Distribution and Expansion of Alien Fish Species in the Karun River Basin, Iran
Abstract
1. Introduction
2. Materials and Methods
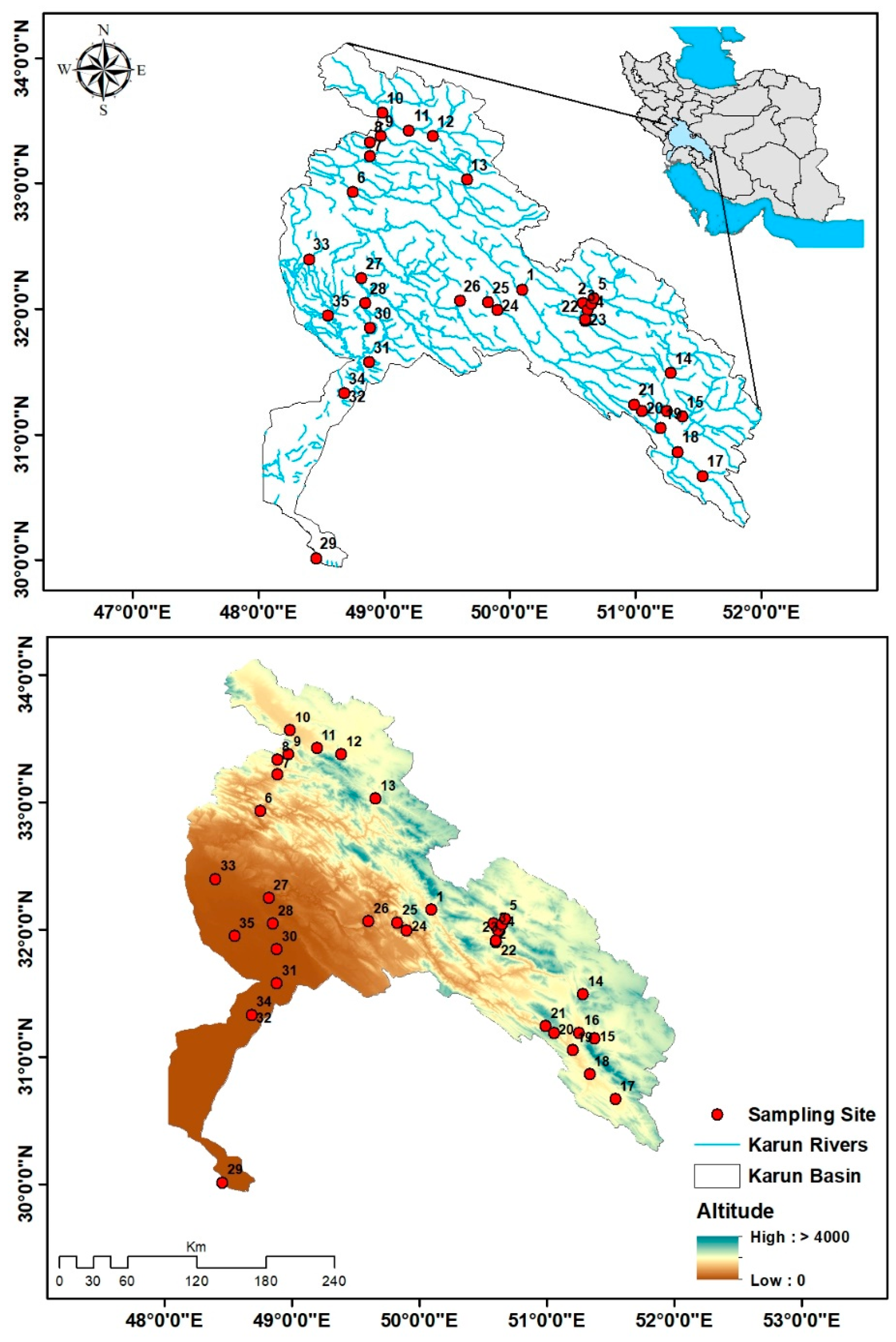
2.1. Study Area
2.2. Fish Sampling
2.3. Water Sampling
2.4. Data Analysis
3. Results
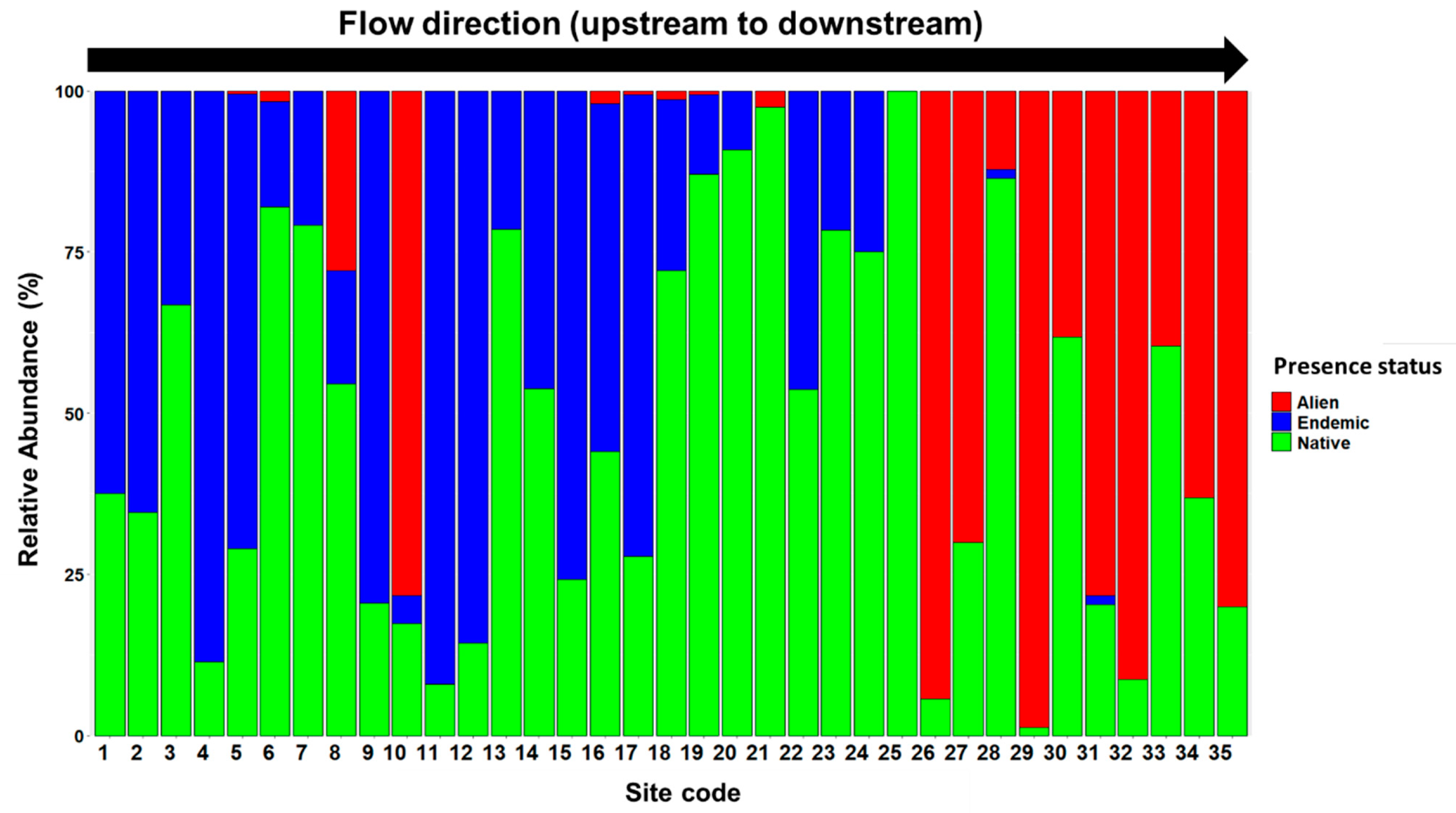
3.1. Alien Fish Species Composition
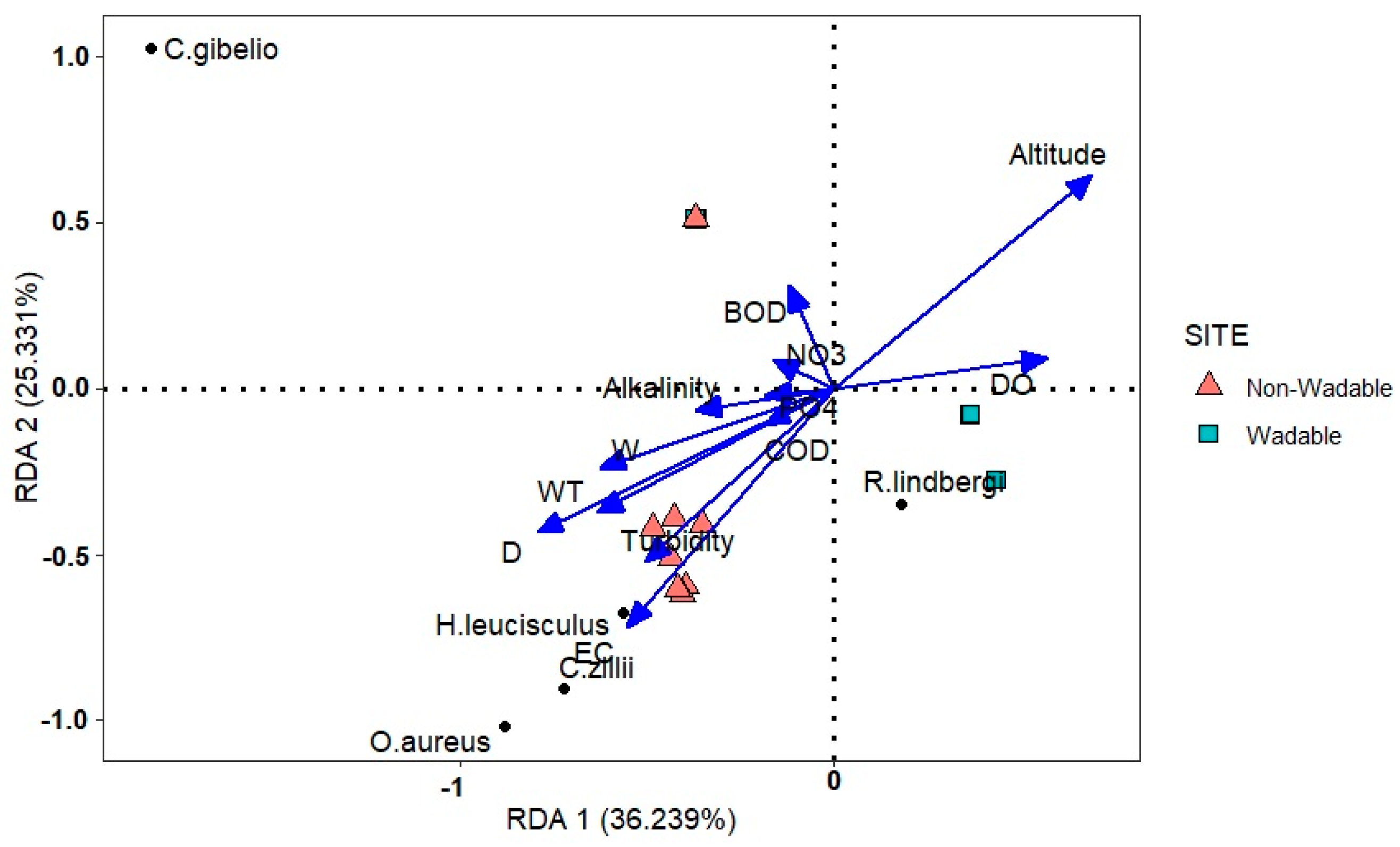
3.2. Relationships between Alien Fish Assemblages and Environmental Variables
4. Discussion
Potential Origin, Possible Destructive Effects, and Management of Alien Fish Species Observed in the Karun River Basin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

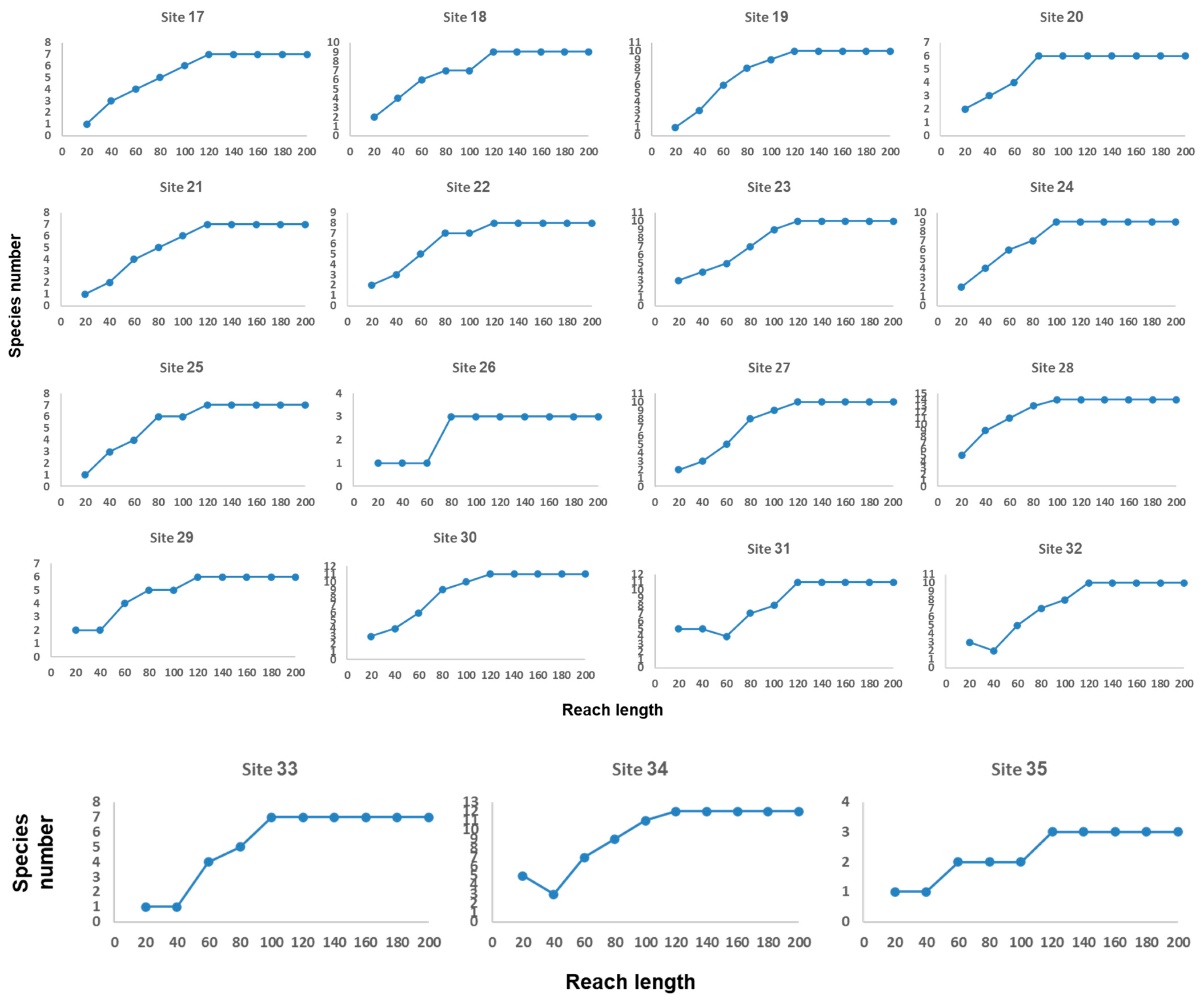
Appendix B
| Family | Cyprinidae | Leuciscidae | Xenocyprididae | Nemacheilidae | Sisoridae | Mugilidae | Aphanidae | Mastacembelidae | Salmonidae | Gobiidae | Gobionidae | Poeciliidae | Cichlidae | Danionidae | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Capoeta coadi | Capoeta aculeata | Capoeta trutta | Carassius gibelio | Arabibarbus grypus | Cyprinus carpio | Garra rufa | Garra gymnothorax | Barbus lacerta | Barbus karunensis | Luciobarbus barbulus | Carasobarbus luteus | Carasobarbus kosswigi | Cyprion macrostomus | Chondrostoma regium | Squalius berak | Squalius lepidus | Acanthobrama marmid | Alburnus sellal | Alburnus caeruleus | Alburnus doriae | Alburnoides idignesis | Hemiculter leucisculus | Ctenopharyngodon idella | Turcinoemacheilus saadii | Turcinoemacheilus hafezi | Oxynoemacheilus euphraticus | Glyptothorax galaxias | Glyptothorax alidaeii | PlaniPlaniliza abu | Esmaeilius vladykovi | Mastacembelus mastacembelus | Oncorhynchus mykiss | Rhinogobius lindbergi | Pseudorasbora parva | Gambusia holbrooki | Oreochromis aureus | Coptodon zillii | Bariliusmesopotamicus |
| 1 | + | + | + | - | - | - | - | - | - | - | - | - | - | - | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2 | + | + | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| 3 | + | - | - | - | - | - | + | + | + | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - |
| 4 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 5 | + | + | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - |
| 6 | + | - | - | + | - | - | + | + | -- | - | + | - | - | + | - | - | - | - | + | - | - | - | - | - | - | - | - | + | + | + | - | - | - | - | - | - | - | - | - |
| 7 | + | - | + | - | + | - | - | - | - | - | - | - | + | + | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 8 | + | - | + | + | - | - | + | + | + | - | - | - | - | - | + | - | - | - | + | - | - | + | - | - | - | - | -- | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | + | - | + | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | + | - | + | + | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 10 | + | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | + | + | - | - | - |
| 11 | + | - | + | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 12 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 13 | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - |
| 14 | + | - | - | - | - | - | + | + | - | - | - | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 15 | + | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| 16 | + | + | -- | - | - | - | + | + | - | + | + | - | - | - | + | + | + | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - |
| 17 | + | + | - | + | - | - | + | + | - | + | - | - | - | - | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 18 | + | + | - | + | - | - | + | + | - | - | - | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 19 | + | + | + | + | - | - | + | + | - | + | - | - | - | + | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 20 | + | + | + | - | - | - | + | + | - | - | + | - | - | - | + | + | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 21 | + | - | + | + | - | - | + | + | - | - | + | - | - | - | + | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 22 | - | - | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | - | + | - | + | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| 23 | + | + | - | - | - | - | - | - | - | - | + | - | - | - | + | + | + | - | - | - | + | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| 24 | - | + | + | - | - | - | + | + | - | - | - | - | - | - | + | + | + | - | + | - | + | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - | - | - |
