Insights of Otoliths Morphology to Reveal Patterns of Teleostean Fishes in the Southern Atlantic
Abstract
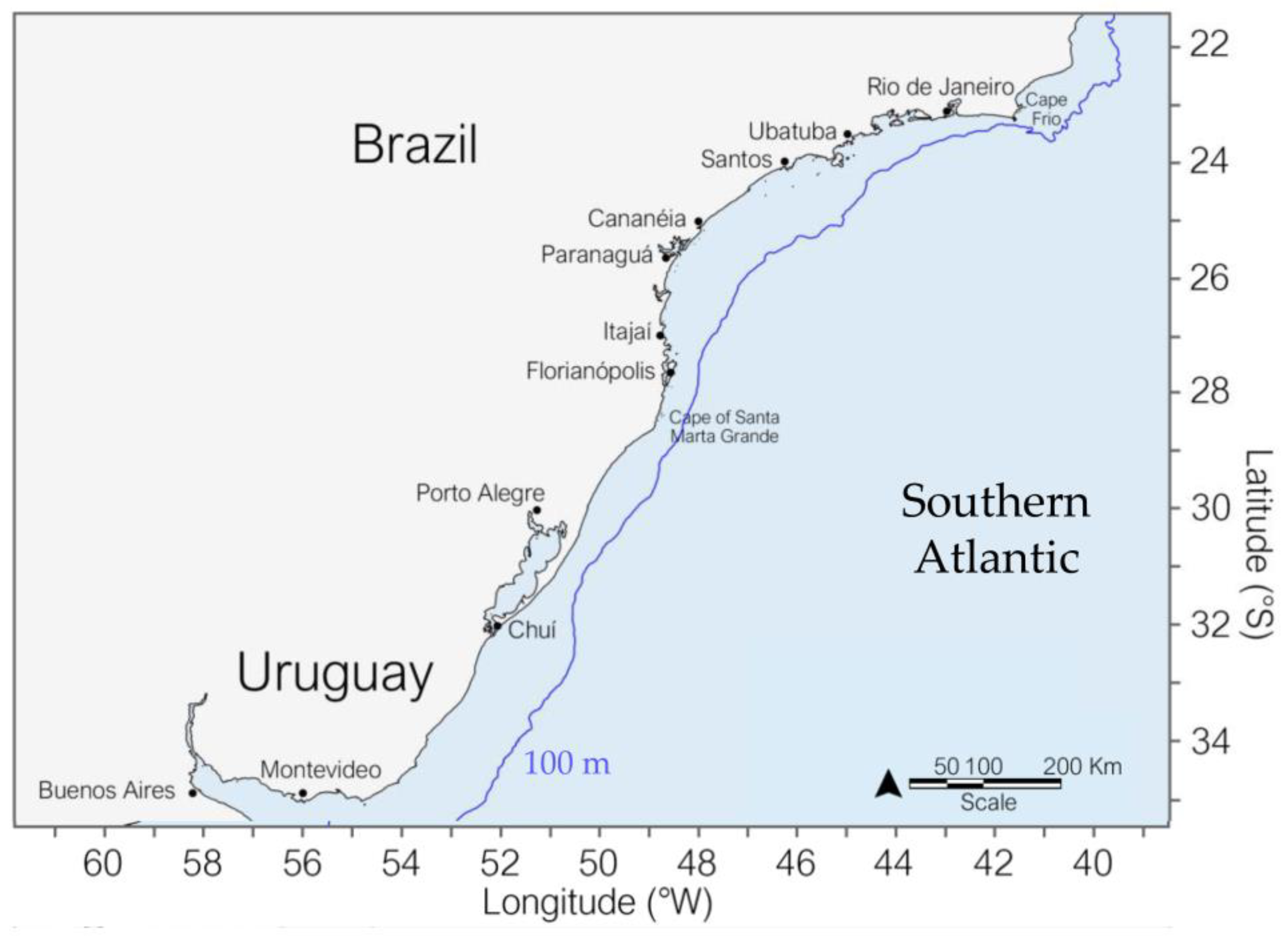
:1. Introduction
2. Materials and Methods
3. Results
- Colliculum absent or present (heteromorphic, holomorphic, homomorphic, monomorphic, unimorphic).
- Sulcus acusticus morphology (archaesulcoid, heterosulcoid, homosulcoid, pseudo-archaesulcoid).
- Sulcus acusticus position (inframedian, median, supramedian).
- Otolith profile (biconvex, concave-convex, flattened, plane-convex).
- Sulcus acusticus orientation (ascending, slightly ascending, descending, horizontal).
- Sulcus acusticus opening (caudal, mesial, ostial, ostio-caudal, para-ostial, pseudo-ostial).
- Rostrum absent or present (developed, underdeveloped/undeveloped).
- Ostium absent or present (bent, bent-concave, discoidal, elliptic, funnel like, lateral, oval, rectangular, round-oval, tubular).
- Otolith shape (34 categories).
- Sulcus acusticus cauda absent or present (elliptic, funnel like, oval-circular, round-oval, slightly curved, and tubular and eight more variations).
