Gonad and Germ Cell Development and Maturation Characteristics of the Pot-Bellied Seahorse (Hippocampus abdominalis) under Captive Breeding Conditions in Northern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Histology Analysis
2.3. Immunofluorescence
2.4. Statistical Analysis
3. Results
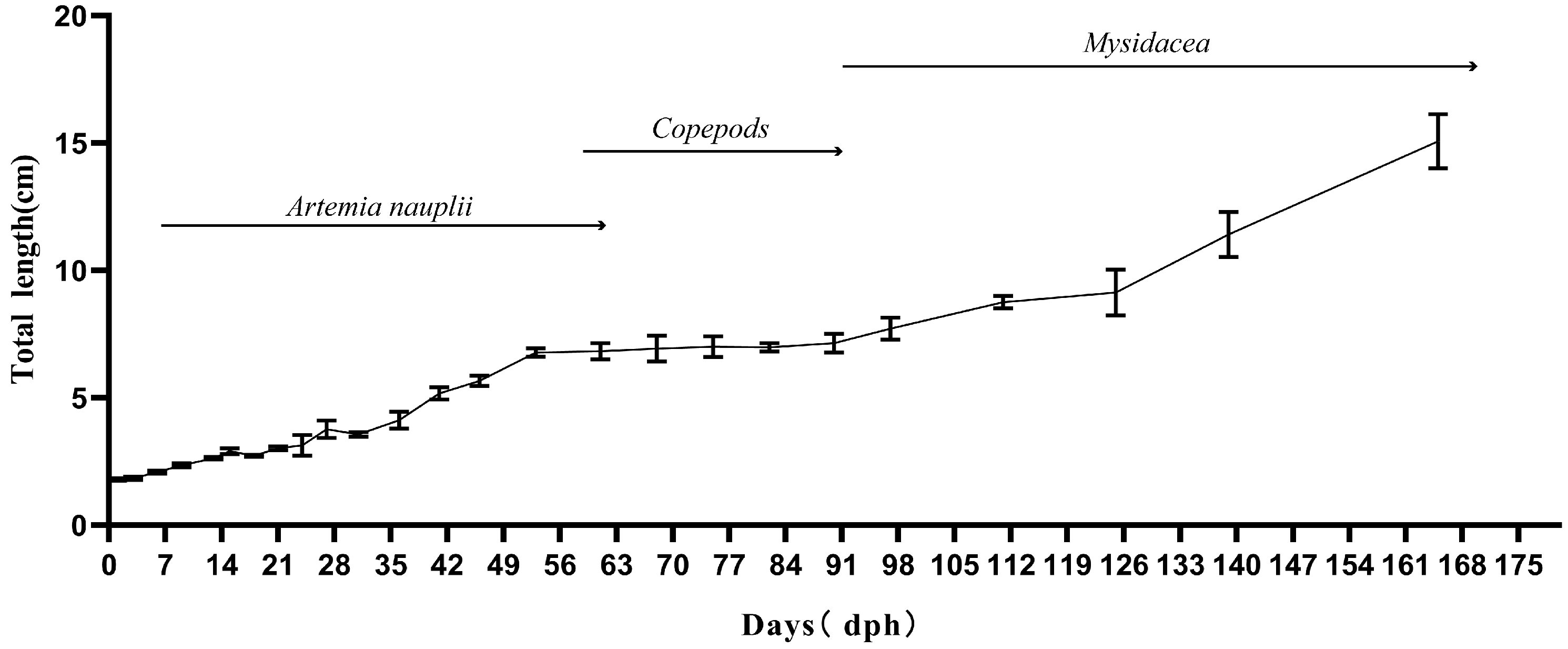
3.1. Growth
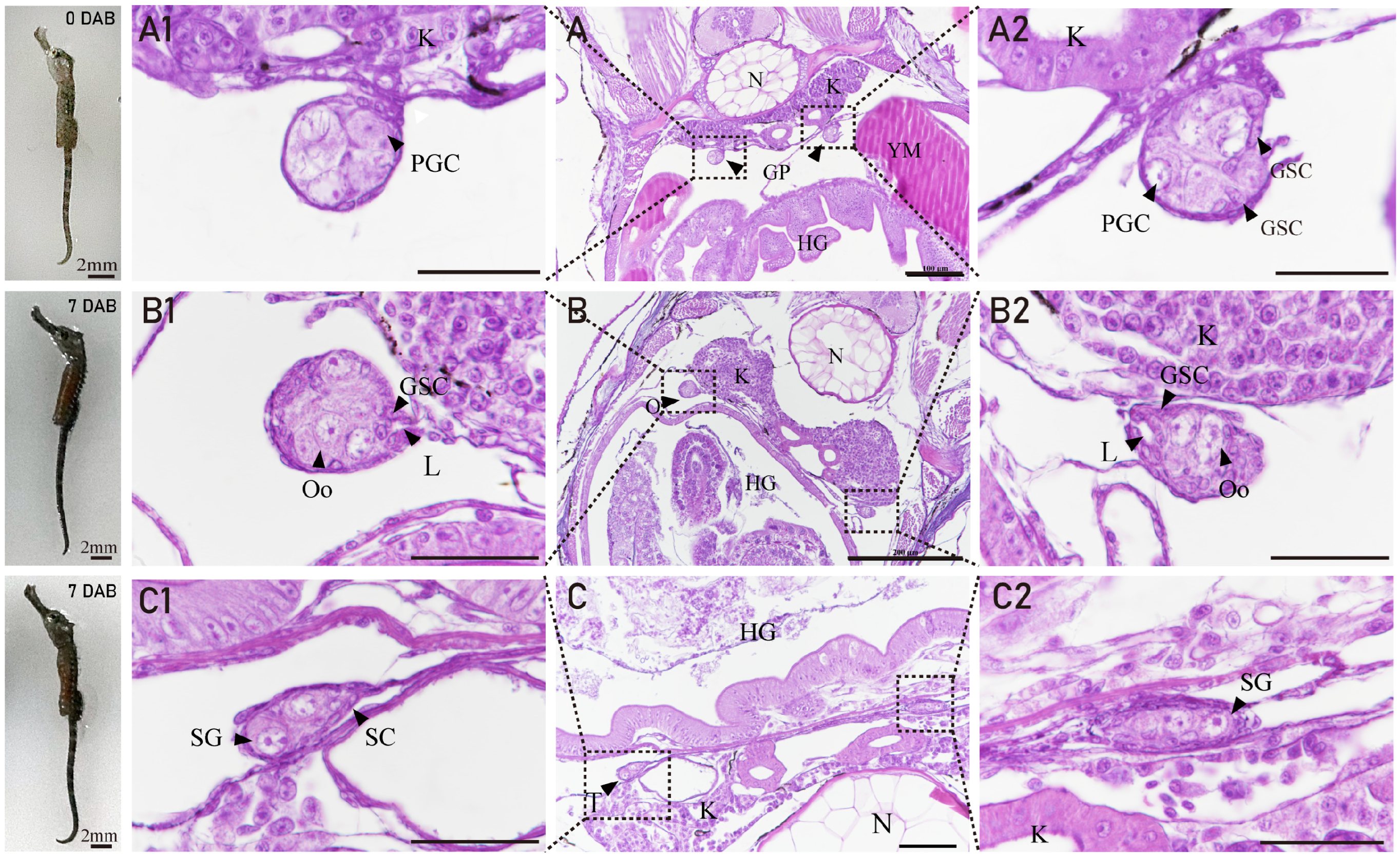
3.2. The Gonadal Primordium and Ovarian Differentiation of H. abdominalis
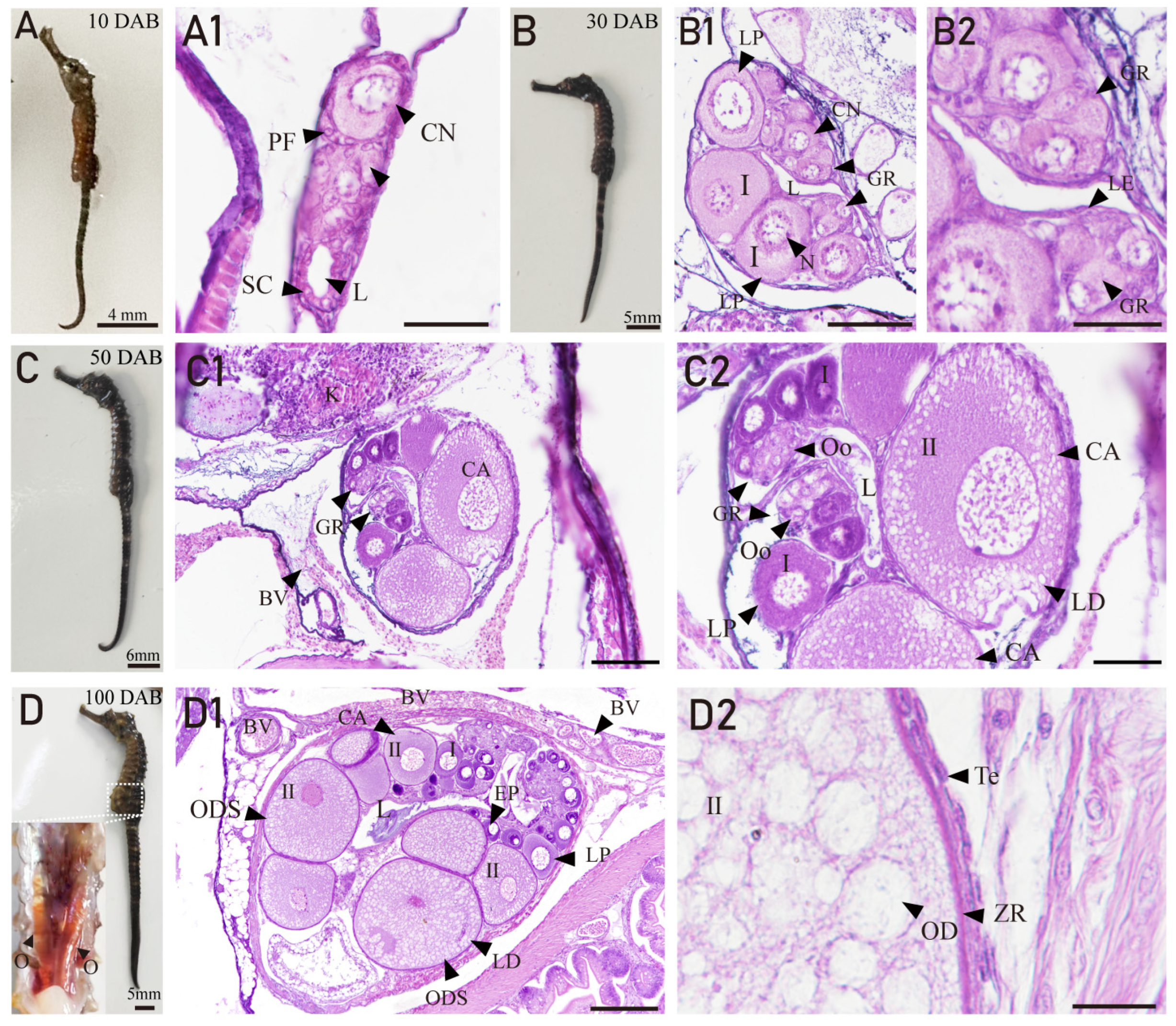
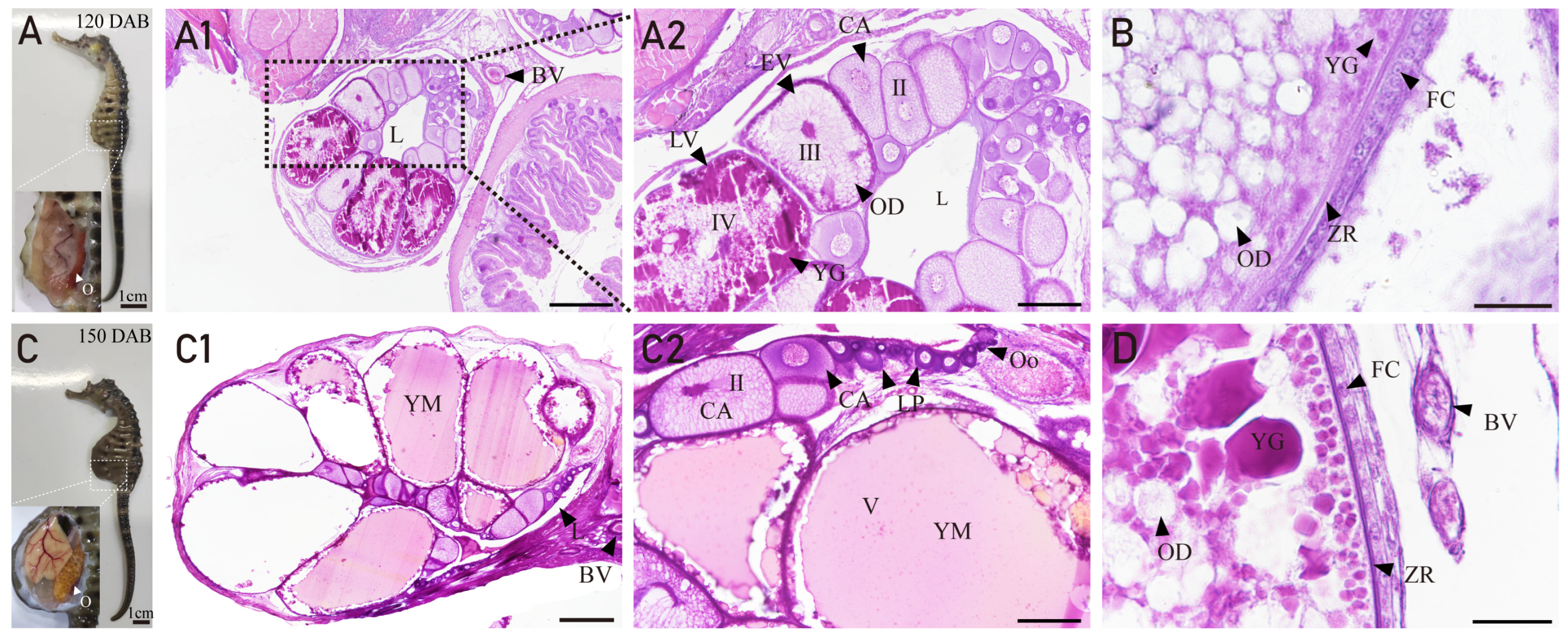
3.3. Ovarian Developmental Characteristics of H. abdominals
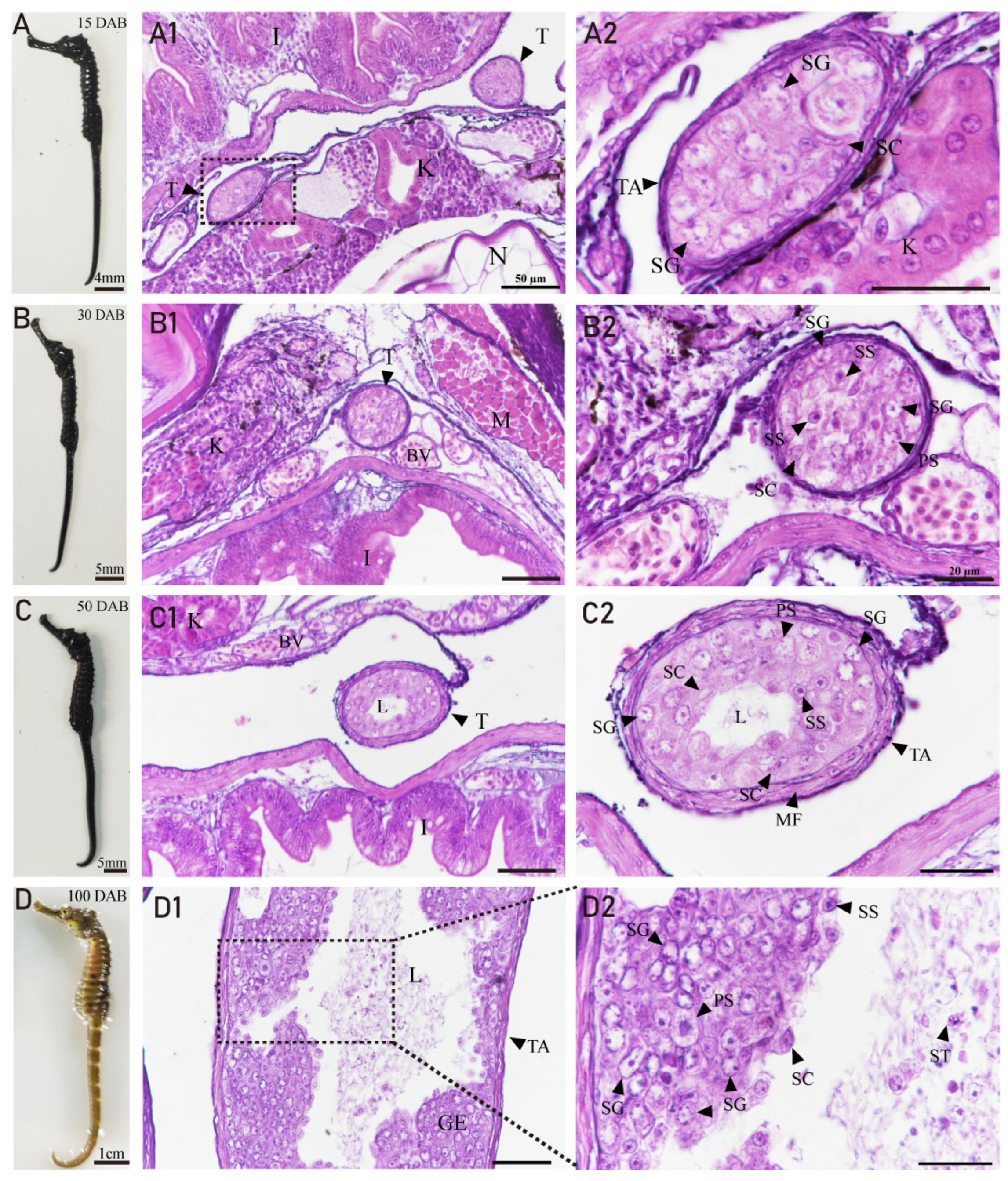
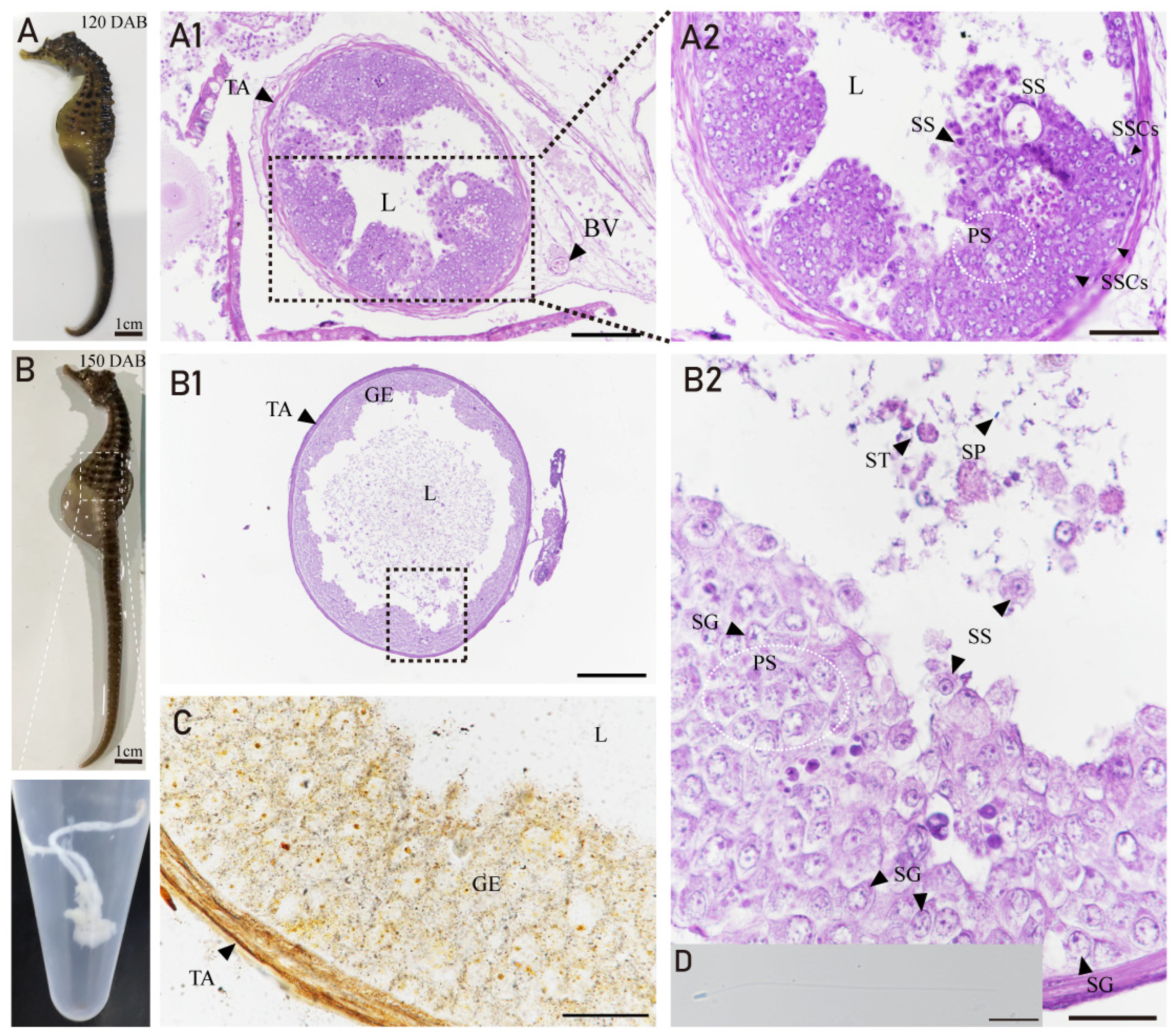
3.4. Developmental Characteristics of the Testis in H. abdominalis
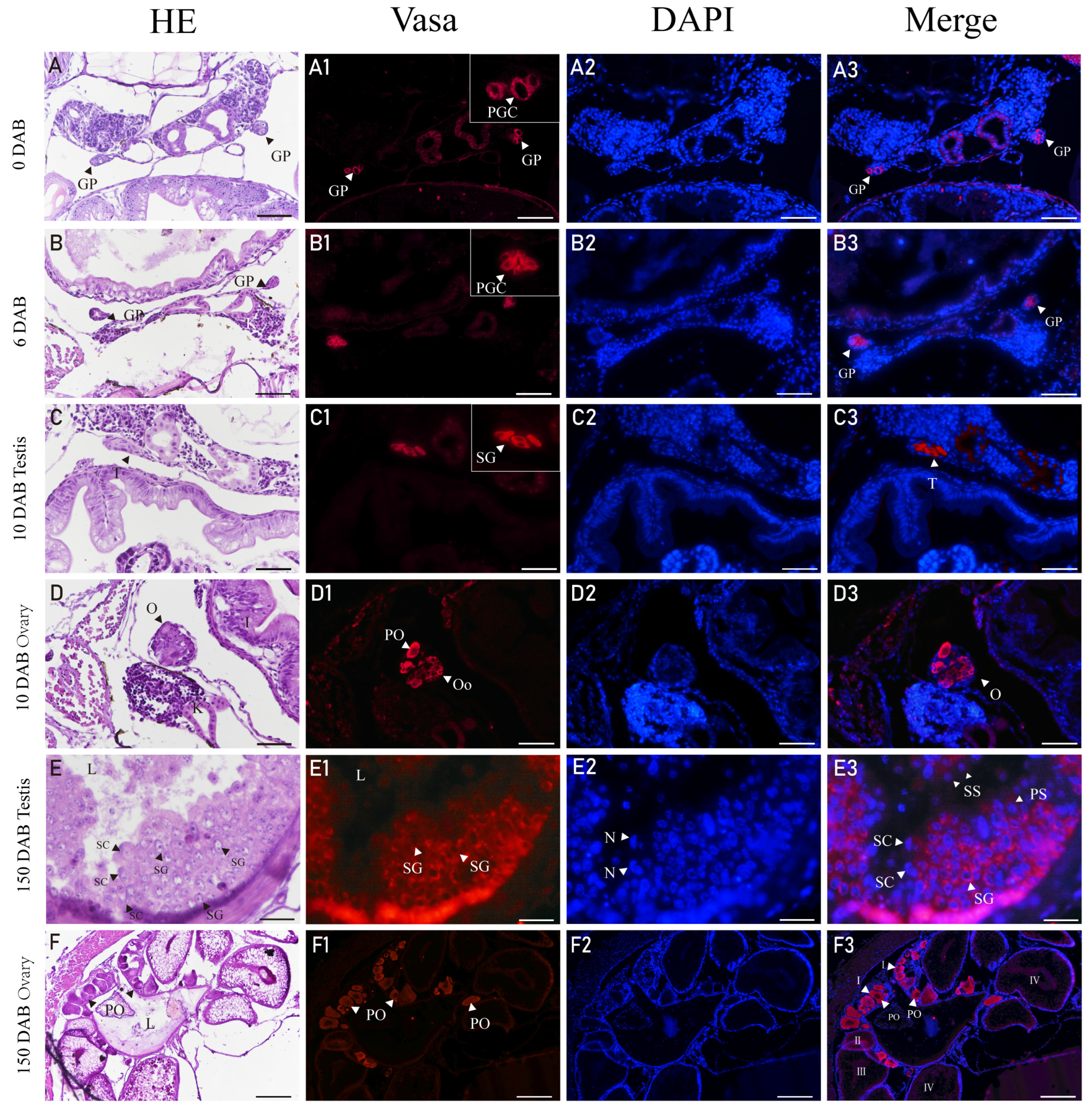
3.5. Expression of Vasa in Gonads of H. abdominalis by Immunofluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Strüssmann, C.A.; Nakamura, M. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol. Biochem. 2002, 26, 13–29. [Google Scholar] [CrossRef]
- Li, J.; Lyu, L.; Wen, H.; Li, Y.; Wang, X.; Zhang, Y.; Yao, Y.; Qi, X. Comparative transcriptomic analysis of gonadal development and renewal in the ovoviviparous black rockfish (Sebastes schlegelii). BMC Genom. 2021, 22, 874. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.W.; de Franca, L.R.; Lareyre, J.J.; Le Gac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Xiao, Y.; Wang, X.; An, H.; Song, Z.; You, F.; Wang, Y.; Ma, D.; Li, J. Germ Cell Migration, Proliferation and Differentiation during Gonadal Morphogenesis in All-Female Japanese Flounder (Paralichthys olivaceus). Anat. Rec. 2018, 301, 727–741. [Google Scholar] [CrossRef]
