Abstract
This study aimed to explore the gill developmental mechanism of the high mortality rate of Paramisgurnus dabryanus larvae in the early life-history process. Based on histological observations with a optical microscope, we studied the ontogeny of the gill of P. dabryanus. The result indicated that external gills first appeared 12 h after hatching. The number of external branchial filaments greatly increased at 2 d after hatching (DAH). The internal branchial filament primordial was formed in the oropharyngeal cavity at 5 DAH. At 9 DAH, none of external branchial filaments were observed outside, but the fish operculum was completed covered. From 15 DAH forward, the filaments and lamellae of gills increased in number and length prominently, and the gills of the larvae were similar to those of juvenile fish. The specific activities of Na+/K+-ATPase steadily increased from 0 to 12 DAH and peaked at 15 DAH. After 15 DAH, the specific activities of Na+/K+-ATPase slightly decreased and then tended to remain steadily stable until the end of the experiment. There were abundant gill lamellae on the gill filaments at 40 DAH. The sensitive period of the development of larval and juvenile P. dabryanus was from 6 to 15 DAH. Our results provide information that may be useful for improving our understanding of the life history and ecology of loaches.
1. Introduction
Larva development is regarded as one of the most important study topics of the early life history of fish. There is a positive correlation between the morphological development and growth of fish larvae and their survival [1,2,3]. Not only is our understanding of the morphological development process of fish the basis of fish natural resource protection, but it also provides useful information regarding fish biology and ecology [4,5]. During early ontogeny in fish, there are rapid changes in morphology, behavior, and physiology [6,7]. Morphological development is intensive and influences fish survival during this sensitive period [8]. It was reported that there are five sensitive stages in the early life history of fish (incubation, opening, gill filament formation, bladder inflation, and deformation) [5]. The fish morphology transition from larvae to juveniles, which is called metamorphosis, is usually violent. The yolk sac and metamorphosis stage has been well-recognized as sensitive, dangerous, and critical in early history. Larval fish metamorphosis is a sensitive period, which can result in larval mortality [9]. The metamorphosis stage is easily observed in species with obvious external morphological changes, such as the eel and flounder [5]. However, the metamorphosis of some cultured freshwater fishes in the early development stage has been neglected in the literature, thereby hindering aquaculture development.
Yang [5] stated that the appearance of metamorphosis includes the absence and location change of some organs; the disappearance of fin folds, external gills, body transparency, and other larval organs and features; and the formation of fins and scales. The external gill is an important breathing organ of fish in the early stage, as it allows them to obtain oxygen from water and to adjust to osmotic pressure changes [10]. During early fish development, the shape, size, area, quantity, and microstructure changes of the external and internal gills are significant factors, indicating respiratory function development stages [5,11]. However, the morphological structure of the gills of fish under various environmental conditions, at different developmental stages, shows obvious differentiation. Studying the structural development of the gills during the early ontogeny of fish can increase our understanding of fish respiratory mechanism development. Na+/K+-ATPase is one of the most important enzymes in fish respiratory metabolism, and it was found to regulate the process of Na+, K+ ion transport across the basolateral membrane through active transport [12,13]. It is located on both sides of the basement membrane of certain cells, e.g., chlorine-secreting cells, mitochondria-rich cells, and ion cells. In these cells, the basement membrane is rich in Na+/K+-ATPase [14,15]. Previous studies have shown that the change in dissolved oxygen in the environment can affect Na+/K+-ATPase activity in many fish species [16].
The air-breathing loach Paramisgurnus dabryanus (Cypriniformes; Cobitidae) is an omnivorous freshwater fish found in East Asia. It mainly occurs in the middle and lower reaches of the Yangtze River and the Pearl River Basin in China [17]. P. dabryanus frequently occurs in hypoxic environments, such as swamps, paddy fields, and ponds. It prefers to swallow air bubbles and relies on the intestinal tract as an accessory organ to carry out respiration [18,19,20]. P. dabryanus has multiple respiratory patterns and breathes via external gills in the early stage of development. When the external gill degenerates, the internal gill assumes the main respiratory function. Amphibian and some fish larvae, such as the South American lungfish (Lepidosiren paradoxa), have feathery “outer gills”, which protrude from the gill area to the outside and lack supporting bone structure [21]. The artificial cultivation of P. dabryanus is increasing, and the demand for P. dabryanus larvae is also increasing. However, metamorphosis is the peak stage of larval death, and this dangerous stage is in the early stage of gill development of larve in the breeding stage [18]. The high mortality rate at the seedling stage limits large-scale artificial production. In the present study, morphological, histological, and enzymological methods were used to study the changes in gill structure and function in the early development of P. dabryanus. The change in respiratory function often indicates a sensitive period in the early development of fish. Therefore, understanding the formation and development process of the respiratory function of P. dabryanus can help support large-scale seedling cultivation and management strategies.
2. Materials and Methods
2.1. Larval Rearing and Experiment Conditions
Fertilized eggs of the loach (P. dabryanus) were obtained from the Dianjiang County Fishery Station of Chongqing City (32°20′N; 107°21′E) in May 2018. The eggs were incubated at 21.3 ± 1.6 °C, pH of 7.3 ± 0.2, salinity of (0.01±0.002)‰, and an oxygen concentration of 7.22 ± 0.61 mg/L in a 600 L tank. The newly hatched larvae were transferred into three separate replicate 400 L cylindroconical tanks at a rearing density of 2000 larvae/m3. We changed sixty percent of water in the tanks once a day. The ammonia levels were checked with a Hach Colorimeter DBR200 (Danaher Co., Ltd., New York, USA), and they were maintained below 0.02 mg/L. The photoperiod was 12:12 h (L: D) and the light intensity was 2000–4000 lux. Larvae were fed three times a day (at 07:00, 12:00, and 17:00) at 4 d after hatching (DAH). All of the food residues were removed before feeding. The larvae were first fed Artemia nauplii (Tianjin Danyang Aquatic Technology Co., Ltd., Tianjin, P. R. China). After 10 DAH, the larvae were fed mixed bait (Artemia nauplii and Chironomidae larvae). After 20 DAH, they were all fed a microparticulate diet (Shandong Shengsuo Fishery Feed Research Center, Shandong, P.R. China). The nutritional contents of the different diets are shown in Table 1.

Table 1.
Main nutritional components of diets.
2.2. Sampling
Samples were collected from the first brooding fish (just out of the membrane), once before feeding at 8:00 a.m. before 10 DAH (including 10 DAH) and once every 2–5 d after 10 DAH, and the total length of 10 tails was measured. The samples were collected until 40 d after the membrane release of the larvae. Each time, 10 tissue samples were taken, fixed for 24 h at 4 °C in Bouin’s fixative, and then preserved in 70% ethanol. Samples of the Na+/K+-ATPase (NKA) activity test (n = 10–200, depending on their size) were randomly collected from the three separate tanks at 0, 0.5, 1, 2, 3, 4, 5, 6, 7, 9, 10, 12, 15, 20, 25, 30, 35, and 40 DAH. From 0 to 20 DAH, whole fish were sampled. After 20 DAH, the gills of larvae were sampled directly. All the collected samples were immediately frozen in liquid nitrogen and stored in a cryogenic freezer at −80 °C until the enzyme assays.
2.3. Histological Analysis
The samples were gradually dehydrated in an ethanol gradient, embedded with routine paraffin, and sectioned in transverse sections (5 μm thick) using a Leica RM 2135 rotary microtome (Leica Ltd., Wetzlar, Germany). The sections were stained with hematoxylin and eosin or using Alcian blue–periodic acid–Schiff (AB-PAS) staining. The mucous cells of P. dabryanus were divided into four types by Alcian blue–periodic acid–Schiff (AB-PAS) staining [22]: type I is red, AB is negative, and PAS is positive, containing neutral mucosaccharide; type II is blue, AB is positive, PAS is negative, containing acid mucopolysaccharide; type III is purplish red, AB and PAS reactions are positive, mainly containing neutral mucopolysaccharide; type IV is blue-purple, AB and PAS reactions are positive, mainly containing acid mucopolysaccharide. Then, the sections were visualized with a Eclipse 80i-NIKON Instruments (Tokyo, Japan) micro-imaging system and photographed.