- Antirostrum absent or present (developed, underdeveloped, undeveloped).
- Anterior and posterior regions (irregular, angled, blunt, double-peaked, flattened, lanceolate, notched, oblique, peaked, round, and their combinations).
- Rostrum antirostrum orientation: when applicable, in agreement and in disagreement (not in agreement).
- Dorsal edge (entire, lobed, sinuate, irregular, and their combination).
- Pseudorostrum and pseudo-antirostrum absent or present (developed and underdeveloped/undeveloped).
- Ventral edge (crenate, dentate, entire, irregular, lobed, round, serrate, sinuate, and their combinations).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Orders. | Inner-Neritic | Rocky-Neritic | Neritic | Oceanic | Deep Ocean | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | D | P | D | P | B | D | P | P | B | D | P | |
| Albuliformes | 1 | |||||||||||
| Anguilliformes | 2 | 2 | ||||||||||
| Clupeiformes | 10 | 2 | ||||||||||
| Argentiniformes | 1 | |||||||||||
| Stomiatiformes | 1 | |||||||||||
| Aulopiformes | 1 | 4 | ||||||||||
| Myctophiformes | 9 | |||||||||||
| Zeiformes | 2 | |||||||||||
| Gadiformes | 11 | |||||||||||
| Polimixiiformes | 1 | |||||||||||
| Beryciformes | 1 | |||||||||||
| Trachichthyiformes | 1 | |||||||||||
| Ophidiiformes | 2 | 1 | ||||||||||
| Batrachoidiformes | 1 | |||||||||||
| Scombriformes | 1 | 7 | 1 | 3 | 1 | |||||||
| Syngnathiformes | 3 | 2 | ||||||||||
| Gobiiformes | 3 | |||||||||||
| Ordem Incertae Sedis Carangaria | 3 | 3 | ||||||||||
| Carangiformes | 5 | 13 | ||||||||||
| Atheriniformes | 1 | |||||||||||
| Beloniformes | 5 | |||||||||||
| Mugiliformes | 2 | |||||||||||
| Bleniiformes | 1 | |||||||||||
| Ordem Incertae Sedis Eupercaria | 17 | 1 | ||||||||||
| Gerreiformes | 2 | 3 | ||||||||||
| Uranoscopiformes | 1 | |||||||||||
| Labriformes | 1 | |||||||||||
| Ephippiformes | 1 | |||||||||||
| Chaetodontiformes | 1 | |||||||||||
| Lutjaniformes | 3 | 5 | 1 | |||||||||
| Spariformes | 1 | 2 | ||||||||||
| Priacanthiformes | 1 | 1 | ||||||||||
| Caproiformes | 1 | |||||||||||
| Lophiiformes | 1 | 2 | ||||||||||
| Tetraodontiformes | 1 | 4 | 1 | |||||||||
| Pempheriformes | 1 | 3 | ||||||||||
| Centrachiformes | 2 | |||||||||||
| Perciformes | 14 | 1 | ||||||||||
| Total | 5 | 11 | 29 | 5 | 6 | 4 | 44 | 27 | 1 | 5 | 28 | 14 |
Appendix B
- Inner-neritic species presented the highest correspondence between given and predicted categories, sustained mainly by Tetraodontiformes, Beloniformes, Anguiliformes, and Priacanthiformes, all of them matching 100%. For other orders, the mismatches were not a problem, once they occurred in the closed category neritic (e.g., Clupeiformes, Carangiformes, Mugiliformes). An interesting disagreement comprised Lutjaniformes and Gerreiformes, reclassified as rocky-neritic, a usual pattern for some species of these orders. This evidence shows the role of genetic determinants, mainly for Lutjaniformes.
- Rocky-neritic species were the less numerous. The Ephippiformes and most Lutjaniformes (Lutjanus spp., Anisotremus surinamensis) were properly assigned by discriminant analysis. Probably due to the reduced number of species, Bleniiformes, Chaetodontiformes, and Centrachiformes were erroneously reclassified as neritic.
- Neritic species correctly reclassified comprised mainly Perciformes (Serranidae), Carangiformes (Carangidae), and the order incertae sedis Eupercaria (Sciaenidae), the most typical group distributed along the continental shelf. Reclassifications as inner-neritic species were not a problem. The wrong reclassifications as rocky-neritic species comprised a miscellanea with no clear pattern, demanding further investigations. Some Perciformes (Triglidae) and Spariformes (Sparidae) were attributed to deep-ocean category by jackknifing, a pertinent classification considering some species of these groups not recorded here.
- Deep-ocean species presented high variability in terms of reclassification. The small size orders (Stomiatiformes, Myctophiformes) matched, sustaining the emergent pattern of relative proportions herein described. Other groups also reinforced the deep-ocean pattern (Argentiniformes, Zeiformes, Polymixiiformes, Beryciformes). Misclassifications were detected in relation to Gadiformes, Lophiiformes, and Pempheriformes (varied patterns). The remarkable mistake comprised the order Aulopiformes, all of them reclassified as rocky-neritic species.
