Dietary Tryptophan Supplementation Implications on Performance, Plasma Metabolites, and Amino Acid Catabolism Enzymes in Meagre (Argyrosomus regius)
Abstract
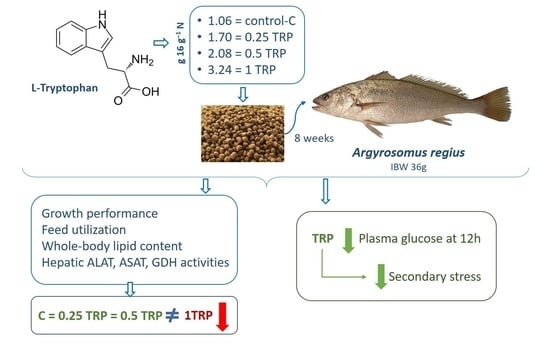
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Growth Trial
2.3. Sampling
2.4. Analytical Methods
2.4.1. Proximate Analysis
2.4.2. Plasma Metabolites
2.4.3. Enzyme Activity
2.5. Statistical Analysis
3. Results
3.1. Growth Trial
3.2. Plasma Metabolites
3.3. Hepatic Protein Catabolism Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Souza, C.F.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential Oils as Stress-Reducing Agents for Fish Aquaculture: A Review. Front. Physiol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Gonzalez-Silvera, D.; Herrera, M.; Giráldez, I.; Esteban, M.A. Effects of Dietary Tryptophan and Aspartate on the Immune Response of Meagre (Argyrosomus regius) after stress. Fishes 2018, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Chen, R.; Goodwin, A.; Praven, K.; Dabrowski, K.; Lee, K.J. Effects of dietary vitamins C and E on alternative complement activity, hematology, tissue composition, vitamin concentrations and response to heat stress in juvenile golden shiner (Notemigonus crysoleucas). Aquaculture 2004, 242, 553–569. [Google Scholar] [CrossRef]
- Salze, G.; McLean, E.; Schwarz, M.H.; Craig, S.R. Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 2008, 274, 148–152. [Google Scholar] [CrossRef]
- Teixeira, C.; Rodrigues, P.; Serrão, P.; Figueira, L.; Guimarães, L.; Teles, L.O.; Peres, H.; Carvalho, A.P. Dietary tryptophan supplementation does not affect growth but increases brain serotonin level and modulates the expression of some liver genes in zebrafish (Danio rerio). Fish Physiol. Biochem. 2021, 47, 1541–1558. [Google Scholar] [CrossRef]
- Hseu, J.R.; Lu, F.I.; Su, H.M.; Wang, L.S.; Tsai, C.L.; Hwang, P.P. Effects of exogenous tryptophan on cannibalism, survival and growth in juvenile grouper, Epinephelus coiodes. Aquac. Eng. 2003, 218, 251–263. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Hosseini, S.A.; Soudagar, M. Dietary tryptophan changes serum stress markers, enzyme activity and ions concentration of wild common carp, Cyprinus carpio exposed to ambient copper. Fish Physiol. Biochem. 2012, 38, 1419–1426. [Google Scholar] [CrossRef]
- Höglund, E.; Bakke, M.J.; Øverli, Ø.; Winberg, S.; Nilsson, G.E. Suppression of aggressive behavior in juvenile Atlantic cod (Gadus morhua) by L-tryptophan supplementation. Aquaculture 2005, 249, 525–531. [Google Scholar] [CrossRef]
- Höglund, E.; Sørensen, C.; Bakke, M.J.; Nilsson, G.E.; Øverli, Ø. Attenuation of stress-induced anorexia in brown trout (Salmo trutta) by pre-treatment with dietary L-tryptophan. Br. J. Nutr. 2007, 97, 786–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winberg, S.; Øverli, Ø.; Lepage, O. Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary L-tryptophan. J. Exp. Biol. 2001, 204, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Wolkers, C.P.B.; Serra, M.; Hoshiba, M.A.; Urbinati, E.C. Dietary L-tryptophan alters aggression in juvenile matrinxa Brycon amazonicus. Fish Physiol. Biochem. 2012, 38, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.L.; Atkinson, J.L.; Hilton, J.W.; Were, K.E. Effect of dietary tryptophan on plasma and brain tryptophan, brain serotonin and brain 5-hydroxyindoleacetic acid in rainbow trout. J. Nutr. Biochem. 1990, 1, 49–54. [Google Scholar] [CrossRef]