2.4. Enzyme Assays
The samples were removed from the freezer and placed on ice to thaw. After thawing was complete, the samples were homogenized with an F6/10 Fluko homogenizer at 12,000× g for 2 min on ice in 0.2 M NaCl, and the supernatant was incubated on ice until analysis. Na+/K+ ATPase (NKA) (EC 3.6.1.3) was measured in fish by using an NKA assay kit (NO: A070, Nanjing Jiancheng Bioengineering Institute, PR China). The soluble protein (g/L) contents were determined with the Bradford method using bovine serum albumin as the standard (0.563 g/L) (Bradford 1976). All assays were performed on duplicate samples using a Thermo Multiskan spectrophotometer (Thermo Fisher Scientific, MA, USA). UV-permeable Corning 96-well microplates (Corning Incorporated, Corning, USA) were used for all assays. All reactions were run at the saturating substrate concentrations determined for each enzyme.
2.5. Data Analysis
External gill filament length was measured using Iamge-Pro Plus image analysis software. Three slides were prepared for each intestinal section of P. dabryanus, and ten fields were randomly selected for observation for a total of thirty measurements per fish. The normality of the data and homogeneity of variance were assessed with the Kolmogorov–Smirnov test and Leven’s test, respectively. The NKA activities were analysed with one-way ANOVA to evaluate the differences between the different age groups. All statistical values are expressed as the mean ± SD, and the Student–Newman–Keuls test was used to detect significant differences (p < 0.05). The recorded data analysis was performed with STATISTICA 6.0 (StatSoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Larval Growth and External Gill Morphology
