1. Introduction
One of the main problems of ichthyology associated with the conservation of biodiversity and the cultivation of fish for the aquaculture purposes is a deep understanding of the physiological and biochemical functions of the main systems of their metabolisms and, in particular, the physiological function of the blood, and the gas exchange [
1].
Cartilaginous fishes are ancient vertebrates that evolved from armored jawless fish at the end of the Silurian period, more than 420 million years ago [
2]. These animals were living witnesses to many events and the biological evolution of our planet. Their DNA “remembered” the splitting of a single land on modern continents and the birth of the ocean. During their long history, cartilaginous and bony fishes have seen significant transformation for oxygen level variations in the atmosphere and water, and warm climatic conditions have been changed more than once by ice ages. In connection with the social problems of global warming and changes in the oxygen regime, it is necessary to understand what molecular biochemical changes occur with such high vitality [
3].
Cartilaginous and bony fish species are interesting not only from an evolutionary point of view, but also because they have high morphophysiological and biochemical plasticity. This plasticity is due to the fact that fish live in rapidly changing conditions: changes in the degree of absorption and salinity that occur in nature, hypoxia, environmental change, environmental impact, exposure to abiogenic and biogenic nature, etc., properties on the proximity of particles. All this determines the uniqueness of the circulatory system in cartilaginous and bony fish species and, above all, the properties of their erythrocytes, the composition of blood plasma, etc. It is well known that in the circulatory system the efficiency of using blood depends on its rheological properties. In this case, the integral rheological value that determines the decisive role in the efficiency of oxygen transport and other manifestations, blood viscosity, which is the determining factor, are the following factors: (1) plasma viscosity; (2) haematocrit; (3) aggregation of blood cells; (4) deformation properties of erythrocytes [
4]. Such a characteristic of the viscosity properties of blood, strictly speaking, is valid only for large vessels. In the microvascular bed, neither haematocrit nor cell aggregation no longer play a significant role, and plasma viscosity and deformation characteristics of erythrocytes become very important. Currently, there is no scientific description of the movement of blood in the capillaries [
5]. Moreover, the process of erythrocytes passage through the capillary sections of a vessel with a smaller diameter than the size of the cells themselves does not fit into the formalization. Such a passage through the capillaries exposes the erythrocytes to significant deformation loads and, apparently, occurs at an energy cost with the help of special mechanisms. These mechanisms are most likely more pronounced in vertebrates that have the largest nucleated erythrocytes [
6] and less deformable compared to non-nuclear RBC in terms of their linear dimensions. The well-known Fareus-Lindqvist phenomenon adds some questions about the movement of erythrocytes in capillaries [
7]. These researchers showed back in 1931 that blood is a non-Newtonian fluid and in vessels less than 300 microns, its viscosity, for unknown reasons, drops up to 20 times. The understanding and research for mechanisms that facilitate the passage of erythrocytes through the capillaries is still relevant today.
The physical basis for the overall improvement of rheology in capillaries, as we believe, can be local thermal heating of the near-wall layer of plasma and the plasma membrane of erythrocytes, which can provide a significant decrease in viscosity and an increase in the deformation capabilities of cells. The prerequisites for such a phenomenon in capillaries are that under conditions of deformation loads and increasing hypoxia, erythrocytes secrete ATP (Adenosine triphosphoric acid), the concentration of which can reach micromolecular concentrations in the whole blood of fish [
8]. In capillaries, considering the small volumes of near-wall plasma, the ATP concentration can be an order of magnitude higher, which determines the importance of this intermediate in the processes of local heat production.
Our earlier studies showed that large, nucleated erythrocytes of the thornback ray (with size of 27 × 14 µm) and scorpionfish (11 × 8 µm of cell size) [
6] had high ecto-ATPase activity. The activity of ecto-ATPase in the thornback ray erythrocytes suspension ranged from 3.0 to 3.9 nmol Fn/min/µL Red Blood Cells (RBC), and in the scorpionfish from 6.0 to 10.0 nmol Fn/min/µL RBC [
7]. This allowed us to suggest that the thermal energy of ATP hydrolysis by ecto-ATPases of fish erythrocytes is used to heat the plasma membrane of red blood cells and the near-wall plasma layer, aimed at improving the rheological characteristics of blood in the capillary blood flow [
6].
