Abstract
Autism is a complex alteration in children’s developing nervous system that manifests in behavioral patterns that do not match those of typical subjects. Moreover, starting at puberty, these children may encounter problems regarding social interaction related to sexual encounters. As studies are scarce, we used the valproate model of autism in the zebrafish to contribute to the knowledge related to sexual behavior in this disorder. Young zebrafish were reproduced, embryos collected, and organized in groups of control or treated with valproic acid, as the autism model. Sexual behavior was recorded in fish from these embryos as they became sexually active. The results show that the zebrafish’s sexual behavior is organized into appetitive, preconsummatory, and consummatory behaviors, as in other vertebrates. In the autism model, the patterns of sexual behavior are present but with significant modifications. The behavior of males was the most affected, while in females, the most affected parameter was oviposition. These results show that, in the autism model of zebrafish, sexual behavior is not suppressed, but it seems that critical changes occur in the neuroendocrine system that reduces reproductive success. Furthermore, the enriched environment was beneficial in maintaining the consummatory behaviors of females and males with autism-like behaviors.
Keywords:
ASD; VPA; autistic; reproduction; sex; fertility; appetitive behavior; preconsummatory behavior; consummatory behavior Key Contribution:
The sexual behavior of the zebrafish is organized into appetitive, preconsummatory, and consummatory behaviors. The display patterns of sexual behavior have distinctive movements for females and males. In the autism model, males exhibit significant modifications in their patterns of sexual behavior, while oviposition in females was the most affected parameter. The subjects escape the autism effect when living in an enriched environment.
1. Introduction
The study of sexual behavior in animals has been relevant within the worldwide literature produced for decades. Notwithstanding, it continues to be a current topic considering the special significance it has for sex and the reproduction of animals per se, but also for supporting some related knowledge in humans. A realm of different behavioral strategies is now described for different species, although some animals are becoming meaningful for science in which the behavioral repertoire is still poorly known. That is the case of the zebrafish, which is consolidating as a promising species for the study of several physiological and behavioral processes, and is now also an appropriate model to investigate the underlying causes of human disorders and diseases, such as the autism spectrum disorder (ASD) or autism in brief.
Autism is a complex alteration in the developing nervous system of children. Such neurodevelopmental modifications appear early in infants and are manifested as behavioral patterns that do not match those of typical girls or boys, of serious concern due to their significant prevalence worldwide. The two domains of autistic behaviors are social and communicative difficulties as well as restricted and repetitive movements [1,2]. Although most studies focus on children [3,4,5], autism is a condition that remains for life; consequently, when reaching puberty, these individuals are faced with their emerging sexual behavior. Reports indicate that subjects in the spectrum, particularly those with high-functioning ASD, can show the corresponding sexual displays during puberty. Furthermore, although they experience sexual arousal, there are gender differences showing problems regarding social interaction and physical contact, which result in increased solitary sexual activities [6,7,8] or even sexual victimization [9]. Despite this sensitive situation, knowledge of appropriate interventions is scarce.
The fact is that sex and reproduction are topics poorly understood in humans with autism, and as it occurs with other topics, studies from animal models are of extreme importance to understand such biological processes. In a previous study, we used the postnatal-treated valproate model of autism in rats to quantify the well-known sexual behavior of males [10,11,12]. We observed that males in this autism model executed all behavioral parameters of sexual behavior, although the quantification revealed significant differences compared to the control subjects. In brief, we reported decreased motivational states and a reduced potency for penile erection and ejaculation [13]. As far as we know, this was the first report addressing sexual behavior in an animal model of autism, but just in males. Now, as the zebrafish is today an expanding model of autism [14], we decided to use this fish species (Danio rerio) to further describe their sexual behavior, as reported elsewhere [15], and to contribute with knowledge related to autism.
In the zebrafish, pheromones are the main attractants to stimulate the first congregate of adult females and males [16]. Such chemicals also regulate female intrasexual competition and allow for males to stimulate females for the production and spawn of viable eggs [17,18]. Mating behavior occurs mainly at dawn in the wild or after the first illumination period in laboratory conditions [19,20], displaying specific patterns reported by females and males during sexual interaction [15]. However, the environment can modify the wild zebrafish’s reproductive and social behaviors [19]. In this study, we identified sexual behavior in two tank conditions, namely plain and enriched environments. Although enriched environments do not match those observed in wild conditions, using tanks with different environments in the laboratory reveals significant effects on behavior and brain organization [21]. Considering the number of reports showing the beneficial effects of enriched environments, from fishes to mammals, here we also sought to describe whether sexual behavior differs between plain and enriched conditions.
2. Materials and Methods
2.1. Subjects
Young adult zebrafish (100 days old Danio rerio) were obtained from the colony of our institute, which was standardized with subjects from an aquarium store. They were maintained in large-size tanks (20 L) with temperature-controlled water (26 °C) and with water appropriately treated for zebrafish maintenance. Females (n = 6) and males (n = 18) were introduced in the same tank but separated by a mesh and were fed twice a day with TetraPro Tropical Crisps (Spectrum Brands, Inc., Middleton, WI, USA). The fish room underwent a light–dark cycle of 14–10 h, respectively (lights on at 08:00 h). Animals were maintained in these conditions until they reached 180 days of age; then, they were used for embryo collection.
2.2. Embryo Collection
A group of 2 females and 3 males were placed above the first mesh-like floor of a lab-made double-bottom tank (40 L). This swimming area was enriched with several artificial plants, and the mesh floor was covered with marbles. Subjects were placed in the tank during the afternoon (about 16:00 h) and removed the next day (about 11:00 h). Embryos were collected from the second bottom and transferred to cell culture plates of 6 wells (15 embryos per well; Thermo Fisher Scientific Inc., Waltham, MA, USA). Wells for control (Ctrl) embryos were filled with water from the reproduction tank, while experimental embryos were immersed in a solution of Valproic Acid Sodium Salt (VPA) dissolved in water from the reproduction tank (48 µM; Sigma-Aldrich, Naucalpan, Mexico). Ctrl and VPA embryos were maintained in the wells for 48 h.
2.3. Larval Rearing and Environment Conditions
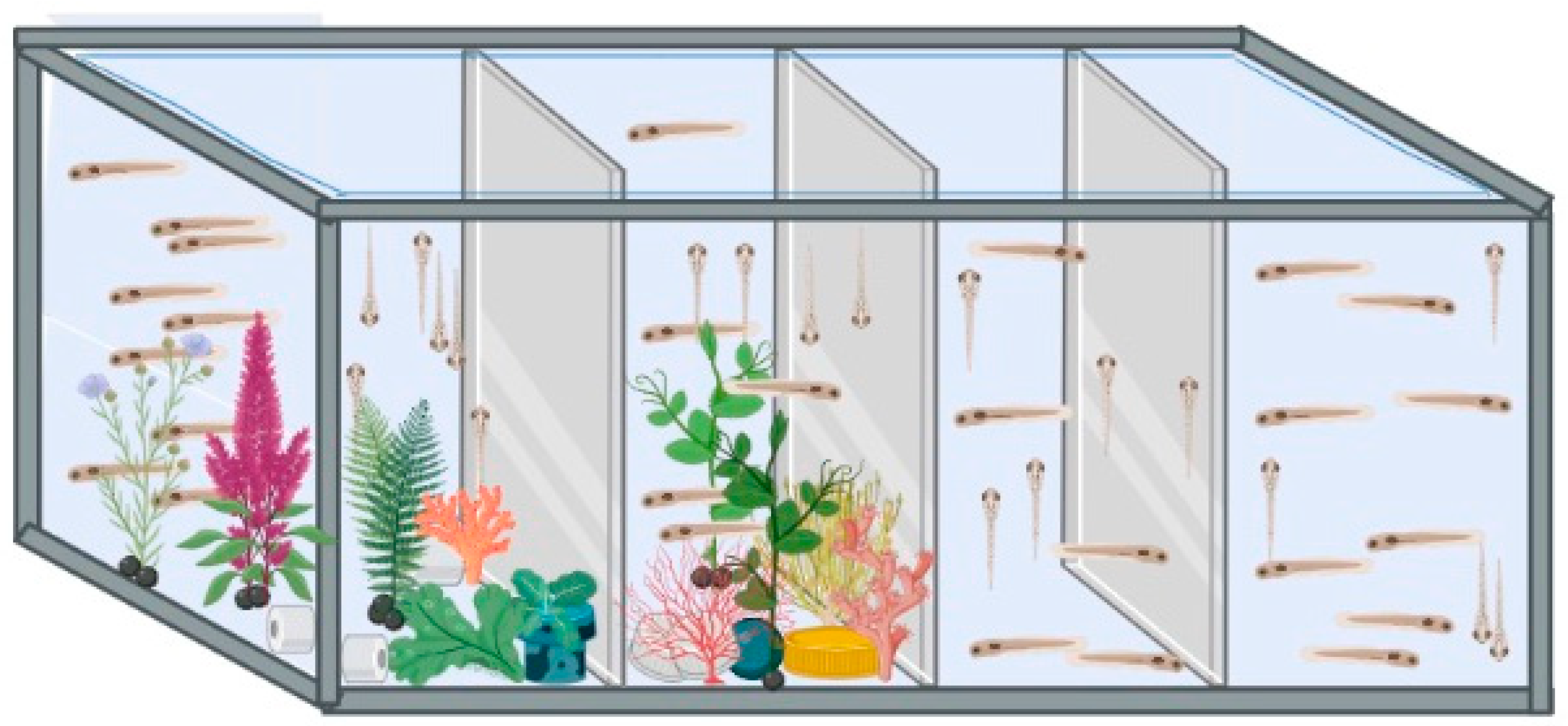
Following the period in the culture wells, Ctrl and VPA embryos were transferred to hatch tanks (small-size tanks, 1 L). Two hatch tanks were used, one with a plain environment (PL) and one with an enriched environment (EE). Hence, four groups were organized, namely Ctrl and VPA in a plain environment and Ctrl and VPA in an enriched environment. Hatch occurred in these tanks, and larvae were fed with Artemia starting on the 6th day post-fertilization (dpf). At 30 dpf, they were moved to medium-size tanks (5 L). At 90 dpf, they were moved to large-size tanks (20 L), and these tanks had a middle mesh to keep females and males separated. The observational study of sexual behavior was initiated when animals reached 180 dpf. Subjects developed from the VPA-treated embryos were considered as a model of ASD as described elsewhere [14,22]. Tanks with the PL condition had just water, while those with the EE condition were assembled with a complex of artificial aquarium plants that were changed twice a week (Figure 1).