| 25 | + | - | + | - | - | - | + | + | - | - | - | - | - | + | + | - | - | - | + | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - |
| 26 | - | - | + | - | + | - | + | + | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | - | - |
| 27 | - | - | - | + | - | - | + | + | - | - | - | - | - | + | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - | - | + | + | - |
| 28 | - | - | - | + | - | - | - | - | - | - | - | + | - | + | + | - | - | - | + | - | + | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | + | + | + |
| 29 | - | - | + | + | - | - | + | + | - | - | - | - | - | + | + | - | - | + | - | - | - | - | + | - | - | - | - | - | - | +- | - | - | - | - | - | - | + | + | - |
| 30 | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | + | - | - | - | - | + | + | + |
| 31 | - | - | - | + | - | - | + | + | - | - | - | - | - | + | + | - | - | + | + | - | + | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | + | + | + |
| 32 | - | - | - | + | - | - | - | - | - | - | - | + | - | + | + | - | - | ++ | - | - | - | - | + | - | - | - | - | - | - | + | - | - | - | - | - | - | + | + | +- |
| 33 | - | - | - | + | - | - | + | + | - | - | - | + | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 34 | - | - | + | + | - | + | - | - | - | - | - | - | - | + | + | - | - | + | + | - | - | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | + | + | - |
| 35 | - | - | + | + | - | - | + | + | - | - | - | + | - | + | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Appendix C
Appendix D
| Site Code | Substrate Characteristics |
|---|---|
| 1 | Cobbles |
| 2 | Cobbles |
| 3 | Boulders (small) |
| 4 | Boulders (small) |
| 5 | Boulders (small) |
| 6 | Cobbles |
| 7 | Cobbles |
| 8 | Cobbles |
| 9 | Cobbles |
| 10 | Gravel (Fine) |
| 11 | Boulders (small) |
| 12 | Cobbles |
| 13 | Boulders (small) |
| 14 | Cobbles |
| 15 | Boulders (small) |
| 16 | Cobbles |
| 17 | Cobbles |
| 18 | Cobbles |
| 19 | Boulders (small) |
| 20 | Boulders (small) |
| 21 | Cobbles |
| 22 | Cobbles |
| 23 | Cobbles |
| 24 | Boulders (small) |
| 25 | Boulders (small) |
| 26 | Cobbles |
| 27 | Sand |
| 28 | Sand |
| 29 | Mud |
| 30 | Mud |
| 31 | Mud |
| 32 | Mud |
| 33 | Mud |
| 34 | Gravel (Coarse) |
| 35 | Sand |
References
- Bănăduc, D.; Simić, V.M.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simi, S.B. Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef]
- Wahltinez, S.J.; Kroll, K.J.; Behringer, D.C.; Arnold, J.E.; Whitaker, B.; Newton, A.L.; Edmiston, K.; Hewson, I.; Stacy, N.I. Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors. Fishes 2023, 8, 51. [Google Scholar] [CrossRef]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple freshwater stressors-Key drivers for the future of freshwater environments. Front. Environ. Sci. 2023, 11, 92. [Google Scholar] [CrossRef]
- Navarro-Ortega, A.; Acuña, V.; Bellin, A.; Burek, P.; Cassiani, G.; Choukr-Allah, R.; Dolédec, S.; Elosegi, A.; Ferrari, F.; Ginebreda, A.; et al. Managing the effects of multiple stressors on aquatic ecosystems under water scarcity. The GLOBAQUA project. Sci. Total Environ. 2015, 503–504, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Curtean-Bănăduc, A.; Olosutean, H.; Bănăduc, D. Influence of Environmental Variables on the Structure and Diversity of Ephemeropteran Communities: A Case Study of the Timiș River, Romania. Acta Zool. Bulg. 2016, 68, 215–224. [Google Scholar]
- Wheeler, C. The Ecosystem Role of Fishes in Lotic Environments. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2014; p. 3694. [Google Scholar]
- Villéger, S.; Brosse, S.; Mouchet, M.A.; Mouillot, D.; Vanni, M.J. Functional ecology of fish: Current approaches and future challenges. Aquat. Sci. 2017, 79, 783–801. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Zubcov, N.; Zubcov, E.; Schnenk, D. The dynamics of metals in fish from Nistru and Prut rivers (Moldova). Transylv. Rev. Syst. Ecol. Res. 2008, 6, 51–58. [Google Scholar]
- Curtean-Bănăduc, A.; Marić, S.; Gabor, G.; Didenko, A.; Rey Planellas, S.; Bănăduc, D. Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians—Conservation elements. Turk. J. Zool. 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Jeeva, V.; Kumar, S.; Verma, D.; Rumana, H.S. River fragmentation and connectivity problems in Gange River of upper Himalayas: The effect on the fish communities (India). Transylv. Rev. Syst. Ecol. Res. 2011, 12, 75–90. [Google Scholar]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The role of aquatic refuge habitats for fish, and threats in the context of climate change and human impact, during seasonal hydrological drought in the Saxon Villages area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Sosai, A.S. Illegal fishing in southern Mannar Island coastal area (Sri Lanka). Transylv. Rev. Syst. Ecol. Res. 2015, 17, 95–108. [Google Scholar]
