Effect of Chronic Hydrogen Peroxide Exposure on Ion Transport in Gills of Common Carp (Cyprinus carpio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experiment Design, and Sampling
2.2. Histological Evaluation of Gills
2.3. Determination of Ion Content in Plasma and Gills
2.4. Determination of Ion-Transport-Related Enzymes in Gills
2.5. Real-Time Quantitative PCR
2.6. Statistical Analysis
3. Results
3.1. Histopathology Observation of Gills after H2O2 Exposure
3.2. Changes in Ion Content in Plasma and Gills after H2O2 Exposure
3.3. Activities of Ion Transport Enzymes in Gills after H2O2 Exposure
3.4. Expression of Ion-Transport-Related Genes in Gills after H2O2 Exposure
3.5. Expression of Calcium Signaling Pathway-Related Genes after H2O2 Exposure
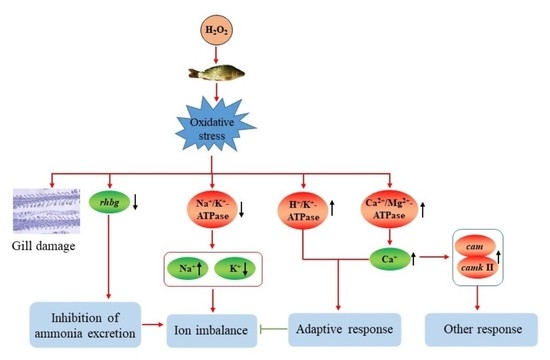
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ueki, R.; Imaizumi, Y.; Iwamoto, Y.; Sakugawa, H.; Takeda, K. Factors controlling the degradation of hydrogen peroxide in river water, and the role of riverbed sand. Sci. Total Environ. 2020, 716, 136971. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.G.; Petasne, R.G.; Plane, J.M.C. Photochemical formation of hydrogen peroxide in natural waters exposed to sunlight. Environ. Sci. Technol. 1988, 22, 1156–1160. [Google Scholar] [CrossRef]
- Kang, C.-M.; Han, J.-S.; Sunwoo, Y. Hydrogen peroxide concentrations in the ambient air of Seoul, Korea. Atmos. Environ. 2002, 36, 5509–5516. [Google Scholar] [CrossRef]
- Cory, R.M.; Davis, T.W.; Dick, G.J.; Johengen, T.H.; Denef, V.J.; Berry, M.A.; Page, S.E.; Watson, S.B.; Yuhas, K.; Kling, G.W. Seasonal dynamics in dissolved organic matter, hydrogen peroxide, and cyanobacterial blooms in Lake Erie. Front. Mar. Sci. 2016, 3, 54. [Google Scholar] [CrossRef]
- Overton, K.; Samsing, F.; Oppedal, F.; Dalvin, S.; Stien, L.H.; Dempster, T. The use and effects of hydrogen peroxide on salmon lice and post-smolt Atlantic salmon. Aquaculture 2018, 486, 246–252. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Magariños, B.; Irgang, R.; Toranzo, A.E. Use of hydrogen peroxide against the fish pathogen Tenacibaculum maritimum and its effect on infected turbot (Scophthalmus maximus). Aquaculture 2006, 257, 104–110. [Google Scholar] [CrossRef]
- Yanong, R.P.E. Use of Hydrogen Peroxide in Finfish Aquaculture; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Bögner, D.; Bögner, M.; Schmachtl, F.; Bill, N.; Halfer, J.; Slater, M.J. Hydrogen peroxide oxygenation and disinfection capacity in recirculating aquaculture systems. Aquac. Eng. 2021, 92, 102140. [Google Scholar] [CrossRef]
- Adams, M.B.; Crosbie, P.B.B.; Nowak, B.F. Preliminary success using hydrogen peroxide to treat Atlantic salmon, Salmo salar L., affected with experimentally induced amoebic gill disease (AGD). J. Fish Dis. 2012, 35, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Helgesen, K.O.; Romstad, H.; Aaen, S.M.R.; Horsberg, T.E. First report of reduced sensitivity towards hydrogen peroxide found in the salmon louse Lepeophtheirus salmonis in Norway. Aquac. Rep. 2015, 1, 37–42. [Google Scholar] [CrossRef]
- Bechmann, R.K.; Arnberg, M.; Gomiero, A.; Westerlund, S.; Lyng, E.; Berry, M.; Agustsson, T.; Jager, T.; Burridge, L.E. Gill damage and delayed mortality of Northern shrimp (Pandalus borealis) after short time exposure to anti-parasitic veterinary medicine containing hydrogen peroxide. Ecotoxicol. Environ. Saf. 2019, 180, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Chen, H.; Wen, Y. Effect of hydrogen peroxide on Microcystic aeruginosa: Role of cytochromes P450. Sci. Total Environ. 2018, 626, 211–218. [Google Scholar] [CrossRef]