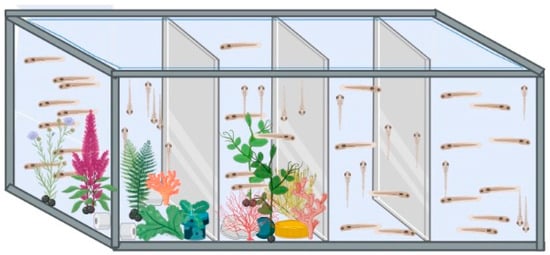
Figure 1.
Environment conditions. The image shows the lab-designed hatch tanks. The two tanks on the left are those with EE environments, and the two on the right are those with the PL environments. Tank dividers were opaque. Larger tanks had a similar arrangement.
2.4. Sexual Behavior Recording
Medium-size tanks were used as arenas to test sexual behavior every three days. Each tank had a slightly enriched environment with marbles on the bottom and a single plant inside. One female and one male were placed in the tank the afternoon before the test (about 16:00 hrs), but a middle mesh separated them. Control and VPA subjects were paired with intact mature subjects. The next day, when lights came on in the fish room (08:00 hrs), the mesh was removed, and behavioral recording started for 30 min (cameras were Sony Handycam HDR-CX675 and HDR-CX440; Sony Mexico, Mexico City, Mexico). Behavior was recorded, and the focal–animal sampling method was used as described elsewhere [23]. Three tests (n = 10 per group) selected subjects displaying sexual-related behaviors while excluding animals with very low or no behavior from the study. The fourth test was from selected sexually active animals (n = 6 per group), and data recorded in this test were analyzed in Section 3.
2.5. Statistics
Results of the total time and frequency of behaviors that occurred in the 30 min tests are presented as means ± SEM. Statistics and graphs were obtained using Prism 9.5 software (GraphPad, San Diego, CA, USA). Behavioral parameters were analyzed by an unpaired t-test comparing control and VPA subjects’ data. Significance was inferred when p < 0.05.
3. Results
3.1. Sexual Behavior
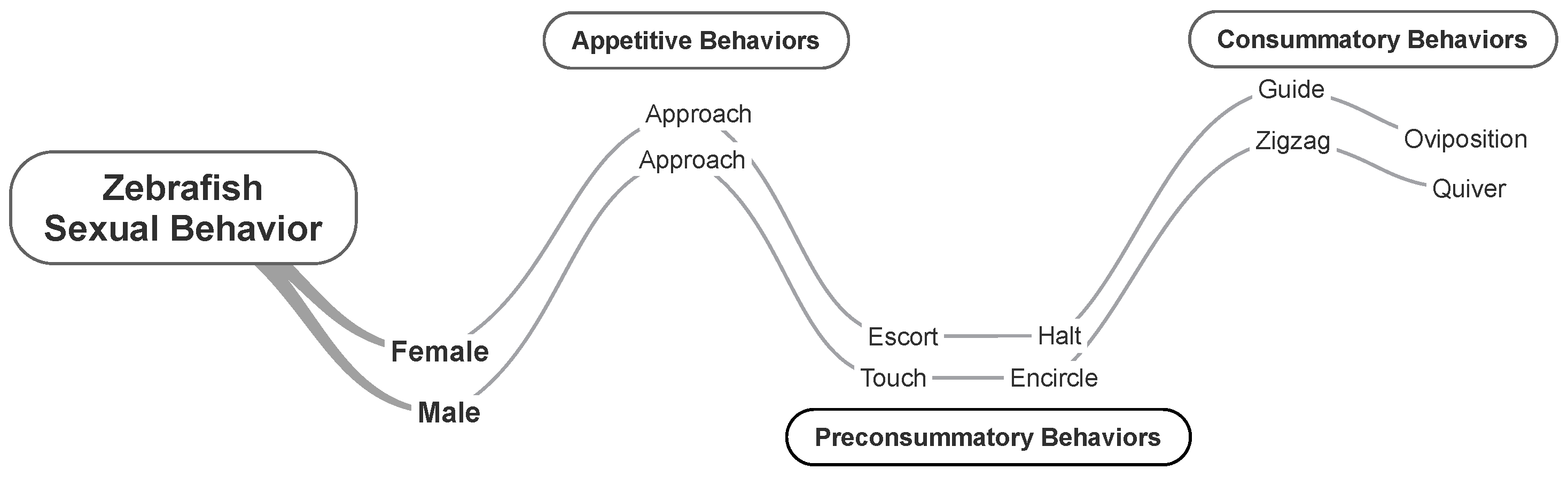
Male and female zebrafish displayed behaviors organized in the sequence groups previously defined for other species, i.e., appetitive, preconsummatory, and consummatory behaviors [24]. In brief, appetitive behavior includes approaching the mating partner to be near each other; preconsummatory behavior includes escort and halt by females and touch and encircle by males; and consummatory behavior includes guide and oviposition by females and zigzag and quiver by males (Figure 2 and Table 1).
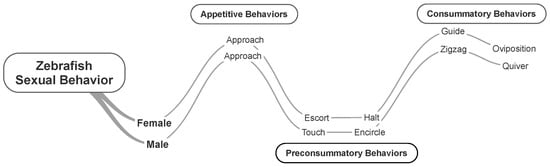
Figure 2.
Zebrafish sexual behavior. Time course (left to right) of appetitive, preconsummatory, and consummatory behaviors. See Table 1 for behavioral descriptions.

Table 1.
Definition of parameters for the zebrafish sexual behavior.
