Effects of Ulva prolifera Degradation on Growth Performance and Antioxidant Capacity of Japanese Flounder (Paralichthys olivaceus) Family
Abstract
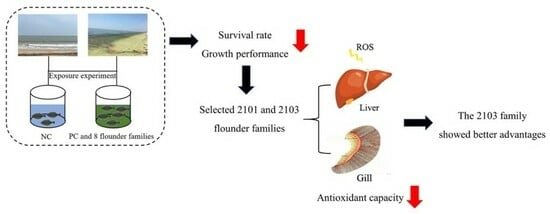
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling Collection
2.3. Chemical Analysis
2.4. Biochemical and Antioxidant Capacity Analysis
2.5. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.6. Calculations and Statistical Analysis
3. Results
3.1. Metal Element Parameters of the Water Solution
3.2. Survival Rate and Growth Performance
3.3. Serum Biochemical Indexes
3.4. Antioxidant Enzyme Activity
3.5. Antioxidant-Related Genes Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global harmful algal bloom status reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Zhang, X. A review of the green tides in the Yellow Sea, China. Mar. Environ. Res. 2016, 119, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Zhang, X.; Mao, Y.; Liang, C.; Xu, D.; Zou, J.; Zhuang, Z.; Wang, Q. ‘Green tides’ are overwhelming the coastline of our blue planet: Taking the world’s largest example. Ecol. Res. 2011, 26, 477–485. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Nelson, T.A.; Ridgway, R.L. Environmental Chemistry and Chemical Ecology of “Green Tide” Seaweed Blooms. Integr. Comp. Biol. 2015, 55, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, K.; Tan, L.; Wang, J. Utilization and release of biogenic elements by macroalgae Ulva prolifera: A mesocosm experiment off the coast of Qingdao, China. Mar. Pollut. Bull. 2021, 170, 112612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X. Release and microbial degradation of dissolved organic matter (DOM) from the macroalgae Ulva prolifera. Mar. Pollut. Bull. 2017, 125, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Arisekar, U.; Shakila, R.J.; Shalini, R.; Jeyasekaran, G.; Padmavathy, P.; Hari, M.S.; Sudhan, C. Accumulation potential of heavy metals at different growth stages of Pacific white leg shrimp, Penaeus vannamei farmed along the Southeast coast of Peninsular India: A report on ecotoxicology and human health risk assessment. Environ. Res. 2022, 212, 113105. [Google Scholar] [CrossRef]
- Talmage, S.C.; Gobler, C.J. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc. Natl. Acad. Sci. USA 2010, 107, 17246–17251. [Google Scholar] [CrossRef]
- Gu, P.; Li, Q.; Zhang, W.; Gao, Y.; Sun, K.; Zhou, L.; Zheng, Z. Biological toxicity of fresh and rotten algae on freshwater fish: LC(50), organ damage and antioxidant response. J. Hazard. Mater. 2021, 407, 124620. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Cui, W.; Cao, L.; Liu, J.; Ren, Z.; Zhao, B.; Dou, S. Effects of seawater acidification and cadmium on the antioxidant defense of flounder Paralichthys olivaceus larvae. Sci. Total Environ. 2020, 718, 137234. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Su, B.; Xue, T.; Cao, M.; Li, C. Genome-wide identification, characterization, and expression of the Toll-like receptors in Japanese flounder (Paralichthys olivaceus). Aquaculture 2021, 545, 737127. [Google Scholar] [CrossRef]
- Fan, Q.; Shi, K.; Zhan, M.; Xu, Q.; Liu, X.; Li, Z.; Liu, H.; Xia, Y.; Chen, Y.; Shi, X.; et al. Acute damage from the degradation of Ulva prolifera on the environmental microbiota, intestinal microbiota and transcriptome of Japanese flounder Paralichthys olivaceus. Environ. Pollut. 2022, 302, 119022. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, H.; Sung, G.; Seo, S.; Kim, K.; Kang, Y.; Kang, J. Toxic effects on hematological parameters and oxidative stress in juvenile olive flounder, Paralichthys olivaceus exposed to waterborne zinc. Aquacult. Rep. 2019, 15, 100225. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, S.; Ji, C.; Li, F.; Cong, M.; Shan, X.; Wu, H. iTRAQ-based proteomic analysis on the mitochondrial responses in gill tissues of juvenile olive flounder Paralichthys olivaceus exposed to cadmium. Environ. Pollut. 2020, 257, 113591. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.A.; Lee, D.J.; Smith, B.C. Are “green tides” harmful algal blooms? Toxic properties of water-soluble extracts from two bloom-forming macroalgae, Ulva fenestrata and Ulvaria obscura (Ulvophyceae). J. Phycol. 2003, 39, 874–879. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Lu, S.; Tian, Y.; Yang, Y.; Chen, S. Genetic parameters estimates for growth performance traits at harvest in Japanese flounder (Paralichthys olivaceus). Aquaculture 2018, 489, 56–61. [Google Scholar] [CrossRef]