- Zare-Shahraki, M.; Ebrahimi-Dorche, E.; Bruder, A.; Flotermersch, J.; Blocksom, K.; Bănăduc, D. Fish species composition, distribution and community structure in relation to environmental variation in a semi-arid mountainous river basin, Iran. Water 2022, 14, 2226. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, Z. Effects of environmental pollution on fish: A short review. Transylv. Rev. Syst. Ecol. Res. 2017, 19, 49–60. [Google Scholar] [CrossRef]
- Bourillon, B.; Feunteun, E.; Acou, A.; Trancart, T.; Teichert, N.; Belpaire, C.; Dufour, S.; Bustamante, P.; Aarestrup, K.; Walker, A.; et al. Anthropogenic Contaminants Shape the Fitness of the Endangered European Eel: A Machine Learning Approach. Fishes 2022, 7, 274. [Google Scholar] [CrossRef]
- Baker, S.M.; Reyier, E.A.; Ahr, B.J.; Cook, G.S. Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies. Fishes 2023, 8, 171. [Google Scholar] [CrossRef]
- Kar, D. Wetlands and their fish diversity in Assam (India). Transylv. Rev. Syst. Ecol. Res. 2019, 21, 47–94. [Google Scholar] [CrossRef]
- Bănăduc, D.; Maric, S.; Cianfaglione, K.; Afanasyev, S.; Somogyi, D.; Nyeste, K.; Antal, L.; Kosco, J.; Caleta, M.; Wanzenbock, J.; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species drive a Fish to the Edge of Extinction. Sustainability 2022, 14, 13493. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Ahammad, A.K.S.; Mahalder, B.; Alam, M.M.; Hasan, N.A.; Bashar, A.; Biswas, J.C.; Haque, M.M. Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study. Fishes 2022, 7, 270. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Mihuț, C.; Burcea, A.; McCall, G.S.; Matei, C.; Bănăduc, D. Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem; Chondrostoma nasus (Linnaeus, 1758) Case Study. Water 2023, 15, 1578. [Google Scholar] [CrossRef]
- Taiwo, I.O.; Olopade, O.A.; Bamidele, N.A. Heavy metal concentration in eight fish species from Epe Lagoon (Nigeria). Transylv. Rev. Syst. Ecol. Res. 2019, 21, 69–82. [Google Scholar] [CrossRef][Green Version]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Banaduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea coast area lakes: A broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Mathers, K.L.; Guareschi, S.; Pattison, Z. Biological invasions in rivers and associated ecosystems: New insights, challenges, and methodological advancements. River Res. Appl. 2022, 38, 1351–1355. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Kipp, R. Predicting the number of ecologically harmful exotic species in an aquatic system. Divers. Distrib. 2008, 14, 374–380. [Google Scholar] [CrossRef]
- Fedorenkova, A.; Vonk, J.A.; Breure, A.M.; Hendriks, A.J.; Leuven, R.S.E.W. Tolerance of native and non-native fish species to chemical stress: A case study for the River Rhine. Aquat. Invasions 2013, 8, 231–241. [Google Scholar] [CrossRef]
- Piria, M.; Simonović, P.; Kalogianni, E.; Vardakas, L.; Koutsikos, N.; Zanella, D.; Ristovska, M.; Apostolou, A.; Adrović, A.; Mrdak, D.; et al. Alien freshwater fish species in the Balkans—Vectors and pathways of introduction. Fish Fish. 2018, 19, 138–169. [Google Scholar] [CrossRef]
- WWF. Living Planet Report 2022 – Building a Nature-Positive Society; Almond, R.E.A., Grooten, M., Juffe Bignoli, D., Petersen, T., Eds.; WWF: Gland, Switzerland, 2000. [Google Scholar]
- Gozlan, R.E.; Andreou, D.; Asaeda, T.; Beyer, K.; Bouhadad, R.; Burnard, D.; Caiola, N.; Cakic, P.; Djikanovic, V.; Esmaeili, H.R.; et al. Pan-continental invasion of Pseudorasbora parva: Towards a better understanding of freshwater fish invasions. Fish Fish. 2010, 11, 315–340. [Google Scholar] [CrossRef]
- Falk-Petersen, J.; Bohn, T.; Sandlund, O.T. On the numerous concepts in invasion biology. Biol. Invasions 2006, 8, 1409–1424. [Google Scholar] [CrossRef]
