A New Conditionally Immortalized Nile Tilapia (Oreochromis niloticus) Heart Cell Line: Establishment and Functional Characterization as a Promising Tool for Tilapia Myocarditis Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cell Culture and Routine Maintenance
2.2. Optimization of Cell Culture Condition
2.3. Cryopreservation and Revival of Cell
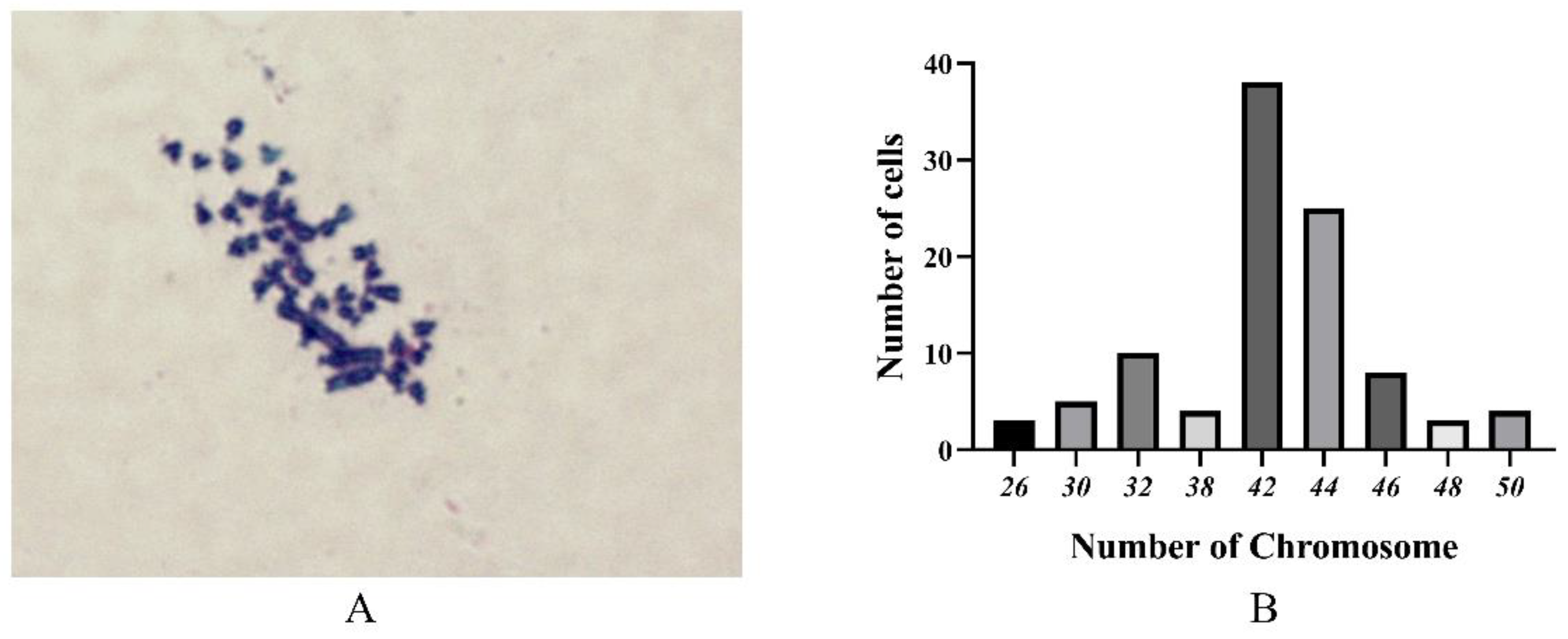