- Gagnon, M.M.; Hodson, P. Field studies using fish biomarkers–How many fish are enough? Mar. Pollut. Bull. 2012, 64, 2871–2876. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.G.; Hansen, P.J.; Engell-Sorensen, K.; Norremark, L.H.; Andersen, P.; Lorenzen, E.; Lorenzen, N. Ichthyotoxicity of the microalga Pseudochattonella farcimen under laboratory and field conditions in Danish waters. Dis. Aquat. Organ. 2015, 116, 165–172. [Google Scholar] [CrossRef] [PubMed]
- John, U.; Supraha, L.; Gran-Stadniczenko, S.; Bunse, C.; Cembella, A.; Eikrem, W.; Janouskovec, J.; Klemm, K.; Kuhne, N.; Naustvoll, L. Spatial and biological oceanographic insights into the massive fish-killing bloom of the haptophyte Chrysochromulina leadbeateri in northern Norway. Harmful Algae 2022, 118, 102287. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Lim, W.A.; Lu, D.; Dai, X.; Orlova, T.; Iwataki, M. Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan, Korea and Russia. Harmful Algae 2021, 102, 101787. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Suresh Kumar, K.; Subba Rao, P.V.; Tsukui, Y.; Bhaskar, N.; Hosokawa, M.; Miyashita, K. Studies on chemical composition of three species of Enteromorpha. Biomed. Prev. Nutr. 2014, 4, 365–369. [Google Scholar] [CrossRef]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Leitch, H.A. Iron overload-induced oxidative stress in myelodysplastic syndromes and its cellular sequelae. Crit. Rev. Oncol. Hematol. 2021, 163, 103367. [Google Scholar] [CrossRef]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef]
- Ahmed, U.; Latham, P.S.; Oates, P.S. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J. Gastroenterol. 2012, 18, 4651–4658. [Google Scholar] [CrossRef]
- Luo, Z.; Zou, G.Y.; Gao, Y.; Ye, H.M.; Xi, W.Q.; Liu, X. Effect of dietary iron (Fe) levels on growth performance, hepatic lipid metabolism and antioxidant responses in juvenile yellow catfish Pelteobagrus fulvidraco. Aquacult. Nutr. 2017, 23, 1475–1482. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Liu, D.; Keesing, J.K.; Gong, J. Macroalgal blooms favor heterotrophic diazotrophic bacteria in nitrogen-rich and phosphorus-limited coastal surface waters in the Yellow Sea. Estuar. Coast. Shelf Sci. 2015, 163, 75–81. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Aly, S.E. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J. Appl. Toxicol. 2005, 25, 218–223. [Google Scholar] [CrossRef]
- Shahraki, J.; Motallebi, A.; Pourahmad, J. Oxidative mechanisms of fish hepatocyte toxicity by the harmful dinoflagellate Cochlodinium polykrikoides. Mar. Environ. Res. 2013, 87–88, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Liu, Z.; Zhao, Z.; Dupont, S.; Wu, F.; Huang, W.; Chen, J.; Hu, M.; Lu, W.; et al. Impact of zinc oxide nanoparticles and ocean acidification on antioxidant responses of Mytilus coruscus. Chemosphere 2018, 196, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Wang, X.; Wang, Z.; Wei, Z.; Wang, L. Metal accumulation and antioxidant defenses in the freshwater fish Carassius auratus in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes. J. Hazard. Mater. 2014, 275, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Pereira, E.; Duarte, A.C.; Ahmad, I. Glutathione and its dependent enzymes’ modulatory responses to toxic metals and metalloids in fish—A review. Environ. Sci. Pollut. Res. 2013, 20, 2133–2149. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Pires, A.; Velez, C.; Almeida, Â.; Moreira, A.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E. Effects of seawater acidification on Diopatra neapolitana (Polychaete, Onuphidae): Biochemical and regenerative capacity responses. Ecol. Indic. 2016, 60, 152–161. [Google Scholar] [CrossRef]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction Techniques, Biological Activities and Health Benefits of Marine Algae Enteromorpha prolifera Polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef]