- Herrero, M.J.; Martínez, F.J.; Miguez, J.M.; Madrid, J.A. Response of plasma and gastrointestinal melatonin, plasma cortisol and activity rhythms of European sea bass (Dicentrarchus labrax) to dietary supplementation with tryptophan and melatonin. J. Comp. Physiol. B 2007, 177, 319–326. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- Lepage, O.; Tottmar, O.; Winberg, S. Elevated dietary intake of L-tryptophan counteracts the stress-induced elevation of plasma cortisol in rainbow trout (Oncoryhnchus mykiss). J. Exp. Biol. 2002, 205, 3679–3687. [Google Scholar] [CrossRef]
- Herrera, M.; Fernández-Alacid, L.; Sanahuja, I.; Ibaraz, A.; Salamanca, N.; Morales, E.; Giráldez, I. Physiological and metabolic effects of a tryptophan-enriched diet to face up chronic stress in meagre (Argyrosomus regius). Aquaculture 2020, 522, 735102. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C.C. Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- Monfort, M.C. Present Market Situation and Prospects of Meagre (Argyosomus regius), as an Emerging Species in Mediterranean Aquaculture. Studies and Reviews; General Fisheries Commission for the Mediterranean: Rome, Italy, 2010; p. 28. Available online: https://www.fao.org/documents/card/en/c/415d0c69-c173-5dc4-8b79-3ac0f17190ab/ (accessed on 9 February 2023).
- Duncan, N.J.; Estévez, A.; Fernández-Palacios, H.; Gairin, I.; Hernández-Cruz, C.M.; Roo, J.; Schuchardt, D.; Vallés, R. Aquaculture production of meager (Argyrosomus regius): Hatchery techniques, ongrowing and market. In Advances in Aquaculture Hatchery Technology; Alan, G., Gavin, B., Eds.; Woodhead: Cambridge, UK, 2013; pp. 519–541. [Google Scholar] [CrossRef]
- Parisi, G.; Terova, G.; Gasco, L.; Piccolo, G.; Roncarati, A.; Moretti, V.M.; Centoducati, G.; Gatta, P.P.; Pais, A. Current status and future perspectives of Italian finfish aquaculture. Rev. Fish Biol. Fish. 2014, 24, 15–73. [Google Scholar] [CrossRef]
- Fountoulaki, E.; Grigorakis, K.; Kounna, C.; Rigos, G.; Papandroulakis, N.; Diakogeorgakis, J.; Kokou, F. Growth performance and product quality of meagre (Argyrosomus regius) fed diets of different protein/lipid levels at industrial scale. Ital. J. Anim. Sci. 2017, 16, 685–694. [Google Scholar] [CrossRef] [Green Version]
- APROMAR OPP 30 (2021) Informe 2021—La Acuicultura en España. Asociación Empresarial de Acuicultura de España. September 2021. Available online: www.apromar.es. (accessed on 9 February 2023).
- Fernández-Alacid, L.; Sanahuja, I.; Ordóñez-Grande, B.; Sanchéz-Nuño, S.; Gisbert, E.; Herrera, M.; Ibarz, A. Skin mucus metabolites and cortisol in meagre fed acute stress-attenuating diets: Correlations between plasma and mucus. Aquaculture 2019, 499, 185–194. [Google Scholar] [CrossRef]
- AOAC. Official methods of Analysis of AOAC International AOAC 2005. Available online: http://www.aoac.org/omarev1/front_18th_ed.pdf (accessed on 9 February 2023).
