Variation in the Physiological Condition of Common Coral Trout (Plectropomus leopardus) Unrelated to Coral Cover on the Great Barrier Reef, Australia
Abstract
:1. Introduction
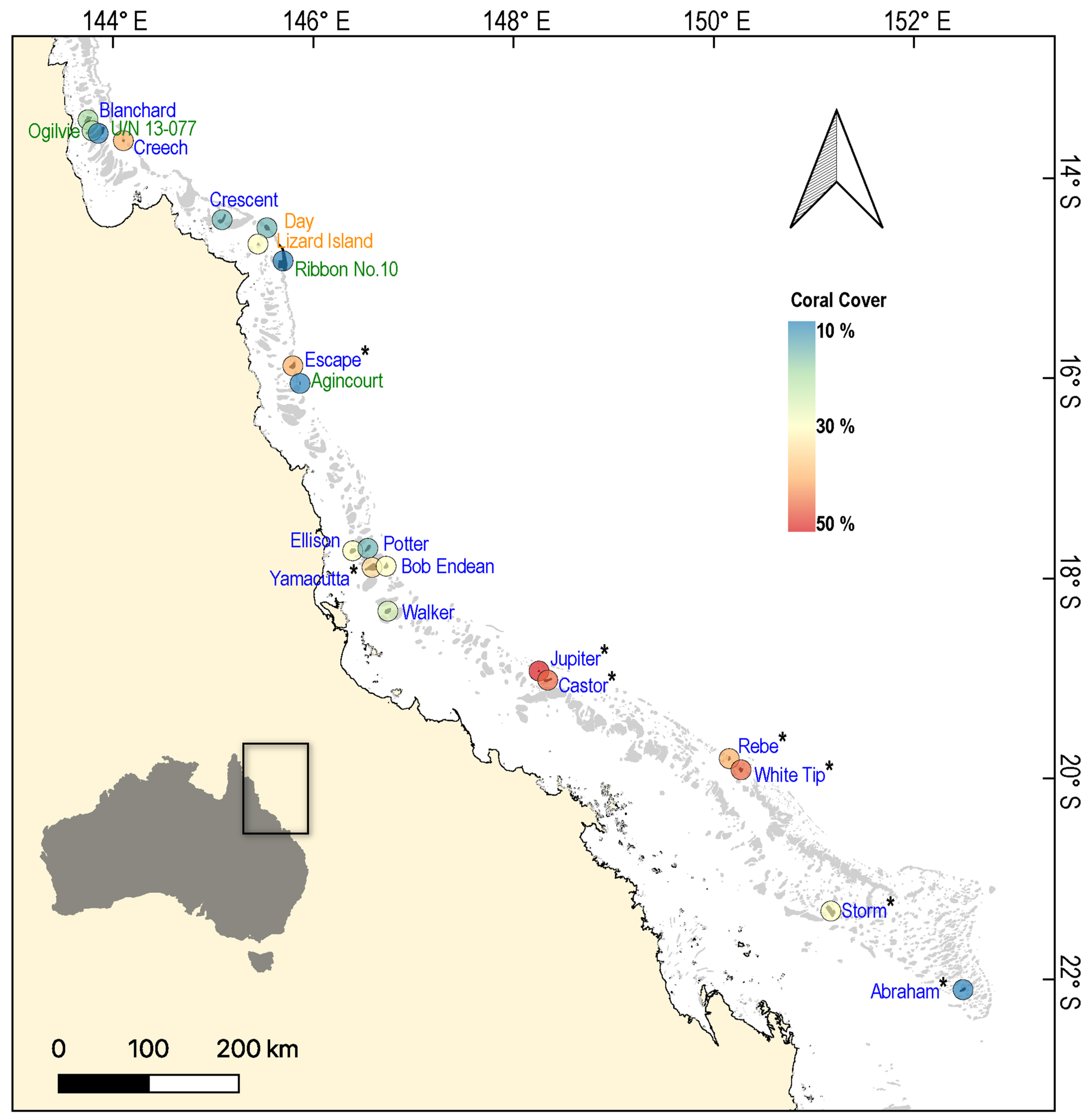
2. Materials and Methods
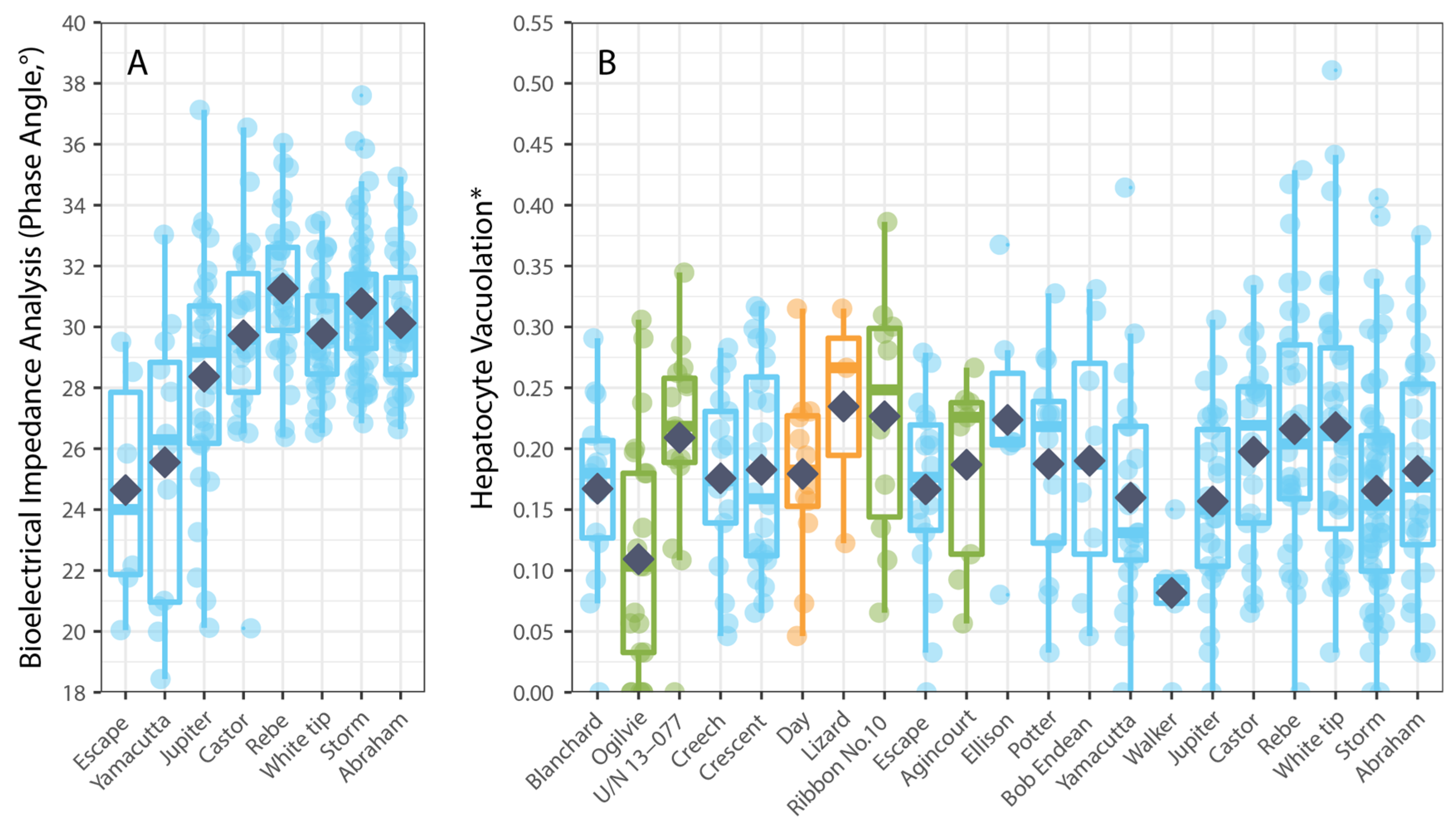
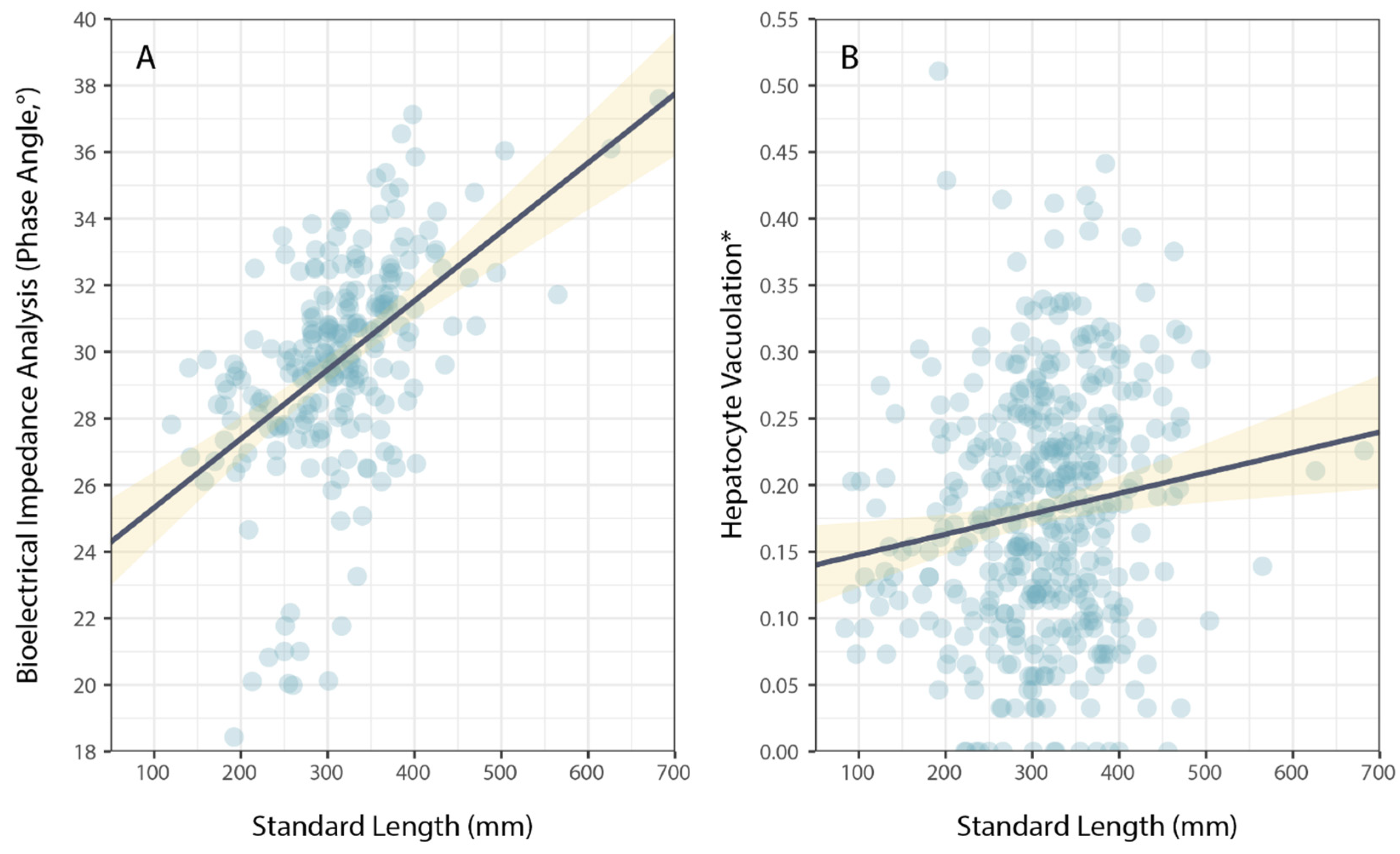
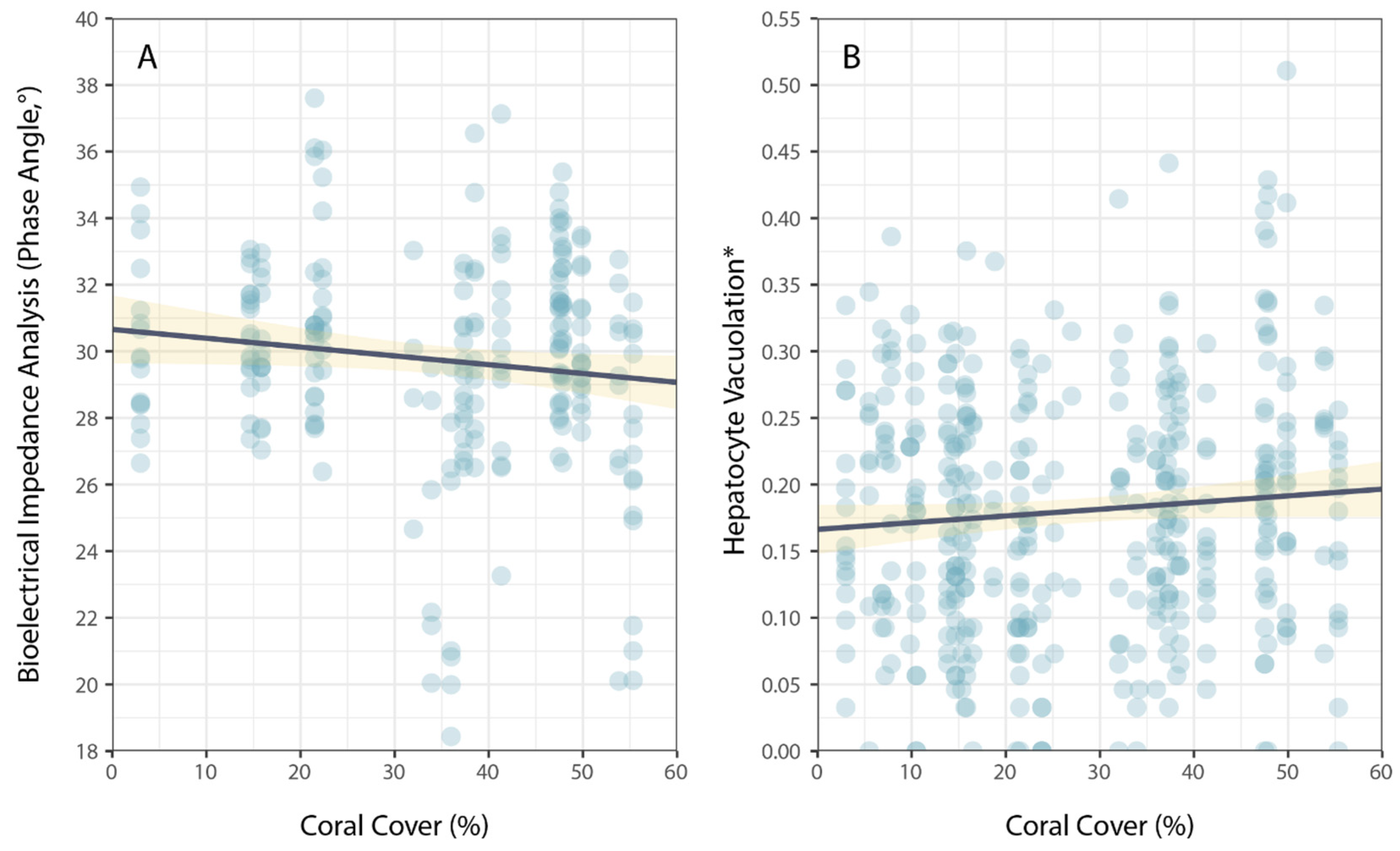
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, W.W.; Sarmiento, J.L.; Dunne, J.; Frölicher, T.L.; Lam, V.W.; Deng Palomares, M.L.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Brown, C.J.; Mellin, C.; Edgar, G.J.; Campbell, M.D.; Stuart-Smith, R.D. Direct and indirect effects of heatwaves on a coral reef fishery. Glob. Change Biol. 2021, 27, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Munday, P.L.; Wilson, S.K.; Graham, N.A.; Cinner, J.E.; Bellwood, D.R.; Jones, G.P.; Polunin, N.V.; McClanahan, T.R. Effects of climate-induced coral bleaching on coral-reef fishes—Ecological and economic consequences. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 257–302. [Google Scholar]
- Bell, J.D.; Ganachaud, A.; Gehrke, P.C.; Griffiths, S.P.; Hobday, A.J.; Hoegh-Guldberg, O.; Johnson, J.E.; Le Borgne, R.; Lehodey, P.; Lough, J.M.; et al. Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nat. Clim. Change 2013, 3, 591–599. [Google Scholar] [CrossRef]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Fisheries productivity under progressive coral reef degradation. J. Appl. Ecol. 2018, 55, 1041–1049. [Google Scholar] [CrossRef]
- Russ, G.R.; Rizzari, J.R.; Abesamis, R.A.; Alcala, A.C. Coral cover a stronger driver of reef fish trophic biomass than fishing. Ecol. Appl. 2021, 31, e02224. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Pratchett, M.S.; Morrison, T.H.; Gurney, G.G.; Hughes, T.P.; Álvarez-Romero, J.G.; Day, J.C.; Grantham, R.; Grech, A.; Hoey, A.S.; et al. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Conserv. 2019, 236, 604–615. [Google Scholar] [CrossRef]
- Eddy, T.D.; Lam, V.W.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Greer, K.; Palomares, M.L.D.; Bruno, J.F.; Ota, Y.; Cheung, W.W. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]
- Ortiz, J.C.; Wolff, N.H.; Anthony, K.R.; Devlin, M.; Lewis, S.; Mumby, P.J. Impaired recovery of the Great Barrier Reef under cumulative stress. Sci. Adv. 2018, 4, eaar6127. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Thompson, C.A.; Hoey, A.S.; Cowman, P.F.; Wilson, S.K. Effects of coral bleaching and coral loss on the structure and function of reef fish assemblages. In Coral Bleaching: Patterns, Processes, Causes and Consequences, 2nd ed.; van Oppen, M.J.H., Lough, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 265–293. [Google Scholar]
- Sadovy de Mitcheson, Y.; Craig, M.T.; Bertoncini, A.A.; Carpenter, K.E.; Cheung, W.W.; Choat, J.H.; Cornish, A.S.; Fennessy, S.T.; Ferreira, B.P.; Heemstra, P.C.; et al. Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 2013, 14, 119–136. [Google Scholar] [CrossRef]
- Frisch, A.J.; Cameron, D.S.; Pratchett, M.S.; Williamson, D.H.; Williams, A.J.; Reynolds, A.D.; Hoey, A.S.; Rizzari, J.R.; Evans, L.; Kerrigan, B.; et al. Key aspects of the biology, fisheries and management of Coral grouper. Rev. Fish Biol. Fish. 2016, 26, 303–325. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Cameron, D.S.; Donelson, J.; Evans, L.; Frisch, A.J.; Hobday, A.J.; Hoey, A.S.; Marshall, N.A.; Messmer, V.; Munday, P.L.; et al. Effects of climate change on coral grouper (Plectropomus spp.) and possible adaptation options. Rev. Fish Biol. Fish. 2017, 27, 297–316. [Google Scholar] [CrossRef]
- Fox, A.R.; Campbell, A.B.; Zieth, J.D. Stock Assessment of Queensland East Coast Common Coral Trout (Plectropomus leopardus), Australia, with Data to December 2021; Queensland Government: Brisbane, Australia, 2022; p. 83. Available online: https://era.daf.qld.gov.au/id/eprint/8618/ (accessed on 1 May 2023).