- Benthic species comprised mainly Gobiiformes, Anguilliformes, and Lophiiformes, properly assigned after jackknifing. There was no trend in relation to the benthic mismatches.
- Demersal species presented the highest absolute number of matches, including representative orders such as Gadiformes (most part), Perciformes, Mugiliformes, and Eupercaria. Some species recategorized as pelagic ones comprised a number of Gadiformes (Macrouridae) and other groups (e.g., Aulopiformes, Gerreiformes) whose species are “more swimmer” in the water column, reflecting in the otoliths relative proportions.
- Pelagic species (Carangiformes, Clupeiformes, Scombriformes and others) were correctly classified in the given group. Uncorrected reclassifications as benthic species did not evidence a pattern, except for some Tetraodontiformes and Beloniformes, because they are “maneuver” fishes. The pelagic species attributed as demersal ones by discriminant analysis also did not evidence any trend.
| Habitat | Predicted Groups (Jackknifed) | Total | |||
| inner-neritic | rocky-neritic | neritic | deep-ocean | ||
| inner-neritic | 25 (55.6%) | 8 (17.8%) | 8 (17.8%) | 4 (8.9%) | 45 |
| rocky-neritic | 1 (9.1%) | 4 (36.4%) | 4 (36.4%) | 2 (18.2%) | 11 |
| neritic | 13 (17.3%) | 20 (26.7%) | 36 (48.0%) | 6 (8.0%) | 75 |
| deep-ocean | 8 (17.0%) | 13 (27.7%) | 9 (19.1%) | 17 (36.2%) | 47 |
| Total | 47 | 45 | 57 | 29 | 178 |
| Habit | Predicted Groups (Jackknifed) | Total | |||
| benthic | demersal | pelagic | |||
| benthic | 9 (64.3%) | 3 (21.4%) | 2 (14.3%) | --- | 14 |
| demersal | 11 (12.5%) | 55 (62.5%) | 22 (25.0%) | --- | 88 |
| pelagic | 23 (30.3%) | 17 (22.4%) | 36 (47.3%) | --- | 76 |
| Total | 44 | 75 | 59 | --- | 178 |
References
- Popper, A.N.; Lu, Z. Structure-function relationships in fish otolith organs. Fish. Res. 2000, 46, 15–25. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Vaz-dos-Santos, A.M. Generalidades de los otolitos. In Métodos de Estudios con Otolitos: Principios y Aplicaciones; Volpedo, A.V., Vaz-dos-Santos, A.M., Eds.; CAFP-PIESCI: Buenos Aires, Argentina, 2015; pp. 21–28. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72 (Suppl. 1), 7–198. [Google Scholar] [CrossRef]
- Lombarte, A.; Tuset, V.M. Morfometría de otolitos. In Métodos de Estudios con Otolitos: Principios y Aplicaciones; Volpedo, A.V., Vaz-dos-Santos, A.M., Eds.; CAFP-PIESCI: Buenos Aires, Argentina, 2015; pp. 269–303. [Google Scholar]
- Martinez-Pérez, J.A.; León, M.R.K.M.; Tafolla, B.F.; Alemán, M.B.; Torres, A.G.; Carrara, X.C. Catálogo de Otólitos Sagitta de Peces del GOLFO de México; UNAM/Mérida: Yucatán, Mexico, 2018; 199p. [Google Scholar]
- Campana, S.E. Photographic Atlas of Fish Otoliths of the Northwest Atlantic Ocean; NRC Research Press: Ottawa, Canada, 2004; 284p. [Google Scholar]
- Baremore, I.E.; Bethea, D.M. A Guide to Otoliths from Fishes of the Gulf of Mexico; NOAA Technical Memorandum NMFS-SEFSC-599: Panama City, Panama, 2010; 102p. [Google Scholar]
- Oré-Villalba, D.O. Catálogo fotográfico de otolitos de peces marinos y dulceacuícolas del Perú. Bol. Inst. Mar Perú 2017, 32, 136–213. [Google Scholar]
- Smale, M.J.; Watson, G.; Hecht, T. Otolith Atlas of Southern African Marine Fishes; J.L.B. Smith Institute of Ichthyology: Grahamstown, South Africa, 1995; 253p. [Google Scholar]
- Morrow, J.E. Preliminary Keys to Otoliths of Some Adult Fishes of the Gulf of Alaska, Bering Sea, and Beaufort Sea; NOAA Technical Report NMFS Circular 420; National Oceanic and Atmospheric Administration: Rockville, MD, USA, 1979; 32p. [Google Scholar]
- Volpedo, A.V.; Echeverría, D.D. Catálogo y Claves de Otolitos para la Identificación de Peces del Mar Argentino. 1. Peces de Importancia Comercial; Editorial Dunken: Buenos Aires, Argentina, 2000; 88p. [Google Scholar]
- Svetocheva, O.; Stasenkova, N.; Fooks, G. Guide to the bony fishes’ otoliths of the White Sea; IMR/PINRO Joint Report Series; IMR: Bergen, Norway; PINRO: Murmansk, Russia, 2007; Volume 3, 46p. [Google Scholar]
