Phylogeographic Analyses of the Shortfin Mako, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamniformes) from the Central Mediterranean Sea, a Critically Endangered Species in the Region
Abstract
:1. Introduction
2. Materials and Methods
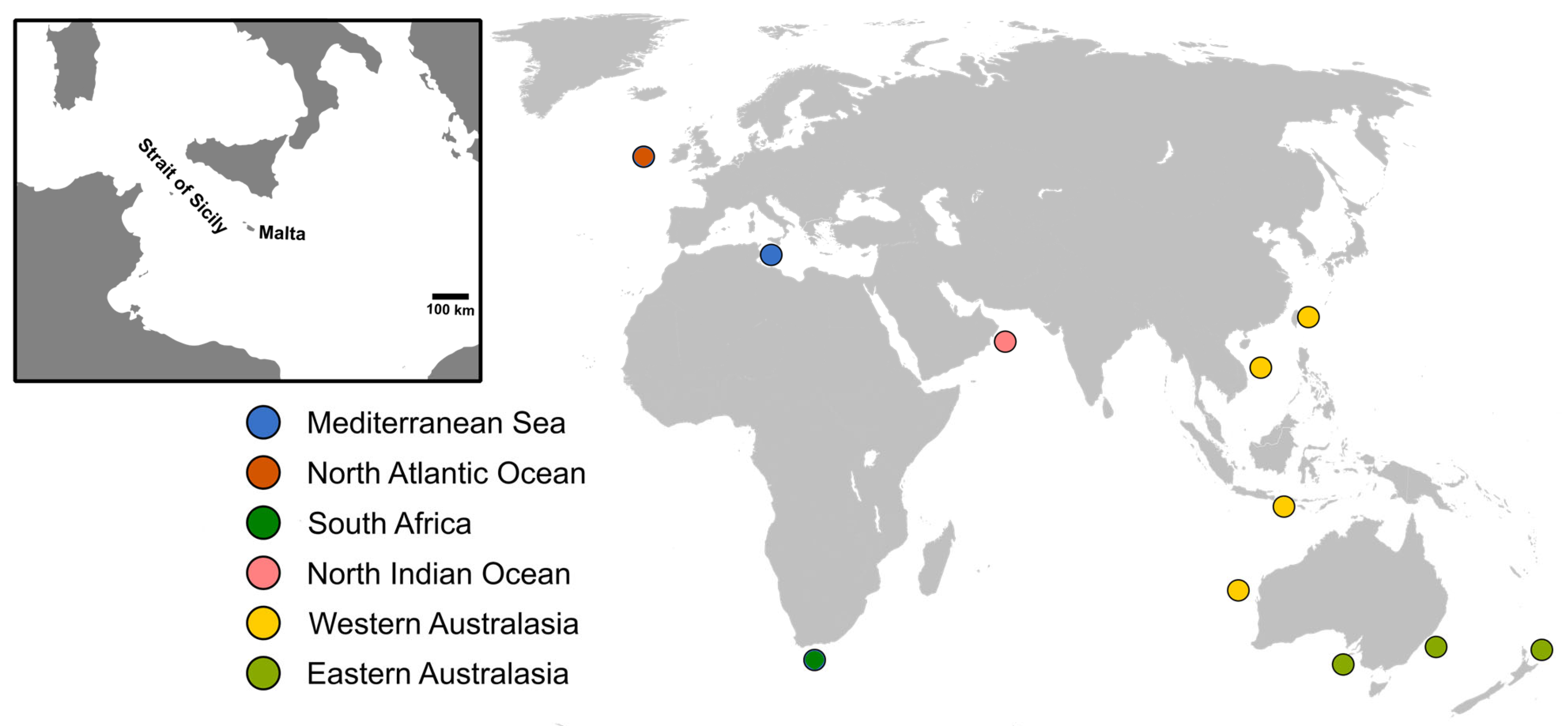
2.1. Sampling
2.2. DNA Extraction, PCR Amplification and Sequencing
2.3. Statistical Analyses
3. Results
3.1. Specimens Sampled
3.2. Genetic Variation
3.3. Phylogeographic Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, P.J.; Huveneers, C.; Page, B.; Goldsworthy, S.D.; Coyne, M.; Lowther, A.D.; Mitchell, J.G.; Seuront, L. Living on the continental shelf edge: Habitat use of juvenile shortfin makos Isurus oxyrinchus in the Great Australian Bight, southern Australia. Fish. Oceanogr. 2015, 24, 205–218. [Google Scholar] [CrossRef]
- Santos, C.; Domingo, A.; Carlson, J.; Natanson, L.; Cortés, E.; Miller, P.; Hazin, F.; Travassos, P.; Mas, F.; Coelho, R. Habitat use and migrations of shortfin mako in the Atlantic using satellite telemetry. Collect. Vol. Sci. Pap. ICCAT 2018, 75, 445–456. [Google Scholar]
- Nasby-Lucas, N.; Dewar, H.; Sosa-Nishizaki, O.; Wilson, C.; Hyde, J.R.; Vetter, R.D.; Wraith, J.; Block, B.A.; Kinney, M.J.; Sippel, T.; et al. Movements of electronically tagged shortfin mako sharks (Isurus oxyrinchus) in the eastern North Pacific Ocean. Anim. Biotelemetry 2019, 7, 12. [Google Scholar] [CrossRef]
- Vaudo, J.J.; Byrne, M.E.; Wetherbee, B.M.; Harvey, G.M.; Shivji, M.S. Long-term satellite tracking reveals region-specific movements of a large pelagic predator, the shortfin mako shark, in the western North Atlantic Ocean. J. Appl. Ecol. 2017, 54, 1765–1775. [Google Scholar] [CrossRef]
- Natanson, L.J.; Kohler, N.E.; Ardizzone, D.; Cailliet, G.M.; Wintner, S.P.; Mollet, H.F. Validated age and growth estimates for the shortfin mako, Isurus oxyrinchus, in the North Atlantic Ocean. Environ. Biol. Fishes 2006, 77, 367–383. [Google Scholar] [CrossRef]