- Sinha, A.K.; Eggleton, M.A.; Lochmann, R.T. An environmentally friendly approach for mitigating cyanobacterial bloom and their toxins in hypereutrophic ponds: Potentiality of a newly developed granular hydrogen peroxide-based compound. Sci. Total Environ. 2018, 637–638, 524–537. [Google Scholar] [CrossRef]
- Abele-Oeschger, D.; Tug, H.; Rottgers, R. Dynamics of UV-Driven Hydrogen Peroxide Formation on an Intertidal Sandflat. Limnol. Oceanogr. 1997, 42, 1406–1415. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.G. Photochemical formation of hydrogen peroxide in surface and ground waters exposed to sunlight. Science 1983, 220, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Sinel’nikov, V.E. Hydrogen peroxide level in river water, and methods for detecting it. Gidrobiol. Zh. 1971, 7, 115–119. [Google Scholar]
- Ndungu, L.K.; Steele, J.H.; Hancock, T.L.; Bartleson, R.D.; Milbrandt, E.C.; Parsons, M.L.; Urakawa, H. Hydrogen peroxide measurements in subtropical aquatic systems and their implications for cyanobacterial blooms. Ecol. Eng. 2019, 138, 444–453. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ushiroda, T.; Takeda, K.; Kumamoto, Y.; Tsubota, H. Diurnal and seasonal distribution of hydrogen peroxide in seawater of the Seto Inland Sea. Geochem. J. 1993, 27, 103–115. [Google Scholar] [CrossRef]
- Sakugawa, H.; Kaplan, I.R.; Tsai, W.; Cohen, Y. Atmospheric hydrogen peroxide. Environ. Sci. Technol. 1990, 24, 1452–1462. [Google Scholar] [CrossRef]
- Schmidt, L.J.; Gaikowski, M.P.; Gingerich, W.H. Environmental Assessment for the Use of Hydrogen Peroxide in Aquaculture for Treating External Fungal and Bacterial Diseases of Cultured Fish and Fish Eggs; USGS: Washington DC, USA, 2006.
- Rach, J.J.; Schreier, T.M.; Howe, G.E.; Redman, S.D. Effect of Species, Life Stage, and Water Temperature on the Toxicity of Hydrogen Peroxide to Fish. Progress. Fish-Cult. 1997, 59, 41–46. [Google Scholar] [CrossRef]
- Tort, M.J.; Hurley, D.; Fernandez-Cobas, C.; Wooster, G.A.; Bowser, P.R. Effects of hydrogen peroxide treatments on catalase and glutathione activity in Walleye Sander vitreus. J. World Aquac. Soc. 2005, 36, 577–586. [Google Scholar] [CrossRef]
- Ana, R.; Hijranyavuzcan, Y.; Ignacio, C.; Neil, D. Physiological stress responses of sea bass (Dicentrarchus labrax) to hydrogen peroxide (H₂O₂) exposure. Aquaculture 2010, 304, 104–107. [Google Scholar]
- Mansour, A.T.; Espinosa, C.; García-Beltrán, J.M.; Miao, L.; Ceballos Francisco, D.C.; Alsaqufi, A.S.; Esteban, M.Á. Dietary supplementation of drumstick tree, Moringa oleifera, improves mucosal immune response in skin and gills of seabream, Sparus aurata, and attenuates the effect of hydrogen peroxide exposure. Fish Physiol. Biochem. 2020, 46, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.O.; Kim, Y.K.; Nam, Y.K. Effect of hydrogen peroxide exposures on mucous cells and lysozymes of gill tissues of olive flounder Paralichthys olivaeceus. Aquac. Res. 2016, 47, 433–444. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, L.; Feng, L.; Jiang, J.; Zhou, X. Protective Effect of Vitamin C on Oxidative Damage in Intestinal Epithelial Cells of Jian Carp (Cyprinus carpio var. Jian). Chin. J. Anim. Nutr. 2012, 24, 1503–1511. [Google Scholar]