- Gong, G.; Xiong, Y.; Xiao, S.; Li, X.Y.; Huang, P.; Liao, Q.; Han, Q.; Lin, Q.; Dan, C.; Zhou, L.; et al. Origin and chromatin remodeling of young X/Y sex chromosomes in catfish with sexual plasticity. Natl. Sci. Rev. 2023, 10, nwac239. [Google Scholar] [CrossRef]
- Planas, J.V.; Swanson, P. Physiological function of gonadotropins in fish. In Fish Reproduction; CRC Press: Boca Raton, FL, USA, 2008; pp. 37–66. [Google Scholar]
- Lyu, L.; Wen, H.; Li, Y.; Li, J.; Zhao, J.; Zhang, S.; Song, M.; Wang, X. Deep Transcriptomic Analysis of Black Rockfish (Sebastes schlegelii) Provides New Insights on Responses to Acute Temperature Stress. Sci. Rep. 2018, 8, 9113. [Google Scholar] [CrossRef]
- Mori, H.; Nakagawa, M.; Soyano, K.; Koya, Y. Annual reproductive cycle of black rockfish Sebastes schlegeli in captivity. Fish. Sci. 2003, 69, 910–923. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, S.; Zhang, Y.; Xu, M.; Zhang, H.; Yang, Y.; Lee, A.P.; Woltering, J.M.; Ravi, V.; Gunter, H.M.; et al. The seahorse genome and the evolution of its specialized morphology. Nature 2016, 540, 395–399. [Google Scholar] [CrossRef]
- Stolting, K.N.; Wilson, A.B. Male pregnancy in seahorses and pipefish: Beyond the mammalian model. Bioessays 2007, 29, 884–896. [Google Scholar] [CrossRef]
- Selman, K.; Wallace, R.A.; Player, D. Ovary of the seahorse, Hippocampus erectus. J. Morphol. 1991, 209, 285–304. [Google Scholar] [CrossRef]
- Piras, F.; Biagi, F.; Taddei, A.R.; Fausto, A.M.; Farina, V.; Zedda, M.; Floris, A.; Franzoi, P.; Carcupino, M. Male gonads morphology, spermatogenesis and sperm ultrastructure of the seahorse Hippocampus guttulatus (Syngnathidae). Acta Zool. 2015, 97, 325–333. [Google Scholar] [CrossRef]