- De Vries, J.W.; Koshi, C.M.; Egberg, D.C.; Larson, P.A. Comparison between a spectrophotometric and high-pressure liquid chromatography method for determining tryptophan in food products. J. Agric. Food Chem. 1980, 28, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Herrera, M.; Mancera, J.M.; Costas, B. The use of dietary additives in fish stress mitigation: Comparative endocrine and physiological responses. Front. Endocrinol. 2019, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Lepage, O.; Larson, E.T.; Mayer, I.; Winberg, S. Tryptophan affects both gastrointestinal melatonin interregnal activity in stressed and nonstressed rainbow trout. J. Pineal Res. 2005, 144, 180–187. [Google Scholar] [CrossRef]
- Tejpal, C.S.; Pal, A.K.; Sahu, N.P.; Kumar, J.A.; Muthappa, N.A.; Vidya, S.; Rajan, M.G. Dietary supplementation of L-tryptophan mitigates crowding stress and augments the growth in Cirrhinus mrigala fingerlings. Aquaculture 2009, 293, 272–277. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Silva, P.I.M.; Costas, B.; Larsen, B.K.; Santos, G.A.; Conceição, L.E.C.; Dias, J.; Øverli, Ø.; Höglund, E.; Scharma, J.W. The effect of tryptophan supplemented diets on brain serotonergic activity and plasma cortisol under undisturbed and stressed conditions in grouped-housed Nile Tilapia Oreochromis Niloticus. Aquaculture 2013, 400, 129–134. [Google Scholar] [CrossRef]
- Winberg, S.; Thörnqvist, P.O. Role of brain serotonin in modulating fish behavior. Curr. Zool. 2016, 62, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, S.M.; Pérez-Jiménez, A.; Costas, B.; Azeredo, R.; Gesto, M. Physiological roles of tryptophan in teleosts: Current knowledge and perspectives for future studies. Rev. Aquac. 2019, 11, 3–24. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Research Council of the National Academies: Washington, DC, USA, 2011; p. 360.
- Rubio, V.C.; Sánchez-Vásquez, F.J.; Madrid, J.A. Oral administration of melatonin reduces food intake and modifies macronutrient selection in European sea bass (Dicentrarchus labrax L.). J. Pineal Res. 2004, 34, 42–47. [Google Scholar] [CrossRef] [PubMed]
- López-Olmeda, J.F.; Madrid, J.A.; Sánchez-Vásquez, F.J. Melatonin effects on food intake and activity rhythms in two fish species with different activity patterns: Diurnal (goldfish) and nocturnal (tench). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.S.; Varghese, T.J. Dietary tryptophan requirement for the growth of rohu, Labeo Rohita. J. Appl. Aquac. 1997, 7, 71–79. [Google Scholar] [CrossRef]
- Abidi, S.F.; Khan, M.A. Dietary tryptophan requirement of fingerling rohu, Labeo rohita (Hamilton), based on growth and body composition. J. World Aquac. Soc. 2010, 41, 700–709. [Google Scholar] [CrossRef]
- Ahmed, I.; Khan, M.A. Dietary tryptophan requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquac. Res. 2005, 36, 687–695. [Google Scholar] [CrossRef]