- Jia, R.; Du, J.; Cao, L.; Li, Y.; Johnson, O.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, inflammatory and immune responses in hydrogen peroxide-induced liver injury of tilapia (GIFT, Oreochromis niloticus). Fish Shellfish. Immunol. 2019, 84, 894–905. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Chronic exposure of hydrogen peroxide alters redox state, apoptosis and endoplasmic reticulum stress in common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 229, 105657. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Application of transcriptome analysis to understand the adverse effects of hydrogen peroxide exposure on brain function in common carp (Cyprinus carpio). Environ. Pollut. 2021, 286, 117240. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Immune, inflammatory, autophagic and DNA damage responses to long-term H2O2 exposure in different tissues of common carp (Cyprinus carpio). Sci. Total Environ. 2021, 757, 143831. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Ding, Q.; Teame, T.; Yang, Y.; Ran, C.; Zhang, Z.; Zhou, Z. Research advances in the structure, function, and regulation of the gill barrier in teleost fish. Water Biol. Secur. 2023, 100139. [Google Scholar] [CrossRef]
- Wu, P.; Pan, F.-Y.; Feng, L.; Jiang, W.-D.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Methionine hydroxy analogue supplementation modulates gill immunological and barrier health status of grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 74, 637–648. [Google Scholar] [CrossRef]
- Seker, E.; Ispir, U.; Yonar, S.M.; Yonar, M.E.; Turk, C. Antioxidant responses of rainbow trout (Oncorhynchus mykiss) gills after exposure to hydrogen peroxide. Fresenius Environ. Bull. 2015, 24, 1837–1840. [Google Scholar]
- Tort, M.J.; Jenningsbashore, C.; Wilson, D.; Wooster, G.A.; Bowser, P.R. Assessing the Effects of Increasing Hydrogen Peroxide Dosage on Rainbow Trout Gills Utilizing a Digitized Scoring Methodology. J. Aquat. Anim. Health 2002, 14, 95–103. [Google Scholar] [CrossRef]
- Fernandez-Senac, C.; Monaghan, S.J.; Mascolo, D.; Baily, J.L.; Betancor, M.; Chalmers, L.; Paladini, G.; Adams, A.; Fridman, S.; Bron, J.E. Investigating the impacts of H2O2 treatment on gills of healthy Atlantic salmon reveals potential changes to mucus production with implications on immune activity. Fish Shellfish Immunol. 2022, 128, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Fujikata, A. Variation of carp serum constituent levels with time for storage of whole blood. Nsugaf 1984, 50, 1337–1340. [Google Scholar] [CrossRef]
- Spears, J.; Kamunde, C.; Stevens, E.D. Effect of TRIS and bicarbonate as buffers on anesthetic efficacy of tricaine methane sulfonate in zebrafish (Danio rerio). Zebrafish 2014, 11, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Nunes, B.; Campos, J.C.; Gomes, R.; Braga, M.R.; Ramos, A.S.; Antunes, S.C.; Correia, A.T. Ecotoxicological effects of salicylic acid in the freshwater fish Salmo trutta fario: Antioxidant mechanisms and histological alterations. Environ. Sci. Pollut. Res. 2015, 22, 667–678. [Google Scholar] [CrossRef]
- Li, D. The Invention Relates to a Method and a Kit for Detecting Sodium Ions. Patent CN 201110132290.1, 20 May 2011. [Google Scholar]
- Pan, Y.; Li, W. Clinical application of serum potassium ion determination. Contemp. Med. 2008, 14, 54. [Google Scholar]
- Liu, D.; Zhang, E.; Pu, Y.; Dong, X. Blockage role of self-designed multiple orgen preservation solution on mitochondrial calcium influx during cryopreservation of rabbit kidney. J. Med. Sci. Yanbian Univ. 2013, 36, 88–91. [Google Scholar]