- Johansen, J.L.; Messmer, V.; Coker, D.J.; Hoey, A.S.; Pratchett, M.S. Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob. Change Biol. 2014, 20, 1067–1074. [Google Scholar] [CrossRef]
- Johansen, J.L.; Pratchett, M.S.; Messmer, V.; Coker, D.J.; Tobin, A.J.; Hoey, A.S. Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci. Rep. 2015, 5, 13830. [Google Scholar] [CrossRef]
- Sun, Z.; Xia, S.; Feng, S.; Zhang, Z.; Rahman, M.M.; Rajkumar, M.; Jiang, S. Effects of water temperature on survival, growth, digestive enzyme activities, and body composition of the leopard coral grouper Plectropomus leopardus. Fish. Sci. 2015, 81, 107–112. [Google Scholar] [CrossRef]
- Messmer, V.; Pratchett, M.S.; Hoey, A.S.; Tobin, A.J.; Coker, D.J.; Cooke, S.J.; Clark, T.D. Global warming may disproportionately affect larger adults in a predatory coral reef fish. Glob. Change Biol. 2017, 23, 2230–2240. [Google Scholar] [CrossRef]
- Clark, T.D.; Messmer, V.; Tobin, A.J.; Hoey, A.S.; Pratchett, M.S. Rising temperatures may drive fishing-induced selection of low-performance phenotypes. Sci. Rep. 2017, 7, 40571. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Heupel, M.; Tobin, A.; Pratchett, M.S. A large predatory reef fish species moderates feeding and activity patterns in response to seasonal and latitudinal temperature variation. Sci. Rep. 2017, 7, 12966. [Google Scholar] [CrossRef]
- Payet, S.D.; Lowe, J.R.; Mapstone, B.D.; Pratchett, M.S.; Sinclair-Taylor, T.H.; Taylor, B.M.; Waldie, P.A.; Harrison, H.B. Comparative demography of commercially important species of coral grouper, Plectropomus leopardus and P. laevis, from Australia’s great barrier reef and Coral Sea marine parks. J. Fish Biol. 2020, 97, 1165–1176. [Google Scholar] [CrossRef]
- Williamson, D.H.; Ceccarelli, D.M.; Evans, R.D.; Jones, G.P.; Russ, G.R. Habitat dynamics, marine reserve status, and the decline and recovery of coral reef fish communities. Ecol. Evol. 2014, 4, 337–354. [Google Scholar] [CrossRef]
- Emslie, M.J.; Cheal, A.J.; Logan, M. The distribution and abundance of reef-associated predatory fishes on the Great Barrier Reef. Coral Reefs 2017, 36, 829–846. [Google Scholar] [CrossRef]
- Hempson, T.N.; Graham, N.A.; MacNeil, M.A.; Williamson, D.H.; Jones, G.P.; Almany, G.R. Coral reef mesopredators switch prey, shortening food chains, in response to habitat degradation. Ecol. Evol. 2017, 7, 2626–2635. [Google Scholar] [CrossRef]
- Kingsford, M.J. Spatial and temporal variation in predation on reef fishes by coral trout (Plectropomus leopardus, Serranidae). Coral Reefs 1992, 11, 193–198. [Google Scholar] [CrossRef]
- Graham, N.A.; Wilson, S.K.; Jennings, S.; Polunin, N.V.; Robinson, J.A.N.; Bijoux, J.P.; Daw, T.M. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 2007, 21, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Heron, S.F.; Mellin, C.; Cumming, G.S. Recurrent mass-bleaching and the potential for ecosystem collapse on Australia’s Great Barrier Reef. In Ecosystem Collapse and Climate Change; Canadell, J.G., Jacksonn, R.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 265–289. [Google Scholar]
- Scott, M.E.; Heupel, M.R.; Simpfendorfer, C.A.; Matley, J.K.; Pratchett, M.S. Latitudinal and seasonal variation in space use by a large, predatory reef fish, Plectropomus leopardus. Funct. Ecol. 2017, 33, 670–680. [Google Scholar] [CrossRef]
- Taylor, B.M.; Gourley, J.; Trianni, M.S. Age, growth, reproductive biology and spawning periodicity of the forktail rabbitfish (Siganus argenteus) from the Mariana Islands. Mar. Freshw. Res. 2016, 68, 1088–1097. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Wilson, S.K.; Berumen, M.L.; McCormick, M.I. Sublethal effects of coral bleaching on an obligate coral feeding butterflyfish. Coral Reefs 2004, 23, 352–356. [Google Scholar] [CrossRef]