- Ahmed, I. Dietary amino acid L-tryptophan requirement of fingerling catfish, Heteropneustes fossilis (Bloch), estimated by growth and haematolo-biochemical parameters. Fish Physiol. Biochem. 2012, 38, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino Acids- Biochemistry and Nutrition, 2nd ed.; CRC: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Oldendorf, W.H. Transport of metabolic substrates through the blood-brain barrier. J. Neurochem. 1977, 8, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Voilqué, G.; Odle, J.; Kim, S.W. Dietary L-tryptophan supplementation with reduced large neutral amino acids enhances feed efficiency and decreases hormone secretion in nursery pigs under social mixing stress. J. Nutr. 2012, 142, 1540–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Z.; Yang, Y.; Wen, Y.; Zhou, Y.; Fu, X.; Ding, S.; Liu, G.; Yao, K.; Wu, X.; Deng, Z.; et al. Metabolomic analysis of amino acid and fat metabolism in rats with L-tryptophan supplementation. Amino acids 2014, 46, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Pal, A.K.; Das, T.; Manush, S.M.; Sarma, K.; Venkateshwarlu, G.; Mukherjee, S.C. Secondary stress response in Indian major carps, Labeo rohita (Hamilton), Catla catla (Hamilton) and Cirrhinus mrigala (Hamilton) fry to increasing packing densities. Aquac. Res. 2006, 37, 472–476. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response in juvenile Chinook salmon (Oncorhynchus tshawystscha). Aquaculture 1991, 62, 299–310. [Google Scholar] [CrossRef]
- Monteiro, M.; Sousa, C.; Coutinho, F.; Castro, C.; Fontinha, F.; Guerreiro, I.; Pousão, P.; Matos, E.; Díaz-Rosales, P.; Oliva-Teles, A.; et al. Functional feeds to tackle meagre (Argyosomus regius) stress: Physiological responses under acute stressful handling conditions. Mar. Drugs 2021, 19, 598. [Google Scholar] [CrossRef] [PubMed]
- Tort, L.; Pavlidis, M.A.; Woo, N.Y.S. Stress and Welfare in Sparid Fishes. In Sparidae: Biology and Aquaculture of Gilthead Sea Bream and Other Species; Pavlidis, M.A., Mylonas, C.C., Eds.; Woley-Blackwell: Oxford, UK, 2011; pp. 199–232. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Saurabh, S.; Pal, A.K.; Sahu, N.P.; Arasu, A.R.T. Stress mitigation and growth enhancing effect of dietary tryptophan in rohu (Labeo rohita, Hamilton, 1822) fingerlings. Fish Physiol. Biochem. 2014, 40, 1325–1338. [Google Scholar] [CrossRef]
- Diógenes, A.F.; Teixeira, C.; Almeida, E.; Skrzynska, A.; Costas, B.; Oliva-Teles, A.; Peres, H. Effects of dietary tryptophan and chronic stress in gilthead seabream (Sparus aurata) juveniles fed corn distillers dried grains with solubles (DDGS) based diets. Aquaculture 2019, 498, 396–404. [Google Scholar] [CrossRef]
- Lepage, O.; Vilchez, I.M.; Pottinger, T.G.; Winberg, S. Time-course of the effect of dietary L-tryptophan on plasma cortisol in rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 2003, 206, 3589–3599. [Google Scholar] [CrossRef] [Green Version]