- Alves, C.B.M.; Vieira, F.; Magalhaes, A.L.B.; Brito, M.F.G. Impacts of Non-Native Fish Species in Minas Gerais, Brazil: Present Situation and Prospects. In Ecological and Genetic Implications of Aquaculture Activities; Springer: Dordrecht, The Netherlands, 2007; pp. 291–314. [Google Scholar]
- Jawad, L.A. (Ed.) Tigris and Euphrates Rivers: Their Environment from Headwaters to Mouth; Aquatic Ec. Springer: Auckland, New Zealand, 2021. [Google Scholar]
- Veer, G.; Nentwig, W. Environmental and economic impact assessment of alien and invasive fish species in Europe using the generic impact scoring system. Ecol. Freshw. Fish 2015, 24, 646–656. [Google Scholar] [CrossRef]
- Mousavi-Sabet, H. Exotic ornamental fishes in Iranian inland water basins: An updated checklist. J. Anim. Divers. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Patimar, R. Fish species diversity in the lakes of Alma-Gol, Adji-Gol, and Ala-Gol, Golestan province, northern Iran. J. Ichthyol. 2008, 48, 911–917. [Google Scholar] [CrossRef]
- Jha, D.N.; Joshi, K.D.; Alam, M.A.; Das, S.C.S.; Kumar, V. Dominance of exotic fishes in the River Ganga at Allahabad stretch. J. Kalash Sci. 2016, 4, 1–6. [Google Scholar]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Leprieur, F.; Beauchard, O.; Blanchet, S.; Oberdorff, T.; Brosse, S. Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biol. 2008, 6, 404–410. [Google Scholar] [CrossRef]
- Rahel, F.J.; Bierwagen, B.; Taniguchi, Y. Managing aquatic species of conservation concern in the face of climate change and invasive species. Conserv. Biol. 2008, 22, 551–561. [Google Scholar] [CrossRef]
- Bani, L.; Orioli, V.; Trasforini, S.; Puzzi, C.M.; Sibilia, A.; Dondina, O.; Tirozzi, P. The spread of exotic fish species in Italian rivers and their effect on native fish fauna since 1990. Biodiversity 2021, 22, 4–12. [Google Scholar] [CrossRef]
- Adha, K.; Esa, Y.; Arshad, A. The Influence of Alien Fish Species on Native Fish Community Structue in Malaysian Waters. Kuroshio Sci. 2013, 7, 81–93. [Google Scholar]
- Britton, J.R.; Davies, G.D.; Harrod, C. Trophic interactions and consequent impacts of the invasive fish Pseudorasbora parva in a native aquatic foodweb: A field investigation in the UK. Biol. Invasions 2010, 12, 1533–1542. [Google Scholar] [CrossRef]
- Gozlan, R.; Hilaire, S.; Feist, S.W.; Martin, P.; Kent, M.L. Biodiversity: Disease threat to European fish. Nature 2005, 435, 1046. [Google Scholar] [CrossRef]
- Leuven, R.; Hendriks, A.; Huijbregts, M.; Lenders, H.; Matthews, J.; Van Der Velde, G. Differences in sensitivity of native and exotic fish species to changes in river temperature. Curr. Zool. 2011, 57, 852–862. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, D.; Srivastava, S.C.; Ansari, A.; Jena, J.K.; Sarkar, U.K. Invasion and impacts of alien fish species in the Ganga River, India. Aquat. Ecosyst. Health Manag. 2013, 16, 408–414. [Google Scholar] [CrossRef]
- Kennard, M.J.; Arthington, A.H.; Pusey, J.; Harch, B.D. Are alien fish a reliable indicator of river health? Freshw. Biol. 2005, 50, 174–193. [Google Scholar] [CrossRef]
- Jouladeh Roudbar, A.; Ghanavi, H.R.; Doadrio, I. Ichthyofauna from iranian freshwater: Annotated checklist, diagnosis, taxonomy, distribution and conservation assessment. Zool. Stud. 2020, 59, 1–303. [Google Scholar] [CrossRef]
- Tabasian, H.; Abdoli, A.; Khorasani, N.; Dehghan Madiseh, S. The reproductive biology of the invasive redbelly tilapia, Coptodon zillii, case study: Shadegan Wetland, Iran. J. Wildl. Biodivers. 2022, 6, 244971122. [Google Scholar]
- Altun, T. Tilapia Culture and Its Problems in Turkey. J. Fish Aquat. Sci. 2006, 23, 473–478. [Google Scholar]
- Al-Faisal, A.J.; Mutlak, F.M.; Abdullah, S.A. Exotic freshwater fishes in the southern Iraq. Marsh Bull. 2014, 9, 65–78. [Google Scholar]
- Mohamed, A.-R.; Abood, A.N. Dispersal of the exotic fish in the Shatt Al-Arab. J. Agric. Vet. Sci. 2017, 10, 50–57. [Google Scholar]
- Coad, B.; Abdoli, A. Exotic fish species in the fresh waters of Iran. Zool. Middle East 1993, 9, 65–80. [Google Scholar]
- Waldron, A.; Miller, D.C.; Redding, D.; Mooers, A.; Kuhn, T.S.; Nibbelink, N.; Roberts, J.T.; Tobias, J.A.; Gittleman, J.L. Reductions in global biodiversity loss predicted from conservation spending. Nature 2017, 551, 364–367. [Google Scholar] [CrossRef]
- Coelho, P.N.; Henry, R. The small foreigner: New laws will promote the introduction of non-native zooplankton in Brazilian aquatic environments. Acta Limnol. Bras. 2017, 29, e7. [Google Scholar] [CrossRef]