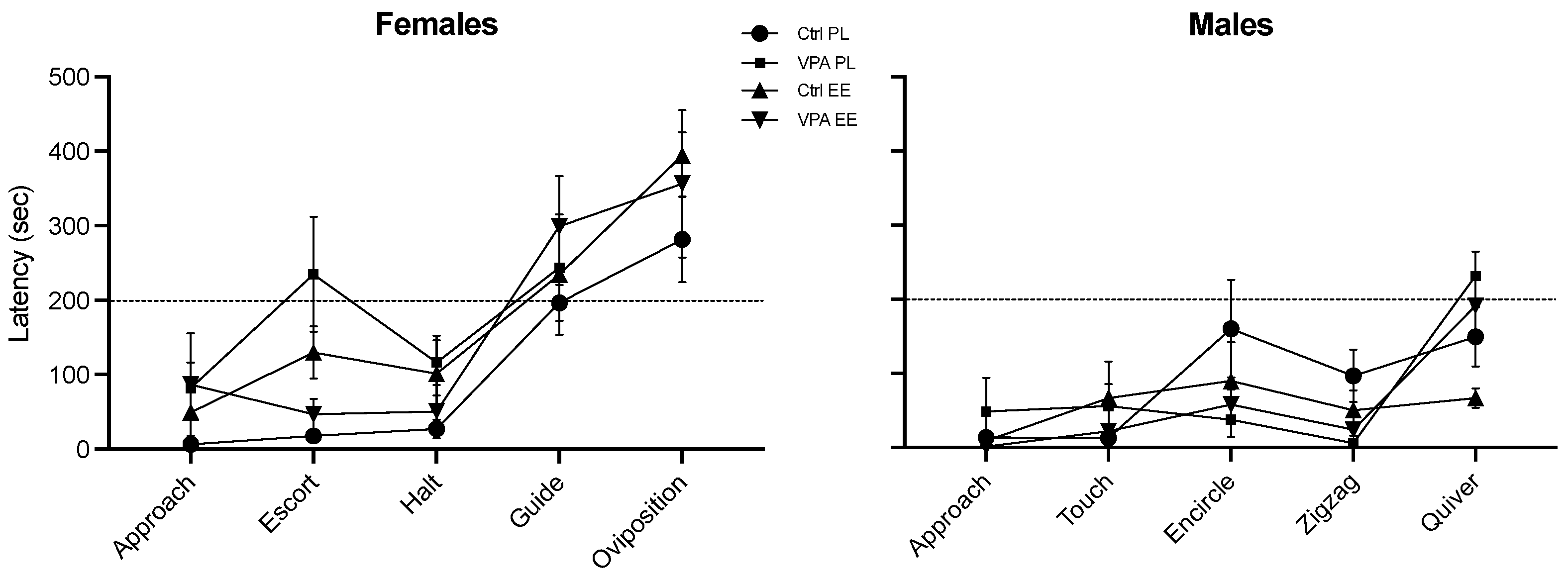
The latency to start each behavior did not display significant differences among the groups. Nevertheless, this parameter revealed that males execute the whole behavioral sequence in about 200 s (about 3 min) after initiating sexual behavior, while females use more time to execute consummatory behaviors (Figure 3).
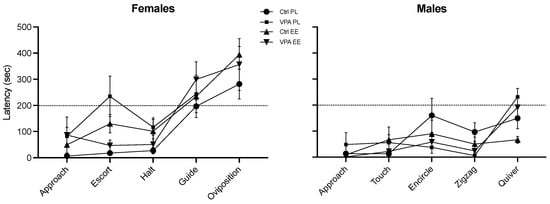
Figure 3.
Latency to initiate appetitive, preconsummatory, and consummatory behaviors. Neither treatment nor environment produced significant changes between groups. The female consummatory behaviors were the last executed in all four groups during interplay. Data show the mean ± SEM.
3.2. Appetitive Behavior
Approach
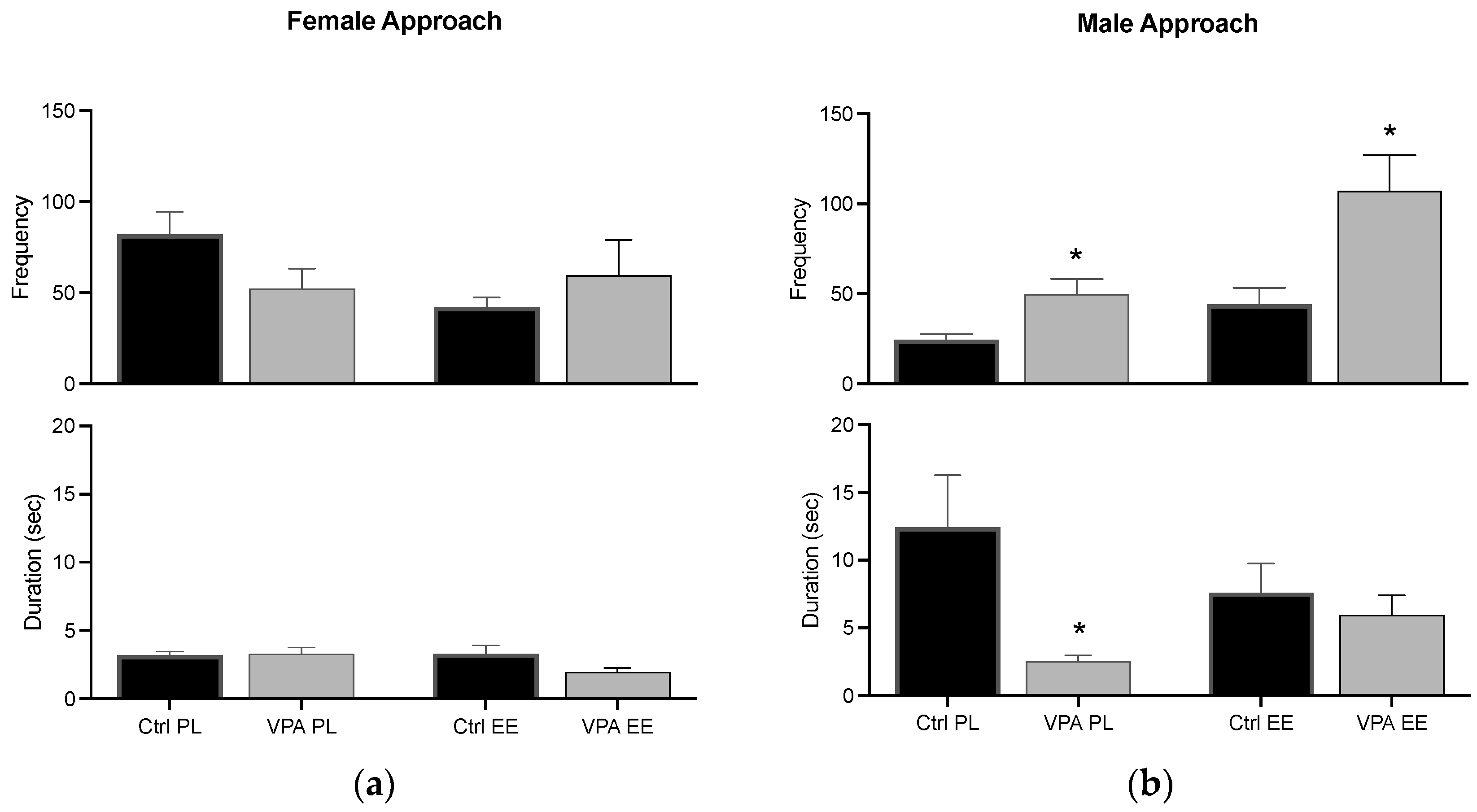
The beginning of a sexual encounter by female and male zebrafish triggered appetitive behavior, i.e., swimming near each other. Both sexes displayed the behavior in an approach–withdrawn pattern, and we quantified the frequency of approach behavior and the seconds each subject stayed near before withdrawing. Ctrl data in the PL environment show that females execute the behavior with a higher frequency than males and males do with a higher duration than females. VPA had no effect in PL females, but the values were inverted in males. The EE condition did not affect female behavior, while the males’ EE environment supported the duration parameter to remain at the Ctrl level (Figure 4).
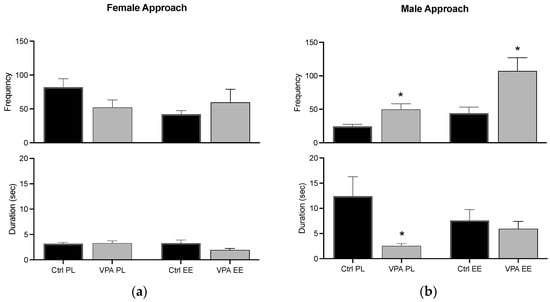
Figure 4.
Appetitive behavior. The graph shows how often the female (a) and male (b) approached each other (frequency), and how long they stayed near each other before withdrawing (duration). Data show the mean ± SEM. * = p < 0.05.