- Tang, Y. Determination of the structure and stability constant of the final product of serum mercuric chlorothiocyanate method. Chin. J. Lab. Med. 2003, 4, 30–31. [Google Scholar]
- Zhang, Y.; Sun, H.J.; Zhang, J.Y.; Ndayambaje, E.; Lin, H.; Chen, J.; Hong, H. Chronic exposure to dichloroacetamide induces biochemical and histopathological changes in the gills of zebrafish. Environ. Toxicol. 2019, 34, 781–787. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Malll, A.K.; Klenk, D.C. Measurement of protein using BCA. Anal. Biochem. 1985, 150, 76–185. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.-P.; Du, J.-L.; He, Q.; Gu, Z.-Y.; Jeney, G.; Xu, P.; Yin, G.-J. Effects of High-Fat Diet on Steatosis, Endoplasmic Reticulum Stress and Autophagy in Liver of Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Jia, R.; Gu, Z.; He, Q.; Du, J.; Cao, L.; Jeney, G.; Xu, P.; Yin, G. Anti-oxidative, anti-inflammatory and hepatoprotective effects of Radix Bupleuri extract against oxidative damage in tilapia (Oreochromis niloticus) via Nrf2 and TLRs signaling pathway. Fish Shellfish Immunol. 2019, 93, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods-A Companion Methods Enzymol. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Kapotwe, M.; Dabi, S.B.; Montes, C.d.S.; Shrivastava, J.; Blust, R.; Boeck, G.D. Differential modulation of ammonia excretion, Rhesus glycoproteins and ion-regulation in common carp (Cyprinus carpio) following individual and combined exposure to waterborne copper and ammonia. Aquat. Toxicol. 2016, 170, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, J.; Xu, P.; Li, J.; Li, H.; Ren, H. Identification of housekeeping genes suitable for gene expression analysis in Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2012, 33, 775–779. [Google Scholar] [CrossRef]
- Ribeiro, C.; Schreiner, M.; Iannini, C.; Gomes, A.D.; Moreira, R.G. Acute and chronic effects of temperature on membrane adjustments in the gills of a neotropical catfish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110625. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Evans, D.H. The fish gill: Site of action and model for toxic effects of environmental pollutants. Environ. Health Perspect. 1987, 71, 47–58. [Google Scholar] [CrossRef]
- Pritchard, J.B. The gill and homeostasis: Transport under stress. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R1269–R1271. [Google Scholar] [CrossRef]
- Bowers, J.; Speare, D.J.; Burka, J.F. The effects of hydrogen peroxide on the stress response of Atlantic Salmon (Salmo salar). J. Vet. Pharmacol. Ther. 2002, 25, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Tort, M.J.; Wooster, G.A.; Bowser, P.R. Effects of hydrogen peroxide on hematology and blood chemistry parameters of walleye Stizostedion vitreum. J. World Aquac. Soc. 2003, 34, 236–242. [Google Scholar] [CrossRef]
- Hwang, P.-P.; Lee, T.-H.; Lin, L.-Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef] [PubMed]
- Doğanli, C.; Oxvig, C.; Lykke-Hartmann, K. Zebrafish as a novel model to assess Na+/K+-ATPase-related neurological disorders. Neurosci. Biobehav. Rev. 2013, 37, 2774–2787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Q.; Liu, C.; Xie, J.X.; Yan, Y.; Lai, F.; Duan, Q.; Li, X.; Tian, J.; Xie, Z. Involvement of Na/K-ATPase in hydrogen peroxide-induced activation of the Src/ERK pathway in LLC-PK1 cells. Free. Radic. Biol. Med. 2014, 71, 415–426. [Google Scholar] [CrossRef]