- Wilson, A.B.; Vincent, A.; Ahnesjö, I.; Meyer, A. Male Pregnancy in Seahorses and Pipefishes (Family Syngnathidae): Rapid Diversification of Paternal Brood Pouch Morphology Inferred from a Molecular Phylogeny. J. Hered. 2001, 92, 159–166. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.M.; Vincent, A.C.J. Assessing East African trade in seahorse species as a basis for conservation under international controls. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, 521–538. [Google Scholar] [CrossRef]
- Giles, B.G.; Ky, T.S.; Hoang, D.H.; Vincent, A.C.J. The catch and trade of seahorses in Vietnam. Biodivers. Conserv. 2006, 15, 2497–2513. [Google Scholar] [CrossRef]
- Rosa, I.L.; Oliveira, T.P.R.; Osório, F.M.; Moraes, L.E.; Castro, A.L.C.; Barros, G.M.L.; Alves, R.R.N. Fisheries and trade of seahorses in Brazil: Historical perspective, current trends, and future directions. Biodivers. Conserv. 2011, 20, 1951–1971. [Google Scholar] [CrossRef]
- Vincent, A.G.; Vincent, A.C.J.; Vincent, A.; Vincentsalomon, A. The International Trade in Seahorses; TRAFFIC International: Cambridge, UK, 1996. [Google Scholar]
- Novelli, B.; Socorro, J.A.; Caballero, M.J.; Otero-Ferrer, F.; Segade-Botella, A.; Molina Dominguez, L. Development of seahorse (Hippocampus reidi, Ginsburg 1933): Histological and histochemical study. Fish. Physiol. Biochem. 2015, 41, 1233–1251. [Google Scholar] [CrossRef]
- Kuiter, R.H. A new pygmy seahorse (Pisces: Syngnathidae: Hippocampus) from Lord Howe Island. Rec. Aust. Mus. 2003, 55, 113–116. [Google Scholar] [CrossRef]
- Lourie, S.A.; Foster, S.J.; Cooper, E.W.T.; Vincent, A.C. A Guide to The Identification of Seahorses. Proj. Seahorse TRAFFIC N. Am. 2004, 114, 1–120. [Google Scholar]
- Koldewey, H.J.; Martin-Smith, K.M. A global review of seahorse aquaculture. Aquaculture 2010, 302, 131–152. [Google Scholar] [CrossRef]