3.3. Preconsummatory Behaviors
3.3.1. Female Escort and Halt
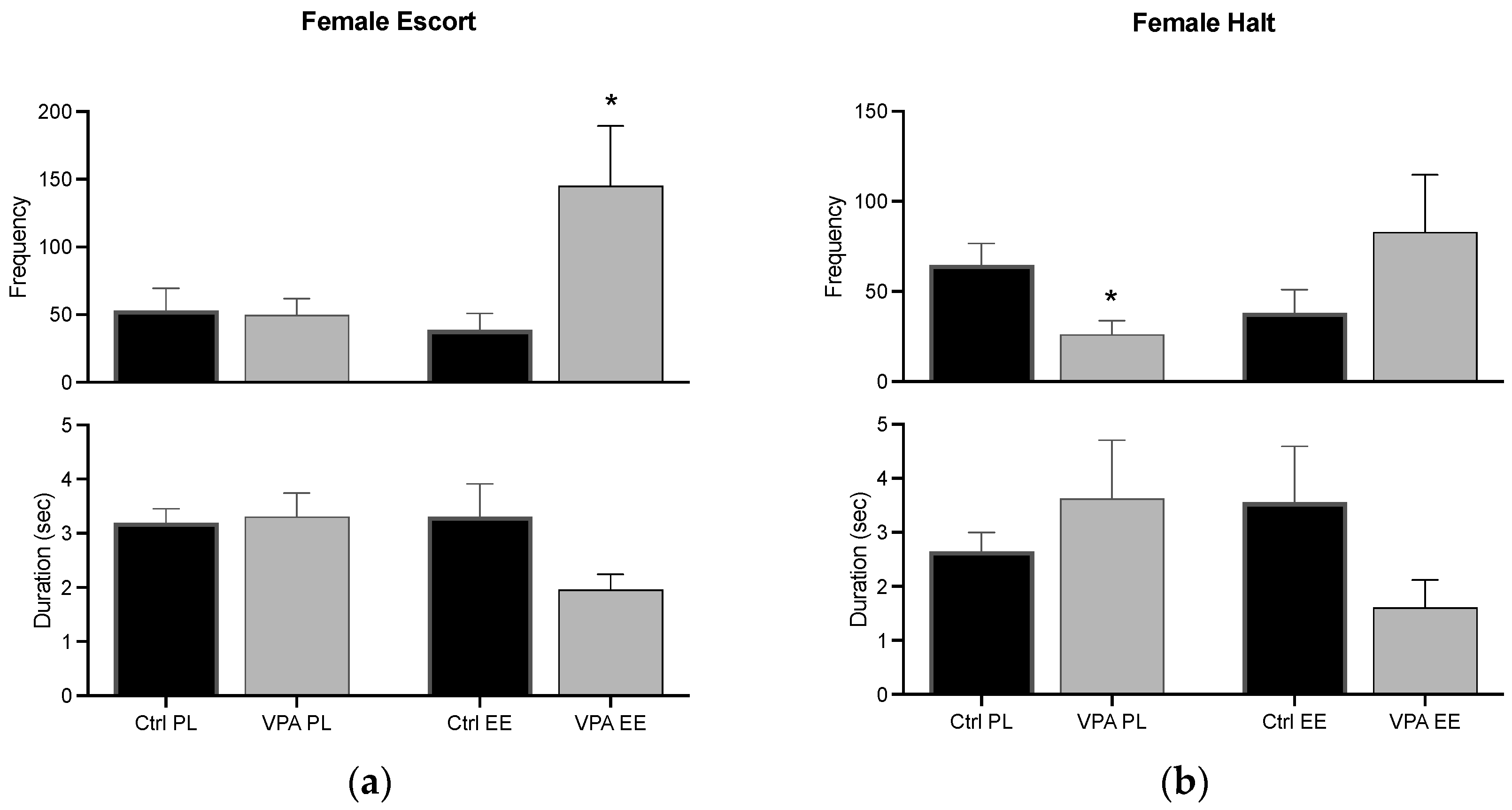
For the PL environment, data show that females execute a similar escort behavior in Ctrl and VPA subjects, while the EE environment significantly increased its frequency. The halt behavior demonstrated a decreased frequency in VPA females in the PL environment, and the EE environment prevented such a decrease (Figure 5).
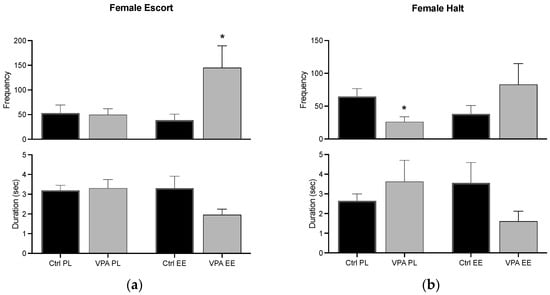
Figure 5.
Female preconsummatory behaviors. The graph shows how many times the female escort (a) and halt (b) the male (frequency), and how long such behaviors were displayed for (duration). Data show the mean ± SEM. * = p < 0.05.
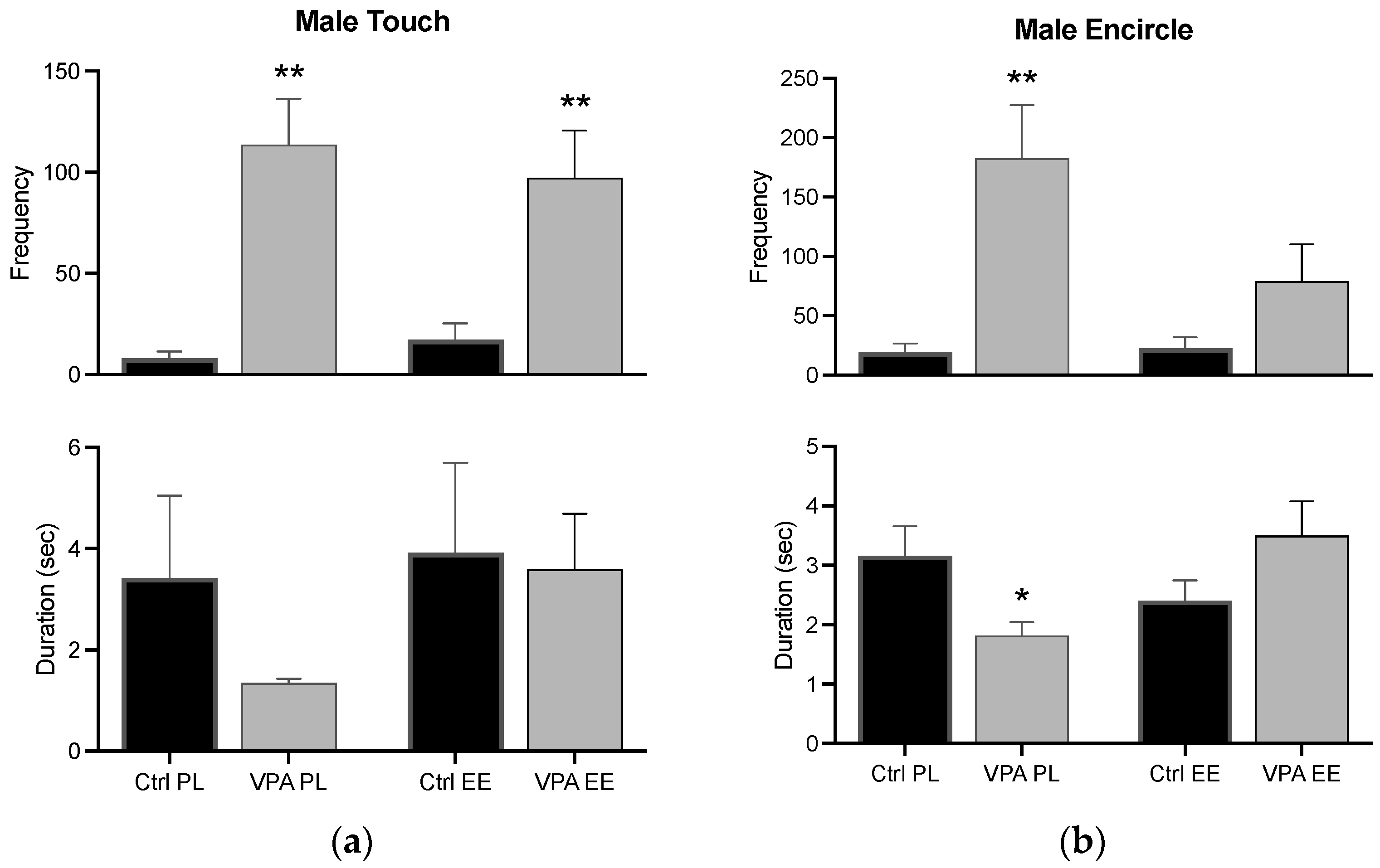
3.3.2. Male Touch and Encircle
The frequency of the touch behavior was significantly increased in VPA-treated males in both PL and EE environments, while its duration was unvarying. In the PL environment, the frequency of the encircle behavior increased while its duration decreased. Notwithstanding, changes in the encircle behavior were prevented in males of the EE environment (Figure 6).
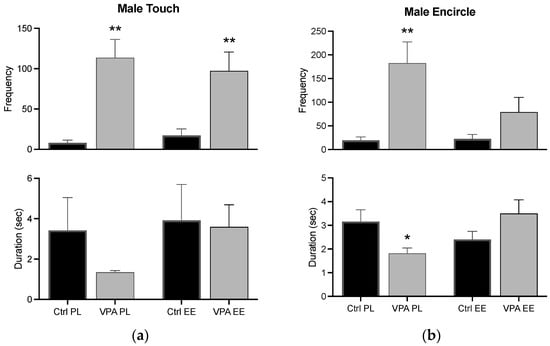
Figure 6.