- Rossi-Wongtschowski, C.L.D.B.; Siliprandi, C.C.; Brenha, M.R.; Gonsales, S.A.; Santificetur, C.; Vaz-dos-Santos, A.M. Atlas of marine bony fish otoliths (sagittae) of Southeastern-Southern Brazil part I: Gadiformes (Macrouridae, Moridae, Bregmacerotidae, Phycidae and Merlucciidae); part II: Perciformes (Carangidae, Sciaenidae, Scombridae and Serranidae). Braz. J. Oceanogr. 2014, 62 (special issue), 1–103. [Google Scholar] [CrossRef] [Green Version]
- Gauldie, R.W.; Crampton, J.S. An eco-morphological explanation of individual variability in the shape of the Fish otolith: Comparison of the otolith of Hoplostethus atlanticus with other species by depth. J. Fish Biol. 2002, 60, 1204–1221. [Google Scholar] [CrossRef]
- Vignon, M.; Morat, F. Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Mar. Ecol. Prog. Ser. 2010, 411, 231–241. [Google Scholar] [CrossRef]
- Abaad, M.; Tuset, V.M.; Montero, D.; Lombarte, A.; Otero-Ferrer, J.L.; Haroun, R. Phenotypic plasticity in wild marine fishes associated with fish-cage aquaculture. Hydrobiologia 2016, 765, 343–358. [Google Scholar] [CrossRef]
- Bose, A.P.; Zimmermann, H.; Winkler, G.; Kaufmann, A.; Strohmeier, T.; Koblmüller, S.; Sefc, K.M. Congruent geographic variation in saccular otolith shape across multiple species of African cichlids. Sci. Rep. 2020, 10, 12820. [Google Scholar] [CrossRef]
- Cruz, A.; Lombarte, A. Otolith size and its relationship with colour patterns and sound production. J. Fish Biol. 2004, 65, 1512–1525. [Google Scholar] [CrossRef]
- Bani, A.; Poursaeid, S.; Tuset, V.M. Comparative morphology of the sagittal otolith in three species of south Caspian gobies. J. Fish Biol. 2013, 82, 1321–1332. [Google Scholar] [CrossRef]
- Jaramillo, A.M.; Tombari, A.D.; Benedito Dura, V.; Rodrigo Santamalia, M.; Volpedo, A.V. Otolith eco-morphological patterns of benthic fishes from the coast of Valencia (Spain). Thalassas 2014, 30, 57–66. [Google Scholar]
- Assis, I.O.; Da Silva, V.E.; Souto-Vieira, D.; Lozano, A.P.; Volpedo, A.V.; Fabré, N.N. Ecomorphological patterns in otoliths of tropical fishes: Assessing trophic groups and depth strata preference by shape. Environ. Biol. Fishes 2020, 103, 349–361. [Google Scholar] [CrossRef]
- Lombarte, A.; Cruz, A. Otolith size trends in marine fish communities from different depth strata. J. Fish Biol. 2007, 71, 53–76. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Tombari, A.D.; Echeverría, D.D. Eco-morphological patterns of the sagitta of Antarctic fish. Polar Biol. 2008, 31, 635–640. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Paola, D.D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef]
- Lombarte, A.; Lleonart, J. Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fishes 1993, 37, 297–306. [Google Scholar] [CrossRef]
- Paxton, J.R. Fish otoliths: Do sizes correlate with taxonomic group, habitat and/or luminescence? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1299–1303. [Google Scholar] [CrossRef]
- Vignon, M. Ontogenetic trajectories of otolith shape during shift in habitat use: Interaction between otolith growth and environment. J. Exp. Mar. Biol. Ecol. 2012, 420–421, 26–32. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Echeverria, D.D. Ecomorphological patterns of the sagitta in fish on the continental shelf off Argentine. Fish. Res. 2003, 60, 551–560. [Google Scholar] [CrossRef]
- Reid, K. A Guide to the Use of Otoliths in the Study of Predators at South Georgia; British Antarctic Survey: Cambridge, UK, 1996; 40p. [Google Scholar]
- Lombarte, A.; Olaso, I.; Bozzano, A. Ecomorphological trends in the Artedidraconidae (Pisces: Perciformes: Notothenioidei) of the Weddell Sea. Antarct. Sci. 2003, 15, 211–218. [Google Scholar] [CrossRef]
- Lombarte, A.; Gordoa, A.; Whitfield, A.K.; James, N.C.; Tuset, V.M. Ecomorphological analysis as a complementary tool to detect changes in fish communities following major perturbations in two South African estuarine systems. Environ. Biol. Fishes 2012, 94, 601–614. [Google Scholar] [CrossRef]