- Crowe, B.H.; Harris, J.O.; Bansemer, M.S.; Stone, D.A. Restricted feeding and dietary energy levels affect liver structure in cultured Yellowtail Kingfish (Seriola lalandi, Valenciennes) at summer water temperatures. Aquac. Res. 2021, 52, 6074–6086. [Google Scholar] [CrossRef]
- Champion, C.; Hobday, A.J.; Pecl, G.T.; Tracey, S.R. Maximising the utility of bioelectrical impedance analysis for measuring fish condition requires identifying and controlling for sources of error. Fish. Res. 2020, 229, 105575. [Google Scholar] [CrossRef]
- Cox, M.K.; Hartman, K.J. Nonlethal estimation of proximate composition in fish. Can. J. Fish. Aquat. Sci. 2015, 62, 269–275. [Google Scholar] [CrossRef]
- Mellin, C.; Matthews, S.; Anthony, K.R.; Brown, S.C.; Caley, M.J.; Johns, K.A.; Osborne, K.; Puotinen, M.; Thompson, A.; Wolff, N.H.; et al. Spatial resilience of the Great Barrier Reef under cumulative disturbance impacts. Glob. Change Biol. 2019, 25, 2431–2445. [Google Scholar] [CrossRef]
- Ling, S.D.; Cowan, Z.L.; Boada, J.; Flukes, E.B.; Pratchett, M.S. Homing behaviour by destructive crown-of-thorns starfish is triggered by local availability of coral prey. Proc. R. Soc. B 2020, 287, 20201341. [Google Scholar] [CrossRef]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Change Biol. 2006, 12, 2220–2234. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K. Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr. Opin. Environ. Sustain. 2014, 7, 37–43. [Google Scholar] [CrossRef]
- Morais, R.A.; Depczynski, M.; Fulton, C.; Marnane, M.; Narvaez, P.; Huertas, V.; Brandl, S.J.; Bellwood, D.R. Severe coral loss shifts energetic dynamics on a coral reef. Funct. Ecol. 2020, 34, 1507–1518. [Google Scholar] [CrossRef]
- Emslie, M.J.; Logan, M.; Cheal, A.J. The distribution of planktivorous damselfishes (Pomacentridae) on the Great Barrier Reef and the relative influences of habitat and predation. Diversity 2019, 11, 33. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Ackerman, J.L.; Depczynski, M. Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob. Change Biol. 2006, 12, 1587–1594. [Google Scholar] [CrossRef]
- Wilson, S.K.; Fisher, R.; Pratchett, M.S.; Graham, N.A.J.; Dulvy, N.K.; Turner, R.A.; Cakacaka, A.; Polunin, N.V.; Rushton, S.P. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Change Biol. 2008, 14, 2796–2809. [Google Scholar] [CrossRef]
- Hoey, A.S.; Howells, E.; Johansen, J.L.; Hobbs, J.P.A.; Messmer, V.; McCowan, D.M.; Wilson, S.K.; Pratchett, M.S. Recent advances in understanding the effects of climate change on coral reefs. Diversity 2016, 8, 12. [Google Scholar] [CrossRef]
- Clark, T.D.; Scheuffele, H.; Pratchett, M.S.; Skeeles, M.R. Behavioural temperature regulation is a low priority in a coral reef fish (Plectropomus leopardus): Insights from a novel behavioural thermoregulation system. J. Exp. Biol. 2022, 225, 244212. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.E.; Tebbett, S.B.; Whitman, K.L.; Thompson, C.A.; Mancini, F.B.; Heupel, M.R.; Pratchett, M.S. Variation in abundance, diversity and composition of coral reef fishes with increasing depth at a submerged shoal in the northern Great Barrier Reef. Rev. Fish Biol. Fish. 2022, 32, 941–962. [Google Scholar] [CrossRef]
- Wen, C.K.; Pratchett, M.S.; Almany, G.; Jones, G.P. Patterns of recruitment and microhabitat associations for three predatory coral reef fishes on the southern Great Barrier Reef, Australia. Coral Reefs 2013, 32, 389–398. [Google Scholar] [CrossRef]