Male preconsummatory behaviors. The graph shows how many times the male touched (a) and encircled (b) the female (frequency) and how long he displayed such behaviors for (duration). Data show the mean ± SEM. * = p < 0.05; ** = p < 0.01.
3.4. Consummatory Behaviors
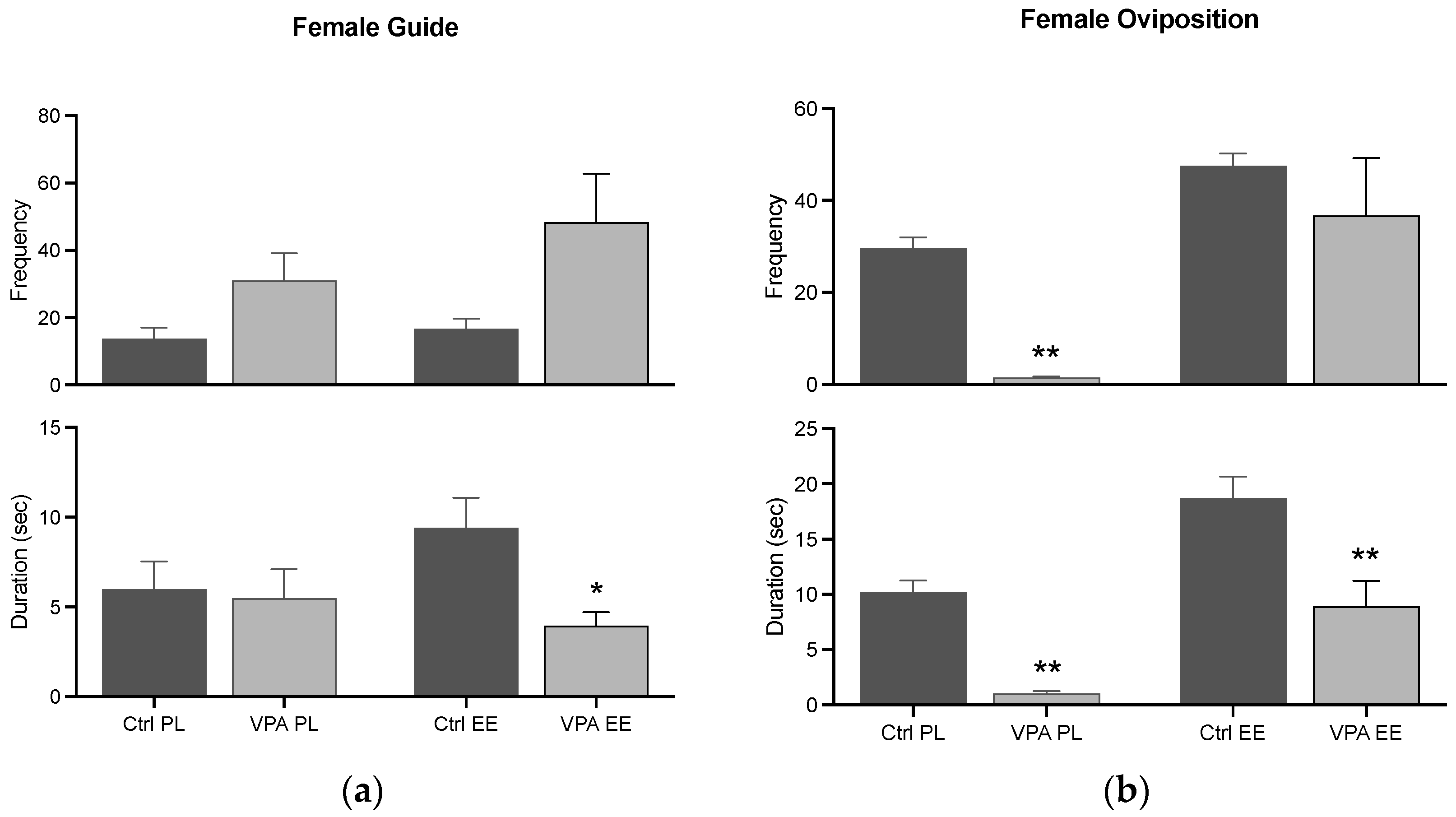
3.4.1. Female Guide and Oviposition
The data showed that the guide behavior is similar in Ctrl and VPA subjects in both PL and EE environments, while the EE environment prevented a significant duration. Additionally, oviposition in the PL environment was significantly reduced in terms of frequency and duration, while the EE environment prevented this effect in the frequency parameter (Figure 7).
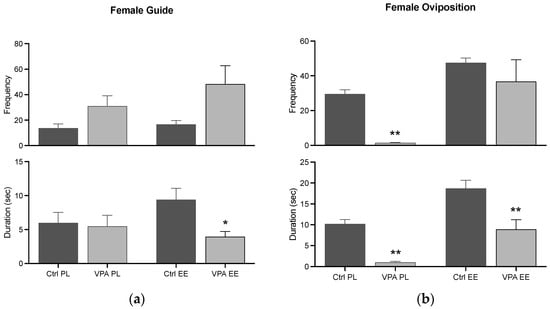
Figure 7.
Female consummatory behaviors. The graph shows how many times the female guided the male (a) and displayed oviposition (b) (frequency), and how long she displayed such behaviors for (duration). Data show the mean ± SEM. * = p < 0.05; ** = p < 0.01.
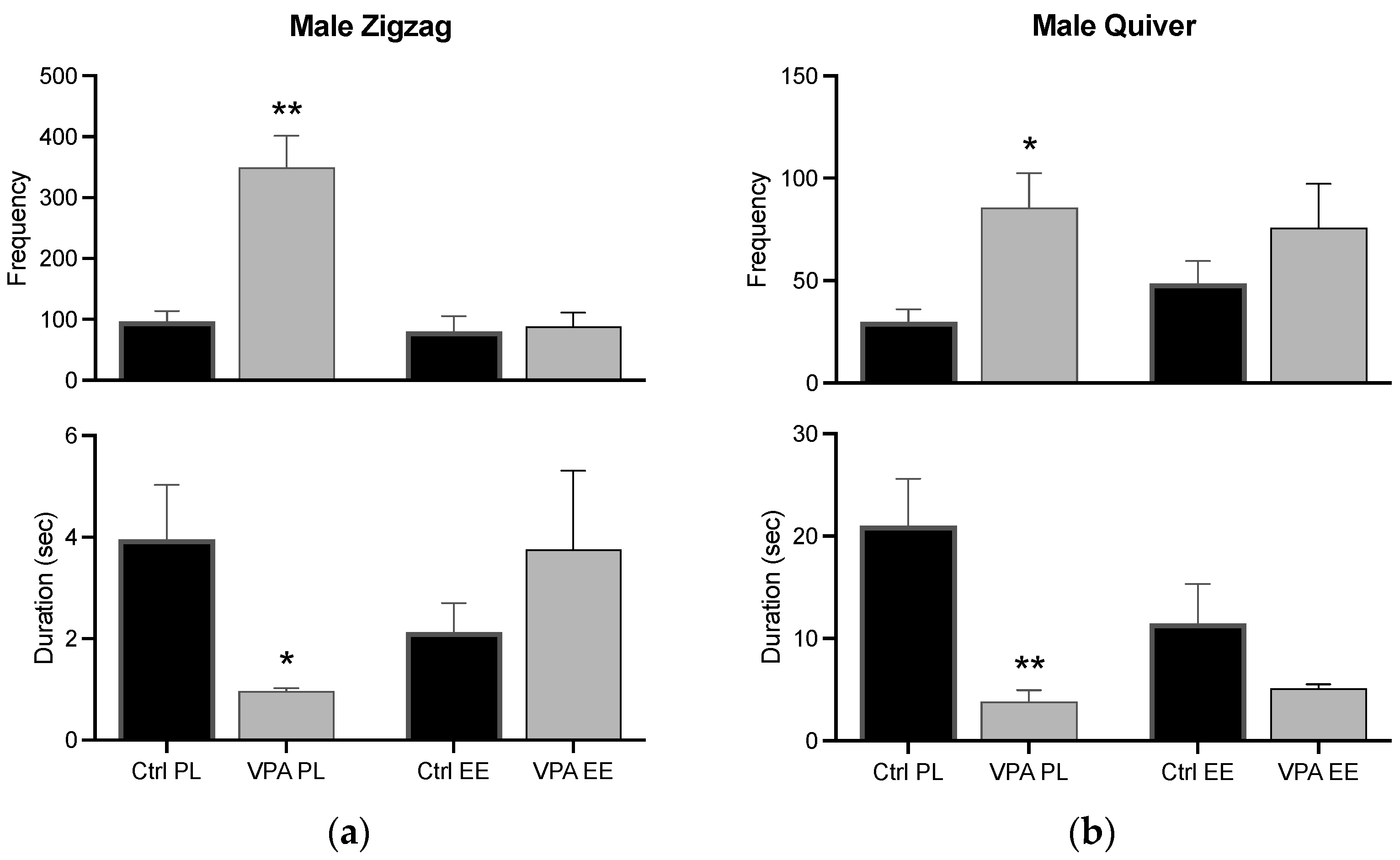
3.4.2. Male Zigzag and Quiver
Zigzag and quiver behaviors had a similar result, i.e., the frequency was increased in the PL environment, while the duration was decreased; however, both changes were prevented in the EE environment (Figure 8).