- Tuset, V.M.; Imondi, R.; Aguado, G.; Otero-Ferrer, J.L.; Santschi, L.; Lombarte, A.; Love, M. Otolith patterns of rockfishes from the Northeastern Pacific. J. Morphol. 2015, 276, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.O.; Martínez, V.H. Morphological variations of the three otoliths of some species of the family Loricariidae (Ostariophysi: Siluriformes). Neotrop. Ichthyol. 2017, 15, e160058. [Google Scholar] [CrossRef] [Green Version]
- Moura, A.; Dias, E.; López, R.; Antunes, C. Regional population structure of the European eel at the Southern limit of its distribution revealed by otolith shape signature. Fishes 2022, 7, 135. [Google Scholar] [CrossRef]
- Rossi-Wongtschowski, C.L.D.B. Morfología de otolitos. In Métodos de Estudios con Otolitos: Principios y Aplicaciones; Volpedo, A.V., Vaz-dos-Santos, A.M., Eds.; CAFP-PIESCI: Buenos Aires, Argentina, 2015; pp. 238–266. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; González, J.A.; Pertusa, J.F.; Lorente, M.J. Comparative morphology of the sagitta otolith in Serranus spp. J. Fish Biol. 2003, 63, 1491–1504. [Google Scholar] [CrossRef]
- Ponton, D. Is geometric morphometrics efficient for comparing otolith shape of different fish species? J. Morphol. 2006, 267, 750–757. [Google Scholar] [CrossRef]
- Perin, S.; Vaz-dos-Santos, A.M. Morphometry and relative growth of the Brazilian sardine, Sardinella brasiliensis (Steindachner, 1879) in the Southeastern Brazilian Bight. Arq. Zool. 2014, 45, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Fan, Y.; Ye, Z.; Li, Z.; Yu, H. Identification of five Pampus species from the coast of China based on sagittal otolith morphology analysis. Acta Oceanol. Sin. 2017, 36, 51–56. [Google Scholar] [CrossRef]
- Tuset, V.M.; Otero-Ferrer, J.L.; Siliprandi, C.C.; Manjabacas, A.; Marti-Puig, P.; Lombarte, A. Paradox of otolith shape indices: Routine but overestimated use. Can. J. Fish. Aquat. Sci. 2021, 78, 681–692. [Google Scholar] [CrossRef]
- MMA—Ministério do Meio Ambiente. Programa REVIZEE: Avaliação do Potencial Sustentável de Recursos Vivos da Zona Econômica Exclusiva do Brasil—Relatório Executivo; MMA: Brasília, Brasil, 2006; 280p. [Google Scholar]
- Vaz-dos-Santos, A.M.; Rossi-Wongtschowski, C.L.D.B. The forgotten requirement: Age and growth in fisheries resources from the Southwestern Atlantic. In Growth in Fisheries Resources from the Southwestern Atlantic; Vaz-dos-Santos, A.M., Rossi-Wongtschowski, C.L.D.B., Eds.; Instituto Oceanográfico—USP: São Paulo, Brasil, 2019; pp. 20–27. [Google Scholar]
- Menezes, N.A. Checklist of marine fishes from São Paulo State, Brazil. Biota Neotrop. 2011, 11, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Siliprandi, C.C.; Brenha-Nunes, M.R.; Rossi-Wongtschowski, C.L.D.B.; Santificetur, C.; Conversani, V.R.M. Atlas of marine bony fish otoliths (sagittae) of Southeastern-Southern Brazil part III: Clupeiformes (Clupeidae, Engraulidae, Pristigasteridae). Braz. J. Oceanogr. 2016, 64(special issue 1), 1–22. [Google Scholar] [CrossRef] [Green Version]
- Brenha-Nunes, M.R.; Santifecetur, C.; Conversani, V.R.M.; Giaretta, M.B.; Rossi-Wongtschowski, C.L.D.B.; Siliprandi, C.C. Atlas of marine bony fish otoliths (sagittae) of Southeastern-Southern Brazil Part IV: Perciformes (Centropomidae, Acropomatidae, Serranidae, Priacanthidae, Malacanthidae, Pomatomidae, Carangidae, Lutjanidae, Gerreidae and Haemulidae). Braz. J. Oceanogr. 2016, 64(special issue 1), 23–75. [Google Scholar] [CrossRef]
- Santifecetur, C.; Conversani, V.R.M.; Brenha-Nunes, M.R.; Giaretta, M.B.; Siliprandi, C.C.; Rossi-Wongtschowski, C.L.D.B. Atlas of marine bony fish otoliths (sagittae) of Southeastern-Southern Brazil Part V: Perciformes (Sparidae, Sciaenidae, Polynemidae, Mullidae, Kyphosidae, Chaetodontidae, Mugilidae, Scaridae, Percophidae, Pinguipedidae, Blenniidae, Gobiidae, Ephippidae, Sphyraenidae, Gempylidae, Trichiuridae, Scombridae, Ariommatidae, Stromateidae and Caproidae). Braz. J. Oceanogr. 2017, 65, 201–257. [Google Scholar] [CrossRef]