- Höglund, E.; Øverli, Ø.; Andersson, M.Å.; Silva, P.; Laursen, D.C.; Moltesen, M.M.; Krogdahl, Å.; Schjolden, J.; Winberg, S.; Vindas, M.A.; et al. Dietary L-tryptophan leaves a lasting impression on the brain and stress response. Br. J. Nutr. 2017, 117, 1351–1357. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.P. Amino acids and proteins. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Academic: San Diego, CA, USA; pp. 143–179.
- Herrera, M.; Herves, M.A.; Giráldez, I.; Skar, K.; Mogren, H.; Mortensen, A.; Puvanendran, V. Effects of amino acid supplementations on metabolic and physiological parameters in Atlantic cod (Gadus morhua) under stress. Fish Physiol. Biochem. 2017, 43, 591–602. [Google Scholar] [CrossRef]
- Herrera, M.; Miró, J.M.; Giráldez, I.; Salamanca, N.; Martos-Sitcha, J.A.; Mancera, J.M.; López, J.R. Metabolic and stress responses in Senegalese soles (Solea senegalensis Kaup) fed tryptophan supplements: Effects of concentration and feeding period. Animals 2019, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Melo, J.F.B.; Lundstedt, L.M.; Metón, I.; Baanante, I.V.; Moares, G. Effects of dietary levels of protein on nitrogenous metabolism of Rhamdia quelen (Teleostei: Pimelodidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 145, 181–187. [Google Scholar] [CrossRef] [Green Version]
| Diets | 0Trp | 0.25Trp | 0.5Trp | 1Trp |
|---|---|---|---|---|
| Ingredients (% DM) | ||||
| Fish meal 1 | 35.0 | 35.0 | 35.0 | 35.0 |
| Corn gluten 2 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean meal 3 | 18.1 | 17.7 | 17.3 | 16.4 |
| Wheat meal 4 | 20.6 | 20.8 | 20.9 | 21.3 |
| Fish oil | 11.8 | 11.8 | 11.8 | 11.8 |
| Vitamin premix 5 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride (50%) | 0.5 | 0.5 | 0.5 | 0.5 |
| Mineral premix 6 | 1.0 | 1.0 | 1.0 | 1.0 |
| Binder 7 | 1.0 | 1.0 | 1.0 | 1.0 |
| Agar | 1.0 | 1.0 | 1.0 | 1.0 |
| L-tryptophan | - | 0.25 | 0.50 | 1.00 |
| Proximate analyses (% dry weight) | ||||
| Dry matter (%) | 96.6 | 95.8 | 95.7 | 95.7 |
| Crude protein | 46.3 | 45.8 | 45.7 | 45.9 |
| Crude lipid | 16.5 | 16.3 | 16.5 | 16.5 |
| Ash | 10.0 | 10.2 | 10.1 | 10.2 |
| Diets | 0Trp | 0.25Trp | 0.5Trp | 1Trp | |
|---|---|---|---|---|---|
| Essential amino acids | |||||
| Histidine | 3.05 | 3.09 | 3.04 | 2.99 | |
| Arginine | 6.09 | 6.10 | 6.12 | 6.14 | |
| Isoleucine | 4.32 | 4.43 | 4.50 | 4.33 | |
| Leucine | 8.83 | 8.82 | 8.91 | 8.99 | |
| Valine | 5.56 | 5.45 | 5.36 | 5.24 | |
| Lysine | 6.34 | 6.43 | 6.20 | 6.26 | |
| Methionine | 2.67 | 2.67 | 2.50 | 2.69 | |
| Phenylalanine | 4.87 | 4.74 | 4.85 | 4.61 | |
| Threonine | 4.32 | 4.27 | 4.24 | 4.25 | |
| Tryptophan | 1.06 | 1.70 | 2.08 | 3.24 | |
| Nonessential amino acids | |||||
| Aspartic Acid | 9.00 | 9.02 | 9.93 | 9.97 | |
| Glutamic Acid | 16.4 | 16.3 | 16.4 | 16.2 | |