- Francis, M.P.; Duffy, C. Length at maturity in three pelagic sharks (Lamna nasus, Isurus oxyrinchus, and Prionace glauca) from New Zealand. Fish. Bull. 2005, 103, 489–500. [Google Scholar]
- Natanson, L.J.; Winton, M.; Bowlby, H.; Joyce, W.; Deacy, B.; Coelho, R.; Rosa, D. Updated reproductive parameters for the shortfin mako (Isurus oxyrinchus) in the north atlantic ocean with inferences of distribution by sex and reproductive stage. Fish. Bull. 2020, 118, 21–36. [Google Scholar] [CrossRef]
- Joung, S.J.; Hsu, H.H. Reproduction and embryonic development of the shortfin mako, Isurus oxyrinchus Rafinesque, 1810, in the Northwestern Pacific. Zool. Stud. 2005, 44, 487–496. [Google Scholar]
- Mollet, H.F.; Cliff, G.; Pratt, H.L.; Stevens, J.D. Reproductive biology of the female shortfin mako, Isurus oxyrinchus Rafinesque, 1810, with comments on the embryonic development of lamnoids. Fish. Bull. 2000, 98, 299–318. [Google Scholar]
- O’Farrell, H.B.; Babcock, E.A. Shortfin mako hot sets–Defining high bycatch conditions as a basis for bycatch mitigation. Fish. Res. 2021, 244, 106123. [Google Scholar] [CrossRef]
- Erguden, D.; Ayas, D.; Kabasakal, H. Recent Occurrence of Shortfin Mako Shark, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamnidae), from the North-Eastern Mediterranean Coast of Turkey. COMU J. Mar. Sci. Fish. 2021, 4, 79–85. [Google Scholar] [CrossRef]
- Barreto, R.R.; De Farias, W.K.T.; Andrade, H.; Santana, F.M.; Lessa, R. Age, growth and spatial distribution of the life stages of the shortfin mako, Isurus oxyrinchus (Rafinesque, 1810) caught in the western and central Atlantic. PLoS ONE 2016, 11, e0153062. [Google Scholar] [CrossRef] [PubMed]
- Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Jabado, R.W.; Liu, K.M.; Marshall, A.; Pacoureau, N.; et al. Isurus oxyrinchus. The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/39341/2903170 (accessed on 8 August 2023).