- Li, C.; Olave, M.; Hou, Y.; Qin, G.; Schneider, R.F.; Gao, Z.; Tu, X.; Wang, X.; Qi, F.; Nater, A.; et al. Genome sequences reveal global dispersal routes and suggest convergent genetic adaptations in seahorse evolution. Nat. Commun. 2021, 12, 1094. [Google Scholar] [CrossRef]
- Teske, P.R.; Cherry, M.I.; Matthee, C.A. The evolutionary history of seahorses (Syngnathidae: Hippocampus): Molecular data suggest a West Pacific origin and two invasions of the Atlantic Ocean. Mol. Phylogenet. Evol. 2004, 30, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Wiswedel, S.; Vincent, A. Opportunities and challenges for analysis of wildlife trade using CITES data—Seahorses as a case study. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 154–172. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, Y.; Zhang, B.; Zhang, Y.; Liu, Y.; Lin, Q. Environmental estrogens and progestins disturb testis and brood pouch development with modifying transcriptomes in male-pregnancy lined seahorse Hippocampus erectus. Sci. Total Environ. 2020, 715, 136840. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liu, Y.L.; Qin, G.; Lin, Q.; Zhang, Y.H. Effects of tributyltin on gonad and brood pouch development of male pregnant lined seahorse (Hippocampus erectus) at environmentally relevant concentrations. J. Hazard. Mater. 2021, 408, 124854. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Okubo, R.; Harada, A.; Miyasaka, K.; Takada, K.; Hiroi, J.; Yasumasu, S. Morphology of brood pouch formation in the pot-bellied seahorse Hippocampus abdominalis. Zool. Lett. 2017, 3, 19. [Google Scholar] [CrossRef]
- Dudley, J.S.; Hannaford, P.; Dowland, S.N.; Lindsay, L.A.; Thompson, M.B.; Murphy, C.R.; Van Dyke, J.U.; Whittington, C.M. Structural changes to the brood pouch of male pregnant seahorses (Hippocampus abdominalis) facilitate exchange between father and embryos. Placenta 2021, 114, 115–123. [Google Scholar] [CrossRef]