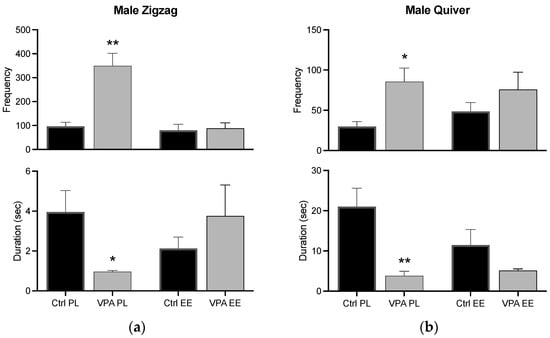
Figure 8.
Zigzag and quiver behaviors in males. The graph shows how many times the male executed the zigzag (a) and quiver (b) behaviors (frequency), and how long he displayed such behaviors for (duration). Data show the mean ± SEM. * = p < 0.05; ** = p < 0.01.
4. Discussion
The sexual behavior of the zebrafish recorded in this study follows those reported previously [15]. Notwithstanding, we propose that future studies should also consider the organization of behavior in appetitive, preconsummatory, and consummatory behaviors, as shown in Figure 2 and reported elsewhere [24]. The trace in Figure 2 shows the stream of patterns that could be useful for a comparative analysis and could also serve to understand zebrafish sexual behavior from an evolutionary perspective. Hence, when studying the sexual behavior of zebrafish, we should recognize that its behavior is homologous to those displayed by other vertebrates. To date, we know that zebrafish is consolidating as a notable species to understand behavior from molecules, and undoubtedly, its sexual behavior will reveal further meaningful information.
Social interactions in the zebrafish start right after hatching, and as it occurs in children, impairment in social behavior is one of the critical domains when modeling autism in this species [25]. Here, we showed that sexual interactions in the zebrafish VPA model of autism have some modified parameters but also have parameters that remain unaltered. The fact that the latency to start each behavior did not show significant differences among groups suggests that the triggering mechanisms for appetitive, preconsummatory, and consummatory behaviors depend on stable physiological systems that respond with the same magnitude no matter the VPA treatment or environmental conditions. We observed increased latencies for mounts and intromissions in the VPA model in male rats, perhaps due to a reduced olfactory sensitivity [13]. Although the zebrafish has a complex olfactory system [26], it seems that the zebrafish relies on both olfactory and visual stimuli for behavioral responses [27]. In humans, the use of antidepressants or antihypertensive drugs has side effects on sexual behavior, but as shown in animal models, such drugs have impacts on behavioral responses but not in the latency to initiate the behavior [28]. In brief, the afferent–efferent pathways to trigger sexual behavior in female and male zebrafish, as occurs in other species, seems to be highly resistant to external drugs or environments.
The approach behavior is a similar appetitive display in females and males. However, females execute the behavior with a higher frequency than males, but males stay close to females for a more extended period of time (Ctrl in PL environment). These actions suggest that zebrafish females are very active in stimulating the following preconsummatory behaviors, also indicating that they pace sexual behavior similarly to that reported for female rats [29]. VPA females display the behavior just as control females do; hence, the motivational aspect of sexual behavior is not modified by the VPA treatment. This result is equivalent to those reported for girls [30] and female mice [31], indicating that zebrafish females are also resistant to some external insults leading to autism-related behaviors. Furthermore, this parameter was also stable in EE females, suggesting that the behavior is also unvarying in different environmental conditions. Males exhibited a different pattern; the VPA treatment in PL and EE environments produced a reversal modification of the approach behavior, i.e., resembling females. Do VPA males acquire a female-like appetitive behavior? It is known that peacock blenny females are very active during courtship, while sneaker males display female-like behaviors as a strategy to reproduce [32], and all this reversal of behavior depends on endocrine changes [33]. Furthermore, these behavioral modifications have a neuroendocrine basis, as shown in the bluehead wrasses [34]. There are no reports in the zebrafish of a similar reversal in behavior as a reproductive strategy, but endocrine-disrupting chemicals [35] or antidepressants [36] affect the courtship behavior. Thus, the male zebrafish is sensitive to chemicals during development, suggesting that the valproate insult modifies development and seems to induce the appearance of female-like behaviors during the appetitive phase of sexual interaction.
The female preconsummatory behavior displayed an increased frequency in the escorting behavior of the EE environment and a decreased frequency in the halt behavior of the PL environment. The constant of such changes is that both occurred in VPA-treated subjects. Together, these data suggest that VPA females increase their mobility around males, which could indicate an increase in proceptivity or solicitation behavior as described for other vertebrates [37,38]. The underlying basis of these behaviors includes changes in neural and endocrine pathways and aims to increase sexual interactions because they are associated with the female’s sexual motivation to enhance the motivation of the male [39]. In our study, VPA produced significant changes in both behaviors, suggesting an increase in female motivation, although further studies are needed to know the effect on the behavioral responses of the male. On the other hand, regarding consummatory behaviors, oviposition was the most affected parameter in VPA females. The reduced frequency of egg release reduced the number of released eggs. It is known that the couple’s body contacts and postures in consummatory behaviors produce the release of gametes [40]. As the male executed the proper movements, we suggest that this effect could be due to female posture and physiological modifications. Posture is a specific parameter that deserves further research, while physiology could be due to the zinc transporter ZIP9 that has androgen receptor activity and, when absent, affects the underlying physiology of zebrafish eggs [41]. Thus, detailed research is needed to determine the causes of reduced oviposition in VPA zebrafish females. What is known is that autism in humans results in reduced reproductive success [42], and with the reduced oviposition, we found that VPA-treated zebrafish females reproduce such complications.
Preconsummatory and consummatory behaviors in VPA males showed that the frequency increased while the duration decreased. Considering both parameters, we suggest that the neural structures that control timing in sexual behavior are affected by the induced condition in the zebrafish male. Time is essential for sexual behavior and reproduction, and in male rats this was reported several years ago [43], with the temporal pattern of sexual behavior then used as a fundamental parameter to comprehend the neuroendocrine basis of reproduction. In the male rat, we have shown that timing in sexual behavior has a neural basis in the cerebellum that shows activity in parallel with the executed behavior [44], that this timing is essential for neuroendocrine processes and the activation of sexual glands [45], and that the lesion of specific nerves alter that timing in sexual behavior [46,47]. Furthermore, in the VPA male rat, we also showed that males still execute sexual behavior, but the frequency and duration of the behavior are affected [13], just as for zebrafish VPA males. Thus, the valproate condition in the zebrafish indicates that sexual behavior is present in males, but the temporal pattern is altered, perhaps with significant effects on reproductive success as it occurred in females. Furthermore, a detailed observation showed no physical anomalies in males; thus, the behavioral findings must result from a disorganized neuroendocrine system. In brief, the valproate condition does not suppress sexual behavior; however, it changes the temporal pattern for a proper display, which implies significant changes in the physiological mechanisms that underlie sexual behavior and reproduction in the autism model of the male zebrafish. On the other hand, for a long time, different reports have shown that in humans, the prevalence of autism is significantly higher in boys, and a recent report further supports this fact [48]. Although several hypotheses are under study, analyzing genetic, neural, endocrine, and environmental causes, the topic remains unknown [49]. However, our data show that the VPA treatment’s environmental effect also had a male tendency regarding sexual behavior. Thus, this zebrafish model of autism and their sexual responses are an excellent model to analyze the underlying causes of the higher prevalence of autism in males.