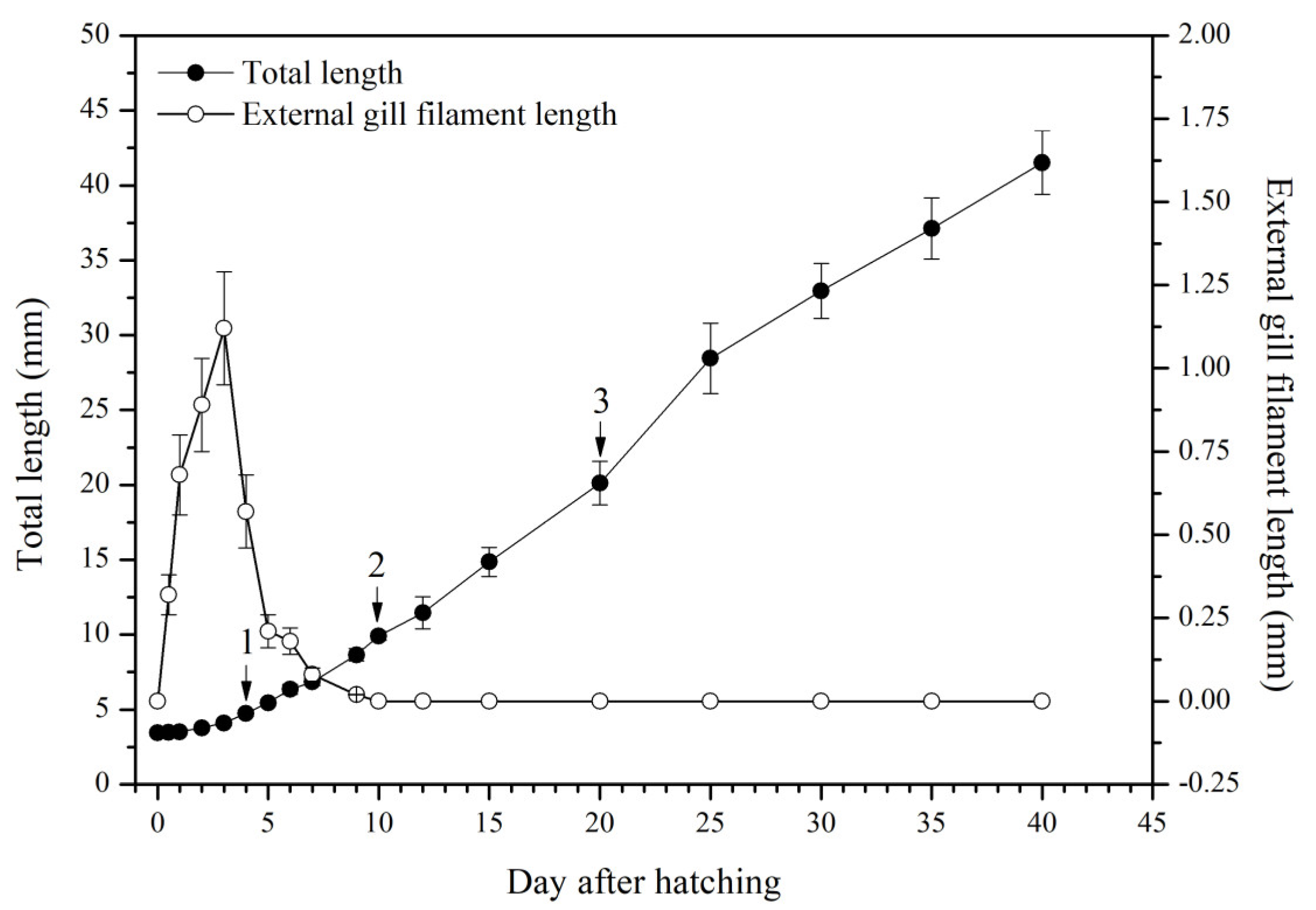
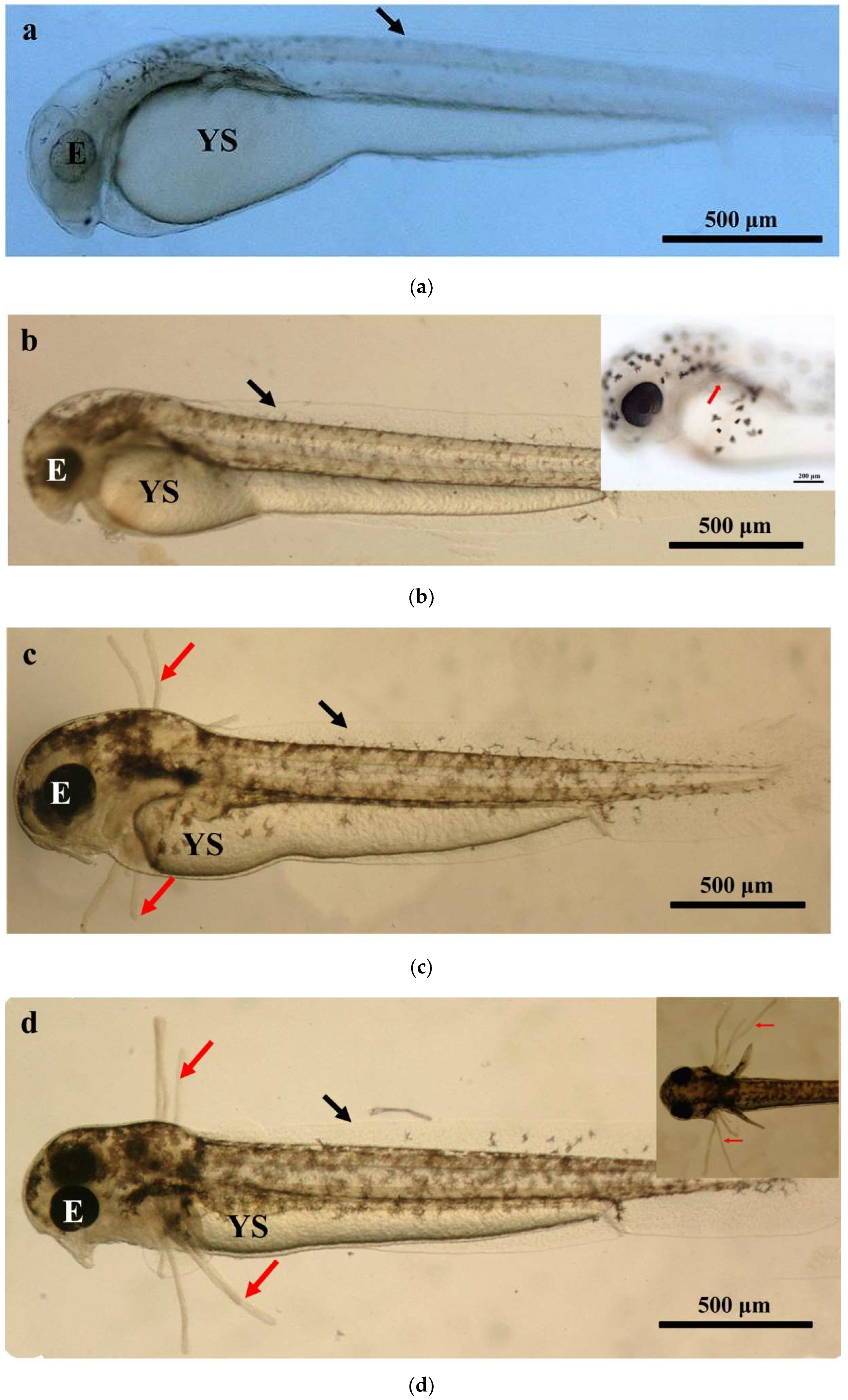
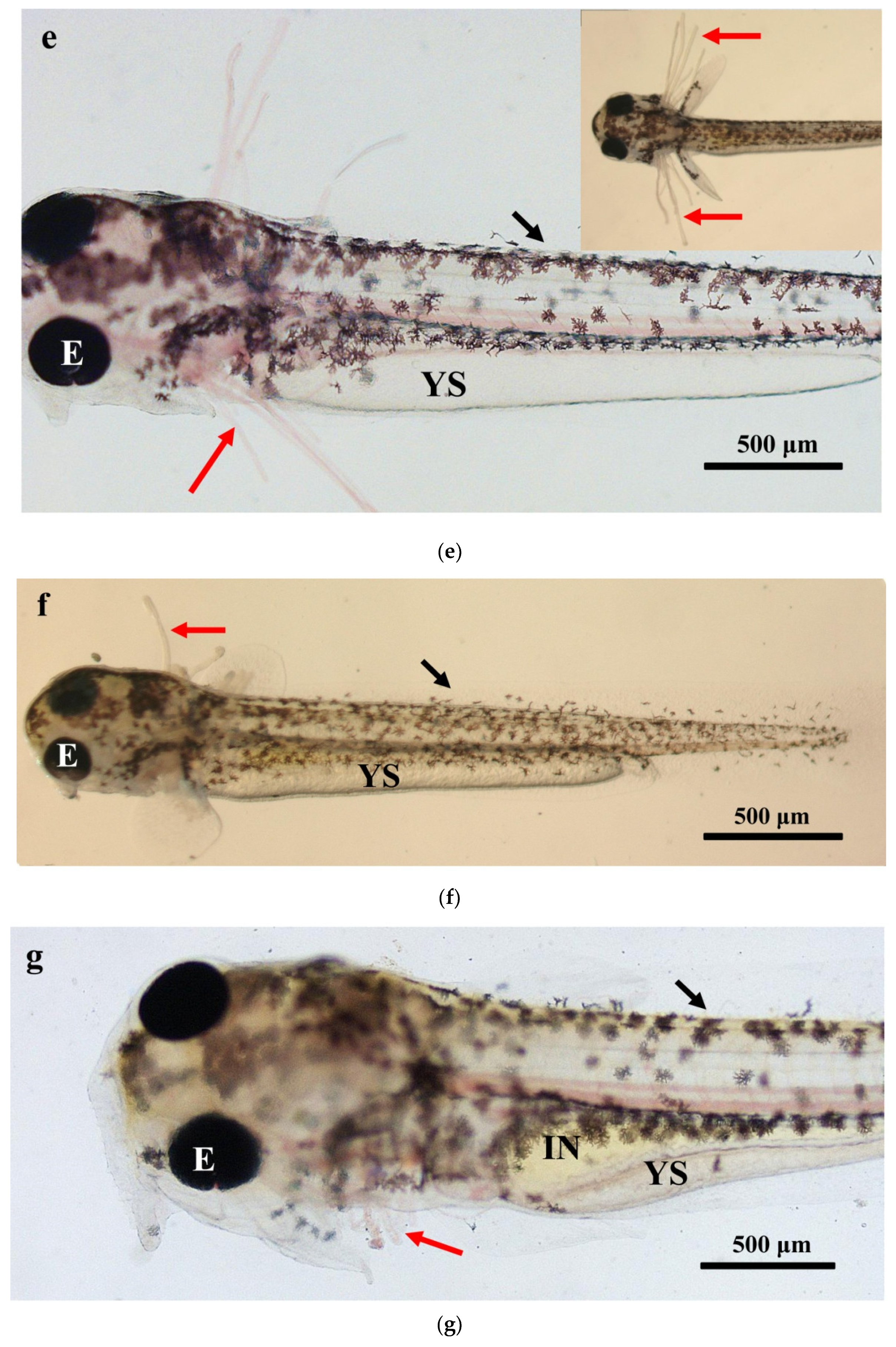
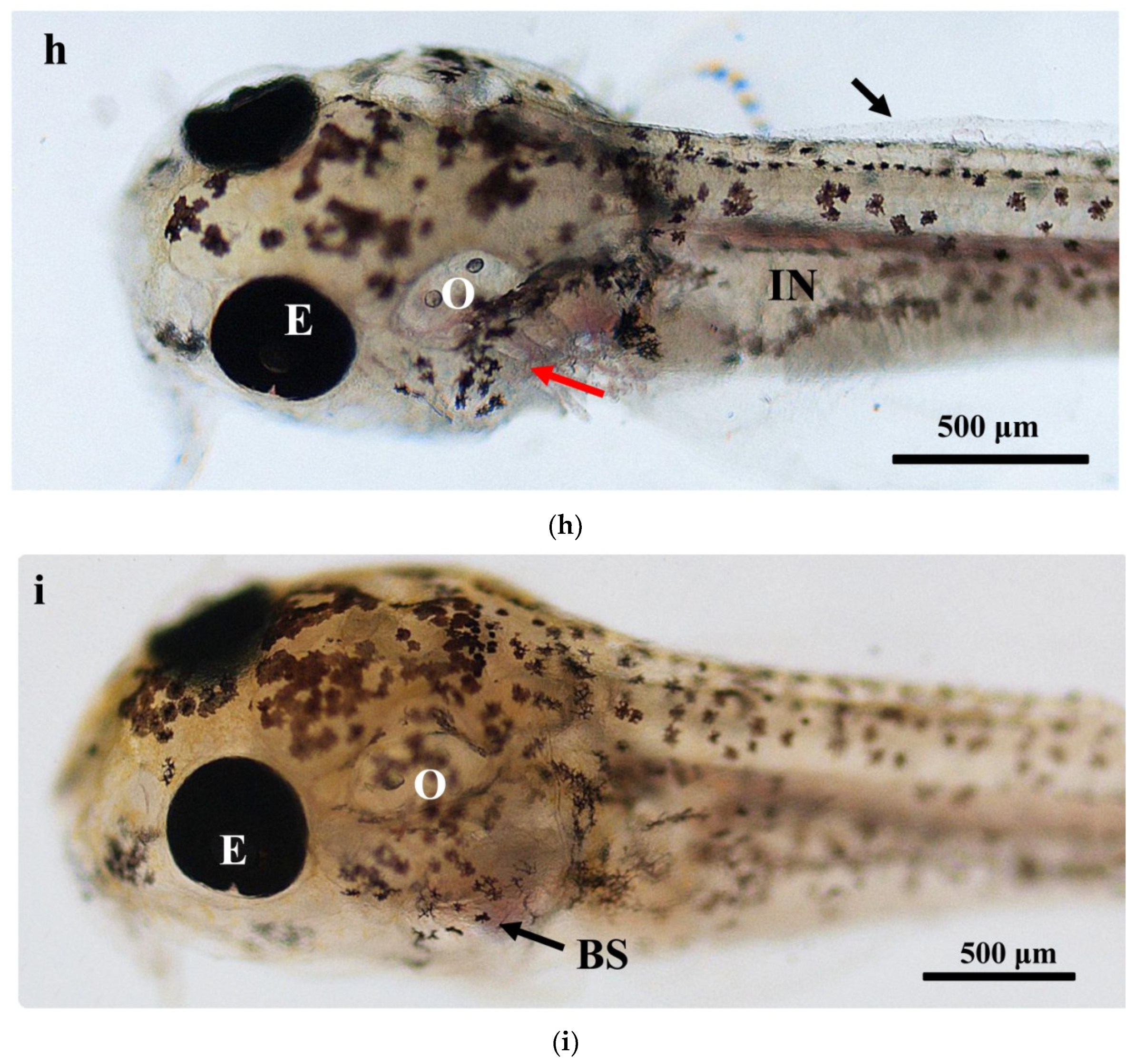
The larval loach growth, in terms of total length and weight, is shown in Figure 1. Newly hatched larvae were 3.45 mm in total length. After rearing for 40 days, the total length of the larvae increased from 3.45 mm to 41.51 mm. During development, the total increase in length was linear, described by the equation y = 0.69x + 2.61 (R2 = 0.885). After the introduction of Chironomidae larvae to their diet, the loaches showed quickly growth from 10 DAH. The length of external gill filament increased significantly after new hatching, peaked at 4 DAH, and then sharply decreased. After 10 DAH, the external gill tended to be disappeared (Figure 1). Observation of external gill development in P. dabryanus during different developmental stages is shown in Figure 2. New hatching larvae were observed to have no external gills (Figure 2a). An external gill primordium first appeared 12 h (0.5 DAH) after hatching (Figure 2b). The external branchial filaments were folded in a tubular fashion, and a small amount of blood flow was observed. After 1 DAH (Figure 2c), four external branchial filaments and obvious blood flow in the dorsal aorta were observed in the fish. The length and number of external branchial filaments increased at 2 DAH, and blood circulation of the external gill gradually accelerated