- Esmaeili, H.R.; Teimori, A.; Owfi, F.; Abbasi, K.; Coad, B.W. Alien and invasive freshwater fish species in Iran: Diversity, environmental impacts and management. Iran. J. Ichthyol. 2014, 1, 61–72. [Google Scholar]
- Naddafi, K.; Honari, H.; Ahmadi, M. Water quality trend analysis for the Karoon River in Iran. Environ. Monit. Assess. 2007, 134, 305–312. [Google Scholar] [CrossRef]
- Fathi, P.; Dorche, E.E.; Shahraki, M.Z.; Stribling, J.; Kashkooli, O.B.; Ofogh, A.E.; Bruder, A. Revised Iranian Water Quality Index (RIWQI): A tool for the assessment and management of water quality in Iran. Environ. Monit. Assess. 2022, 194, 504. [Google Scholar] [CrossRef]
- Rahimi, D.; Hasheminasab, S.; Abdollahi, K. Assessment of temperature and rainfall changes in the Karoun River basin. Theor. Appl. Climatol. 2019, 137, 2829–2839. [Google Scholar] [CrossRef]
- Rahimi, Y.; Saghafian, B.; Banihashemi, M.A. Hydrological and Hydraulic Uncertainty Analysis in Probabilistic Design of Flood Diversion Systems Using NSGAII and Bivariate Frequency Analysis. Iran. J. Sci. Technol. Trans. Civ. Eng. 2020, 45, 2651–2662. [Google Scholar] [CrossRef]
- Sadeghianl, M.S.; Hassunizadeh, H.; McNaughton, A.N. Optimising the River Karun system, Iran. Trans. Ecol. Environ. 2003, 60, 109–118. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Khosravi, M.; Siadatmousavi, S.M.; Yari, S.; Azizpour, J. Observation of currents in Karun River. Res. Mar. Sci. 2017, 2, 50–58. [Google Scholar]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The Relation Between the Number of Species and the Number of Individuals in a Random Sample of an Animal Population. J. Anim. Ecol. 1943, 12, 42. [Google Scholar] [CrossRef]
- Zare Shahraki, M.; Ebrahimi Dorche, E.; Keivany, Y.; Blocksom, K.A.; Bruder, A.; Flotemersch, J.E. A fish-based multi-metric assessment index in the Karun River Basin, Iran. River Res. Appl. 2022, 38, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Keivany, Y.; Nasri, M.; Abbasi, K.; Abdoli, A. Atlas of Inland Water Fishes of Iran; Iran Department of Environment: Tehran, Iran, 2016. [Google Scholar]
- Froese, R.; Pauly, D. Fish Base. World Wide Web Electronic Publication. Version (12/2019). 2019. Available online: www.fishbase.org (accessed on 20 March 2019).
- Ter Braak, C.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, Version 4.5; 2002; (Microcomputer Power); Available online: www.canoco.com; https://edepot.wur.nl/405659; (accessed on 12 October 2023). [Google Scholar]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using Canoco 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Milardi, M.; Gavioli, A.; Soininen, J.; Castaldelli, G. Exotic species invasions undermine regional functional diversity of freshwater fish. Sci. Rep. 2019, 9, 17921. [Google Scholar] [CrossRef]
- Milardi, M.; Aschonitis, V.; Gavioli, A.; Lanzoni, M.; Fano, E.A.; Castaldelli, G. Run to the hills: Exotic fish invasions and water quality degradation drive native fish to higher altitudes. Sci. Total Environ. 2018, 624, 1325–1335. [Google Scholar] [CrossRef]
- Huang, J.; Huang, L.; Wu, Z.; Mo, Y.; Zou, Q.; Wu, N.; Chen, Z. Correlation of fish assemblages with habitat and environmental variables in a headwater stream section of Lijiang River, China. Sustainability 2019, 11, 1135. [Google Scholar] [CrossRef]
- Purevdorj, Z.; Munkhbayar, M.; Paek, W.K.; Ganbold, O.; Jargalsaikhan, A.; Purevee, E.; Amartuvshin, T.; Genenjamba, U.; Nyam, B.; Lee, J.W. Relationships between Bird Assemblages and Habitat Variables in a Boreal Forest of the Khentii Mountain, Northern Mongolia. Forests 2022, 13, 1037. [Google Scholar] [CrossRef]
- Blocksom, K.A.; Johnson, B.R. Development of a regional macroinvertebrate index for large river bioassessment. Ecol. Indic. 2009, 9, 313–328. [Google Scholar] [CrossRef]
- Shahraki, M.Z.; Dorche, E.E.; Fathi, P.; Flotemersch, J.; Blocksom, K.; Stribling, J.; Keivany, Y.; Kashkooli, O.B.; Scown, M.; Bruder, A. Defining a Disturbance Gradient in a Middle-Eastern River Basin. Limnologica 2021, 91, 125923. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, F. Vegan: Community Ecology Package; 2017; R package Version 2.4-3; Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 October 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 12 October 2023).
