J.; Jakobsen, J.V.; Krogdahl, Å. Microbiota in intestinal digesta of Atlantic salmon (Salmo salar), observed from late freshwater stage until one year in seawater, and effects of functional ingredients: A case study from a commercial sized research site in the Arctic region. Anim. Microbiome 2021, 3, 14. [Google Scholar] [CrossRef]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Li, P.; Huang, X.; Wang, C.H.; Li, M.; Xu, Z.Z. Zebrafish model for human gut microbiome-related studies: Advantages and limitations. Med. Microecol. 2021, 8, 100042. [Google Scholar] [CrossRef]
- Xiao, F.; Zhu, W.; Yu, Y.; He, Z.; Wu, B.; Wang, C.; Shu, L.; Li, X.; Yin, H.; Wang, J.; et al. Host development overwhelms environmental dispersal in governing the ecological succession of zebrafish gut microbiota. NPJ Biofilms Microbiomes 2021, 7, 5. [Google Scholar] [CrossRef]
- Cornuault, J.K.; Byatt, G.; Paquet, M.-E.; De Koninck, P.; Moineau, S. Zebrafish: A big fish in the study of the gut microbiota. Curr. Opin. Biotechnol. 2022, 73, 308–313. [Google Scholar] [CrossRef]
- Ward, N.L.; Steven, B.; Penn, K.; Methé, B.A.; Detrich, W.H. Characterization of the intestinal microbiota of two Antarctic notothenioid fish species. Extremophiles 2009, 13, 679–685. [Google Scholar] [CrossRef]
- Song, W.; Li, L.; Huang, H.; Jiang, K.; Zhang, F.; Chen, X.; Zhao, M.; Ma, L. The gut microbial community of antarctic fish detected by 16S rRNA gene sequence analysis. BioMed Res. Int. 2016, 2016, 3241529. [Google Scholar] [CrossRef]
- Gallet, A.; Koubbi, P.; Léger, N.; Scheifler, M.; Ruiz-Rodriguez, M.; Suzuki, M.T.; Desdevises, Y.; Duperron, S. Low-diversity bacterial microbiota in Southern Ocean representatives of lanternfish genera Electrona, Protomyctophum and Gymnoscopelus (family Myctophidae). PLoS ONE 2019, 14, e0226159. [Google Scholar] [CrossRef] [PubMed]
- Kock, K.-H.; Everson, I. Biology and ecology of mackerel icefish, Champsocephalus gunnari: An Antarctic fish lacking hemoglobin. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 1067–1077. [Google Scholar] [CrossRef]
- di Prisco, G.; Cocca, E.; Parker, S.K.; Detrich, H.W. Tracking the evolutionary loss of hemoglobin expression by the white-blooded Antarctic icefishes. Gene 2002, 295, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Koo, M.H.; Han, D.-W.; Kim, I.-C.; Lee, J.H.; Kim, J.-H.; Sultana, R.; Kim, S.Y.; Youn, U.J.; Kim, J.-H. Comparison of Fatty Acid Contents and MMP-1 Inhibitory Effects of the Two Antarctic Fish, Notothenia rossii and Champsocephalus gunnari. Molecules 2022, 27, 4554. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- CCAMLR. Map of the CAMLR Convention Area. 2017. Available online: www.ccamlr.org/node/86816 (accessed on 1 December 2022).