with the increase in heartbeat frequency (Figure 2d). At 3 DAH, 10 external branchial filaments with different lengths were found in the larvae (Figure 2e). External branchial filaments shortened, whereas the pectoral fin of fish developed quickly at 4 DAH (Figure 2f). At 5 DAH, the yolk sac was almost absorbed, while the gill cover grew gradually, and external branchial filaments continued to shorten and degenerate (Figure 2g). The opercula extended backward and gradually hardened, the external branchial filaments shortened but were still partially exposed, and the blood flow intensity on the outer abalone filaments began to weaken at 6 DAH (Figure 2h). At 9 DAH, none of external branchial filaments were observed outside, but the opercula of the fish were completed covered (Figure 2i).
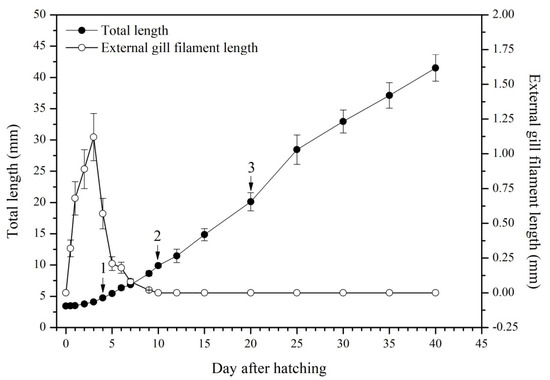
Figure 1.
Total length and external gill filament length of P. dabryanus larvae and juveniles during the experiment. ‘1’: first fed Artemia nauplii; ‘2’: fed mixed bait (Artemia nauplii and Chironomidae larvae); ‘3’: fed a microparticulate diet.
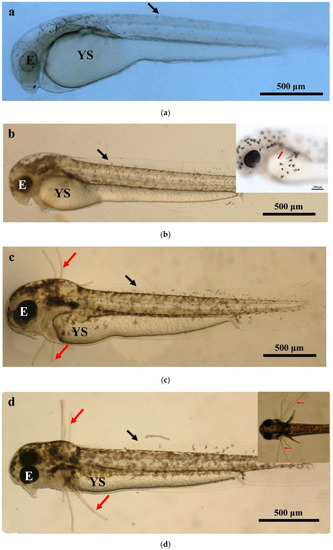
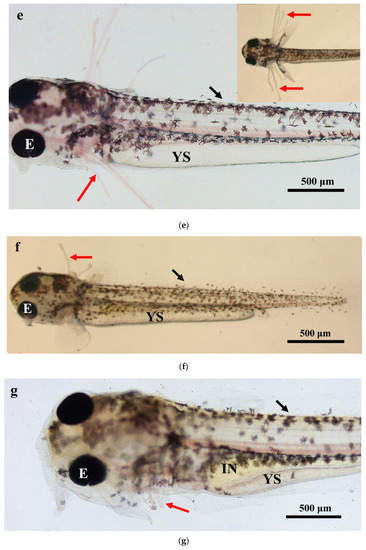
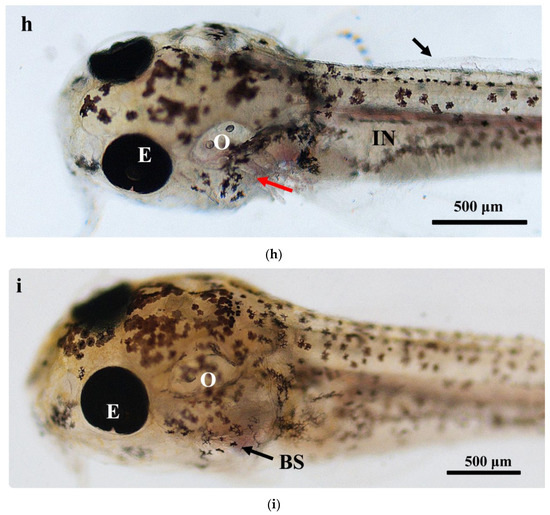
Figure 2.
Morphological observation of ontogenetic development of external gill in P. dabryanus: (a) newly hatched larva; (b) 0.5 DAH larva; (c) 1 DAH larva; (d) 2 DAH larva; (e) 3 DAH larva; (f) 4 DAH larva; (g) 5 DAH larva; (h) 6 DAH larva; (i) 9 DAH juvenile. Red arrows: external gill; E: eye; IN: intestine; GA: gill arch; GR: gill raker; O: otolith; SF: secondary filament; YS: yolk sac.