- Dudley, J.S.; Paul, J.W.; Teh, V.; Mackenzie, T.E.; Butler, T.A.; Tolosa, J.M.; Smith, R.; Foley, M.; Dowland, S.; Thompson, M.B.; et al. Seahorse brood pouch morphology and control of male parturition in Hippocampus abdominalis. Placenta 2022, 127, 88–94. [Google Scholar] [CrossRef]
- Harada, A.; Shiota, R.; Okubo, R.; Yorifuji, M.; Sogabe, A.; Motomura, H.; Hiroi, J.; Yasumasu, S.; Kawaguchi, M. Brood pouch evolution in pipefish and seahorse based on histological observation. Placenta 2022, 120, 88–96. [Google Scholar] [CrossRef]
- Whittington, C.M.; Griffith, O.W.; Qi, W.; Thompson, M.B.; Wilson, A.B. Seahorse Brood Pouch Transcriptome Reveals Common Genes Associated with Vertebrate Pregnancy. Mol. Biol. Evol. 2015, 32, 3114–3131. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Qin, G.; Chen, Z.; Qu, M.; Zhang, B.; Han, X.; Wang, X.; Qian, P.Y.; Lin, Q. Regulatory Role of Retinoic Acid in Male Pregnancy of the Seahorse. Innovation 2020, 1, 100052. [Google Scholar] [CrossRef]
- Qin, G.; Luo, W.; Tan, S.; Zhang, B.; Ma, S.; Lin, Q. Dimorphism of sex and gonad-development-related genes in male and female lined seahorse, Hippocampus erectus, based on transcriptome analyses. Genomics 2019, 111, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Scobell, S.K.; Mackenzie, D.S. Reproductive endocrinology of Syngnathidae. J. Fish. Biol. 2011, 78, 1662–1680. [Google Scholar] [CrossRef]
- Van Look, K.J.; Dzyuba, B.; Cliffe, A.; Koldewey, H.J.; Holt, W.V. Dimorphic sperm and the unlikely route to fertilisation in the yellow seahorse. J. Exp. Biol. 2007, 210, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.; Cursons, R. Genetic diversity and population structure of the pot-belly seahorse Hippocampus abdominalis in New Zealand. N. Z. J. Mar. Freshw. Res. 2012, 46, 207–218. [Google Scholar] [CrossRef]