- Dobrota, D.; Matejovicova, M.; Kurella, E.G.; Boldyrev, A.A. Na/K-ATPase under oxidative stress: Molecular mechanisms of injury. Cell. Mol. Neurobiol. 1999, 19, 141–149. [Google Scholar] [CrossRef]
- Liu, J.; Kennedy, D.J.; Yan, Y.; Shapiro, J.I. Reactive Oxygen Species Modulation of Na/K-ATPase Regulates Fibrosis and Renal Proximal Tubular Sodium Handling. Int. J. Nephrol. 2012, 2012, 381320. [Google Scholar] [CrossRef]
- Chkadua, G.; Nozadze, E.; Tsakadze, L.; Shioshvili, L.; Arutinova, N.; Leladze, M.; Dzneladze, S.; Javakhishvili, M. Effect of H2O2 on Na,K-ATPase. J. Bioenerg. Biomembr. 2022, 54, 241–249. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, J. Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian). Aquaculture 2009, 288, 285–289. [Google Scholar] [CrossRef]
- Deng, X.-s.; Meng, X.; Fullerton, D.; Stone, M.; Iguidbashian, J.; Jaggers, J. Complement Cross Talks With H-K-ATPase to Upregulate Runx2 in Human Aortic Valve Interstitial Cells. J. Surg. Res. 2023, 286, 118–126. [Google Scholar] [CrossRef]
- Barnawi, E.A.; Doherty, J.E.; Ferreira, P.G.; Wilson, J.M. Extra-gastric expression of the proton pump H+/K+-ATPase in the gills and kidney of the teleost Oreochromis niloticus. J. Exp. Biol. 2020, 223, jeb214890. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.P.; Verlander, J.W.; Wingo, C.S.; Evans, D.H. A putative H+-K+-ATPase in the Atlantic stingray, Dasyatis sabina: Primary sequence and expression in gills. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R981–R991. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jarabo, I.; Paullada-Salmeron, J.A.; Jerez-Cepa, I.; Neto, J.B.G.; Bystriansky, J.S.; Mancera, J.M. Acute Stress in Lesser-Spotted Catshark (Scyliorhinus canicula Linnaeus, 1758) Promotes Amino Acid Catabolism and Osmoregulatory Imbalances. Animals 2022, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.; Wood, C. An analysis of branchial ammonia excretion in the freshwater rainbow trout: Effects of environmental pH change and sodium uptake blockade. J. Exp. Biol. 1985, 114, 329–353. [Google Scholar] [CrossRef]
- Nakada, T.; Westhoff, C.M.; Kato, A.; Hirose, S. Ammonia secretion from fish gill depends on a set of Rh glycoproteins. FASEB J. 2007, 21, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.M.; Wright, P.A.; Wood, C.M. Ammonia and urea handling by early life stages of fishes. J. Exp. Biol. 2017, 220, 3843–3855. [Google Scholar] [CrossRef]
- Nawata, C.M.; Hung, C.C.; Tsui, T.K.; Wilson, J.M.; Wright, P.A.; Wood, C.M. Ammonia excretion in rainbow trout (Oncorhynchus mykiss): Evidence for Rh glycoprotein and H+-ATPase involvement. Physiol. Genom. 2007, 31, 463–474. [Google Scholar] [CrossRef]
- Lim, M.Y.-T.; Zimmer, A.M.; Wood, C.M. Acute exposure to waterborne copper inhibits both the excretion and uptake of ammonia in freshwater rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2015, 168, 48–54. [Google Scholar] [CrossRef]
- Shang, Z.-H.; Huang, M.; Wu, M.-X.; Mi, D.; You, K.; Zhang, Y.-L. Transcriptomic analyses of the acute aerial and ammonia stress response in the gill and liver of large-scale loach (Paramisgurnus dabryanus). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2021, 250, 109185. [Google Scholar] [CrossRef]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. In Calcium Signaling, 2nd ed.; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1131, pp. 1–6. [Google Scholar]
- Verkhratsky, A. Calcium and cell death. In Calcium Signalling and Disease: Molecular Pathology of Calcium; Carafoli, E., Brini, M., Eds.; Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2007; Volume 45, pp. 465–480. [Google Scholar]