- Vella, A.; Vella, N.; Schembri, S. A molecular approach towards taxonomic identification of elasmobranch species from Maltese fisheries landings. Mar. Genom. 2017, 36, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Cortés, E.; Vaudo, J.J.; Harvey, G.C.M.N.; Sampson, M.; Wetherbee, B.M.; Shivji, M. Satellite telemetry reveals higher fishing mortality rates than previously estimated, suggesting overfishing of an apex marine predator. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170658. [Google Scholar] [CrossRef] [PubMed]
- Walls, R.H.L.; Soldo, A. Isurus oxyrinchus (Mediterranean Assessment). The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/39341/16527941 (accessed on 8 August 2023).
- UNCLOS United Nations Convention on the Law of the Sea-Annex I. Available online: https://www.un.org/depts/los/convention_agreements/texts/unclos/annex1.htm (accessed on 8 August 2023).
- CMS Appendices I and II of the Convention on the Conservation of Migratory Species of Wild Animals. Available online: https://www.cms.int/sites/default/files/basic_page_documents/appendices_cop13_e_0.pdf (accessed on 8 August 2023).
- Convention on the Conservation of European Wildlife and Natural Habitats (Bern Convention) Annex III. Available online: https://rm.coe.int/168097eb57 (accessed on 8 August 2023).
- CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora. Appendices I, II and III. Available online: https://cites.org/sites/default/files/eng/app/2023/E-Appendices-2023-05-21.pdf (accessed on 8 August 2023).
- UNEP/MAP-SPA/RAC SPA-BD Protocol Annex II: List of Endangered or Threatened Species. Available online: https://www.rac-spa.org/sites/default/files/annex/annex_2_en_20182.pdf (accessed on 8 August 2023).
- GFCM Recommendation GFCM/36/2012/3 on Fisheries Management Measures for the Conservation of Sharks and Rays in the GFCM Area of Application; GFCM: Rome, Italy, 2012.
- ICCAT Recommendation by ICCAT on Shortfin Mako Caught in Association with ICCAT Fisheries (14-06 BYC); ICCAT: Madrid, Spain, 2014.
- ICCAT Recommendation by ICCAT on the Conservation of the North Atlantic Stock of Shortfin Mako Caught in Association with ICCAT Fisheries (17-08 BYC); ICCAT: Madrid, Spain, 2017.
- ICCAT Recommendation by ICCAT on the Conservation of the North Atlantic Stock of Shortfin Mako Caught in Association with ICCAT Fisheries (21-09 BYC); ICCAT: Madrid, Spain, 2021.
- Kabasakal, H. Occurrence of shortfin mako shark, Isurus oxyrinchus Rafinesque, 1810, off Turkey’s coast. Mar. Biodivers. Rec. 2015, 8, e134. [Google Scholar] [CrossRef]
- Bradai, M.N.; Saidi, B.; Enajjar, S. Elasmobranches of the Mediterranean and Black Sea: Status, ecology and biology. Bibliographic analysis. In Studies and Reviews. General Fisheries Commission for the Mediterranean; FAO: Rome, Italy, 2012; Volume 91, ISBN 9786021018187. [Google Scholar]
- Maia, A.; Queiroz, N.; Correia, J.P.; Cabral, H. Food habits of the shortfin mako, Isurus oxyrinchus, off the southwest coast of Portugal. Environ. Biol. Fishes 2006, 77, 157–167. [Google Scholar] [CrossRef]
- Legislation Malta Subsidiary Legislation 549.44: Flora, Fauna, and Natural Habitats Protection Regulations. Available online: https://legislation.mt/eli/sl/549.44/eng/pdf (accessed on 8 August 2023).