The enriched environment protected several parameters from the effects of the VPA treatment; the most remarkable benefits were in the consummatory behaviors of females and males. Thus, it seems that an EE has the potency to maintain the reproductive success of zebrafish subjects with autism-like behaviors. This situation is similar to those reported in captive animals [50] or laboratory zebrafish [51]. We suggest that such results depend on the neural plasticity effect of the EE in several brain nuclei [21,52]. A critical region altered in autism is the cerebellum. Although very little information is available for zebrafish and autism [53,54], we have shown alterations of this structure in the rat and mice models of autism [55,56] and their modifications by sexual experience [44,57]. Data from experience experiments suggest that the increased sensory stimulation provided by the EE is also significant in protecting changes in the central structures underlying sexual behavior. Furthermore, the neurochemistry of specific brain pathways, such as the histamine in the hypothalamus or androgen receptors in the cerebellum, is also altered in autism and changes social behavior [55,58], indicating that the EE could also benefit neurochemistry aspects. Then, our data support several reports (an appropriate review can be found in reference [59]) showing that enriched environments are preferred by zebrafish and that the impact is on neural structures and behavior. Here, we suggest that the benefit is for autism-induced behaviors but could also be for other neurodevelopmental disorders.
5. Conclusion
The zebrafish sexual behavior is organized into appetitive, preconsummatory, and consummatory behaviors, which are homologous to those displayed by other vertebrates. In the VPA model of autism, the latency to start such behaviors remains stable in both sexes; thus, this parameter resists the impact of external drugs or environments. However, once initiated, the execution of the sexual behavior of males appears deeply affected, while females are affected mainly in the consummatory parameters. Notwithstanding, subjects of both sexes escape the autism effect when living in an enriched environment.
Author Contributions
Conceptualization, J.M. and X.V.-L.; methodology, P.C. and M.R.T.-C.; validation, M.E.H.-A. and D.H.-C.; formal analysis, G.A.C.-A.; investigation, X.V-L. and J.M.; resources, J.M. and L.I.G.; writing—original draft preparation, J.M.; writing—review and editing, all authors; visualization, M.E.H.-A. and M.R.T.-C.; project administration, J.M.; funding acquisition, X.V.-L. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conacyt Fellowship 315027 to X.V.-L., and by the Coveicydet Grant 09 1238/2021 to J.M.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee (CICUAL) of INSTITUTO DE INVESTIGACIONES CEREBRALES (BRAIN RESEARCH INSTITUTE) (protocol code 2021-009a and date of approval: 19 November 2021).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Samantha Sofía Ferez-Ramos for her excellent administrative support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Manzo, J. Un Segundo Espectro Del Autismo: De La Conducta a La Neurona. eNeurobiol 2019, 23, 1501. [Google Scholar] [CrossRef]
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of Brain Overgrowth in the First Year of Life in Autism. JAMA 2003, 290, 337–344. [Google Scholar] [CrossRef]
- Fernández-Lechuga, A.I.; Nuñez-Arcos, L.Y.; Carrillo, P.; García, L.I.; Coria-Ávila, G.A.; Toledo, R.; Hernández, M.E.; Manzo, J. Reduction of Cutaneous von Frey Thresholds in Boys with Autism Following a Year of Tactile and Emotional Stimulation. Rev. Mex. Neuroc. 2021, 22, 85–88. [Google Scholar] [CrossRef]
- Kanner, L. Autistic Disturbances of Affective Contact. Nervous Child 1943, 2, 217–250. [Google Scholar]
- Byers, E.S.; Nichols, S.; Voyer, S.D. Challenging Stereotypes: Sexual Functioning of Single Adults with High Functioning Autism Spectrum Disorder. J. Autism Dev. Disord. 2013, 43, 2617–2627. [Google Scholar] [CrossRef]
- Kellaher, D.C. Sexual Behavior and Autism Spectrum Disorders: An Update and Discussion. Curr. Psychiat. Rep. 2015, 17, 562. [Google Scholar] [CrossRef] [PubMed]
- Dewinter, J.; Vermeiren, R.; Vanwesenbeeck, I.; Lobbestael, J.; Nieuwenhuizen, C.V. Sexuality in Adolescent Boys with Autism Spectrum Disorder: Self-Reported Behaviours and Attitudes. J. Autism Dev. Disord. 2015, 45, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Brown-Lavoie, S.M.; Viecili, M.A.; Weiss, J.A. Sexual Knowledge and Victimization in Adults with Autism Spectrum Disorders. J. Autism Dev. Disord. 2014, 44, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Ågmo, A. Male Rat Sexual Behavior. Brain Res. Protoc. 2019, 1, 203–209. [Google Scholar] [CrossRef]
- Meisel, R.L.; Sachs, B.D. The Physiology of Male Sexual Behavior. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; Volume 2, pp. 3–105. [Google Scholar]
- Manzo, J.; Carrillo, P.; Coria-Avila, G.A.; Garcia, L.I. The Sexual Cerebellum. In Behavioral Neuroendocrinology; Komisaruk, B.R., González-Mariscal, G., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 103–112. [Google Scholar]
- Manzo, J.; Coria-Avila, G.A.; Garcia, L.I.; Hernández-Aguilar, M.E.; Herrera-Covarrubias, D.; Toledo, R.; Monje-Reyna, D.; Santamaría, F. Male Sexual Behavior and Prostate Histology in a Rat Model of Autism. Eneurobiología 2019, 25, 280919. [Google Scholar] [CrossRef]
- Chen, J.; Lei, L.; Tian, L.; Hou, F.; Roper, C.; Ge, X.; Zhao, Y.; Chen, Y.; Dong, Q.; Tanguay, R.L.; et al. Developmental and Behavioral Alterations in Zebrafish Embryonically Exposed to Valproic Acid (VPA): An Aquatic Model for Autism. Neurotoxicol. Teratol. 2018, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Darrow, K.O.; Harris, W.A. Characterization and Development of Courtship in Zebrafish, Danio rerio. Zebrafish 2004, 1, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Bloom, H.D.; Perlmutter, A. A Sexual Aggregating Pheromone System in the Zebrafish, Brachydanio rerio (Hamilton-buchanan). J. Exp. Zool. 1977, 199, 215–226. [Google Scholar] [CrossRef]
- Gerlach, G. Pheromonal Regulation of Reproductive Success in Female Zebrafish: Female Suppression and Male Enhancement. Anim. Behav. 2006, 72, 1119–1124. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The Behaviour and Ecology of the Zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Hutter; Magee; Zala; Penn Reproductive Behaviour of Wild Zebrafish (Danio rerio) in Large Tanks. Behaviour 2010, 147, 641–660. [CrossRef]
- Ghoshal, A.; Daniel, D.K.; Bhat, A. Temporal Patterns and Sex Differences in Dyadic Interactions in a Wild Zebrafish Population. Behav. Process 2019, 166, 103896. [Google Scholar] [CrossRef] [PubMed]
- DePasquale, C.; Neuberger, T.; Hirrlinger, A.M.; Braithwaite, V.A. The Influence of Complex and Threatening Environments in Early Life on Brain Size and Behaviour. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152564. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Leite, C.E.; Cognato, G.D.P.; Bonan, C.D. Embryological Exposure to Valproic Acid Induces Social Interaction Deficits in Zebrafish (Danio rerio): A Developmental Behavior Analysis. Neurotoxicol. Teratol. 2015, 52, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Kippin, T.E.; Coria-Avila, G. What Can Animal Models Tell Us about Human Sexual Response? Annu. Rev. Sex Res. 2003, 14, 1–63. [Google Scholar] [PubMed]