- Mazzoni, T.S.; Grier, H.J.; Quagio-Grassiotto, I. Germline cysts and the formation of the germinal epithelium during the female gonadal morphogenesis in Cyprinus carpio (Teleostei: Ostariophysi: Cypriniformes). Anat. Rec. 2010, 293, 1581–1606. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.A.; Bubner, E.J.; Takeuchi, Y.; Yoshizaki, G.; Wang, T.; Cummins, S.F.; Elizur, A. Primordial germ cell migration in the yellowtail kingfish (Seriola lalandi) and identification of stromal cell-derived factor 1. Gen. Comp. Endocrinol. 2015, 213, 16–23. [Google Scholar] [CrossRef]
- Saito, T.; Otani, S.; Fujimoto, T.; Suzuki, T.; Nakatsuji, T.; Arai, K.; Yamaha, E. The germ line lineage in ukigori, Gymnogobius species (Teleostei: Gobiidae) during embryonic development. Int. J. Dev. Biol. 2004, 48, 1079–1085. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, S.; Liu, Y.; Wang, Y.; Liu, Q.; Li, J. Gonadogenesis analysis and sex differentiation in cultured turbot (Scophthalmus maximus). Fish. Physiol. Biochem. 2017, 43, 265–278. [Google Scholar] [CrossRef]
- Rasmussen, T.H.; Jespersen, A.; Korsgaard, B. Gonadal morphogenesis and sex differentiation in intraovarian embryos of the viviparous fish Zoarces viviparus (Teleostei, Perciformes, Zoarcidae): A histological and ultrastructural study. J. Morphol. 2006, 267, 1032–1047. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, T.; Chang, X.-T.; Nagahama, Y. Gonadal sex differentiation in teleost fish. J. Exp. Zool. 1998, 281, 362–372. [Google Scholar] [CrossRef]
