Hematological Response of Juvenile Cobia to Three Anesthetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, System and Husbandry
2.2. Anesthesia, Sample Collection and Data Analysis
2.2.1. Dose Response
2.2.2. Blood Collection, Handling, and Analysis
2.3. Statistical Analysis
3. Results
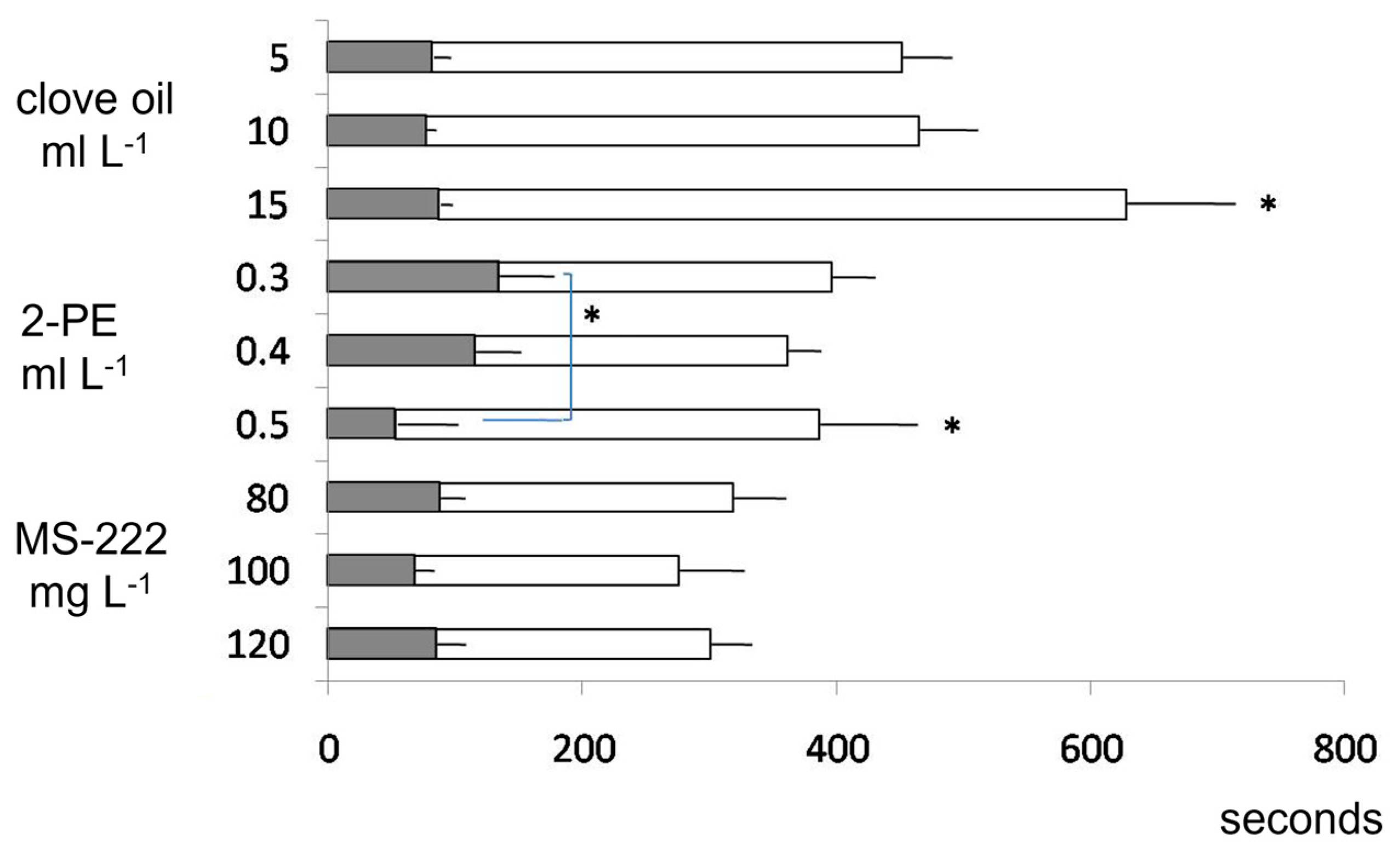
3.1. Dose Response
3.2. Effect of Anesthesia on Hematological Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ostenfeld, T.; Thomsen, S.; Ingolfsdottir, S.; Rønsholdt, B.; McLean, E. Evaluation of the effect of live haulage on metabolites and fillet texture of rainbow trout (Oncorhynchus mykiss). Wat. Sci. Technol. 1995, 31, 233–237. [Google Scholar] [CrossRef]
- Schroeder, P.; Lloyd, R.; McKimm, R.; Metselaar, M.; Navarro, J.; O’Farrell, M.; Readman, G.D.; Speilberg, L.; Mocho, J.-P. Anaesthesia of laboratory, aquaculture and ornamental fish: Proceedings of the first LASA-FVS Symposium. Lab. Anim. 2021, 55, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.G.; Ross, B. Anesthetic and Sedative Techniques for Aquatic Animals, 3rd ed.; Blackwell Publishing Ltd.: Oxford, UK, 2008; 222p. [Google Scholar]
- Neiffer, D.L.; Stamper, M.A. Fish sedation, anesthesia, analgesia, and euthanasia: Considerations, methods, and types of drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A review of tricaine methanesulfonate for anesthesia of fish. Rev. Fish Biol. Fish. 2011, 21, 51–59. [Google Scholar] [CrossRef]
- Sneddon, L.U. Clinical anesthesia and analgesia in fish. J. Exot. Pet Med. 2012, 21, 32–43. [Google Scholar] [CrossRef]
- Seibel, H.; Baßmann, B.; Rebl, A. Blood will tell: What hematological analyses can reveal about fish welfare. Vet. Sci. 2021, 8, 616955. [Google Scholar] [CrossRef]
- Vergneau-Grosset, C.; Benedetti, I.-C.C. Fish sedation and anesthesia. Vet. Clin. Exot. Anim. 2022, 25, 13–29. [Google Scholar] [CrossRef]
- Holloway, A.C.; Keene, J.L.; Noakes, D.G.; Moccia, R.D. Effects of clove oil and MS-222 on blood hormone profiles in rainbow trout Onchorhynchus mykiss, Walbaum. Aquacult. Res. 2004, 35, 1025–1030. [Google Scholar] [CrossRef]
- Benetti, D.D.; Suarez, J.; Camperio, J.; Hoenig, R.H.; Tudela, C.E.; Daugherty, Z.; McGuigan, C.J.; Mathur, S.; Anchieta, L.; Buchalla, Y.; et al. A review on cobia, Rachycentron canadum, aquaculture. J. World Aquacult. Soc. 2021, 52, 691–709. [Google Scholar] [CrossRef]
- Tveterås, R.; Jory, D.E.; Nystoyl, R. GOAL 2019: Global Finfish Production Review and Forecast. Available online: globalseafood.org/advocate/goal-2019-global-finfish-production-review-and-forecast/ (accessed on 16 November 2022).
- Webb, K.A., Jr.; Hitzfelder, G.M.; Faulk, C.K.; Holt, G.J. Growth of juvenile cobia, Rachycentron canadum, at three different densities in a recirculating aquaculture system. Aquaculture 2007, 264, 223–227. [Google Scholar] [CrossRef]