- Santifecetur, C.; Giaretta, M.B.; Conversani, V.R.M.; Brenha-Nunes, M.R.; Siliprandi, C.C.; Rossi-Wongtschowski, C.L.D.B. Atlas of marine bony fish otoliths of Southeastern-Southern Brazil Part VIII: Siluriformes (Ariidae) and Pleuronectiformes (Achiridae, Paralichthyidae, Cynoglossidae). Braz. J. Oceanogr. 2017, 65, 448–494. [Google Scholar] [CrossRef] [Green Version]
- Giaretta, M.B.; Siliprandi, C.C.; Santificetur, C.; Brenha-Nunes, M.R.; Conversani, V.R.M.; Rossi-Wongstschowski, C.L.D.B. Atlas of marine bony fish otoliths (sagittae) of Southeastern-Southern Brazil Part VI: Albuliformes, Anguiliformes, Osmeriformes, Stomiiformes, Aulopiformes, Myctophiformes, Ophidiiformes, Polimixiiformes, Batrachoidiformes and Lophiformes. Braz. J. Oceanogr. 2017, 65, 258–308. [Google Scholar] [CrossRef] [Green Version]
- Conversani, V.R.M.; Brenha-Nunes, M.R.; Santificetur, C.; Giaretta, M.B.; Siliprandi, C.C.; Rossi-Wongtschowski, C.L.D.B. Atlas of marine bony fish otoliths (sagittae) of Southeastern—Southern Brazil Part VII: Atheriniformes, Beloniformes, Beryciformes, Zeiformes, Syngnathiformes, Scorpaeniformes and Tetraodontiformes. Braz. J. Oceanogr. 2017, 65, 400–447. [Google Scholar] [CrossRef] [Green Version]
- Lemos, P.H.B.; Corrêa, M.F.M.; Abilhôa, V. Catálogo de otólitos de Gerreidae (Osteichthyes-Perciformes) do litoral do Estado do Paraná, Brasil. Nerítica 1992, 7, 109–117. [Google Scholar]
- Lemos, P.H.B.; Corrêa, M.F.M.; Pinheiro, P.C. Catálogo de otólitos de Clupeidae (Clupeiformes-Osteichthyes) do Litoral do Estado do Paraná, Brasil. Arq. Biol. Tec. 1995, 38, 747–759. [Google Scholar]
- Lemos, P.H.B.; Corrêa, M.F.M.; Pinheiro, P.C. Catálogo de otólitos de Engraulidae (Clupeiformes-Osteichthyes) do litoral do Estado do Paraná, Brasil. Arq. Biol. Tec. 1995, 38, 731–745. [Google Scholar]
- Corrêa, M.F.M.; Vianna, M.S. Catálogo de otólitos de Sciaenidae (Osteichthyes-Perciformes) do litoral do Estado do Paraná, Brasil. Nerítica 1992, 7, 13–41. [Google Scholar]
- Assis, C.A. Guia para a Identificação de Algumas Famílias de Peixes Ósseos de Portugal Continental, Através da Morfologia dos Seus Otólitos Sagitta; Câmara Municipal de Cascais: Cascais, Portugal, 2004; 190p. [Google Scholar]
- Betancur, R.R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Ortí, G. Phylogenetic classification of bony fishes. BMC Evol. Biol. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricke, R.; Eschmeyer, W.; Van Der Laan, R. Eschmeyer’s catalog of fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 13 April 2022).
- Figueiredo, J.L.; Menezes, N.A. Manual de Peixes Marinhos do Sudeste do Brasil. II. Teleostei (1); Museu de Zoologia da Universidade de São Paulo: São Paulo, Brasil, 1978; 110p. [Google Scholar]
- Figueiredo, J.L.; Menezes, N.A. Manual de Peixes Marinhos do Sudeste do Brasil. III. Teleostei (2); Museu de Zoologia da Universidade de São Paulo: São Paulo, Brasil, 1980; 90p. [Google Scholar]
- Figueiredo, J.L.; Menezes, N.A. Manual de Peixes Marinhos do Sudeste do Brasil. VI. Teleostei (5); Museu de Zoologia da Universidade de São Paulo: São Paulo, Brasil, 2000; 116p. [Google Scholar]
- Menezes, N.A.; Figueiredo, J.L. Manual de Peixes Marinhos do Sudeste do Brasil. IV. Teleostei (3); Museu de Zoologia da Universidade de São Paulo: São Paulo, Brasil, 1980; 96p. [Google Scholar]
- Menezes, N.A.; Figueiredo, J.L. Manual de Peixes Marinhos do Sudeste do Brasil. V. Teleostei (4); Museu de Zoologia da Universidade de São Paulo: São Paulo, Brasil, 1985; 105p. [Google Scholar]
- Lalli, C.M.; Parsons, T.R. Biological Oceanography: An Introduction, 2nd ed; The Open University: Vancouver, BC, Canada, 2006; 314p. [Google Scholar]
- Semmar, N. Native Statistics for Natural Sciences; Nova Science Publishers: New York, NY, USA, 2013; 515p. [Google Scholar]
- Taguchi, Y.H.; Oono, Y. Relational patterns of gene expression via non-metric multidimensional scaling analysis. Bioinformatics 2005, 21, 730–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legendre, P.; Legendre, L.F.J. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 990p. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Naveda, I.G.G. Patrones morfológicos del otolito sagitta de algunos peces óseos del mar peruano. Bol. Inst. Mar Perú 2001, 20, 1–83. [Google Scholar]