3.2. Histological Observation
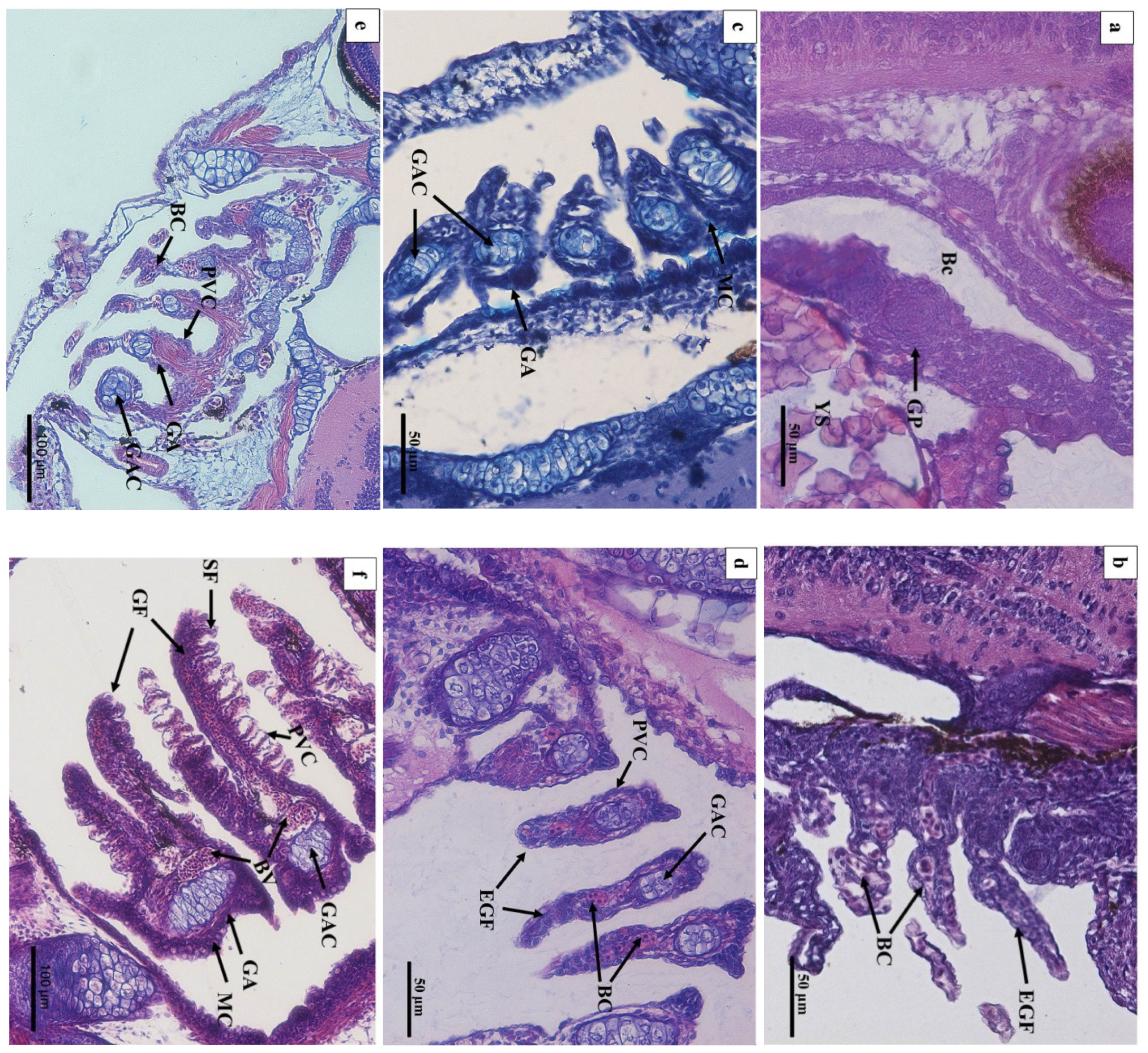
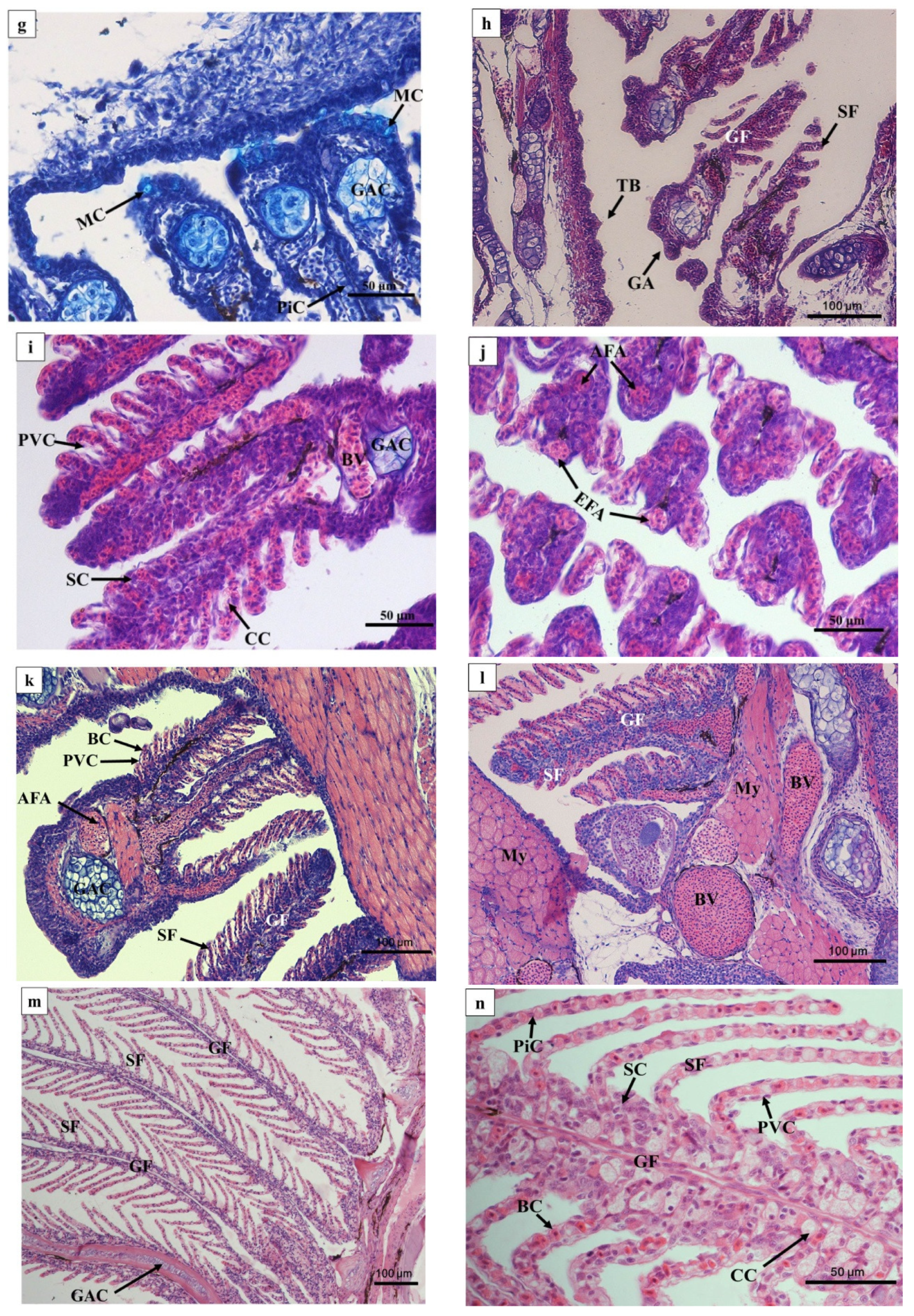
In 1 DAH larvae, oropharyngeal primordia appeared in front of the digestive tube, and a row of gill primordia was seen at the rear of the cavity (Figure 3a). The oropharyngeal cavity of 2 DAH larvae was formed, and the oropharyngeal cavity was differentiated by the activity of abdominal side branchial primordia forming external branchial filaments, which were rich in blood cells (Figure 3b). At 3 DAH, five pairs of primitive branchial arches were observed, formed by some undifferentiated epithelial cells surrounding the center of chondroblast cells, and acidic mucus cells (type II) appeared (Figure 3c). The primordial branchial filaments of 5 DAH larvae were formed in the oropharyngeal cavity, the primitive vascular system appeared on the branchial arch, and sporadic blood cells were observed (Figure 3d). At 7 DAH, muscle fibers emerged on the branchial arch, blood cells gradually increased, and flat cells appeared at the base of the branchial filament; mucus cells could be seen inside (Figure 3e). At 12 DAH, the length of branchial filaments increased, and the number of branchial fillets increased. Capillary vessels were clearly observed in the center of branchial fillets, and the branchial arch cartilage thickened and lengthened (Figure 3f,g). The gill rake of 15 DAH juvenile loaches was oval, and seven to nine branchial filaments were arranged in a comb shape (Figure 3h). By 20 DAH, the gill structure had developed completely; the artery vessels were thick and extended into the branchial filaments (Figure 3i). After 25 DAH, the number of branchial patches had increased, and the branchial filaments were vertically arranged on both sides (Figure 3j). At 30 DAH, the end of the branchial lamella was enlarged. It was composed of upper and lower monolayer respiratory epithelium and supporting columnar cells, and the subsequent development of gill tissue was mainly quantitative (Figure 3k). There were abundant gill lamellae on the gill filaments of the loaches at 40 DAH (Figure 3l). No significant differences were found in tissue structure between the adult loach and loach at 40 DAH (Figure 3m,n).