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.; Gorley, R. PRIMER v6: User Manual/Tutorial; PRIMER-e Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Kim, P.S.; Shin, N.-R.; Lee, J.-B.; Kim, M.-S.; Whon, T.W.; Hyun, D.-W.; Yun, J.-H.; Jung, M.-J.; Kim, J.Y.; Bae, J.-W. Host habitat is the major determinant of the gut microbiome of fish. Microbiome 2021, 9, 166. [Google Scholar] [CrossRef]
- He, S.; Ran, C.; Qin, C.; Li, S.; Zhang, H.; de Vos, W.M.; Ringø, E.; Zhou, Z. Anti-infective effect of adhesive probiotic lactobacillus in fish is correlated with their spatial distribution in the intestinal tissue. Sci. Rep. 2017, 7, 13195. [Google Scholar] [CrossRef]
- Burtseva, O.; Kublanovskaya, A.; Fedorenko, T.; Lobakova, E.; Chekanov, K. Gut microbiome of the White Sea fish revealed by 16S rRNA metabarcoding. Aquaculture 2021, 533, 736175. [Google Scholar] [CrossRef]
- Uren Webster, T.M.; Rodriguez-Barreto, D.; Consuegra, S.; Garcia de Leaniz, C. Cortisol-Related Signatures of Stress in the Fish Microbiome. Front. Microbiol. 2020, 11, 1621. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.J. Bacteria. In Taxonomic Guide to Infectious Diseases, 2nd ed.; Berman, J.J., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 3; pp. 39–119. [Google Scholar]
- Tran, N.T.; Li, Z.; Ma, H.; Zhang, Y.; Zheng, H.; Gong, Y.; Li, S. Clostridium butyricum: A promising probiotic confers positive health benefits in aquatic animals. Rev. Aquac. 2020, 12, 2573–2589. [Google Scholar] [CrossRef]
- Ramesh, A.; Venugopalan, V. Role of luminous bacteria in chitin degradation in the intestine of fish. MIRCEN J. Appl. Microbiol. Biotechnol. 1989, 5, 55–59. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.; Miao, J.; Leng, K. Chitin from Antarctic krill shell: Eco-preparation, detection, and characterization. Int. J. Biol. Macromol. 2020, 164, 4125–4137. [Google Scholar] [CrossRef] [PubMed]
- Sasi Jyothsna, T.S.; Tushar, L.; Sasikala, C.; Ramana, C.V. Paraclostridium benzoelyticum gen. nov., sp. nov., isolated from marine sediment and reclassification of Clostridium bifermentans as Paraclostridium bifermentans comb. nov. Proposal of a new genus Paeniclostridium gen. nov. to accommodate Clostridium sordellii and Clostridium ghonii. Int. J. Syst. Evol. Microbiol. 2016, 66, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.B.; Song, H.S.; Chung, W.-H.; Lee, C.; Ahn, S.W.; Lee, S.H.; Jung, M.Y.; Kim, T.-W.; Nam, Y.-D.; et al. Genomic Analysis of a Pathogenic Bacterium, Paeniclostridium sordellii CBA7122 Containing the Highest Number of rRNA Operons, Isolated from a Human Stool Sample. Front. Pharmacol. 2017, 8, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landsberg, J.H.; Tabuchi, M.; Rotstein, D.S.; Subramaniam, K.; Rodrigues, T.C.S.; Waltzek, T.B.; Stacy, N.I.; Wilson, P.W.; Kiryu, Y.; Uzal, F.A.; et al. Novel Lethal Clostridial Infection in Florida Manatees (Trichechus manatus latirostris): Cause of the 2013 unusual mortality event in the Indian River Lagoon. Front. Mar. Sci. 2022, 9, 195. [Google Scholar] [CrossRef]
- Dehler, C.E.; Secombes, C.J.; Martin, S.A.M. Seawater transfer alters the intestinal microbiota profiles of Atlantic salmon (Salmo salar L.). Sci. Rep. 2017, 7, 13877. [Google Scholar] [CrossRef] [Green Version]