- Caldarone, E.M.; MacLean, S.A.; Sharack, B. Evaluation of bioelectrical impedance analysis and Fulton’s condition factor as nonlethal techniques for estimating short-term responses in postsmolt Atlantic salmon (Salmo salar) to food availability. Fish. Bull. 2012, 110, 257–270. [Google Scholar]
- Donelson, J.M.; McCormick, M.I.; Munday, P.L. Parental condition affects early life-history of a coral reef fish. J. Exp. Mar. Biol. Ecol. 2008, 360, 109–116. [Google Scholar] [CrossRef]
- Willis, J.; Hobday, A.J. Application of bioelectrical impedance analysis as a method for estimating composition and metabolic condition of southern bluefin tuna (Thunnus maccoyii) during conventional tagging. Fish. Res. 2008, 93, 64–71. [Google Scholar] [CrossRef]
- Fitzhugh, G.R.; Wuenschel, M.J.; McBride, R.S. Evaluation of bioelectrical impedance analysis (BIA) to measure condition and energy allocated to reproduction in marine fishes. J. Phys. Conf. Ser. 2010, 224, 012137. [Google Scholar] [CrossRef]

| Source | Estimate | SE | t | p-Value |
|---|---|---|---|---|
| Phase angle | ||||
| Latitude | 7.68 × 10−1 | 1.24 × 10−1 | 6.183 | <0.001 |
| Coral Cover | −1.23 × 10−2 | 1.49 × 10−2 | −0.824 | 0.411 |
| Size | 2.19 × 10−2 | 2.73 × 10−3 | 8.030 | <0.001 |
| Latitude × Coral Cover | 3.67 × 10−2 | 9.16 × 10−3 | 4.001 | <0.001 |
| Latitude × Size | −5.15 × 10−3 | 1.93 × 10−3 | −2.667 | 0.008 |
| Coral Cover × Size | −3.26 × 10−5 | 2.20 × 10−4 | −0.148 | 0.883 |
| Latitude × Coral Cover × Size | 1.13 × 10−6 | 1.34 × 10−4 | 0.008 | 0.993 |
| Hepatocyte vacuolation | ||||
| Latitude | 3.03 × 10−3 | 1.82 × 10−3 | 1.667 | 0.096 |
| Coral Cover | 2.58 × 10−4 | 3.24 × 10−4 | 0.797 | 0.426 |
| Size | 1.50 × 10−4 | 6.28 × 10−5 | 2.387 | 0.018 |
| Latitude × Coral Cover | 2.64 × 10−4 | 1.18 × 10−4 | 2.234 | 0.026 |
| Latitude × Size | −2.31 × 10−5 | 2.49 × 10−5 | −0.926 | 0.355 |
| Coral Cover × Size | 7.28 × 10−8 | 4.40 × 10−6 | 0.017 | 0.987 |
| Latitude × Coral Cover × Size | −1.94 × 10−7 | 1.66 × 10−6 | −0.117 | 0.907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratchett, M.S.; Caballes, C.F.; Hobbs, J.-P.A.; DiBattista, J.D.; Bergseth, B.; Waldie, P.; Champion, C.; Mc Cormack, S.P.; Hoey, A.S. Variation in the Physiological Condition of Common Coral Trout (Plectropomus leopardus) Unrelated to Coral Cover on the Great Barrier Reef, Australia. Fishes 2023, 8, 497. https://doi.org/10.3390/fishes8100497
Pratchett MS, Caballes CF, Hobbs J-PA, DiBattista JD, Bergseth B, Waldie P, Champion C, Mc Cormack SP, Hoey AS. Variation in the Physiological Condition of Common Coral Trout (Plectropomus leopardus) Unrelated to Coral Cover on the Great Barrier Reef, Australia. Fishes. 2023; 8(10):497. https://doi.org/10.3390/fishes8100497
Chicago/Turabian StylePratchett, Morgan S., Ciemon F. Caballes, Jean-Paul A. Hobbs, Joseph D. DiBattista, Brock Bergseth, Peter Waldie, Curtis Champion, Samuel P. Mc Cormack, and Andrew S. Hoey. 2023. "Variation in the Physiological Condition of Common Coral Trout (Plectropomus leopardus) Unrelated to Coral Cover on the Great Barrier Reef, Australia" Fishes 8, no. 10: 497. https://doi.org/10.3390/fishes8100497
APA StylePratchett, M. S., Caballes, C. F., Hobbs, J.-P. A., DiBattista, J. D., Bergseth, B., Waldie, P., Champion, C., Mc Cormack, S. P., & Hoey, A. S. (2023). Variation in the Physiological Condition of Common Coral Trout (Plectropomus leopardus) Unrelated to Coral Cover on the Great Barrier Reef, Australia. Fishes, 8(10), 497. https://doi.org/10.3390/fishes8100497