- Garcia, D.; Lima, D.; da Silva, D.G.H.; de Almeida, E.A. Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: Evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water. Ecotoxicol. Environ. Saf. 2020, 190, 110107. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, S.; Wu, S. Modulation of the growth performance, body composition and nonspecific immunity of crucian carp Carassius auratus upon Enteromorpha prolifera polysaccharide. Int. J. Biol. Macromol. 2020, 147, 29–33. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Nam, S.E.; Shin, Y.K.; Rhee, J.S. The dinoflagellate Alexandrium affine acutely induces significant modulations on innate immunity, hepatic function, and antioxidant defense system in the gill and liver tissues of red seabream. Aquat. Toxicol. 2021, 240, 105985. [Google Scholar] [CrossRef] [PubMed]






| Gene | Sequence (5′–3′) | GenBank Accession Number |
|---|---|---|
| β-actin-F | GGAAATCGTGCGTGACATTAAG | XM_020109620.1 |
| β-actin-R | CCTCTGGACAACGGAACCTCT | |
| sod-F | CGTTGGAGACCTGGGGAATGTG | EF681883.1 |
| sod-R | ATCGTCAGCCTTCTCGTGGATC | |
| cat-F | CACGGACCAGATGAAGCAGTG | XM_020079314.1 |
| cat-R | CCTTGGAGTAGCGGGTAATGTC |
| Index | Types | ||
|---|---|---|---|
| UG | NC | p Value | |
| Fe (μg/L) | 7.4 ± 0.79 * | 5.4 ± 0.17 | 0.013 |
| Ca (mg/L) | 330.3 ± 1.16 | 375.0 ± 29.46 | 0.12 |
| K (mg/L) | 439.0 ± 3.67 ** | 378.7 ± 18.56 | 0.005 |
| Mg (mg/L) | 1075.7 ± 4.04 ** | 970.7 ± 25.54 | 0.002 |
| Na (mg/L) | 9904.7 ± 65.07 ** | 8953.7 ± 258.72 | 0.000 |
| Group | IBW (g) | FBW (g) | SR (%) | SGR (%/day) |
|---|---|---|---|---|
| NC | 2.5 ± 0.02 | 52.9 ± 0.87 a | 88.7 ± 1.16 a | 5.1 ± 0.04 a |
| PC | 2.5 ± 0.01 | 40.8 ± 0.64 bc | 48.0 ± 3.46 c | 4.6 ± 0.01 bc |
| 2101 | 2.5 ± 0.01 | 42.7 ± 1.83 bc | 77.3 ± 9.24 ab | 4.8 ± 0.07 b |
| 2102 | 2.5 ± 0.01 | 44.6 ± 3.1 b | 53.3 ± 9.87 c | 4.8 ± 0.12 b |
| 2103 | 2.5 ± 0.03 | 43.2 ± 2.29 bc | 75.3 ± 1.15 ab | 4.8 ± 0.04 b |
| 2104 | 2.5 ± 0.01 | 43.6 ± 1.89 b | 58.7 ± 4.16 bc | 4.8 ± 0.7 b |
| 2105 | 2.5 ± 0.01 | 42.2 ± 3.32 bc | 56.0 ± 15.62 bc | 4.7 ± 0.14 bc |
| 2106 | 2.5 ± 0.01 | 39.9 ± 4.68 bc | 63.3 ± 8.08 bc | 4.6 ± 0.19 bc |
| 2107 | 2.5 ± 0.02 | 36.6 ± 1.04 c | 61.3 ± 10.07 bc | 4.5 ± 0.04 c |
| 2108 | 2.5 ± 0.02 | 38.3 ± 1.87 bc | 52.7 ± 4.16 c | 4.6 ± 0.08 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, W.; Wang, R.; Xu, D.; Chen, Y.; Cui, Z.; Chen, S. Effects of Ulva prolifera Degradation on Growth Performance and Antioxidant Capacity of Japanese Flounder (Paralichthys olivaceus) Family. Fishes 2023, 8, 598. https://doi.org/10.3390/fishes8120598
Yang Y, Li W, Wang R, Xu D, Chen Y, Cui Z, Chen S. Effects of Ulva prolifera Degradation on Growth Performance and Antioxidant Capacity of Japanese Flounder (Paralichthys olivaceus) Family. Fishes. 2023; 8(12):598. https://doi.org/10.3390/fishes8120598
Chicago/Turabian StyleYang, Yingming, Wenlong Li, Run Wang, Dan Xu, Yadong Chen, Zhongkai Cui, and Songlin Chen. 2023. "Effects of Ulva prolifera Degradation on Growth Performance and Antioxidant Capacity of Japanese Flounder (Paralichthys olivaceus) Family" Fishes 8, no. 12: 598. https://doi.org/10.3390/fishes8120598
APA StyleYang, Y., Li, W., Wang, R., Xu, D., Chen, Y., Cui, Z., & Chen, S. (2023). Effects of Ulva prolifera Degradation on Growth Performance and Antioxidant Capacity of Japanese Flounder (Paralichthys olivaceus) Family. Fishes, 8(12), 598. https://doi.org/10.3390/fishes8120598