- Ofelio, C.; Diaz, A.O.; Radaelli, G.; Planas, M. Histological development of the long-snouted seahorse Hippocampus guttulatus during ontogeny. J. Fish. Biol. 2018, 93, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Ma, D.; Xiao, Y.; Xu, S.; Wang, X.; Song, Z.; You, F.; Li, J. Spermatogonial stem cells differentiation and testicular lobules formation in a seasonal breeding teleost: The evidence from the heat-induced masculinization of genetically female Japanese flounder (Paralichthys olivaceus). Theriogenology 2018, 120, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Takahashi, H. Gonadal Sex Differentiation in Tilapia mossambica, with Special Regard to the Time of Estrogen Treatment Effective in Inducing Complete Feminization of Genetic Males. Bull. Fac. Fish. Hokkaido Univ. 1973, 24, 1–13. [Google Scholar]
- Mattei, C.; Mattei, X. Spermiogenesis in a teleost fish (Lepadogaster lepadogaster). I. The spermatid. Biol. Cell. 1978, 32, 257–266. [Google Scholar]
- Carcupino, M.; Baldacci, A.; Corso, G.; Franzoi, P.; Pala, M.; Mazzini, M. Testis structure and symplastic spermatid formation during spermatogenesis of pipefishes. J. Fish Biol. 1999, 55, 344–353. [Google Scholar] [CrossRef]
- Biagi, F.; Piras, F.; Farina, V.; Zedda, M.; Mura, E.; Floris, A.; Franzoi, P.; Fausto, A.M.; Taddei, A.R.; Carcupino, M. Testis structure, spermatogenesis and sperm morphology in pipefishes of the genus Syngnathus. Acta Zool. 2014, 97, 90–101. [Google Scholar] [CrossRef]
- Kaur, L.; Raj, R.; Thakur, A.K.; Singh, I. Development of chitosan-catechol conjugates as mucoadhesive polymer: Assessment of acute oral toxicity in mice. Environ. Anal. Health Toxicol. 2020, 35, e2020014. [Google Scholar] [CrossRef]
- Mazzoni, T.S.; Grier, H.J.; Quagio-Grassiotto, I. Male gonadal differentiation and the paedomorphic evolution of the testis in Teleostei. Anat. Rec. 2014, 297, 1137–1162. [Google Scholar] [CrossRef]