The hydrolytic activity of this enzyme with the release of thermal energy could contribute to the heating of the near-wall plasma layer and change the viscoelastic characteristics of the erythrocyte plasma membrane. One of the factors contributing to this assumption is the presence in the plasma of a significant amount of ATP, which can significantly affect the functioning of the entire circulatory system of marine fish. The presence of its micromolar concentrations in the blood plasma of fish is maintained due to the release of macroenergetic substrates into it, mainly by erythrocytes. This process is especially enhanced with an increase in hypoxia in the capillary blood flow, where erythrocytes are subjected to strong deformation loads [
9]. The reasons for the high concentration of extracellular ATP in the bloodstream have not been precisely established, and this issue is open for more detailed study [
10]. As already mentioned, ATP in blood plasma constantly undergoes enzymatic hydrolysis, mainly due to extracellular ATPases of erythrocytes of vascular endothelial cells, and other unidentified “participants” of this process.
Based on the abovementioned, the aim of this research is to study the heat production of fish erythrocyte suspensions under conditions of the experimental addition of extracellular ATP to cell suspensions in vitro. The objectives of the study included a comparative analysis of the heat-producing activity of erythrocyte suspensions of cartilaginous fish species—the Black Sea thornback ray sea (Raja clavata L.) and bony fish species—black scorpionfish (Scorpaena porcus L.).
2. Material and Methods
2.1. Experimental Design
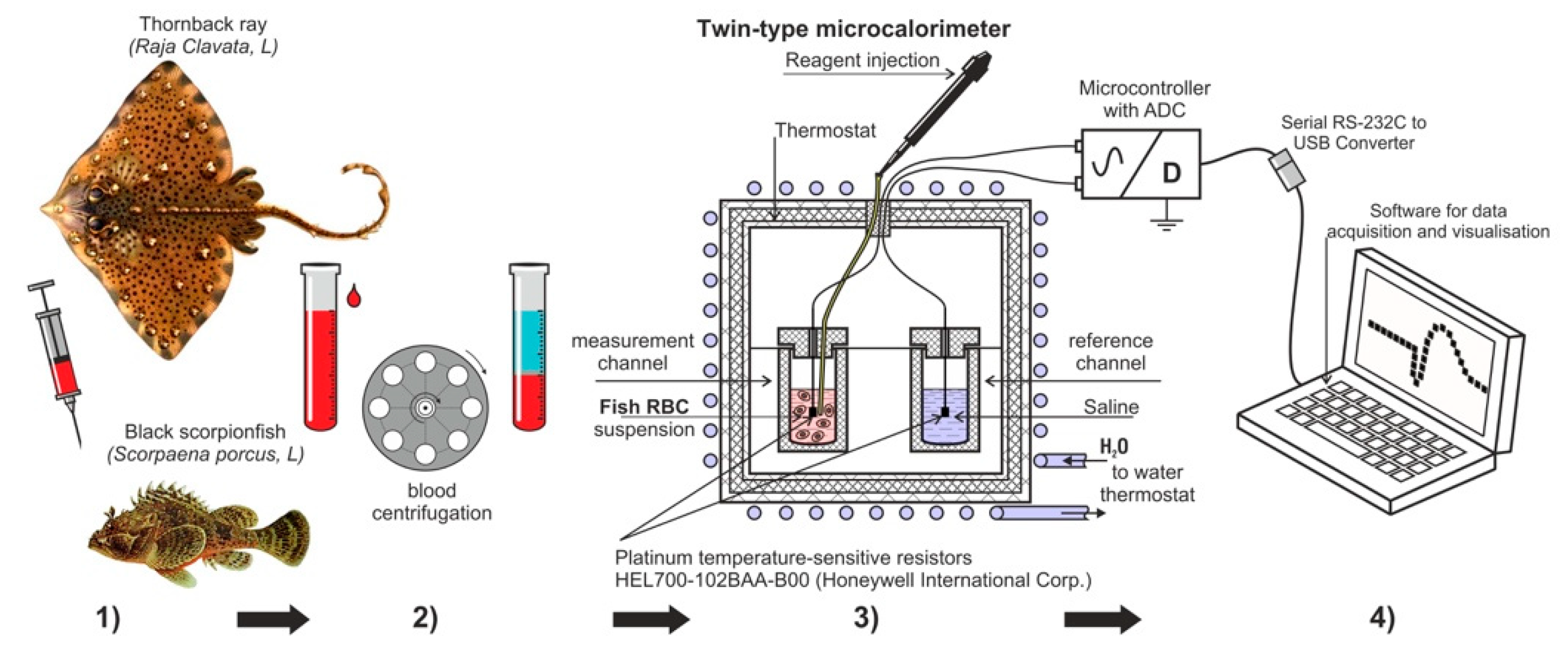
The experimental design used in this study is presented in
Figure 1. Four consecutive steps were followed in order to obtain the experimental results. Firstly, the blood was taken from acclimated fishes (more details are in
Section 2.2). Then the second step was to prepare the suspension of fish erythrocytes washed from plasma. Thirdly, the measurements of the temperature dynamic of erythrocytes suspension were registered, using specially designed cell and injection of reagents (more details are in
Section 2.4) the fourth step was to record and analyse the obtained data.