- French, R.P.; Lyle, J.; Tracey, S.; Currie, S.; Semmens, J.M. High survivorship after catch-and-release fishing suggests physiological resilience in the endothermic shortfin mako shark (Isurus oxyrinchus). Conserv. Physiol. 2015, 3, cov044. [Google Scholar] [CrossRef]
- Keller, B.; Swimmer, Y.; Brown, C. Review on the effect of hook type on the catchability, hooking location, and post-capture mortality of the shortfin mako, Isurus oxyrinchus. Collect. Vol. Sci. Pap. ICCAT 2020, 77, 240–251. [Google Scholar]
- Campana, S.E.; Joyce, W.; Fowler, M.; Showell, M. Discards, hooking, and post-release mortality of porbeagle (Lamna nasus), shortfin mako (Isurus oxyrinchus), and blue shark (Prionace glauca) in the Canadian pelagic longline fishery. ICES J. Mar. Sci. 2016, 72, 520–528. [Google Scholar] [CrossRef]
- Rogers, P.; Ward, T.; van Ruth, P.; Williams, A.; Bruce, B.; Connell, S.; Currie, D.; Davies, C.; Evans, K.; Gillanders, B.; et al. Physical Processes, Biodiversity and Ecology of the Great Australian Bight Region: A Literaure Review; CSIRO: Canberra, Australia, 2013. [Google Scholar]
- Musyl, M.K.; Brill, R.W.; Curran, D.S.; Fragoso, N.M.; McNaughton, L.M.; Nielsen, A.; Kikkawa, B.S.; Moyes, C.D. Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish. Bull. 2011, 109, 341–368. [Google Scholar]
- Taguchi, M.; Kitamura, T.; Yokawa, K. Genetic population structure of shortfin mako (Isurus oxyrinchus) inferred from mitochondrial DNA on inter-oceanic scale. Isc. Ac. Affrc. Go. Jp. 2011, 11, 19–21. [Google Scholar]
- Corrigan, S.; Lowther, A.D.; Beheregaray, L.B.; Bruce, B.D.; Cliff, G.; Duffy, D.J.; Foulis, A.; Francis, M.P.; Goldsworthy, S.D.; Hyde, J.R.; et al. Population Connectivity of the Highly Migratory Shortfin Mako (Isurus oxyrinchus Rafinesque 1810) and Implications for Management in the Southern Hemisphere. Front. Ecol. Evol. 2018, 6, 187. [Google Scholar] [CrossRef]
- González, M.T.; Leiva, N.V.; Zárate, P.M.; Baeza, J.A. Regional (south-eastern Pacific Ocean) population genetics and global phylogeography of two endangered highly migratory pelagic sharks, the blue shark Prionace glauca and shortfin mako Isurus oxyrinchus. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 1098–1115. [Google Scholar] [CrossRef]
- Kacev, D. Understanding Population Connectivity in Shortfin Mako Shark (Isurus oxyrinchus) at Multiple Spatial Scales. Ph.D. Thesis, University of California Davis and San Diego State University, San Diego, CA, USA, 2015. [Google Scholar]
- Vella, N.; Vella, A. Population genetics of the deep-sea bluntnose sixgill shark, Hexanchus griseus, revealing spatial genetic heterogeneity. Mar. Genom. 2017, 36, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, C.L.; Blower, D.C.; Broderick, D.; Giles, J.L.; Holmes, B.J.; Kashiwagi, T.; Krück, N.C.; Morgan, J.A.T.; Tillett, B.J.; Ovenden, J.R. A review of the application of molecular genetics for fisheries management and conservation of sharks and rays. J. Fish Biol. 2012, 80, 1789–1843. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.J.; Streich, M.K.; Topping, T.S.; Stunz, G.W. New insights into the seasonal movement patterns of Shortfin mako sharks in the Gulf of Mexico. Front. Mar. Sci. 2021, 8, 623104. [Google Scholar] [CrossRef]