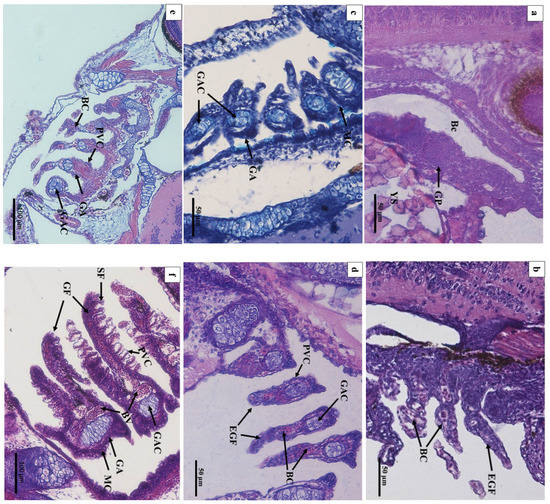
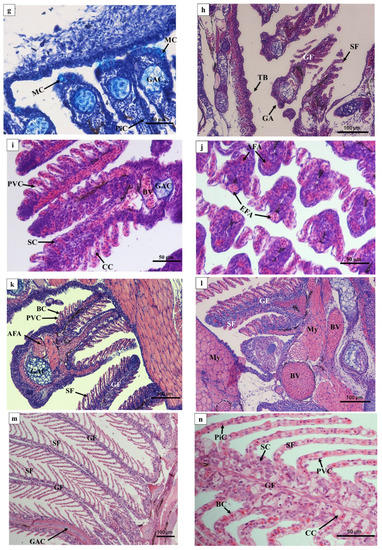
Figure 3.
Histology of the external and internal gill development of P. dabryanus after hatching: (a) longitudinal section of 1 DAH larva (HE); (b) longitudinal section of 2 DAH larva (HE); (c) longitudinal section of 3 DAH larva (AB-PAS); (d) longitudinal section of 5 DAH larva (HE); (e) longitudinal section of 7 DAH juvenile (HE); (f) longitudinal section of 12 DAH juvenile (HE); (g) longitudinal section of 12 DAH juvenile (AB-PAS); (h) longitudinal section of 15 DAH juvenile (HE); (i) longitudinal section of 20 DAH juvenile (HE); (j) cross-section of 25 DAH juvenile (HE); (k) cross-section of 30 DAH juvenile (HE); (l) cross-section of 40 DAH juvenile (HE); (m) longitudinal section of adult gill (objective lens ×10) (HE); (n) longitudinal section of adult gill (objective lens ×40) (HE). Bc: buccopharynx cavity; GP: gill primordium; EGF: external gill filament; BC: blood cell; GAC: gill arch cartilage; PVC: pavement cells; MC: mucous cells; SF: secondary filament; GF: gill filament; BV: blood vessel; TB: taste bud; CC: chloride cell; SC: Sertoli cell; EFA: efferent filament artery; AFA: afferent filament artery.
3.3. NKA Activity
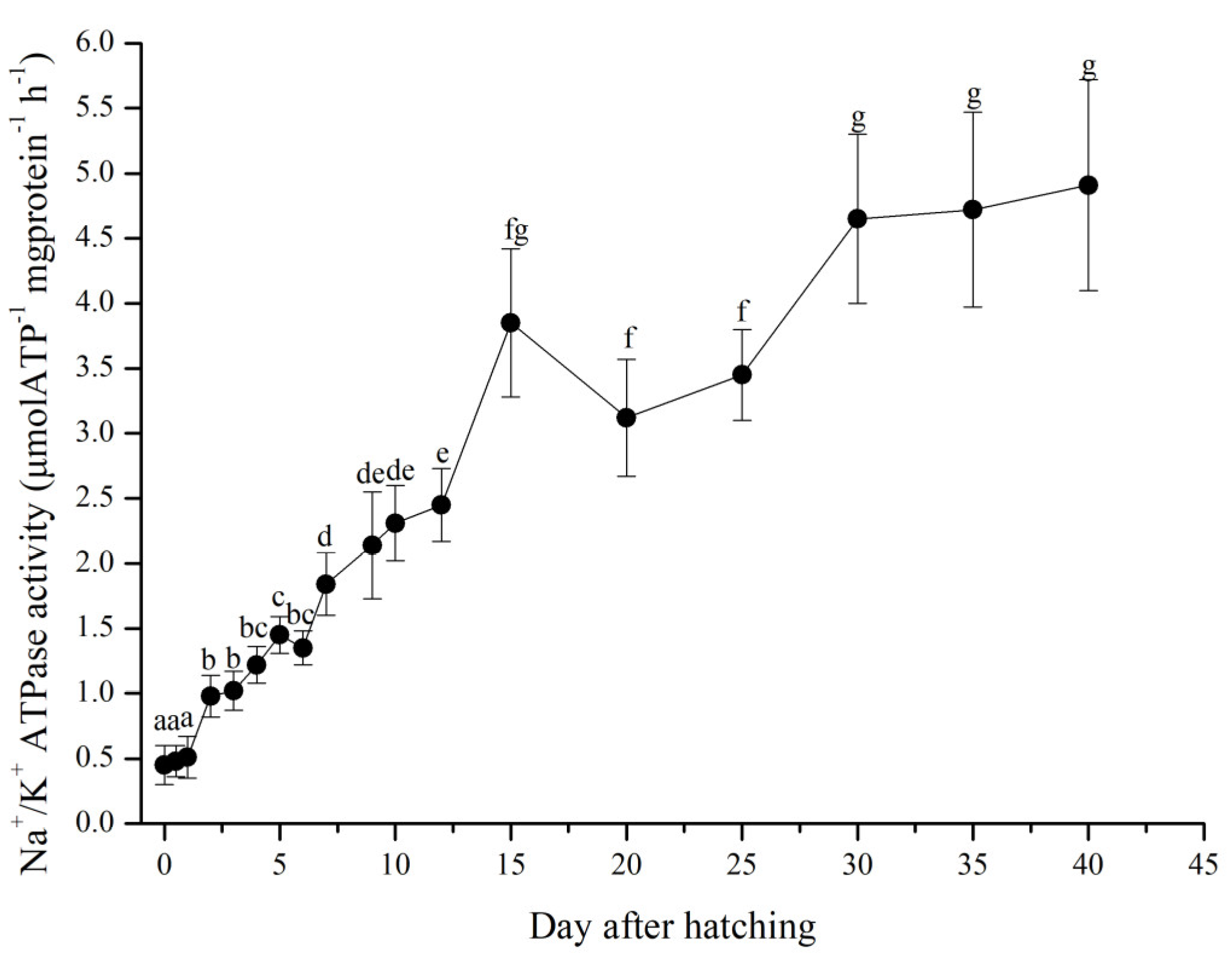
The fluctuations in the specific activities of NKA in P. dabryanus are shown in Figure 4. NKA activity was detected in the newly hatched larvae, remained at a low level until 2 DAH, and then displayed a significant increase (p < 0.05). Another obvious rise in NKA activity was observed from 6 to 7 DAH (p < 0.05). Generally, the specific activities of NKA steadily increased from 0 to 12 DAH and peaked at 15 DAH. After 15 DAH, the specific activities of NKA slightly decreased and then tended to remain steadily stable until the end of the experiment.
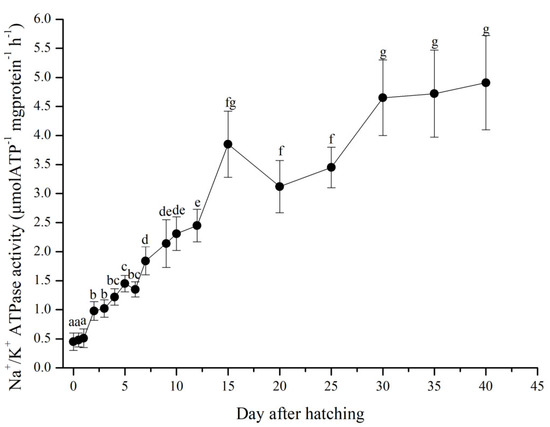
Figure 4.