2.2. Species Selection, Capture and Conditions of Keeping Fish
The studies were carried out in May 2021 on erythrocytes of the Black Sea thornback ray and black scorpionfish, with their natural habitat, living in the shelf zone of the South-Eastern Crimea. Black Sea thornback ray is a widespread species of stingray. Females reach a length of 125 cm, and males—70–85 cm. The weight of the caught individuals was 1.5–2.5 kg. The Black Sea thornback ray is a cold-water-loving species, rarely approaching places with shallow depths, more often it stays at a depth of 50 to 70 m with an optimal temperature range for this species, equal to +8 °C–+18 °C. The black scorpionfish is a heat-loving ambush predator, the temperature halo of habitat is from 12 °C to 19 °C. It spends most of its life in a motionless state among stones and underwater vegetation. This fish makes sharp jerks when catching prey. The black scorpionfish belongs to the benthic fish group and is widely distributed in the coastal zone of the Black Sea [
11]. The sizes of the black scorpionfish caught for this study were 230.0–270 g in weight and 25.0–27.0 cm in length.
The rays were caught with hooks, and scorpionfishes—with a set seine. In specially constructed tanks, the fish were transported to aquatic pools (1000 L) for acclimation before the experiments. Acclimation of fish was carried out in stagnant, air-aerated pools for 2–4 days at temperatures from +14 °C to +18 °C. All rays and scorpionfishes taken in the experiment were females of class II and III stages of maturity. To obtain a sufficient number of erythrocytes for the experiment, blood was taken from 3 stingrays and 5 scorpionfishes.
2.3. Preparation of Fish Erythrocytes Suspensions
Blood was obtained from stingrays by puncture of the heart ventricle, and from scorpionfish, by puncture of the caudal aorta. For the experiment, a mixture of blood (21 mL) taken from 3 rays was used, since the collection of no more than 7 mL of blood from one individual fish gave the most favorable prognosis for the subsequent survival of the fish. In scorpionfishes, 3 mL of blood was taken from one individual, and a mixture of blood from 5 fish (15 mL) was used for the experiment.
The collected fish blood was transferred into a cooled (+4 °C) solution for washing erythrocytes from plasma in a ratio of 1:10. Heparin (50 units/mL of blood) was used as an anticoagulant. Erythrocytes were washed three times from plasma in a tenfold volume of saline having the following composition for rays (mmol/L): 220 NaCl, 300 urea, 10 Tris-HCl buffer, (pH 7.3–7.4). The physiological solution for washing scorpionfish erythrocytes had (mmol/L): 180 NaCl, 10 Tris-HCl-buffer, (pH 7.3–7.4). Cell sedimentation was carried out on a K-23 centrifuge (Germany) (1500 rpm, 2 min at +4 °C). The supernatant was decanted. The layer of leukocytes located on the surface of the precipitated erythrocytes was carefully removed with filter paper. The washed erythrocytes were resuspended in a small amount of physiological saline, and the hematocrit (Ht) was determined in the resulting erythrocyte suspension using a TL-11 hematocrit centrifuge (Germany). Cell suspensions with established hematocrit were diluted with saline to a value equal to 50% for rays and 30% for scorpionfish, and these prepared suspensions were used for further experiments.