- Feldheim, K.A.; Gruber, S.H.; Dibattista, J.D.; Babcock, E.A.; Kessel, S.T.; Hendry, A.P. Two decades of genetic profiling yields first evidence of natal philopatry and long-term fidelity to parturition sites in sharks. Mol. Ecol. 2014, 23, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gubili, C.; Bilgin, R.; Kalkan, E.; Karhan, S.Ü.; Jones, C.S.; Sims, D.W.; Kabasakal, H.; Martin, A.P.; Noble, L.R. Antipodean white sharks on a Mediterranean walkabout? historical dispersal leads to genetic discontinuity and an endangered anomalous population. Proc. R. Soc. B Biol. Sci. 2011, 278, 1679–1686. [Google Scholar] [CrossRef]
- Quattro, J.M.; Stoner, D.S.; Driggers, W.B.; Anderson, C.A.; Priede, K.A.; Hoppmann, E.C.; Campbell, N.H.; Duncan, K.M.; Grady, J.M. Genetic evidence of cryptic speciation within hammerhead sharks (Genus Sphyrna). Mar. Biol. 2006, 148, 1143–1155. [Google Scholar] [CrossRef]
- Gubili, C.; Sims, D.W.; Veríssimo, A.; Domenici, P.; Ellis, J.; Grigoriou, P.; Johnson, A.F.; McHugh, M.; Neat, F.; Satta, A.; et al. A tale of two seas: Contrasting patterns of population structure in the small-spotted catshark across Europe. R. Soc. Open Sci. 2014, 1, 140175. [Google Scholar] [CrossRef]
- Cardeñosa, D.; Hyde, J.; Caballero, S. Genetic Diversity and Population Structure of the Pelagic Thresher Shark (Alopias pelagicus) in the Pacific Ocean: Evidence for Two Evolutionarily Significant Units. PLoS ONE 2014, 9, e110193. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Urso, I.; Damalas, D.; Martinsohn, J.; Zanzi, A.; Mariani, S.; Sperone, E.; Micarelli, P.; Garibaldi, F.; Megalofonou, P.; et al. Genetic differentiation and phylogeography of Mediterranean-North Eastern Atlantic blue shark (Prionace glauca, L. 1758) using mitochondrial DNA: Panmixia or complex stock structure? PeerJ 2017, 5, e4112. [Google Scholar] [CrossRef] [PubMed]
- Keeney, D.B.; Heist, E.J. Worldwide phylogeography of the blacktip shark (Carcharhinus limbatus) inferred from mitochondrial DNA reveals isolation of western Atlantic populations coupled with recent Pacific dispersal. Mol. Ecol. 2006, 15, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Mehlrose, M.R.; Bernard, A.M.; Finnegan, K.A.; Krausfeldt, L.E.; Lopez, J.V.; Shivji, M.S. Three complete mitochondrial genomes of shortfin mako sharks, Isurus oxyrinchus, from the Atlantic and Pacific Oceans. Mitochondrial DNA Part B 2022, 7, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Shao, K.T.; Lin, Y.S.; Tsai, A.Y.; Su, P.X.; Ho, H.C. The complete mitochondrial genome of the shortfin mako, Isurus oxyrinchus (Chondrichthyes, Lamnidae). Mitochondrial DNA 2015, 26, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.; Marra, N.; Shivji, M.S.; Stanhope, M.J. The complete mitochondrial genome of an Atlantic Ocean Shortfin Mako Shark, Isurus oxyrinchus. Mitochondrial DNA Part B 2019, 4, 3642–3643. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.S.; Hung, T.C.; Chang, H.A.; Huang, C.K.; Shiao, J.C. The species and origin of shark fins in Taiwan’s fishing ports, markets, and customs detention: A DNA barcoding analysis. PLoS ONE 2016, 11, e0147290. [Google Scholar] [CrossRef] [PubMed]