- DIVA-GIS. Free Spatial Data by Country. 2017. Available online: http://www.diva-gis.org/gdata (accessed on 15 February 2018).
- ESRI. ArcGIS Desktop; Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- US Geological Survey. U.S. Geological Survey. National Water Information System Data Available on the World Wide Web. 2020. Available online: https://waterdata.usgs.gov/nwis/ (accessed on 30 June 2018).
- Hosseini, S.A.; Mirvaghefi, A.; Alishahi, M.; Genetics, F.; Yasuj, M.; Rastiannasab, A. Measurement Some of Immunological Parameters Cyprinus Carpio in the Polluted (Ahwaz) and Non-Polluted (Shoushtar) Area in Karun Riverian Iran. J. Appl. Biol. Sci. 2014, 8, 81–85. [Google Scholar]
- Arthington, A.; McKenzie, F. Review of Impacts of Displaced/Introduced Fauna Associated with Inland Waters. Australia: State of the Environment Technical Paper Series (Inland waters); Department of the Environment: Canberra, Australia, 1997; 69p. [Google Scholar]
- Chapman, D.V.; WHO; UNESCO; Programme, U.N.E. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring; Chapman, D., Ed.; Chapman and Hall Ltd.: London, UK, 1996; p. 651. [Google Scholar] [CrossRef]
- Rahel, F.J.; Olden, J.D. Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. J. Soc. Conserv. Biol. 2008, 22, 521–533. [Google Scholar] [CrossRef]
- Záhorská, E. Climate warming and invasive fish species: Will they replace native fish species in waters of temperate zones? Biologia 2016, 71, 727–735. [Google Scholar] [CrossRef]
- Okun, N.; Brasil, J.; Attayde, J.; Costa, I. Omnivory does not prevent trophic cascades in pelagic food webs. Freshw. Biol. 2007, 53, 129–138. [Google Scholar] [CrossRef]
- Lusk, S.; Luskova, V.; Hanel, L. Alien fish species in the Czech Republic and their impact on the native fish fauna. Folia Zool. 2010, 59, 57–72. [Google Scholar] [CrossRef]
- Gavioli, A.; Milardi, M.; Castaldelli, G.; Fano, E.A.; Soininen, J. Diversity patterns of native and exotic fish species suggest homogenization processes, but partly fail to highlight extinction threats. Divers. Distrib. 2019, 25, 983–994. [Google Scholar] [CrossRef]
- Taylor, C.A.; Knouft, J.H.; Hiland, T.M. Consequences of stream impoundment on fish communities in a small North American drainage. Regul. Rivers Res. Manag. 2001, 17, 687–698. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Kawanna, M. Optimum water temperature boosts the growth performance of Nile tilapia (Oreochromis niloticus) fry reared in a recycling system. Aquac. Res. 2008, 39, 670–672. [Google Scholar] [CrossRef]
- Jones, P.E.; Tummers, J.S.; Galib, S.M.; Woodford, D.J.; Hume, J.B.; Silva, L.G.M.; Braga, R.R.; de Leaniz, C.G.; Vitule, J.R.S.; Herder, J.E.; et al. The Use of Barriers to Limit the Spread of Aquatic Invasive Animal Species: A Global Review. Front. Ecol. Evol. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Rakocy, J.E. FAO—Oreochromis Niloticus. 2009. Available online: https://www.fao.org/fishery/docs/CDrom/aquaculture/I1129m/file/en/en_niletilapia.htm (accessed on 25 December 2021).
- Zeng, L.; Zhou, L.; Guo, D.-L.; Fu, D.-H.; Xu, P.; Zeng, S.; Tang, Q.-D.; Chen, A.-L.; Chen, F.-Q.; Luo, Y.; et al. Ecological effects of dams, alien fish, and physiochemical environmental factors on homogeneity/heterogeneity of fish community in four tributaries of the Pearl River in China. Ecol. Evol. 2017, 7, 3904–3915. [Google Scholar] [CrossRef]
- Welcomme, R. A history of international introductions of inland aquatic species. Mar. Sci. Symp. 1992, 194, 3–14. [Google Scholar]
- Valikhani, H.; Abdoli, A.; Hassanzade Kiabi, B.; Sadeghsaba, M.; Khosravi, M. A Study on the status of invasive tilapia species (Coptodon zillii Gervais, 1848 and Oreochromis aureus Steindachner, 1864) in aquatic ecosystems of Khuzestan Province, Iran. Environ. Sci. 2018, 15, 29–44. [Google Scholar]
- Cruz, E.M.; Ridha, M. Overwintering tilapia, Oreochromis spilurus (Gunther), fingerlings using warm underground sea water. Aquac. Fish. Manag. 1994, 25, 865–871. [Google Scholar] [CrossRef]
- Khaefi, R.; Esmaeili, H.R.; Zareian, H.; Babaei, S. The first record of the redbelly tilapia, Tilapia zillii (Gervais, 1848), in freshwaters of Iran. Turk. J. Zool. 2014, 38, 96–98. [Google Scholar] [CrossRef]
- Peterson, M.S.; Slack, W.T.; Woodley, C.M. The occurrence of non-indigenous Nile tilapia, Oreochromis niloticus (linnaeus) in coastal Mississippi, USA: Ties to aquaculture and thermal effluent. Wetlands 2005, 25, 112–121. [Google Scholar] [CrossRef]
- Martin, C.W.; Valentine, M.M.; Valentine, J.F. Competitive interactions between invasive nile tilapia and native fish: The potential for altered trophic exchange and modification of food webs. PLoS ONE 2010, 5, e14395. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, W.; Xie, Y.; Xue, H.; Li, J.; Li, Y.; Chen, W.; Huang, Y.; Li, X. Ecological and economic impacts of exotic fish species on fisheries in the Pearl River basin. Manag. Biol. Invasions 2019, 10, 127–138. [Google Scholar] [CrossRef]
- Abdullah, A.H.J.; Abdullah, S.A.; Yaseen, A.T. A composition and abundance of alien fish species in inland waters, southern iraq. Iraqi J. Sci. 2020, 62, 373–386. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Abood, A.N. Compositional change in fish assemblage structure in the Shatt Al-Arab River, Iraq. Asian J. Appl. Sci. 2017, 5, 944–958. [Google Scholar]
- Gu, D.E.; Ma, G.M.; Zhu, Y.J.; Xu, M.; Luo, D.; Li, Y.Y.; Wei, H.; Mu, X.D.; Luo, J.R.; Hu, Y.C. The impacts of invasive Nile tilapia (Oreochromis niloticus) on the fisheries in the main rivers of Guangdong Province, China. Biochem. Syst. Ecol. 2015, 59, 1–7. [Google Scholar] [CrossRef]
- Hashemi, S.; Eskandary, G.; Ansary, H.; Yooneszadeh, M. Stock Assessment and Production of Fish Species in the Shadegan Wetland, Iran. World J. Fish Mar. Sci. 2011, 3, 502–508. [Google Scholar]
- Hashemi, S.A.R.; Ansary, H. Biomass and production of fish species in the Shadegan Wetland, Iran. Glob. Vet. 2012, 9, 123–128. [Google Scholar]
- Razlutskij, V.; Mei, X.; Maisak, N.; Sysova, E.; Lukashanets, D.; Makaranka, A.; Jeppesen, E.; Zhang, X. Omnivorous Carp (Carassius gibelio) Increase Eutrophication in Part by Preventing Development of Large-Bodied Zooplankton and Submerged Macrophytes. Water 2021, 13, 1497. [Google Scholar] [CrossRef]
- Tapkir, S.; Boukal, D.; Kalous, L.; Bartoň, D.; Souza, A.T.; Kolar, V.; Soukalová, K.; Duchet, C.; Gottwald, M.; Šmejkal, M. Invasive gibel carp (Carassius gibelio) outperforms threatened native crucian carp (Carassius carassius) in growth rate and effectiveness of resource use: Field and experimental evidence. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1901–1912. [Google Scholar] [CrossRef]
- Cucherousset, J.; Aymes, J.; Poulet, N.; Santoul, F.; Cereghino, R. Do native brown trout and non-native brook trout interact reproductively? Naturwissenschaften 2008, 95, 647–654. [Google Scholar] [CrossRef]
- El-Matbouli, M.; Soliman, H. Transmission of Cyprinid herpesvirus-3 (CyHV-3) from goldfish to naïve common carp by cohabitation. Res. Vet. Sci. 2011, 90, 536–539. [Google Scholar] [CrossRef]
- Azevedo-Santos, V.M.; Pelicice, F.M.; Lima-Junior, D.P.; Magalhães, A.L.B.; Orsi, M.L.; Vitule, J.R.S.; Agostinho, A.A. How to avoid fish introductions in Brazil: Education and information as alternatives. Nat. E Conserv. 2015, 13, 123–132. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium. 2021. Available online: www.cabi.org/isc (accessed on 10 May 2023).