- McLean, E.; Salze, G.; Schwarz, M.H.; Craig, S.R. Cobia cultivation. In New Technologies in Aquaculture: Improving Production Efficiency, Quality and Environmental Management; Burnell, G., Allan, G., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; 1224p. [Google Scholar]
- Gullian, M.; Villanueva, J. Efficacy of tricaine methanesulphonate and clove oil as anaesthetics for juvenile cobia Rachycentron canadum. Aquacult. Res. 2009, 40, 852–860. [Google Scholar] [CrossRef]
- Trushenski, J.T.; Bowzer, J.C.; Bowker, J.D.; Schwarz, M.H. Chemical and electrical approaches to sedation of cobia: Induction, recovery, and physiological responses to sedation. Mar. Coast. Fish. 2012, 4, 639–650. [Google Scholar] [CrossRef]
- Felix, N.; Bala Murugan, U.; Rajaram, K. Efficacy of phenoxyethanol and clove oil as anaesthetics in marine finfish cobia, Rachycentron canadum. Int. J. Fish. Aquat. Stud. 2013, 1, 46–49. [Google Scholar]
- Pedron, J.S.; Miron, D.S.; Rodrigues, R.V.; Okamoto, M.H.; Tesser, M.B.; Sampaio, L.A. Stress response in transport of juvenile cobia Rachycentron canadum using the anesthetic benzocaine. Lat. Am. J. Aquat. Res. 2016, 44, 638–642. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Cardinaletti, G.; Sigelaki, I.; Polzonetti-Mangi, A. Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) at different temperatures. Aquaculture 2005, 246, 467–481. [Google Scholar] [CrossRef]
- Cotter, P.A.; Rodruck, K.J. Differential effects of anesthetics on electrical properties of the rainbow trout (Oncorhynchus mykiss) heart. Comp. Biochem. Physiol. 2006, 145A, 158–165. [Google Scholar] [CrossRef]
- Keene, J.L.; Noakes, D.L.G.; Moccia, R.D.; Soto, C.G. The efficacy of clove oil as an anesthetic for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquacult. Res. 1998, 29, 89–101. [Google Scholar] [CrossRef]
- Beecham, R.V.; Small, B.C.; Minchew, C.D. Using portable lactate and glucose meters for catfish research: Acceptable alternatives to established laboratory methods? N. Am. J. Aquacult. 2006, 68, 291–295. [Google Scholar] [CrossRef]
- Skår, M.W.; Haugland, G.T.; Powell, M.D.; Wergeland, H.I.; Samuelsen, O.B. Development of anaesthetic protocols for lumpfish (Cyclopterus lumpus L.): Effect of anaesthetic concentrations, sea water temperature and body weight. PLoS ONE 2017, 12, e0179344. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Fernández-Castro, M.; del Santo O’Neill, T.J.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Mancera, J.A.; Ruiz-Jarabo, I. Transport and recovery of gilthead seabream (Sparus aurata L.) sedated with clove oil and MS-222: Effects on stress axis regulation and intermediary metabolism. Front. Physiol. 2019, 10, 612. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Sladky, K.K.; Swanson, C.R.; Stoskopf, M.K.; Loomis, M.R.; Lewbart, G.A. Comparative efficacy of tricaine methanesulfonate and clove oil for use as anesthetics in red pacu (Piaractus brachypomus). Am. J. Vet. Res. 2001, 62, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.J.; Lin, H. Effect of water pH on gas and ion transfer across fish gills. In Fish Ecophysiology; Rankin, J.C., Jensen, F.B., Eds.; Chapman & Hall: London, UK, 1993; pp. 265–275. [Google Scholar]
- Hikasa, Y.; Takase, K.; Ogasawara, T.; Ogawasara, S. Anaesthesia and recovery with tricaine methanesulfonate, eugenol and thiopental sodium in the carp, Cyprinus carpio. Jpn. J. Vet. Sci. 1986, 48, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Velisek, J.; Wlasow, T.; Gomulka, P.; Svobodova, Z.; Novotny, L. Effects of 2-phenoxyethanol anaesthesia on sheatfish (Silurus glanis L.). Vet. Med. 2007, 52, 103–110. [Google Scholar] [CrossRef]
- Houston, A.H.; Madden, J.A.; Woods, R.J.; Miles, H.M. Variations in the blood and tissue chemistry of brook trout, Salvelinus fontinalis, subsequent to handling, anesthesia, and surgery. J. Fish Res. Board Can. 1971, 28, 635–642. [Google Scholar] [CrossRef]
- Soivio, A.; Nyholm, K.; Huhti, M. Effects of anaesthesia with MS 222, neutralized MS 222 and benzocaine on the blood constituents of rainbow trout, Salmo gairdneri. J. Fish Biol. 1977, 10, 91–101. [Google Scholar] [CrossRef]
- Randall, D.J. The control of respiration and circulation in fish during exercise and hypoxia. J. Exp. Biol. 1982, 100, 275–288. [Google Scholar] [CrossRef]
- Lai, J.C.C.; Kakuta, I.; Mok, H.O.L.; Rummer, J.L.; Randall, D.J. Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. J. Exp. Biol. 2006, 209, 2734–2738. [Google Scholar] [CrossRef]
- Rothwell, S.E.; Forster, M.E. Anaesthetic effects on the hepatic portal vein and on the vascular resistance of the tail of the Chinook salmon (Oncorhynchus tshawytscha). Fish Physiol. Biochem. 2005, 31, 11–21. [Google Scholar] [CrossRef]
- Bohr, C.; Hasselbach, K.; Krogh, A. Ueber emen in biologischen Beziehung wichtigen Einfluss, den die Kohlen saurespannung des Blutes anf dessen samerstoffbinding ubt. Skand. Arch. Physiol. 1904, 16, 402–412. [Google Scholar] [CrossRef]
- Tufts, B.L.; Tufts, Y.T.K.; Boutilier, R.G. Exhaustive exercise in “wild” Atlantic salmon (Salmo salar): Acid–base regulation and blood gas transport. Can. J. Fish. Aquat. Sci. 1991, 48, 868–874. [Google Scholar] [CrossRef]
- Marshall, W.S. Transport processes in isolated teleost epithelia: Opercular epithelium and urinary bladder. In Cellular and Molecular Approaches to Fish Ionic Regulation; Wood, C.M., Shuttleworth, T.J., Eds.; Academic Press: London, UK, 1995; pp. 1–23. [Google Scholar]
| Sedation | ||
|---|---|---|
| Stage | Descriptor | Behavior |
| 0 | Normal equilibrium | Reactive to external stimuli with opercular rate and muscle tone normal. |