- Schrey, A.W.; Heist, E.J. Microsatellite analysis of population structure in the shortfin mako (Isurus oxyrinchus). Can. J. Fish. Aquat. Sci. 2003, 60, 670–675. [Google Scholar] [CrossRef]
- Taguchi, M.; Kitamura, T.; Shigenobu, Y.; Ohkubo, M.; Yanagimoto, T.; Sugaya, T.; Nakamura, Y.; Saitoh, K.; Yokawa, K. Development of 15 polymorphic microsatellite markers for the shortfin mako, Isurus oxyrinchus, and cross-species amplification in lamniforme sharks. Conserv. Genet. Resour. 2013, 5, 675–678. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D.; Gwiazdowski, R.A.; Ashlock, D.; Hanner, R. An exploration of sufficient sampling effort to describe intraspecific DNA barcode haplotype diversity: Examples from the ray-finned fishes (Chordata: Actinopterygii). DNA Barcodes 2016, 3, 66–73. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Vella, A.; Vella, N.; Karakulak, F.S.; Oray, I.; Garcia-Tiscar, S.; de Stephanis, R. Population genetics of Atlantic bluefin tuna, Thunnus thynnus (Linnaeus, 1758), in the Mediterranean: Implications for its conservation management. J. Appl. Ichthyol. 2016, 32, 523–531. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kabasakal, H.; de Maddalena, A. A huge shortfin mako shark Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamnidae) from the waters of Marmaris, Turkey. Ann. Ser. Hist. Nat. 2011, 1, 5–8. [Google Scholar]
- Celona, A.; Piscitelli, L.; de Maddalena, A. Two large shortfin makos, Isurus oxyrinchus, Rafinesque, 1809, caught off Sicily, Western Ionian Sea. Ann. Ser. Hist. Nat. 2004, 14, 35–42. [Google Scholar]
- Lopez-Mirones, F.; de Maddalena, A.; Sagarminaga van Buiten, R. On a huge shortfin mako shark Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamnidae) observed at Cabrera Grande, Balearic Islands, Spain. Ann. Ser. Hist. Nat. 2020, 30, 25–30. [Google Scholar]
- Celona, A.; Donato, N.; de Maddalena, A. In relation to the captures of a great white shark, Carcharodon carcharias (Linnaeus, 1758), and a shortfin mako, Isurus oxyrinchus Rafinesque, 1809, in the Messina Strait. Ann. Ser. Hist. Nat. 2001, 11, 13–16. [Google Scholar]
- Maia, A.; Queiroz, N.; Cabral, H.N.; Santos, A.M.; Correia, J.P. Reproductive biology and population dynamics of the shortfin mako, Isurus oxyrinchus Rafinesque, 1810, off the southwest Portuguese coast eastern North Atlantic. J. Appl. Ichthyol. 2007, 23, 246–251. [Google Scholar] [CrossRef]
- Kohler, N.E.; Casey, J.G.; Turner, P.A. Lenght-weight relationships for 13 species of sharks from the western North Atlantic. Fish. Bull. 1995, 93, 412–418. [Google Scholar]