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2015. [Google Scholar]
- Ahmed, K.K.U.; Hambrey, J.B. Studies on the fish catch efficiency of different types of fishing gear in Kaptai Reservoir, Bangladesh. Lakes Reserv. Res. Manag. 2005, 10, 221–234. [Google Scholar] [CrossRef]
- Laxmappa, B.; Bakshi, R.R. Types of fishing gears operating and their impact on Krishna river fishery in Mahabubnagar district, India. Int. J. Fish. Aquat. Stud. 2014, 2, 30–41. [Google Scholar]
- Oliveira, A.G.; Gomes, L.C.; Latini, J.D.; Agostinho, A.A. Implications of using a variety of fishing strategies and sampling techniques across different biotopes to determine fish species composition and diversity. Nat. Conserv. 2014, 12, 112–117. [Google Scholar] [CrossRef]
- Dadebo, E.; Eyayu, A.; Sorsa, S.; Tilahun, G. Food and Feeding Habits of the Common Carp (Cyprinus carpio L. 1758) (Pisces: Cyprinidae) in Lake Koka, Ethiopia. Momona Ethiop. J. Sci. 2015, 7, 16–31. [Google Scholar] [CrossRef]
- Erarto, F.; Getahun, A. Impacts of introductions of alien species with emphasis on fishes. Int. J. Fish. Aquat. Stud. 2020, 8, 207–216. [Google Scholar]
- Eagderi, S.; Nasri, M.; Cicek, E. First record of the Amur goby Rhinogobius lindbergi Berg 1933 (Gobiidae) from the Tigris River drainage, Iran. Int. J. Aquat. Biol. 2018, 6, 202–207. [Google Scholar] [CrossRef]
- Sadeghi, R.; Esmaeili, H.R.; Zarei, F.; Esmaeili, A.; Abbasi, K. The taxonomic status of an introduced freshwater goby of the genus Rhinogobius to Iran (Teleostei: Gobiidae). Zool. Middle East 2019, 65, 51–58. [Google Scholar] [CrossRef]
- Kalogianni, E.; Koutsikos, N.; Vardakas, L.; Giakoumi, S.; Chatzinikolaou, Y.; Oikonomou, A. Impacts of the alien mosquitofish on the abundance and condition of two Mediterranean native fish. Mediterr. Mar. Sci. 2019, 20, 727–735. [Google Scholar] [CrossRef]
- Coad, B.W. Zoogeography of the fishes of the tigris-euphrates basin. Zool. Middle East 1996, 13, 51–70. [Google Scholar] [CrossRef]
- Kumar, A.B. Exotic fishes and freshwater fish diversity. Zoos’ Print J. 2000, 15, 363–367. [Google Scholar] [CrossRef]
- Segev, O.; Mangel, M.; Blaustein, L. Deleterious effects by mosquitofish (Gambusia affinis) on the endangered fire salamander (Salamandra infraimmaculata). Anim. Conserv. 2009, 12, 29–37. [Google Scholar] [CrossRef]
- Ekmekçï, F.; Kirankaya, Ş. Distribution of an Invasive Fish Species, Pseudorasbora parva (Temminck & Schlegel, 1846) in Turkey. Turk. J. Zool. 2006, 30, 329–334. [Google Scholar]
- Taybi, A.F.; Mabrouki, Y.; Doadrio, I. The occurrence, distribution and biology of invasive fish species in fresh and brackish water bodies of ne Morocco. Arxius de Miscellania Zoologica. 2020, 18, 59–73. [Google Scholar] [CrossRef]
- Grabowska, J.; Przybylski, M. Life-history traits of non-native freshwater fish invaders differentiate them from natives in the Central European bioregion. Rev. Fish Biol. Fish. 2015, 25, 165–178. [Google Scholar] [CrossRef]
- Blinn, D.W.; Runck, C.; Clark, D.A.; Rinne, J.N. Notes: Effects of Rainbow Trout Predation on Little Colorado Spinedace. Trans. Am. Fish. Soc. 1993, 122, 139–143. [Google Scholar] [CrossRef]
- Aalipour, M.; Keivany, Y.; Ebrahimi, E. Feeding habits of Rainbow Trout (Oncorhynchus mykiss) in Beheshtabad River of Chaharmahal & Bakhtiari Province. Appl. Biol. 2019, 32, 76–97. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.R.; Ford, T. Effects of flow on the fish communities of a regulated California river: Implications for managing native fishes. River Res. Appl. 2002, 18, 331–342. [Google Scholar] [CrossRef]
- Ostberg, S.; Schaphoff, S.; Lucht, W.; Gerten, D. Three centuries of dual pressure from land use and climate change on the biosphere. Environ. Res. Lett. 2015, 10, 044011. [Google Scholar] [CrossRef]



| Family | Species | IUCN Status | Status | Relative Abundance |
|---|---|---|---|---|
| Xenocyprididae | Hemiculter leucisculus (Basilewsky, 1855) | Least Concern | Alien | 2.18 |
| Ctenopharyngodon idella (Valenciennes, 1844) | Least Concern | Alien | 0.02 | |
| Danionidae | Barilius mesopotamicus (Berg, 1932) | Least Concern | Native | 0.57 |
| Gobionidae | Pseudorasbora parva (Temminck & Schlegel, 1846) | Least Concern | Alien | 0.05 |
| Cyprinidae | Capoeta coadi (Alwan, Zareian & Esmaeili, 2016) | Not Evaluated | Endemic | 13.78 |
| Capoeta aculeata (Valenciennes, 1844) | Not Evaluated | Endemic | 7.32 | |
| Capoeta trutta (Heckel, 1843) | Least Concern | Native | 6.79 | |
| Carassius gibelio (Bloch, 1782) | Not Evaluated | Alien | 5.29 | |
| Arabibarbus grypus (Heckel, 1843) | Vulnerable/Decreasing | Native | 0.14 | |
| Cyprinus carpio (Linnaeus, 1758) | Vulnerable | Alien | 0.08 | |
| Carasobarbus luteus (Heckel, 1843) | Least Concern | Native | 0.14 | |
| Barbus lacerta (Heckel, 1843) | Least Concern | Native | 0.26 | |
| Barbus karunensis (Khaefi, Esmaeili, Geiger & Eagderi, 2017) | Not Evaluated | Endemic | 0.24 | |