- Hecht, T. A guide to the otoliths of Southern Ocean fishes. S. Afr. J. Antarct. Res. 1987, 17, 1–87. [Google Scholar]
- Tombari, A.D.; Volpedo, A.V.; Echeverría, D.D. Desarrollo de la sagitta em juveniles y adultos de Odontesthes argentinensis (Valenciennes, 1835) y O. bonariensis (Valenciennes, 1835) de la provincia de Buenos Aires, Argentina (Teleostei: Atheriniformes). Rev. Chil. Hist. Nat. 2005, 78, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, B.M.; Vaz-dos-Santos, A.M.; Spach, H.L.; Volpedo, A.V. Ontogenetic development of the sagittal otolith of the anchovy, Anchoa tricolor, in a subtropical estuary. Sci. Mar. 2015, 79, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Rondon, A.S.; Vaz-dos-Santos, A.M.; Rossi-Wongtschowski, C.L.D.B. Morfologia e biometria dos otólitos de Beryx splendens e Hoplostethus occidentalis (Beryciformes) no Atlântico Sudoeste. Bol. Inst. Pesca 2014, 40, 195–206. [Google Scholar]
- Dizaj, L.P.; Esmaeili, H.R.; Teimori, A. Comparative otolith morphology of clupeids from the Iranian brackish and marine resources (Teleostei: Clupeiformes). Acta Zool. 2022, 103, 29–47. [Google Scholar] [CrossRef]
- Williams, R.; McEldowney, A. A guide to the fish otoliths from waters off the Australian Antarctic Territory, Heard and Macquarie Islands; Antarctic Division, Department of the Arts, Sport, the Environment, Tourism and Territories: Canberra, Australia, 1990; 173p. [Google Scholar]
- Baldás, M.G.; Pérez Macri, G.; Volpedo, A.V.; Echeverría, D.D. Morfología de Ia sagitta de peces marinos de Ia costa bonaerense de Ia Argentina 1: Carangidae, Sciaenidae, Mullidae. Atlântica 1997, 19, 99–112. [Google Scholar]
- Härkonen, T. Guide to the Otoliths of the Bony Fishes of the Northeast Atlantic; Danbiu ApS—Biological Consultants: Hellerup, Denmark, 1986; 256p. [Google Scholar]
- Hunt, J.J. Morphological characteristics of otoliths for selected fish in the Northwest Atlantic. J. Northwest Atl. Fish. Sci. 1992, 13, 63–75. [Google Scholar] [CrossRef]
- Parmentier, E.; Vandewalle, P.; Lagardère, F. Morpho-anatomy of the otic region in carapid fishes: Eco-morphological study of their otoliths. J. Fish Biol. 2001, 58, 1046–1061. [Google Scholar] [CrossRef]
- Waessle, J.A.; Lasta, C.A.; Favero, M. Otolith morphology and body size relationships for juvenile Sciaenidae in the Río de la Plata estuary (35–36° S). Sci. Mar. 2003, 67, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Vaz-dos-Santos, A.M.; Santos-Cruz, N.N.; Rossi-Wongtschowski, C.L.D.B. Caracterização dos otólitos sagitta do peixe-lagarto Bembrops heterurus Miranda-Ribeiro, 1903 (Teleostei: Percophidae) da região Sudeste-Sul do Brasil. Bioikos 2007, 21, 69–78. [Google Scholar]
- Kalish, J.M.; Beamish, R.J.; Brothers, E.B.; Casselman, J.M.; Francis, R.I.C.C.; Mosegaard, H.; Panfili, J.; Prince, E.D.; Thresher, R.E.; Wilson, C.A.; et al. Glossary. In Recent Developments in Fish Otolith Research; Secor, D.H., Dean, J.M., Campana, S.E., Eds.; University of South Carolina Press: Columbia, SC, USA, 1995; pp. 723–729. [Google Scholar]
- Aguirre, H.; Lombarte, A. Ecomorphological comparisons of sagittae in Mullus barbatus and M. surmuletus. J. Fish Biol. 1999, 55, 105–114. [Google Scholar] [CrossRef]
- Lombarte, A.; Palmer, M.; Matallanas, J.; Gómez-Zurita, J.; Morales-Nin, B. Ecomorphological trends and phylogenetic inertia of otolith sagittae in Nototheniidae. Environ. Biol. Fishes 2010, 89, 607–618. [Google Scholar] [CrossRef]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith analyses highlight morpho-functional differences of three species of mullet (Mugilidae) from transitional water. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Fuchs, D.V. Ecomorphological patterns of the lapilli of Paranoplatense Siluriforms (South America). Fish. Res. 2010, 102, 160–165. [Google Scholar] [CrossRef]
- FishCAST: Collection of Fish Calcified Structures, Version 04/2022. Available online: www.fishcast.info (accessed on 17 October 2022).