- Burns, A.R.; Watral, V.; Sichel, S.; Spagnoli, S.; Banse, A.V.; Mittge, E.; Sharpton, T.J.; Guillemin, K.; Kent, M.L. Transmission of a common intestinal neoplasm in zebrafish by cohabitation. J. Fish Dis. 2018, 41, 569–579. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Q.; Lin, Y.; Hao, J.; Wang, S.; Zhang, J.; Li, A. Taxonomic and Functional Characteristics of the Gill and Gastrointestinal Microbiota and Its Correlation with Intestinal Metabolites in NEW GIFT Strain of Farmed Adult Nile Tilapia (Oreochromis niloticus). Microorganisms 2021, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Mulet, M.; Altun, S.; Saticioglu, I.B.; Ozdemir, B.; Ajmi, N.; Lalucat, J.; García-Valdés, E. The diversity of Pseudomonas species isolated from fish farms in Turkey. Aquaculture 2021, 535, 736369. [Google Scholar] [CrossRef]
- Algammal, A.M.; Mabrok, M.; Sivaramasamy, E.; Youssef, F.M.; Atwa, M.H.; El-kholy, A.W.; Hetta, H.F.; Hozzein, W.N. Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor oprL and toxA virulence genes and blaTEM, blaCTX-M, and tetA antibiotic-resistance genes. Sci. Rep. 2020, 10, 15961. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.T.; Kim, J.H.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Han, S.J.; Kwon, J.; et al. Genetic characterization and pathological analysis of a novel bacterial pathogen, Pseudomonas tructae, in Rainbow Trout (Oncorhynchus mykiss). Microorganisms 2019, 7, 432. [Google Scholar] [CrossRef] [Green Version]
- Korkea-aho, T.L.; Papadopoulou, A.; Heikkinen, J.; von Wright, A.; Adams, A.; Austin, B.; Thompson, K.D. Pseudomonas M162 confers protection against rainbow trout fry syndrome by stimulating immunity. J. Appl. Microbiol. 2012, 113, 24–35. [Google Scholar] [CrossRef]
- Abd El-Rhman, A.M.; Khattab, Y.A.E.; Shalaby, A.M.E. Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2009, 27, 175–180. [Google Scholar] [CrossRef]



| Fish ID | Sample ID * | Total Length (cm) | Standard Length (cm) ** | Wet Weight (g) | Sex (M/F) | Gut Weight (g) |
|---|---|---|---|---|---|---|
| F1 | WI1 | 37.5 | 34 | 431.2 | F | 9.37 |
| F2 | WI2 | 40 | 36 | 515.3 | M | 12.6 |
| F3 | WI3 | 38.5 | 34.2 | 443.56 | M | 12.4 |
| F4 | WI4 | 38.5 | 34.2 | 503.21 | F | 13.38 |
| F5 | IW1, IC1 | 40 | 36 | 512.53 | M | 12.65 |
| F6 | IW2, IC2 | 37.5 | 33.7 | 410.73 | F | 9.28 |
| F7 | IW3, IC3 | 36.5 | 33 | 453.16 | F | 13.11 |
| F8 | IW4, IC4 | 37 | 34 | 429.01 | F | 11.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Lee, S.; Han, D.-W.; Kim, J.-H. Characterization of the Gut Microbiota of Mackerel Icefish, Champsocephalus gunnari. Fishes 2023, 8, 13. https://doi.org/10.3390/fishes8010013
Song H, Lee S, Han D-W, Kim J-H. Characterization of the Gut Microbiota of Mackerel Icefish, Champsocephalus gunnari. Fishes. 2023; 8(1):13. https://doi.org/10.3390/fishes8010013
Chicago/Turabian StyleSong, Hokyung, Seungyeon Lee, Dong-Won Han, and Jin-Hyoung Kim. 2023. "Characterization of the Gut Microbiota of Mackerel Icefish, Champsocephalus gunnari" Fishes 8, no. 1: 13. https://doi.org/10.3390/fishes8010013
APA StyleSong, H., Lee, S., Han, D.-W., & Kim, J.-H. (2023). Characterization of the Gut Microbiota of Mackerel Icefish, Champsocephalus gunnari. Fishes, 8(1), 13. https://doi.org/10.3390/fishes8010013