| Cyprinion macrostomus (Heckel, 1843) | Least Concern | Native | 3.84 | |
| Luciobarbus barbulus (Heckel, 1847) | Not Evaluated | Native | 0.29 | |
| Carasobarbus kosswigi (Ladiges, 1960) | Vulnerable/Decreasing | Native | 0.08 | |
| Garra rufa (Heckel, 1843) | Least Concern | Native | 1.16 | |
| Garra gymnothorax (Berg, 1949) | Not Evaluated | Endemic | 0.8 | |
| Leuciscidae | Alburnus caeruleus (Heckel, 1843) | Least Concern | Native | 0.13 |
| Alburnus sellal (Heckel, 1843) | Least Concern | Native | 19.66 | |
| Alburnus doriae (De Filippi, 1865) | Not Evaluated | Endemic | 2.65 | |
| Alburnoides idignensis (Bogutskaya & Coad, 2009) | Not Evaluated | Endemic | 2.10 | |
| Chondrostoma regium (Heckel, 1843) | Least Concern | Native | 13.95 | |
| Squalius berak (Heckel, 1843) | Least Concern | Native | 0.92 | |
| Squalius lepidus (Heckel, 1843) | Least Concern | Native | 0.51 | |
| Acanthobrama marmid (Heckel, 1843) | Least Concern | Native | 4.94 | |
| Nemacheilidae | Turcinoemacheilus saadii (Esmaeili, Sayyadzadeh, Özulug, Geiger & Freyhof, 2014) | Not Evaluated | Endemic | 0.51 |
| Turcinoemacheilus hafezi (Golzarianpour, Abdoli, Patimar & Freyhof, 2013) | Not Evaluated | Endemic | 0.05 | |
| Oxynoemacheilus euphraticus (Bănărescu & Nalbant, 1964) | Not Evaluated | Endemic | 0.37 | |
| Cichlidae | Oreochromis aureus (Steindachner, 1864) | Not Evaluated | Alien | 4.02 |
| Coptodon zillii (Gervais, 1848) | Least Concern | Alien | 2.98 | |
| Sisoridae | Glyptothorax galaxias (Mousavi-Sabet & Eagderi & Vatandoust & Freyhof, 2021) | Not Evaluated | Endemic | 0.62 |
| Glyptothorax alidaeii (Mousavi-Sabet & Eagderi & Vatandoust & Freyhof, 2021) | Not Evaluated | Endemic | 0.62 | |
| Aphanidae | Esmaeilius vladykovi (Coad, 1988) | Not Evaluated | Endemic | 1.24 |
| Poeciliidae | Gambusia holbrooki (Girard, 1859) | Least Concern | Alien | 0.14 |
| Mugilidae | Planiliza abu (Heckel, 1843) | Least Concern | Native | 0.54 |
| Salmonidae | Oncorhynchus mykiss (Walbaum, 1792) | Not Evaluated | Alien | 0.03 |
| Gobiidae | Rhinogobius lindbergi (Berg, 1933) | Not Evaluated | Alien | 0.92 |
| Mastacembelidae | Mastacembelus mastacembelus (Banks & Solander, 1794) | Least Concern | Native | 0.05 |
| Variable | Unit | Mean ± SD | Range (Min–Max) |
|---|---|---|---|
| Altitude | Meter above sea level | 1061 ± 681 | 1–1961 |
| Depth (D) | Cm | 58 ± 26 | 25–120 |
| Water temperature (WT) | °C | 13.5 ± 3.2 | 7.2–19.6 |
| Electrical conductivity (EC) | (μmho/cm) | 740.7 ± 541.3 | 259–2186 |
| Turbidity | (mg/L) | 68 ± 187 | 16.8–1149 |
| Width (W) | M | 52 ± 48 | 5–170 |
| Dissolved Oxygen (DO) | (mg/L) | 8.4 ± 1.3 | 5.3–12.6 |
| Alkalinity | (mg/L CaCO3) | 220 ± 13 | 201–274 |
| Biological Oxygen Demand (BOD) | (mg/L) | 2.19 ± 0.99 | 0.56–4.7 |
| Nitrate (NO3) | (mg/L) | 8.55 ± 5.4 | 3.7–37 |
| Chemical Oxygen demand (COD) | (mgO2/L) | 14 ± 9.4 | 0.02–41.7 |
| Phosphate (PO4) | (mg/L) | 0.52 ± 0.33 | 0.1–1.89 |
| Variable | Axis1 | Axis 2 | F-Ratio | p-Value |
|---|---|---|---|---|
| Altitude | 0.70 | 0.64 | 12.77 | 0.005 ** |
| Depth (D) | −0.80 | −0.43 | 12.56 | 0.005 ** |
| Electrical conductivity (EC) | −0.55 | −0.72 | 10.79 | 0.005 ** |
| Water temperature (WT) | −0.63 | −0.37 | 7.52 | 0.005 ** |
| Turbidity | −0.50 | −0.52 | 6.74 | 0.005 ** |
| Width (W) | −0.63 | −0.24 | 6.32 | 0.005 ** |
| Dissolved Oxygen (DO) | 0.57 | 0.09 | 4.50 | 0.02 * |
| Alkalinity | −0.37 | −0.06 | 1.78 | 0.165 |
| Biological Oxygen Demand (BOD) | −0.11 | 0.30 | 1.21 | 0.295 |
| Nitrate (NO3) | −0.16 | 0.08 | 0.74 | 0.505 |
| Chemical Oxygen Demand (COD) | −0.18 | −0.10 | 0.56 | 0.69 |
| Phosphate (PO4) | −0.18 | −0.02 | 0.41 | 0.735 |
| Cumulative percentage of the variance of the species abundance | 36.24 | 25.33 | ||
| Cumulative percentage of the relation of species abundance and environmental variables | 53.14 | 37.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahraki, M.Z.; Keivany, Y.; Dorche, E.E.; Blocksom, K.; Bruder, A.; Flotemersch, J.; Bănăduc, D. Distribution and Expansion of Alien Fish Species in the Karun River Basin, Iran. Fishes 2023, 8, 538. https://doi.org/10.3390/fishes8110538
Shahraki MZ, Keivany Y, Dorche EE, Blocksom K, Bruder A, Flotemersch J, Bănăduc D. Distribution and Expansion of Alien Fish Species in the Karun River Basin, Iran. Fishes. 2023; 8(11):538. https://doi.org/10.3390/fishes8110538
Chicago/Turabian StyleShahraki, Mojgan Zare, Yazdan Keivany, Eisa Ebrahimi Dorche, Karen Blocksom, Andreas Bruder, Joseph Flotemersch, and Doru Bănăduc. 2023. "Distribution and Expansion of Alien Fish Species in the Karun River Basin, Iran" Fishes 8, no. 11: 538. https://doi.org/10.3390/fishes8110538
APA StyleShahraki, M. Z., Keivany, Y., Dorche, E. E., Blocksom, K., Bruder, A., Flotemersch, J., & Bănăduc, D. (2023). Distribution and Expansion of Alien Fish Species in the Karun River Basin, Iran. Fishes, 8(11), 538. https://doi.org/10.3390/fishes8110538