- Campana, S.E.; Marks, L.; Joyce, W. The biology and fishery of shortfin mako sharks (Isurus oxyrinchus) in Atlantic Canadian waters. Fish. Res. 2005, 73, 341–352. [Google Scholar] [CrossRef]
- Alahyene, J.; Chiahou, B.; El Habouz, H.; Ben-Bani, A. Sex ratio and male maturity for Shortfin mako shark in the Moroccan central Atlantic coast. Croat. J. Fish. 2022, 80, 67–75. [Google Scholar] [CrossRef]
- Vella, N.; Vella, A. A preliminary study of the Bluntnose sixgill shark, Hexanchus griseus, in the central Mediterranean region around the Maltese Islands. Rapp. Comm. Int. Mer Méditerranée 2010, 39, 695. [Google Scholar]
- Van Der Valk, T.; Sandoval-Castellanos, E.; Caillaud, D.; Ngobobo, U.; Binyinyi, E.; Nishuli, R.; Stoinski, T.; Gilissen, E.; Sonet, G.; Semal, P.; et al. Significant loss of mitochondrial diversity within the last century due to extinction of peripheral populations in eastern gorilla. Sci. Rep. 2018, 8, 6551. [Google Scholar] [CrossRef] [PubMed]
- Patarnello, T.; Volckaert, F.A.M.J.; Castilho, R. Pillars of Hercules: Is the Atlantic-Mediterranean transition a phylogeographical break? Mol. Ecol. 2007, 16, 4426–4444. [Google Scholar] [CrossRef]
- Carruthers, T.; Di Natale, A.; Lauretta, M.; Paga Garcia, A.; Tensek, S. Migratory behaviour of Atlantic Bluefin Tuna entering the Mediterranean. Collect. Vol. Sci. Pap. ICCAT 2018, 74, 3082–3099. [Google Scholar]
- Abid, N.; Bakkali, M.; Tserpes, G.; Idrissi, M. Swordfish growth pattern in the strait of Gibraltar; implications for Atlantic and Mediterranean stock mixing. Mediterr. Mar. Sci. 2014, 15, 135–144. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H.L. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea; IUCN: Malaga, Spain, 2016. [Google Scholar]
- Vella, N.; Vella, A. The complete mitogenome of the Critically Endangered smalltooth sand tiger shark, Odontaspis ferox (Lamniformes: Odontaspididae). Mitochondrial DNA Part B 2020, 5, 3319–3322. [Google Scholar] [CrossRef]


| Specimen ID | Date | Total Length (cm) a | Gender a | Haplotype | Accession Numbers |
|---|---|---|---|---|---|
| CBRG-Ioxy01 | 8 February 2005 | 127 | Male | H1 | OR497223 |
| CBRG-Ioxy10 | 30 April 2005 | 84 | Female | H4 b | OR497232 |
| CBRG-Ioxy11 | 22 June 2005 | 185 | Male | H1 | OR497233 |
| CBRG-Ioxy12 | 25 April 2006 | 323 | Female | H1 | OR497234 |
| CBRG-Ioxy13 | 25 April 2006 | 208 | Female | H4 b | OR497235 |
| CBRG-Ioxy02 | 9 May 2006 | 203 | Male | H5 b | OR497224 |
| CBRG-Ioxy14 | 18 May 2006 | 235 | Male | H2 | OR497236 |
| CBRG-Ioxy15 | 18 May 2006 | 350 | Female | H6 b | OR497237 |
| CBRG-Ioxy16 | 21 May 2006 | 222 | Male | H7 | OR497238 |
| CBRG-Ioxy17 | 31 May 2006 | 184 | Male | H1 | OR497239 |
| CBRG-Ioxy18 | 31 May 2006 | 81 | Female | H1 | OR497240 |
| CBRG-Ioxy03 | 10 June 2006 | 303 | Female | H8 | OR497225 |
| CBRG-Ioxy33 | 22 June 2006 | - | - | H1 | OR497255 |
| CBRG-Ioxy37 | 2 February 2008 | 110 | - | H3 | OR497259 |