| 1 | Light sedation | Slight loss of reactivity to external visual and tactile stimuli. Opercular rate slightly decreased but equilibrium normal. |
| 2 | Deep sedation | Total loss of reactivity to external stimuli except to strong pressure. Slight decrease in opercular rate but equilibrium normal. |
| 3 | Partial decline in equilibrium | Partial loss of muscle tone combined with erratic swimming. Increased opercular rate. Only reactive to strong tactile and vibrational stimuli. |
| 4 | Total loss of equilibrium | Total loss of muscle tone and equilibrium. Slow but regular opercular rate combined with loss of spinal reflexes. |
| 5 | Lack of reflex reactivity | Total loss of reactivity. Opercular reactions slow and irregular. Heart rate very slow and loss of all reflexes. |
| Recovery | ||
| Stage | Behavioral adjustment | |
| 1 | Return of opercular movement. | |
| 2 | Partial recovery of equilibrium and swimming motion. | |
| 3 | Total recovery of equilibrium. | |
| 4 | Revival of avoidance swimming motion and response to external stimuli, but behavioral response impassive. | |
| 5 | Total behavioral recovery. Normal swimming and reflex activity. | |
| Hematocrit | pH | pCO2 | pO2 | |
|---|---|---|---|---|
| Control | 23.2 ± 4.0 | 7.58 ± 0.04 a | 9.0 ± 1.23 c | 30.2 ± 9.5 |
| Clove oil | 29.2 ± 5.3 | 7.27 ± 0.08 b | 15.8 ± 1.48 a | 41.6 ± 13.5 |
| 2-PE | 30.8 ± 4.4 | 7.23 ± 0.06 b | 13.0 ± 1.22 b | 59.0 ± 21.1 |
| MS-222 | 25.8 ± 2.6 | 7.28 ± 0.03 b | 14.4 ± 1.34 ab | 51.6 ± 19.0 |
| p-value | 0.0568 | 0.001 | 0.001 | 0.068 |
| Na+ (mmol L−1) | K+ (mmol L−1) | Ca2+ (mmol L−1) | Glucose (mg dL−1) | |
| Control | 165.8 ± 1.92 c | 4.65 ± 0.41 b | 1.52 ± 0.01 b | 56.2 ± 6.61 b |
| Clove oil | 175.8 ± 4.49 a | 5.44 ± 0.72 ab | 1.76 ± 0.17 a | 54.6 ± 11.74 ab |
| 2-PE | 170.4 ± 4.04 bc | 5.94 ± 0.56 a | 1.64 ± 0.12 ab | 83.8 ± 20.9 a |
| MS-222 | 172.2 ± 3.96 ab | 5.38 ± 0.55 ab | 1.70 ± 0.14 a | 74.8 ± 16.42 ab |
| p-value | 0.005 | 0.036 | 0.052 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorensen, K.; Craig, S.R.; Cnaani, A.; McLean, E. Hematological Response of Juvenile Cobia to Three Anesthetics. Fishes 2023, 8, 31. https://doi.org/10.3390/fishes8010031
Sorensen K, Craig SR, Cnaani A, McLean E. Hematological Response of Juvenile Cobia to Three Anesthetics. Fishes. 2023; 8(1):31. https://doi.org/10.3390/fishes8010031
Chicago/Turabian StyleSorensen, Karl, Steven R. Craig, Avner Cnaani, and Ewen McLean. 2023. "Hematological Response of Juvenile Cobia to Three Anesthetics" Fishes 8, no. 1: 31. https://doi.org/10.3390/fishes8010031
APA StyleSorensen, K., Craig, S. R., Cnaani, A., & McLean, E. (2023). Hematological Response of Juvenile Cobia to Three Anesthetics. Fishes, 8(1), 31. https://doi.org/10.3390/fishes8010031