Fluctuations in the specific activities of Na+/K+ ATPase in P. dabryanus larvae and juveniles during the experiment. Values in the table are means ± SD, n = 3; different superscript letters indicate significant differences on different days, p < 0.05. From 0 to 20 DAH, whole fish were sampled for Na+/K+ ATPase activity test. After 20 DAH, the gills of larvae were sampled directly for Na+/K+ ATPase activity test.
4. Discussion
Unlike most teleost fish, the early life history of P. dabryanus is characterized by a metamorphosis, which is adaptive to its environment. The main breathing patterns and structural characteristics of corresponding respiratory organs differ during the developmental stages of P. dabryanus. In our study, the formation of external gill primordia emerged 12 h after hatching, which was earlier than the development of external gills of loaches reported by Zhang [23]. Liang [24] stated that the development of external gills of P. dabryanus occurs later than 12 h due to the differences in environmental factors such as temperature. Many amphibians also have external gills during early development stages [25]. In our study, before 4 DAH, the function of internal gills in P. dabryanus had not formed, and individuals mainly depended on the external gill and skin for the absorption of dissolved oxygen. During this period, larvae mainly relied on the yolk to provide energy. The external gill filaments of the larvae became shorter after their mouths opened (Figure 1). NKA activity was observed to be at a low level, which verified the weak activity ability of the larvae (Figure 4). NKA is regarded as a key factor in the regulation of fish metabolism ability, especially in active transport [12]. With the introduction of Artemia to their diet, the larvae displayed a significantly increased dissolved oxygen demand to support them in digesting foreign food. Gao [26] found that Misgurnus anguillicaudatus adapts to its environment by changing its morphology and behavioral characteristics. In our study, the internal gill primordia appeared in the oropharyngeal cavity from 4 to 6 DAH (Figure 3e). During this period, the P. dabryanus larvae displayed increased feeding and swimming activity and required more oxygen. The ability to swim is critical to the survival of larval fish transitioning from endogenous to exogenous nutrition [6,9,27]. To meet the increasing demands of metabolic and oxygen consumption, the respiration function of larvae starts to transition from an external gill to an internal gill (Figure 2f–i; Figure 3d–f). This is consistent with the changes in other benthic fish [26,28]. NKA activity also showed an obvious rise during this stage (Figure 4). The functions of NKA activity maintained the ion gradient and membrane potential while adjusting cell volume as well as sugar and amino acid transport [13]. Richards [16] reported that change in the oxygen demand of tissue can influence NKA activity in many fish species. The lamellae are the primary sites for oxygen uptake in fish. In many species of adult fish, the lamellae are also largely responsible for active and passive fluxes of ions and water in and out of the animal [29]. In the current study, the formation of gill lamellae in loaches indicated that the gill had initiated its respiratory function (Figure 3f). The gill lobules of Oplegnathus fasciatus are differentiated from the gill filament at 6 DAH, while the gill lobules of Dentex dentex appear at 15 DAH [30,31]. The gill filaments in Lateolabrax maculatus larvae occur late in the early stage [32]. Consistent with the report written by Liu [19], the posterior intestine of P. dabryanus was observed to display special structural characteristics with the anterior and middle intestine. This indicates that strengthening of the intestinal respiratory function assists in compensating for the loss of gill respiration [33]. Christian [34] found that the air-breathing behavior of some fish is the necessary metabolic pathway to maintain normal life activities. It plays an important role in adapting to environmental changes and harsh environments such as those with low dissolved oxygen. Some fish can obtain oxygen directly from the air by specialized air-breathing organs, which help meet oxygen requirements and increase hypoxia tolerance. Air-breathing is not an essential life activity of Hypostomus plecostomus and Hoplosternum thoracatum when dissolved oxygen in water is adequate [35,36,37]. These species only breathe air when oxygen in the aquatic medium is insufficient, or when they are emersed. Zhang [38] found gut air-breathing significantly developed in mud loach (Misgurnus anguillicaudatus) larvae from 10 to 20 DAH. From 15 to 30 DAH, the gill differentiated into small pieces, the surface of which was covered with pits, gaps, furrows, and ridges. With the increased number of small pieces, the gill became the main respiratory organ of P. dabryanus. A similar relationship was found between the gill breathing surface area and rates of oxygen uptake in other teleost fish species [39,40]. After 15 DAH, the specific activities of NKA tended to remain steadily stable until the end of the experiment, and it was noted that the function of the gill of P. dabryanus approached perfection.
5. Conclusions
The stage (6~15 DAH) during which the breathing patterns of larva change is particularly vulnerable to mortality. This may be related to the sharp increase in blood flow in larvae during this stage, while NKA activity increases relatively slowly. This leads to the osmotic regulation ability of larva not being able to meet the demand of larval first growth. As such, it is necessary to strengthen commercial pond management and to maintain high levels of dissolved oxygen in the water during this period. Our results provide information that may be useful for improving our understanding of the life history and ecology of loaches. We observed and suggested improvements in larval mass production that could also be of value to other suborder larvae.
Author Contributions
Y.L.: Conceptualization, data curation resources, visualization and writing—original draft. Z.W.: supervision and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Science and Technology Program of Guangzhou Grant Number 202201010762; the National Key R&D Program of China Grant Number 2018YFD0900903; Open Fund project of Fishery Resources and Environmental Science Experimental Station of The Upper-Middle Reaches of Yangtze River Ministry of Agriculture, Grant number 0202020017.
Institutional Review Board Statement
The methods involving animals in this study were conducted in accordance with the Laboratory Animal Management Principles of China. All experimental protocols were approved by the Ethics Committee of the Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences (approval code LAEC-PRFRI-2019-01-04, approved on 1 January 2019).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to Jianrong Zhao and Shengtao Gao for experimental assistances in the present study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du, R.B.; Wang, Y.Q.; Jiang, H.B.; Liu, L.M.; Wang, M.J.; Li, T.B.; Zhang, S.B. Embryonic and larval development in barfin flounder Verasper moseri (Jordan and Gilbert). Chin. J. Oceanol. Limn. 2010, 28, 18–25. [Google Scholar] [CrossRef]
- Ogata, Y.; Morioka, S.; Sano, K.; Vongvichith, B.; Eda, H.; Kurokura, H.; Khonglaliane, T. Growth and morphological development of laboratory-reared larvae and juveniles of the Laotioan indigenous cyprinid Hypsibarbus malcolmi. Ichthyol. Res. 2010, 57, 389–397. [Google Scholar] [CrossRef]
- Roca, C.Y.; Rhody, N.R.; Nystrom, M.; Wittenrich, M.L.; Main, K.L. Embryonic and early larval development in hatchery-reared common snook. N. Am. J. Aquac. 2012, 74, 499–511. [Google Scholar] [CrossRef]
- Choo, C.K.; Liew, H.C. Morphological development and allometric growth patterns in the juvenile seahorse Hippocampus kuda Bleeker. J. Fish Biol. 2006, 69, 426–445. [Google Scholar] [CrossRef]