- Watanabe, S.; Hara, M.; Watanabe, Y. Male Internal Fertilization and Introsperm-like Sperm of the Seaweed Pipefish (Syngnathus schlegeli). Zool. Sci. 2000, 17, 759–767. [Google Scholar] [CrossRef]
- Giacomello, E.; Neat, F.C.; Rasotto, M.B. Mechanisms enabling sperm economy in blenniid fishes. Behav. Ecol. Sociobiol. 2007, 62, 671–680. [Google Scholar] [CrossRef]
- Kvarnemo, C.; Simmons, L.W. Testes investment and spawning mode in pipefishes and seahorses (Syngnathidae). Biol. J. Linn. Soc. 2004, 83, 369–376. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qin, S.; Liu, Q.; Wang, W. Gonad and Germ Cell Development and Maturation Characteristics of the Pot-Bellied Seahorse (Hippocampus abdominalis) under Captive Breeding Conditions in Northern China. Fishes 2023, 8, 551. https://doi.org/10.3390/fishes8110551
Zhang Y, Qin S, Liu Q, Wang W. Gonad and Germ Cell Development and Maturation Characteristics of the Pot-Bellied Seahorse (Hippocampus abdominalis) under Captive Breeding Conditions in Northern China. Fishes. 2023; 8(11):551. https://doi.org/10.3390/fishes8110551
Chicago/Turabian StyleZhang, Yichao, Siyong Qin, Qinghua Liu, and Wenqi Wang. 2023. "Gonad and Germ Cell Development and Maturation Characteristics of the Pot-Bellied Seahorse (Hippocampus abdominalis) under Captive Breeding Conditions in Northern China" Fishes 8, no. 11: 551. https://doi.org/10.3390/fishes8110551
APA StyleZhang, Y., Qin, S., Liu, Q., & Wang, W. (2023). Gonad and Germ Cell Development and Maturation Characteristics of the Pot-Bellied Seahorse (Hippocampus abdominalis) under Captive Breeding Conditions in Northern China. Fishes, 8(11), 551. https://doi.org/10.3390/fishes8110551