- Dwivedi, S.; Medishetti, R.; Rani, R.; Sevilimedu, A.; Kulkarni, P.; Yogeeswari, P. Larval Zebrafish Model for Studying the Effects of Valproic Acid on Neurodevelopment: An Approach towards Modeling Autism. J. Pharmacol. Toxicol. 2019, 95, 56–65. [Google Scholar] [CrossRef]
- Korsching, S.I.; Argo, S.; Campenhausen, H.; Friedrich, R.W.; Rummrich, A.; Weth, F. Olfaction in Zebrafish: What Does a Tiny Teleost Tell Us? Semin. Cell Dev. Biol. 1997, 8, 181–187. [Google Scholar] [CrossRef]
- Santacà, M.; Dadda, M.; Bisazza, A. The Role of Visual and Olfactory Cues in Social Decisions of Guppies and Zebrafish. Anim. Behav. 2021, 180, 209–217. [Google Scholar] [CrossRef]
- Pfaus, J.G. Neurobiology of Sexual Behavior. Curr. Opinion. Neurobiol. 1999, 9, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.G.; Vazquez, B. What Do Female Rats like about Sex? Paced Mating. Behav. Brain Res. 1999, 105, 117–127. [Google Scholar] [CrossRef]
- Welberg, L. Autism: The Importance of Getting the Dose Right. Nat. Rev. Neurosci. 2011, 12, 429. [Google Scholar] [CrossRef]
- Grissom, N.M.; McKee, S.E.; Schoch, H.; Bowman, N.; Havekes, R.; O’Brien, W.T.; Mahrt, E.; Siegel, S.; Commons, K.; Portfors, C.; et al. Male-Specific Deficits in Natural Reward Learning in a Mouse Model of Neurodevelopmental Disorders. Mol. Psychiatr. 2018, 23, 544–555. [Google Scholar] [CrossRef]
- Gonçalves, E.J.; Almada, V.C.; Oliveira, R.F.; Santos, A.J. Female Mimicry as a Mating Tactic in Males of the Blenniid Fish Salaria Pavo. J. Mar. Biol. Assoc. UK 1996, 76, 529–538. [Google Scholar] [CrossRef]
- Gonçalves, D.; Alpedrinha, J.; Teles, M.; Oliveira, R.F. Endocrine Control of Sexual Behavior in Sneaker Males of the Peacock Blenny Salaria Pavo: Effects of Castration, Aromatase Inhibition, Testosterone and Estradiol. Horm. Behav. 2007, 51, 534–541. [Google Scholar] [CrossRef]
- Todd, E.V.; Liu, H.; Lamm, M.S.; Thomas, J.T.; Rutherford, K.; Thompson, K.C.; Godwin, J.R.; Gemmell, N.J. Female Mimicry by Sneaker Males Has a Transcriptomic Signature in Both the Brain and the Gonad in a Sex-Changing Fish. Mol. Biol. Evol. 2017, 35, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.-Y.; Li, X.; Zhou, H.-J.; Zhang, S.-H.; Liu, X.-D.; Chen, D.-Y.; Fang, Y.-C.; Feng, X.-Z. Behavioural Effect of Low-Dose BPA on Male Zebrafish: Tuning of Male Mating Competition and Female Mating Preference during Courtship Process. Chemosphere 2017, 169, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fan, Z.; Yang, M.; Zhang, S.; Li, M.; Fang, Y.; Li, J.; Feng, X. Low Concentrations of the Antidepressant Venlafaxine Affect Courtship Behaviour and Alter Serotonin and Dopamine Systems in Zebrafish (Danio rerio). Aquat. Toxicol. 2022, 244, 106082. [Google Scholar] [CrossRef]
- Beach, F.A. Sexual Attractivity, Proceptivity, and Receptivity in Female Mammals. Horm. Behav. 1976, 7, 105–138. [Google Scholar] [CrossRef]
- McClintock, M.K.; Adler, N.T. The Role of the Female During Copulation in Wild and Domestic Norway Rats (Rattus Norvegicus). Behaviour 1978, 67, 67–95. [Google Scholar] [CrossRef]
- Chu, X.; Ågmo, A. Studies of Sociosexual Interactions in Rats in an Externally Valid Procedure: Are They Relevant for Understanding Human Sexual Behavior? Int. J. Psychol. Res. 2016, 9, 76–95. [Google Scholar] [CrossRef]
- Zempo, B.; Tanaka, N.; Daikoku, E.; Ono, F. High-Speed Camera Recordings Uncover Previously Unidentified Elements of Zebrafish Mating Behaviors Integral to Successful Fertilization. Sci. Rep.-UK 2021, 11, 20228. [Google Scholar] [CrossRef] [PubMed]
- Converse, A.; Thomas, P. The Zinc Transporter ZIP9 (Slc39a9) Regulates Zinc Dynamics Essential to Egg Activation in Zebrafish. Sci. Rep.-UK 2020, 10, 15673. [Google Scholar] [CrossRef]
- Ploeger, A.; Galis, F. Evolutionary Approaches to Autism- an Overview and Integration. Mcgill J. Med. 2020, 13, 38. [Google Scholar] [CrossRef]
- Sachs, B.D.; Barfield, R.J. Temporal Patterning of Sexual Behavior in the Male Rat. J. Comp. Physiol. Psych. 1970, 73, 359. [Google Scholar] [CrossRef]
- Manzo, J.; Miquel, M.; Toledo, R.; Mayor-Mar, J.A.; Garcia, L.I.; Aranda-Abreu, G.E.; Caba, M.; Hernandez, M.E. Fos Expression at the Cerebellum Following Non-Contact Arousal and Mating Behavior in Male Rats. Physiol. Behav. 2008, 93, 357–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernandez, M.E.; Soto-Cid, A.; Aranda-Abreu, G.E.; Díaz, R.; Rojas, F.; Garcia, L.I.; Toledo, R.; Manzo, J. A Study of the Prostate, Androgens and Sexual Activity of Male Rats. Reprod. Biol. Endocrin. 2007, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Lucio, R.A.; Manzo, J.; Martínez-Gómez, M.; Sachs, B.D.; Pacheco, P. Participation of Pelvic Nerve Branches in Male Rat Copulatory Behavior. Physiol. Behav. 1994, 55, 241–246. [Google Scholar] [CrossRef]
- Manzo, J.; Vazquez, M.I.; Cruz, M.R.; Hernandez, M.E.; Carrillo, P.; Pacheco, P. Fertility Ratio in Male Rats Effects after Denervation of Two Pelvic Floor Muscles. Physiol. Behav. 2000, 68, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, J.; Frombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Ferri, S.L.; Abel, T.; Brodkin, E.S. Sex Differences in Autism Spectrum Disorder: A Review. Curr. Psych. Rep. 2018, 20, 2–17. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Effects of Environmental Enrichment on Reproduction. Zoo Biol. 1994, 13, 447–458. [Google Scholar] [CrossRef]
- Wafer, L.N.; Jensen, V.B.; Whitney, J.C.; Gomez, T.H.; Flores, R.; Goodwin, B.S. Effects of Environmental Enrichment on the Fertility and Fecundity of Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 291–294. [Google Scholar]
- Sale, A.; Berardi, N.; Maffei, L. Enrich of the Environment to Empower the Brain. Trends Neurosci. 2009, 32, 233–239. [Google Scholar] [CrossRef]
- Antón-Galindo, E.; Vecchia, E.D.; Orlandi, J.G.; Castro, G.; Gualda, E.J.; Young, A.M.J.; Guasch-Piqueras, M.; Arenas, C.; Herrera-Ubeda, C.; Garcia-Fernandez, J.; et al. Deficiency of the ywhaz Gene, Involved in Neurodevelopmental Disorders, Alters Brain aAtivity and Behaviour in Zebrafish. Mol. Psych. 2022, 27, 3739–3748. [Google Scholar] [CrossRef]
- Elsen, G.E.; Choi, L.Y.; Prince, V.E.; Ho, R.K. The Autism Susceptibility Gen met Regulates Zebrafish Cerebellar Development and Facial Motor Neuron Migration. Dev. Biol. 2009, 335, 78–92. [Google Scholar] [CrossRef]
- Perez-Pouchoulen, M.; Miquel, M.; Saft, P.; Brug, B.; Toledo, R.; Hernandez, M.E.; Manzo, J. Prenatal Exposure to Sodium Valproate Alters Androgen Receptor Expression in the Developing Cerebellum in a Region and Age Specific Manner in Male and Female Rats. Int. J. Dev. Neurosci. 2016, 53, 46–52. [Google Scholar] [CrossRef]
- Monje-Reyna, D.; Manzo, J.; Santamaria, F. Effects of Environmental Enrichment and Sexual Dimorphism on the Expression of Cerebellar Receptors in C57BL/6 and BTBR + ltpr3tf/J mice. BMC Res. Notes 2022, 15, 175. [Google Scholar] [CrossRef]
- Perez-Pouchoulen, M.; Toledo, R.; Garcia, L.I.; Perez-Estudillo, C.A.; Coria-Avila, G.A.; Hernandez, M.E.; Carrillo, P.; Manzo, J. Androgen Receptors in Purkinje Neurons are Modulated by Systemic Testosterone and Sexual Training in a Region-Specific Manner in the Male Rat. Physiol. Behav. 2016, 156, 191–198. [Google Scholar] [CrossRef]
- Baronio, D.; Puttonen, H.A.; Sundvik, M.; Semenova, S.; Lehtonen, E.; Panula, P. Embryonic Exposure to Valproic Acid Affects the Histaminergic System and the Social Behaviour of Adult Zebrafish (Danio rerio). Br. J. Pharmacol. 2018, 175, 797–809. [Google Scholar] [CrossRef]
- Stevens, C.H.; Reed, B.T.; Hawkins, P. Enrichment for Laboratory Zebrafish—A Review of the Evidence and the Challenges. Animals 2021, 11, 698. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).