| Serine | 5.56 | 5.43 | 5.70 | 4.75 | |
| Glycine | 5.57 | 5.41 | 5.57 | 5.09 | |
| Tyrosine | 3.88 | 3.93 | 3.75 | 3.73 | |
| Alanine | 6.13 | 6.52 | 6.21 | 5.72 | |
| Proline | 5.20 | 5.12 | 5.12 | 5.43 |
| Diets | 0Trp | 0.25Trp | 0.5Trp | 1Trp |
|---|---|---|---|---|
| Initial body weight (g) | 36.1 ± 0.0 | 36.1 ± 0.0 | 36.2 ± 0.0 | 36.0 ± 0.0 |
| Final body weight (g) | 123.7 ± 7.0 b | 116.5 ± 1.8 b | 114.0 ± 2.9 b | 87.8 ± 3.0 a |
| Weight gain (g kg ABW−1 § day−1) | 19.5 ± 0.7 b | 18.8 ± 0.2 b | 18.5 ± 0.3 b | 14.9 ± 0.5 a |
| Thermal growth coefficient 1 | 0.14 ± 0.0 b | 0.13 ± 0.0 b | 0.13 ± 0.0 b | 0.09 ± 0.0 a |
| Daily growth index 2 | 3.0 ± 0.2 b | 2.8 ± 0.1 b | 2.8 ± 0.1 b | 2.0 ± 0.1 a |
| Feed intake (g kg ABW−1 § day−1) | 17.5 ± 0.4 | 17.4 ± 0.3 | 16.8 ± 0.2 | 15.8 ± 0.6 |
| Feed efficiency 3 | 1.1 ± 0.0 b | 1.1 ± 0.0 b | 1.1 ± 0.0 b | 0.93 ± 0.2 a |
| Protein efficiency ratio 4 | 2.4 ± 0.0 b | 2.4 ± 0.0 b | 2.4 ± 0.0 b | 2.03 ± 0.4 a |
| Mortality (%) | 2.2 ± 2.2 | 0.0 ± 0.0 | 2.2 ± 2.2 | 4.4 ± 2.2 |
| Nitrogen utilization | ||||
| N Intake (g kg ABW−1day−1) 5 | 1.3 ± 0.1 b | 1.3 ± 0.0 ab | 1.2 ± 0.0 ab | 1.2 ± 0.1 a |
| N Retention (g kg ABW−1day−1) 6 | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.4 ± 0.0 a |
| N Retention (% nitrogen intake) 7 | 45.3 ± 3.0 b | 45.0 ± 0.8 b | 45.4 ± 1.3 b | 37.7 ± 2.0 a |
| Diets | Initial | 0Trp | 0.25Trp | 0.5Trp | 1Trp |
|---|---|---|---|---|---|
| Whole-body composition (%) | |||||
| Dry matter (%) | 24.6 | 28.5 ± 0.3 b | 27.81 ± 1.0 ab | 28.3 ± 0.4 ab | 26.4 ± 1.0 a |
| Protein | 15.8 | 17.5 ± 0.4 | 17.9 ± 0.2 | 18.0 ± 0.4 | 17.1 ± 0.3 |
| Lipids | 5.9 | 8.1 ± 0.3 b | 7.2 ± 1.2 ab | 7.8 ± 0.3 ab | 6.1 ± 0.8 a |
| Ash | 4.2 | 4.3 ± 0.4 | 4.1 ± 0.3 | 4.4 ± 0.2 | 4.3 ± 0.1 |
| Body indices | |||||
| Hepatosomatic index 1 | ― | 1.7 ± 0.3 | 1.8 ± 0.3 | 2.0 ± 0.1 | 1.9 ± 0.4 |
| Visceral index 2 | ― | 4.0 ± 0.4 | 3.9 ± 0.4 | 4.1 ± 0.2 | 4.1 ± 0.6 |
| Relative intestinal length 3 | ― | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 |
| Time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diets | 0h | 1h | 3h | 6h | 12h | |||||||
| Protein | ||||||||||||
| 0Trp | 1.8 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.1 | 2.4 ± 0.2 | 2.6 ± 0.4 | |||||||
| 0.25Trp | 1.8 ± 0.2 | 2.3 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.1 | 2.3 ± 0.1 | |||||||
| 0.5Trp | 1.8 ± 0.1 | 2.2 ± 0.2 | 2.5 ± 0.2 | 2.3 ± 0.1 | 2.3 ± 0.2 | |||||||
| 1Trp | 1.8 ± 0.2 | 2.3 ± 0.2 | 2.0 ± 0.4 | 2.2 ± 0.1 | 2.3 ± 0.3 | |||||||
| Glucose | ||||||||||||
| 0Trp | 71.2 ± 23.3 AB | 44.0 ± 9.1 A | 69.1 ± 11.3 bAB | 72.5 ± 23.8 B | 112.4 ± 26.2 bC | |||||||
| 0.25Trp | 77.0 ± 4.8 B | 48.1 ± 9.6 A | 48.6 ± 17.3 aA | 69.9 ± 8.2 AB | 63.7 ± 10.8 aAB | |||||||
| 0.5Trp | 76.4 ± 7.7 | 45.0 ± 11.4 | 49.9 ± 7.8 a | 75.9 ± 16.5 | 63.1 ± 18.4 a | |||||||
| 1Trp | 60.5 ± 3.7 | 46.5 ± 6.7 | 55.5 ± 21.4 ab | 72.7 ± 16.4 | 58.7 ± 15.2 a | |||||||