| Taxa | N sp | Taxa | N sp |
|---|---|---|---|
| Albuliformes | 1 | Carangiformes | 18 |
| Albulidae | 1 | Carangidae | 18 |
| Anguilliformes | 4 | Atheriniformes | 1 |
| Congridae | 2 | Atherinopsidae | 1 |
| Muraenidae | 2 | Beloniformes | 5 |
| Clupeiformes | 12 | Belonidae | 2 |
| Chirocentridae | 1 | Hemiramphidae | 3 |
| Clupeidae | 3 | Mugiliformes | 2 |
| Engraulidae | 7 | Mugilidae | 2 |
| Pristigasteridae | 1 | Bleniiformes | 1 |
| Argentiniformes | 1 | Blenniidae | 1 |
| Argentinidae | 1 | Order Incertae Sedis Eupercaria | 18 |
| Stomiatiformes | 1 | Malacanthidae | 2 |
| Sternoptychidae | 1 | Sciaenidae | 16 |
| Aulopiformes | 5 | Gerreiformes | 5 |
| Cholorophthalmidae | 5 | Gerreidae | 5 |
| Myctophiformes | 9 | Uranoscopiformes | 1 |
| Myctophidae | 8 | Pinguipedidae | 1 |
| Neoscopelidae | 1 | Labriformes | 1 |
| Zeiformes | 2 | Labridae | 1 |
| Grammicolepididae | 1 | Ephippiformes | 1 |
| Zeniontidae | 1 | Ephippidae | 1 |
| Gadiformes | 11 | Chaetodontiformes | 1 |
| Bregmacerotidae | 2 | Chaetodontidae | 1 |
| Macrouridae | 4 | Lutjaniformes | 9 |
| Merlucciidae | 1 | Haemulidae | 6 |
| Moridae | 2 | Lutjanidae | 3 |
| Phycidae | 2 | Spariformes | 3 |
| Polimixiiformes | 1 | Sparidae | 3 |
| Polimixiidae | 1 | Priacanthiformes | 2 |
| Beryciformes | 1 | Priacanthidae | 2 |
| Berycidae | 1 | Caproiformes | 1 |
| Trachichthyiformes | 1 | Caproidae | 1 |
| Trachichthyidae | 1 | Lophiiformes | 3 |
| Ophidiiformes | 3 | Lophiidae | 1 |
| Ophidiidae | 3 | Ogcocephalidae | 2 |
| Batrachoidiformes | 1 | Tetraodontiformes | 6 |
| Batrachoididae | 1 | Diodontidae | 2 |
| Scombriformes | 13 | Monacanthidae | 1 |
| Ariommatidae | 1 | Tetraodontidae | 3 |
| Gempylidae | 1 | Pempheriformes | 4 |
| Pomatomidae | 1 | Acropomatidae | 2 |
| Scombridae | 5 | Percophidae | 2 |
| Stromateidae | 1 | Centrachiformes | 2 |
| Trichiuridae | 4 | Kyphosidae | 2 |
| Syngnathiformes | 5 | Perciformes | 15 |
| Centriscidae | 2 | Peristediidae | 1 |
| Dactylopteridae | 1 | Scorpaenidae | 2 |
| Mullidae | 2 | Sebastidae | 1 |
| Gobiiformes | 3 | Serranidae | 7 |
| Gobiidae | 3 | Setarchidae | 1 |
| Order Incertae Sedis Carangaria | 6 | Triglidae | 3 |
| Centropomidae | 2 | Number of Orders = 40 | |
| Polynemidae | 1 | Number of Families = 69 | |
| Sphyraenidae | 3 | Number of species = 179 | |
| Source | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Habitat | 0.1573 | 3 | 0.0524 | 4.0586 | 0.0006 |
| Habit | 0.1009 | 2 | 0.0505 | 3.9072 | 0.0033 |
| Interaction | 0.3104 | 6 | 0.0517 | 4.0047 | 0.0001 |
| Residual | 2.1441 | 166 | 0.0129 | ||
| Total | 2.7126 | 177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.; Vaz-dos-Santos, A.M. Insights of Otoliths Morphology to Reveal Patterns of Teleostean Fishes in the Southern Atlantic. Fishes 2023, 8, 21. https://doi.org/10.3390/fishes8010021
Santos L, Vaz-dos-Santos AM. Insights of Otoliths Morphology to Reveal Patterns of Teleostean Fishes in the Southern Atlantic. Fishes. 2023; 8(1):21. https://doi.org/10.3390/fishes8010021
Chicago/Turabian StyleSantos, Lucinha, and André Martins Vaz-dos-Santos. 2023. "Insights of Otoliths Morphology to Reveal Patterns of Teleostean Fishes in the Southern Atlantic" Fishes 8, no. 1: 21. https://doi.org/10.3390/fishes8010021
APA StyleSantos, L., & Vaz-dos-Santos, A. M. (2023). Insights of Otoliths Morphology to Reveal Patterns of Teleostean Fishes in the Southern Atlantic. Fishes, 8(1), 21. https://doi.org/10.3390/fishes8010021