| CBRG-Ioxy19 | 26 February 2008 | 170 | Male | H2 | OR497241 |
| CBRG-Ioxy20 | 28 February 2008 | 227 | Male | H1 | OR497242 |
| CBRG-Ioxy21 | 28 February 2008 | 258 | Male | H1 | OR497243 |
| CBRG-Ioxy22 | 8 April 2009 | - | - | H2 | OR497244 |
| CBRG-Ioxy23 | 8 April 2009 | - | - | H2 | OR497245 |
| CBRG-Ioxy04 | 26 April 2009 | 297 | Female | H2 | OR497226 |
| CBRG-Ioxy24 | 8 May 2009 | 232 | Female | H6 b | OR497246 |
| CBRG-Ioxy25 | 12 May 2009 | 217 | - | H4 b | OR497247 |
| CBRG-Ioxy34 | 21 May 2009 | 316 | Female | H2 | OR497256 |
| CBRG-Ioxy35 | 21 May 2009 | 215 | Male | H3 | OR497257 |
| CBRG-Ioxy36 | 21 May 2009 | 213 | Female | H1 | OR497258 |
| CBRG-Ioxy05 | 23 May 2009 | - | - | H1 | OR497227 |
| CBRG-Ioxy26 | 10 July 2009 | 188 | Female | H3 | OR497248 |
| CBRG-Ioxy27 | 10 July 2009 | 132 | Male | H1 | OR497249 |
| CBRG-Ioxy06 | 8 June 2010 | 124 | Female | H2 | OR497228 |
| CBRG-Ioxy28 | 10 July 2010 | - | - | H2 | OR497250 |
| CBRG-Ioxy29 | 9 May 2011 | 223 | Female | H7 | OR497251 |
| CBRG-Ioxy30 | 9 May 2011 | 163 | Male | H9 | OR497252 |
| CBRG-Ioxy31 | 28 May 2011 | 219 | - | H2 | OR497253 |
| CBRG-Ioxy32 | 8 July 2011 | 204 | Male | H1 | OR497254 |
| CBRG-Ioxy07 | 5 December 2011 | 122 | Male | H1 | OR497229 |
| CBRG-Ioxy08 | 24 January 2012 | - | - | H1 | OR497230 |
| CBRG-Ioxy09 | 2 March 2012 | 130 | Male | H2 | OR497231 |
| NAtl (n = 30) | SAfr (n = 92) | NInd (n = 77) | IndP (n = 22) | WAus (n = 45) | EAus (n = 59) | NZea (n = 39) | ||
|---|---|---|---|---|---|---|---|---|
| Med | FST | 0.063 | 0.109 * | 0.325 * | 0.136 | 0.123 * | 0.115 * | 0.133 * |
| (n = 37) | p-value | 0.0067 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| ϕST | 0.052 | 0.181 * | 0.458 * | 0.197 * | 0.168 * | 0.195 * | 0.228 * | |
| p-value | 0.0532 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vella, N.; Vella, A. Phylogeographic Analyses of the Shortfin Mako, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamniformes) from the Central Mediterranean Sea, a Critically Endangered Species in the Region. Fishes 2023, 8, 520. https://doi.org/10.3390/fishes8100520
Vella N, Vella A. Phylogeographic Analyses of the Shortfin Mako, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamniformes) from the Central Mediterranean Sea, a Critically Endangered Species in the Region. Fishes. 2023; 8(10):520. https://doi.org/10.3390/fishes8100520
Chicago/Turabian StyleVella, Noel, and Adriana Vella. 2023. "Phylogeographic Analyses of the Shortfin Mako, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamniformes) from the Central Mediterranean Sea, a Critically Endangered Species in the Region" Fishes 8, no. 10: 520. https://doi.org/10.3390/fishes8100520
APA StyleVella, N., & Vella, A. (2023). Phylogeographic Analyses of the Shortfin Mako, Isurus oxyrinchus Rafinesque, 1810 (Chondrichthyes: Lamniformes) from the Central Mediterranean Sea, a Critically Endangered Species in the Region. Fishes, 8(10), 520. https://doi.org/10.3390/fishes8100520