- Yang, R.; Xie, C.; Fan, Q. Progress in Critical Periods in Early Life History of Fishes. J. Huazhong Agric. Univ. 2008, 27, 161–165. [Google Scholar]
- Gisbert, E.; Merino, G.; Muguet, J.B.; Bush, D.; Piedrahita, R.H.; Conklin, D. Morphological development and allometric growth patterns in hatchery reared California halibut larvae. J. Fish Biol. 2002, 61, 1217–1229. [Google Scholar] [CrossRef]
- Gisbert, E.; Doroshov, S.I. Allometric growth in green sturgeon larvae. J. Appl. Ichthyol. 2006, 22, 202–207. [Google Scholar] [CrossRef]
- Gisbert, E. Early development and allometric growth patterns in Siberian sturgeon and their ecological significance. J. Fish Biol. 1999, 54, 852–862. [Google Scholar] [CrossRef]
- Morioka, S.; Ito, S.; Kitamura, S.; Vongvichith, B. Growth and morphological development of laboratory-reared larval and juvenile climbing perch Anabas testudineus. Ichthyol. Res. 2009, 56, 162–171. [Google Scholar] [CrossRef]
- Ramos, C.A.; da Costa, O.; Duncan, W.; Fernandes, M.N. Morphofunctional description of mucous cells in the gills of the Arapaimidae Arapaima gigas (Cuvier) during its development. Anat. Histol. Embryol. 2018, 47, 330–337. [Google Scholar] [CrossRef]
- Nilsson, G.E. Gill remodeling in fish--a new fashion or an ancient secret? J. Exp. Biol. 2007, 210, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.S.; Simi, S. Hypoxia stress modifies NA+/K+-ATPase, H+/K+-ATPase, NA+/NH4+-ATPase, and nkaα1isoform expression in the brain of immune-challenged airbreathing fish. J. Exp. Neurosci. 2017, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z. Effect of hypoxia and air-breathing restricted on respiratory physiology of air-breathing loach (Paramisgurnus dabryanus). Fish Physiol. Biochem. 2021, 47, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Kaneko, T.; Natio, N.; Takei, Y. Molecular biology of major components of chloride cells. Comp. Biochem. Physiol. B 2003, 136, 593–620. [Google Scholar] [CrossRef]
- Leone, F.A.; Masui, D.C.; de Souza Bezerra, T.M.; Garcon, D.P.; Valenti, W.C.; Augusto, A.S.; McNamara, J.C. Kinetic analysis of gill (Na(+),K(+))-ATPase activity in selected ontogenetic stages of the Amazon River shrimp, Macrobrachium amazonicum (Decapoda, Palaemonidae): Interactions at ATP- and cation-binding sites. J. Membr. Biol. 2012, 245, 201–215. [Google Scholar] [CrossRef]
- Richards, J.G.; Wang, Y.S.; Brauner, C.J.; Gonzalez, R.J.; Patrick, M.L.; Schulte, P.M.; Choppari-Gomes, A.R.; Almeida-Val, V.M.; Val, A.L. Metabolic and ionoregulatory responses of the Amazonian cichlid, Astronotus ocellatus, to severe hypoxia. J. Comp. Physiol. B 2007, 177, 361–374. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wu, Q.W.; Hu, W.H.; Wang, F.; Zhao, Z.B.; He, H.; Fan, Q.X. Changes in digestive enzyme activities during larval development of Chinese loach Paramisgurnus dabryanus (Dabry de Thiersant, 1872). Fish Physiol. Biochem. 2015, 41, 1577–1585. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Zhao, J.; Zhan, S.; Wang, Z. Distribution and Development of Mucous Cells in Digestive Tract of Larvae and Juvenile in Loach (Paramisgurnus dabryanus). Chin. J. Zool. 2016, 51, 623–632. [Google Scholar]
- Liu, Y.; Wang, Z.J. A Study on Structural Characteristics of Intestinal Tract of the air-breathing locah, Paramisgurnus dabryanus (Sauvage, 1878). Pak. J. Zool. 2017, 49, 1223–1230. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, X.H.; Chang, O.Q. Histological observation on the post-embryonic development of the kidney in loach, Paramisgurnus dabryanus. Freshw. Fish. 2018, 48, 30–34. [Google Scholar]
- Moraes, M.F.; Holler, S.; Da Costa, O.; Glass, M.L.; Fernandes , M.N.; Perry , S.F. Morphometric comparison of the respiratory organs in the south american lungfish Lepidosiren paradoxa (Dipnoi). Physiol. Biochem. Zool. 2005, 78, 546–559. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.H.; Wang, Z. Study on distribution characteristic of intestinal mucous cells and digestive enzyme activities in Paramisgurnus dabryanus. Acta Hydrobiol. Sin. 2017, 41, 1048–1053. [Google Scholar]
- Zhang, J. Ontogenies of Digestive System and External Gills in Mud Loach Misgurnus anguillicaudatus Larvae; Huazhong Agricultural University: Wuhan, China, 2014. [Google Scholar]
- Liang, Z.X.; Liang, J.Y.; Chen, C.; Li, Z.J.; Lin, J.H.; Zhang, J.J. The embryonic development and fingerling culture of loach Paramisgurnus dabryanus (Sauvage). Acta Hydrobiol. Sin. 1988, 12, 27–42. [Google Scholar]
- Xie, W.; Li, G.F. Comparative Analysis on the Early Development of Anuran amphibian’s Respiratory System. J. Yulin Norm. Univ. 2009, 30, 95–99. [Google Scholar]
- Gao, L.; Duan, M.; Cheng, F.; Xie, S. Ontogenetic development in the morphology and behavior of loach (Misgurnus anguillicaudatus) during early life stages. Chin. J. Oceanol. Limnol. 2014, 32, 973–981. [Google Scholar] [CrossRef]
- Osse, J.W.; Boogaart, G.M.; Snik, G.M.; Van der Sluys, L. Priorities during early growth of fish larvae. Aquaculture 1997, 155, 249–258. [Google Scholar] [CrossRef]
- Bjelland, R.M.; Skiftesvik, A.B. Larval development in European hake (Merluccius merluccius L.) reared in a semiintensive culture system. Aquac. Res. 2006, 37, 1117–1129. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Dymowska, A.; Stecyk, J.A. New insights into the plasticity of gill structure. Respir. Physiol. Neurobiol. 2012, 184, 214–222. [Google Scholar] [CrossRef]
- Santamaría, C.A.; Marínde, M.M.; Traveset, R.; Sala, R.; Grau, A.; Pastor, E.; Sarasquete, C.; Crespo, S. Larval organogenesis in common dentec, Dentex dentex L. (Sparidae): Histological and histochemical aspects. Aquaculture 2004, 237, 207–228. [Google Scholar] [CrossRef]
- He, T.; Xiao, Z.Z.; Liu, Q.H.; Li, J. Ontogeny of the gill and Na+, K+-ATPase activity of rock bream (Oplegnathus fasciatus). J. Fish. China 2013, 37, 520–525. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, H.S.; Huang, J.; Li, J.; Zhang, M.; Qi, X.; Li, Y. Histological and morphological observations of the gill and swim bladder development of Lateolabrax maculatus. J. Fish. China 2019, 43, 2476–2484. [Google Scholar]
- Liu, Y.; Li, X.H.; Zhao, J.R.; Wang, Z. Effect of intestinal air-breathing restriction on respiratory metabolism and antioxidant capability of loach (Paramisgurnus dabryanus). Chin. J. Zool. 2017, 52, 857–864. [Google Scholar]
- Damsgaard, C.; Gam, L.T.H.; Tuong, D.D.; Thinh, P.V.; Huong Thanh, D.T.; Wang, T.; Bayley, M. High capacity for extracellular acid-base regulationin the air-breathing fish Pangasianodon hypophthalmus. J. Exp. Biol. 2015, 218, 1290–1296. [Google Scholar] [PubMed]
- Perna, S.A.; Fernandes, M.N. Gill Morphometry of the Facultative Air-breathing Loricariid fish, Hypostomus plecostomus (Walbaum) with, special emphasis on aquatic respiration. Fish Physiol. Biochem. 1996, 15, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.; Gee, P. Aquatic Surface Respiration, Buoyancy Control and the Evolution of air-breathingin gobies (Gobiidae: Pisces). J. Exp. Biol. 1995, 198, 79–89. [Google Scholar] [CrossRef]
- Nelson, J.A. Breaking wind to survive: Fishes that breathe air with their gut. J. Fish Biol. 2014, 84, 554–576. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Yang, X.; Fan, Q.; Wang, W. Ontogeny of the digestive tract in mud loach Misgurnus anguillicaudatus larvae. Aquac. Res. 2016, 47, 1180–1190. [Google Scholar] [CrossRef]
- Bernal, D.; Dickson, K.A.; Shadwick, R.E.; Graham, J.B. Analysis of the evolutionary convergence for high performance swimming in lamnid sharks and tunas. Comp. Biochem. Physiol. A 2001, 129, 695–726. [Google Scholar] [CrossRef]
- Frommel, A.Y.; Kwan, G.T.; Prime, K.J.; Tresguerres, M.; Lauridsen, H.; Val, A.L.; Brauner, C. Changes in gill and air-breathing organ characteristics during the transition from water- to air-breathing in juvenile Arapaima gigas. J. Exp. Zool. A Ecol. Integr. Physiol. 2021, 335, 801–813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).