2.4. Study of Heat Production of Erythrocyte Suspensions
The studies were carried out in a microcalorimeter modified for these purposes (
Figure 1) [
12]. The volumes of erythrocyte suspensions of the studied fish were different due to the difficulty of obtaining a sufficient number of erythrocytes. For the ray, the final suspension volume was 5.0 mL (4.5 mL of cell suspension + 0.5 mL of ATP solution), and for scorpionfish, 2.0 mL (1.5 mL of cell suspension + 0.5 mL of ATP solution). In parallel, the same control cell was prepared with physiological saline corresponding to the type of fish. The experiments were carried out at the room temperature of +20 °C. The cell in which the studies were carried out was a plastic test tube with external thermal insulation, 10.5 mL capacity, with a lid made of a material with low thermal conductivity. The injection of additives was carried out using a microdoser directly into the cell with a short opening of the chamber and a thermostated ATP aliquot (1 mg/mL suspension), the temperature of suspension was as close as possible to the temperature of the cell with the erythrocyte suspension. A HEL700-102BAA-B00 platinum resistive thermal sensor was placed in the cell. Resistive thermal sensors from Honeywell with a resistance of 1 kOhm are miniature (1.5–2 mm) and sensitive platinum sensors. They allow measuring the temperature in the installation with an accuracy of ± 0.0001 °C, at a measurement frequency of 0.16 Hz and an accuracy of ± 0.0015 °C, at a maximum measurement frequency of 6.65 Hz. A feature of the differential method is the use of two control (T2) and measuring (T1) cells located in the microcalorimeter, with the exclusion of external and internal heat transfer. We used the measurement ΔT = T1 − T2, which represents the temperature difference in the measuring (T1) and control cells (T2). The use of two cells simultaneously, control and experimental, eliminated artifacts of external thermal effects and increased the reliability of the data obtained. Signals from sensors with a resolution of 6.65 measurements per second were converted into a computer using a special electronic unit and a developed program. In the final form, these measurements were processed using special software and recorded as a data array. The program displayed a graph on the monitor screen, which made it possible to quickly assess the current temperature changes inside the cell. The rate of temperature change in the cell was expressed in °C/h. The data obtained in the experiments were processed using the OriginPro software from OriginLab Corp.
2.5. Statistic
The data were subjected to statistical processing and presented as the arithmetic mean ± standard deviation (x ± S). The collected data were examined for their homogeneity and normal disruption prior to data analysis. Significant differences of the compared results were determined using Student’s t-test. Differences were considered significant at p < 0.05.
4. Discussion
The studies were aimed at modeling the situation of a simultaneous increase in the macroenergetic adenosine triphosphate phosphate in the pericellular space of fish erythrocytes. As already reported above, the final extracellular concentration of ATP as a result of a single injection of its concentrated solution was 1 mg/mL, or about 200 μM. The choice of this concentration is not accidental and is due to an attempt to obtain a quite tangible physiological response to the heat-producing activity of fish erythrocytes, which have an ecto-ATPase with a sufficiently high enzymatic activity on their surface. In the presented experiments, the ecto-ATPase activity of erythrocytes of thornback ray was 3.4 nmol Fn/min/µL RBC, and in scorpion fish it was 6.4 nmol Fn/min/µL RBC, which corresponded, to our earlier measurements, with the values of erythrocyte eco-enzyme activities in these fish [
13]. We assumed that the nonconjugated enzymatic hydrolysis of extracellular ATP by the ecto-ATPase of the erythrocytes of the studied fish should have caused a significant release of heat, which was recorded by a temperature sensor inserted into the cell. The process of ATP hydrolysis can be expressed by the following equation:
The splitting of ATP to ADP generates 7.3 kcal/mol, and ADP to AMP generates another 7.3 kcal/mol. Taking into account the total losses, the energy yield during the hydrolysis of ATP to AMP corresponded to ≈ 12 kcal/mol [
14].
Despite the obvious difference in the amplitude of the temperature response to the addition of ATP to erythrocyte suspensions of cartilaginous and bony fish, it is necessary to recalculate the increase in ∆T of scorpionfish, considering the volume and hematocrit of the cell suspension. The gain obtained must be divided by 2.5 and multiplied by 1.67 (0.22 °C: 2.5 × 1.67 = 0.147 °C). The introduction of corrections is due to the different volumes of suspensions of erythrocytes of the thornback ray and black scorpionfish (5.0 mL: 2.0 mL = 2.5) and, accordingly, their different heat capacity, as well as the difference in hematocrit (50%: 30% = 1.67). The calculation of the heat-producing activity of the erythrocytes of the black scorpionfish showed a 1.8-fold higher value than that of the thornback ray. This result was in good agreement with the multiplicity of the activity of the ectoenzyme (nmolFn/min/µL RBC) in the erythrocytes of the studied fish (6.4:3.4 = 1.88) and testified in favor of the correctness of the obtained experimental data.
Thus, the erythrocytes of the cartilaginous species of thornback ray and bony scorpionfish differed in their ability to generate heat. The erythrocytes of the black scorpionfish in this experiment demonstrated a high heat-producing activity in terms of the calculated amplitude and duration of heat generation.