- Ogi, M.; Yokomori, H.; Inao, M.; Oda, M.; Ishii, H. Hepatic stellate cells express Ca^ pump-ATPase and Ca^-Mg^-ATPase in plasma membrane of caveolae. J. Gastroenterol. 2000, 35, 912–918. [Google Scholar] [CrossRef]
- Sugasini, D.; Lokesh, B.R. Rats fed linseed oil in microemulsion forms enriches the cardiac sarcoplasmic reticulum lipids with docosahexaenoic acid and lower calcium transport. J. Funct. Foods 2013, 5, 1863–1872. [Google Scholar] [CrossRef]
- Kong, X.H.; Wang, G.Z.; Li, S.J. Changes of antioxidant defenses, atpase activity and cell membrane fatty acid composition in gill of scylla serrata under low temperature acclimation. Acta Hydrobiol. Sin. 2007, 12, 708–713. [Google Scholar]
- Fei, Y.; Peng, S.; Peng, S.; Shi, Z. Effects of low salinity stress on the antioxidant enzyme activities in juvenile Pampus argenteus liver and the APTase activities in its gill and kidney. Yingyong Shengtai Xuebao 2011, 22, 1059–1066. [Google Scholar]
- Putney Jr, J.W. Calcium signaling: Up, down, up, down.... What’s the point? Science 1998, 279, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Jana, B. Role of Calcium in Modulating the Conformational Landscape and Peptide Binding Induced Closing of Calmodulin. J. Phys. Chem. B 2021, 125, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Burkitt, M.J.; Kass, G.E.; Dypbukt, J.M.; Nicotera, P. Calcium ions and oxidative cell injury. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1992, 32, S33–S42. [Google Scholar] [CrossRef]
- Toledo, F.D.; Pérez, L.M.; Basiglio, C.L.; Ochoa, J.E.; Sanchez Pozzi, E.J.; Roma, M.G. The Ca(2+)-calmodulin-Ca(2+)/calmodulin-dependent protein kinase II signaling pathway is involved in oxidative stress-induced mitochondrial permeability transition and apoptosis in isolated rat hepatocytes. Arch. Toxicol. 2014, 88, 1695–1709. [Google Scholar] [CrossRef]







| Gene | Primer Sequence (5′–3′) | Genbank Number |
|---|---|---|
| cam | F: CGCGAGGCTTTTCGGGTA | XM_019069985.2 |
| R: ACCATCACCATCTATGTCGGC | ||
| camk Ⅱ | F: GGAATCATCAGAGAGCGCC | XM_042728442.1 |
| R: ACCATCACCATCTATGTCGGC | ||
| nkaa | F: ATGGGTCGTATCGCCACTCT | JX570881.1 |
| R: CCAAGGATCAGGGAGAGAACG | ||
| rhbg | F: TCCCAGTTTCCAGGATGTTC | JX570877 [49] |
| R: TGGAAAAAGCCCTGCATAAG | ||
| rhcg1 | F: ATCCTGAACATCCTCCATGC | JX570878 [49] |
| R: AACTTGGCCAGAACATCCAC | ||
| rhcg2 | F: CACAAAGCCACACACAGTCC | JX570879 [49] |
| R: TCTTTTTCTCGCCGTTCTTG | ||
| β-actin | F: ATCCGTAAAGACCTGTATGCCA | JQ619774.1 [50] |
| R: GGGGAGCAATGATCTTGATCTTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, Y.; Li, B.; Hou, Y.; Jia, R.; Zhu, J. Effect of Chronic Hydrogen Peroxide Exposure on Ion Transport in Gills of Common Carp (Cyprinus carpio). Fishes 2023, 8, 134. https://doi.org/10.3390/fishes8030134
Mou Y, Li B, Hou Y, Jia R, Zhu J. Effect of Chronic Hydrogen Peroxide Exposure on Ion Transport in Gills of Common Carp (Cyprinus carpio). Fishes. 2023; 8(3):134. https://doi.org/10.3390/fishes8030134
Chicago/Turabian StyleMou, Yating, Bing Li, Yiran Hou, Rui Jia, and Jian Zhu. 2023. "Effect of Chronic Hydrogen Peroxide Exposure on Ion Transport in Gills of Common Carp (Cyprinus carpio)" Fishes 8, no. 3: 134. https://doi.org/10.3390/fishes8030134
APA StyleMou, Y., Li, B., Hou, Y., Jia, R., & Zhu, J. (2023). Effect of Chronic Hydrogen Peroxide Exposure on Ion Transport in Gills of Common Carp (Cyprinus carpio). Fishes, 8(3), 134. https://doi.org/10.3390/fishes8030134