| Cholesterol | ||||||||||||
| 0Trp | 67.3 ± 8.0 | 109.8 ± 14.3 | 73.3 ± 5.1 | 78.8 ± 7.5 | 71.3 ± 6.5 | |||||||
| 0.25Trp | 64.1 ± 4.8 | 112.2 ± 10.5 | 87.0 ± 9.9 | 88.8 ± 10.2 | 63.1 ± 5.5 | |||||||
| 0.5Trp | 67.1 ± 7.7 | 112.1 ± 20.5 | 89.9 ± 14.4 | 87.6 ± 12.4 | 66.1 ± 6.1 | |||||||
| 1Trp | 63.1 ± 3.7 | 100.0 ± 19.7 | 80.0 ± 13.4 | 75.6 ± 9.4 | 65.2 ± 10.8 | |||||||
| Triglycerides | ||||||||||||
| 0Trp | 153.6 ± 27.0 A | 735.8 ± 84.4 C | 486.8 ± 66.6 B | 575.1 ± 103.1 B | 541.8 ± 92.6 B | |||||||
| 0.25Trp | 152.5 ± 15.1 A | 607.3 ± 46.6 C | 387.6 ± 48.0 B | 720.1 ± 92.1 C | 534.4 ± 171.7 BC | |||||||
| 0.5Trp | 161.8 ± 20.9 A | 819.5 ± 152.0 D | 637.5 ± 60.2 CD | 595.4 ± 80.0 BC | 274.0 ± 102.7 AB | |||||||
| 1Trp | 137.3 ± 8.2 A | 689.5 ± 95.5 D | 435.4 ± 89.4 C | 636.4 ± 70.3 D | 279.7 ± 66.7 B | |||||||
| Two Way ANOVA | Variation Source | Diet | Time | |||||||||
| Time | Diet | Interaction | 0Trp | 0.25Trp | 0.5Trp | 1Trp | 0h | 1h | 3h | 6h | 12h | |
| Protein | *** | ns | ns | a | a | a | a | a | b | b | b | b |
| Glucose | *** | ** | ** | |||||||||
| Cholesterol | *** | * | ns | ab | ab | b | a | a | c | b | c | a |
| Triglycerides | *** | ns | *** | |||||||||
| Diets | 0Trp | 0.25Trp | 0.5Trp | 1Trp |
|---|---|---|---|---|
| ASAT | 1947.6 ± 198.6 c | 1673.1 ± 104.4 bc | 1529.4 ± 211.7 b | 1231.0 ± 132.7 a |
| ALAT | 666.0 ± 83.4 c | 582.8 ± 38.3 bc | 534.7 ± 88.5 b | 416.0 ± 24.1 a |
| GDH | 830.6 ± 71.6 c | 721.3 ± 53.3 b | 684.2 ± 51.6 ab | 592.3 ± 53.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, C.; Pedrosa, R.; Castro, C.; Magalhães, R.; Matos, E.; Oliva-Teles, A.; Peres, H.; Pérez-Jiménez, A. Dietary Tryptophan Supplementation Implications on Performance, Plasma Metabolites, and Amino Acid Catabolism Enzymes in Meagre (Argyrosomus regius). Fishes 2023, 8, 141. https://doi.org/10.3390/fishes8030141
Teixeira C, Pedrosa R, Castro C, Magalhães R, Matos E, Oliva-Teles A, Peres H, Pérez-Jiménez A. Dietary Tryptophan Supplementation Implications on Performance, Plasma Metabolites, and Amino Acid Catabolism Enzymes in Meagre (Argyrosomus regius). Fishes. 2023; 8(3):141. https://doi.org/10.3390/fishes8030141
Chicago/Turabian StyleTeixeira, Cláudia, Rita Pedrosa, Carolina Castro, Rui Magalhães, Elisabete Matos, Aires Oliva-Teles, Helena Peres, and Amalia Pérez-Jiménez. 2023. "Dietary Tryptophan Supplementation Implications on Performance, Plasma Metabolites, and Amino Acid Catabolism Enzymes in Meagre (Argyrosomus regius)" Fishes 8, no. 3: 141. https://doi.org/10.3390/fishes8030141
APA StyleTeixeira, C., Pedrosa, R., Castro, C., Magalhães, R., Matos, E., Oliva-Teles, A., Peres, H., & Pérez-Jiménez, A. (2023). Dietary Tryptophan Supplementation Implications on Performance, Plasma Metabolites, and Amino Acid Catabolism Enzymes in Meagre (Argyrosomus regius). Fishes, 8(3), 141. https://doi.org/10.3390/fishes8030141