The results obtained confirm our assumptions and testify in favor of enzymatic thermal hydrolysis of ATP by the ecto-ATPase of erythrocytes of thornback ray and scorpionfish. It is important to note that after the end of the experiment in a cell with cells suspension, because of natural sedimentation of erythrocytes, the supernatant was completely transparent without signs of hemolysis. This could be one of the criteria that heat release occurs due to ecto-ATPases, and not due to the work of intracellular Na
+, K
+- and Ca
2+-ATPases. In earlier experiments on black scorpionfish erythrocytes, we showed complete blocking of the heat production process by adding salt of the EDTA-Na
2 (final concentration 1.0 M) in the experimental cell, which occurred due to the binding of bivalent Mg
2+ and Ca
2+ ions by the complex one. The blocking of the ATP hydrolysis process is because the enzyme uses an ATP complex with divalent Ca
2+ or Mg
2+ cations as a substrate [
15].
The importance of experimental confirmation of the ability of fish erythrocytes to generate heat is related to the fact that the development of the question of the role of extracellular ATP and the functions of ecto-ATPases in fish erythrocytes is still at an early stage. In the works of Jensen et al. [
8], it was convincingly shown that fish erythrocytes constantly release ATP into the blood plasma at a concentration of about 3 μM. The results of Jensen et al. [
8] demonstrated the average concentration of extracellular ATP in the blood of trout and did not reflect the concentrations of phosphagen that can occur in local parts of the bloodstream. In arterioles and capillaries, where there was an increase in hypoxic conditions and deformation loads of erythrocytes, the release of ATP by red blood cells can increase sharply [
10,
16]. At the same time, in this section of the blood flow, the concentration of ATP can multiply. Such an increase in the extracellular concentration of ATP in the capillaries can bring us closer to the model of our experiment. In the human circulatory system, the release of ATP under conditions of hypoxia and deformation pressure is aimed at triggering vasodilating mechanisms [
17,
18]. In fish, the same mechanisms for the release of ATP from erythrocytes into the extracellular space could not be associated with the vasodilating function. In any case, the discrepancy between the role of ATP allocated to the extracellular space in vertebrates with nucleated and non-nuclear erythrocytes in response to hypoxia required more additional studies.
The novelty of the performed experiments lies in the confirmation of the proposed assumption that the heat of the hydrolytic activity of surface ecto-ATPases can be used by vertebrates with nucleated erythrocytes much more widely than in mammals [
19]. In these vertebrates, ecto-ATPases are not only participants in the ATP receptor signal transduction chain, but due to the function of local heat generation, they are deeply integrated into the vital activity of the plasma membrane of the cell and the entire blood flow. It can also be emphasized that all species of vertebrates, including fish, which have high ecto-ATPase activity during their life, face periods of existence at temperatures close to zero. Therefore, the magnitude of the hydrolyzing activity of ecto-ATPase serves, as we believe, as a physical basis capable of ensuring not only the passage of capillaries by large erythrocytes, but also a factor that contributes to the survival of these species under hypothermic conditions. Finally, the effect of heat generation on the surface of the plasma membrane of erythrocytes may be of vital importance for the functioning of red blood cells, which opens the prospect of studying this phenomenon for practical use in medical application.
5. Conclusions
The presence of heat-producing activity of suspensions of erythrocytes of the thornback ray and black scorpionfish is the evidence of the possible use of thermal energy of ATP hydrolysis by fish erythrocytes, both to maintain the functional state of the own plasma membrane and to improve rheological characteristics in the capillary section of blood flow. Therefore, we make a conclusion that:
1. Erythrocytes of the thornback ray and black scorpionfish, when extracellular ATP was added, demonstrated heat generation, which was almost four times shorter in duration up to the maximum ∆T in suspension of erythrocytes of the thornback ray than in black scorpionfish.
2. The process of decreasing the temperature in the experimental cell for the erythrocyte suspension of the scorpionfish occurred more than two times slower than in the thornback ray suspension.
3. Calculation of the heat-producing activity of scorpionfish erythrocytes showed a (0.147:0.08) 1.84 times higher value than that of the thornback ray, which was in good agreement with the multiplicity of ecto-ATPase activity (6.4:3.4 = 1.88) (nmol inorganic Phosphorus (Pi)/min/μL of RBC) of plasma membranes of erythrocytes of the studied fishes.
These experiments demonstrate that in nature nothing is lost without a trace. Heat, as a by-product of the hydrolytic activity of surface ecto-ATPases, is used by vertebrates with nucleated erythrocytes much more widely than in mammals. In these vertebrates, ecto-ATPases also conduct the functions of local heat generation and, thus, are deeply integrated into the vital activity of the cell and the entire blood flow as a whole.