2.4. Chromosome Analysis
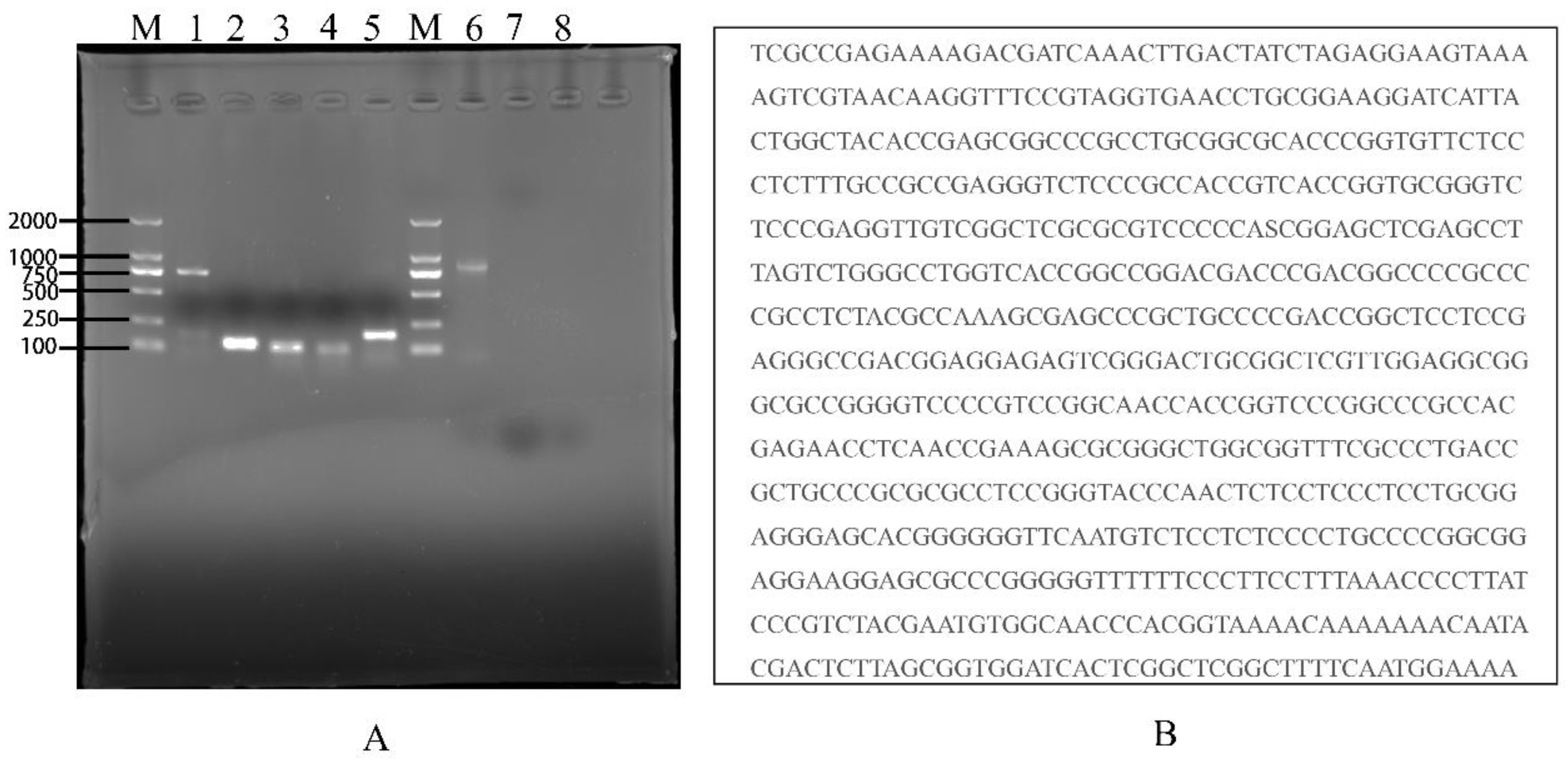
2.5. Identification of TAH-11 and Detection of Mycoplasma Contamination
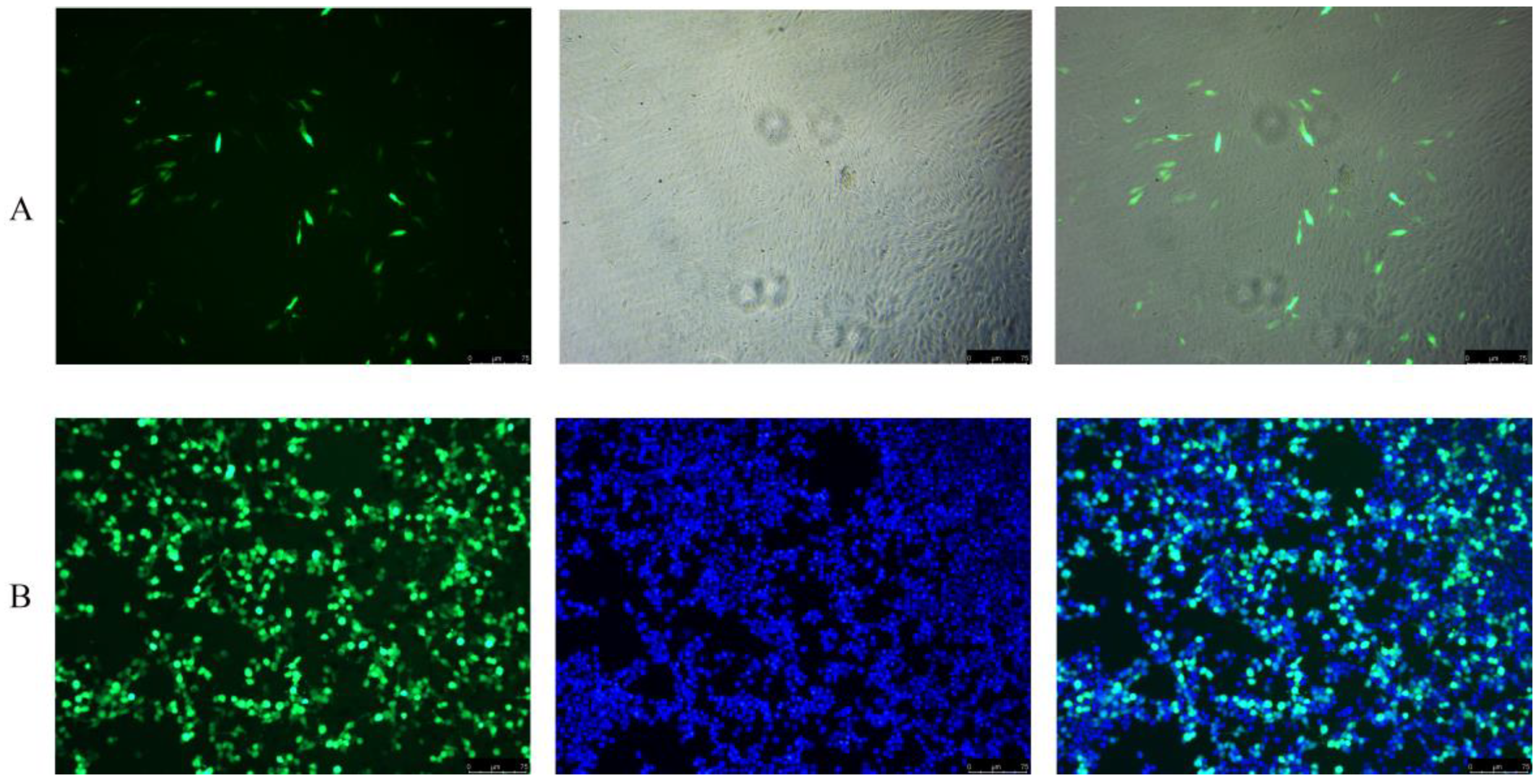
2.6. Transfection Efficiency Analysis
2.7. Virus Susceptibility Analysis
2.8. Transmission Electron Microscopic (TEM) Analysis of CGSIV and Streptococcus spp. to TAH-11
2.9. Adhesion Analysis of Streptococcus spp. to TAH-11
2.10. Analysis of Fish Immune-Related Gene Expression with Streptococcus spp.
3. Results
3.1. Primary Cell Culture and Routine Maintenance
3.2. Optimization of Cell Culture Condition
3.3. Cryopreservation and Revival of Cell
3.4. Chromosome Analysis
3.5. Identification of TAH-11 and Detection of Mycoplasma Contamination
3.6. Transfection Efficiency Analysis
3.7. Virus Susceptibility Analysis
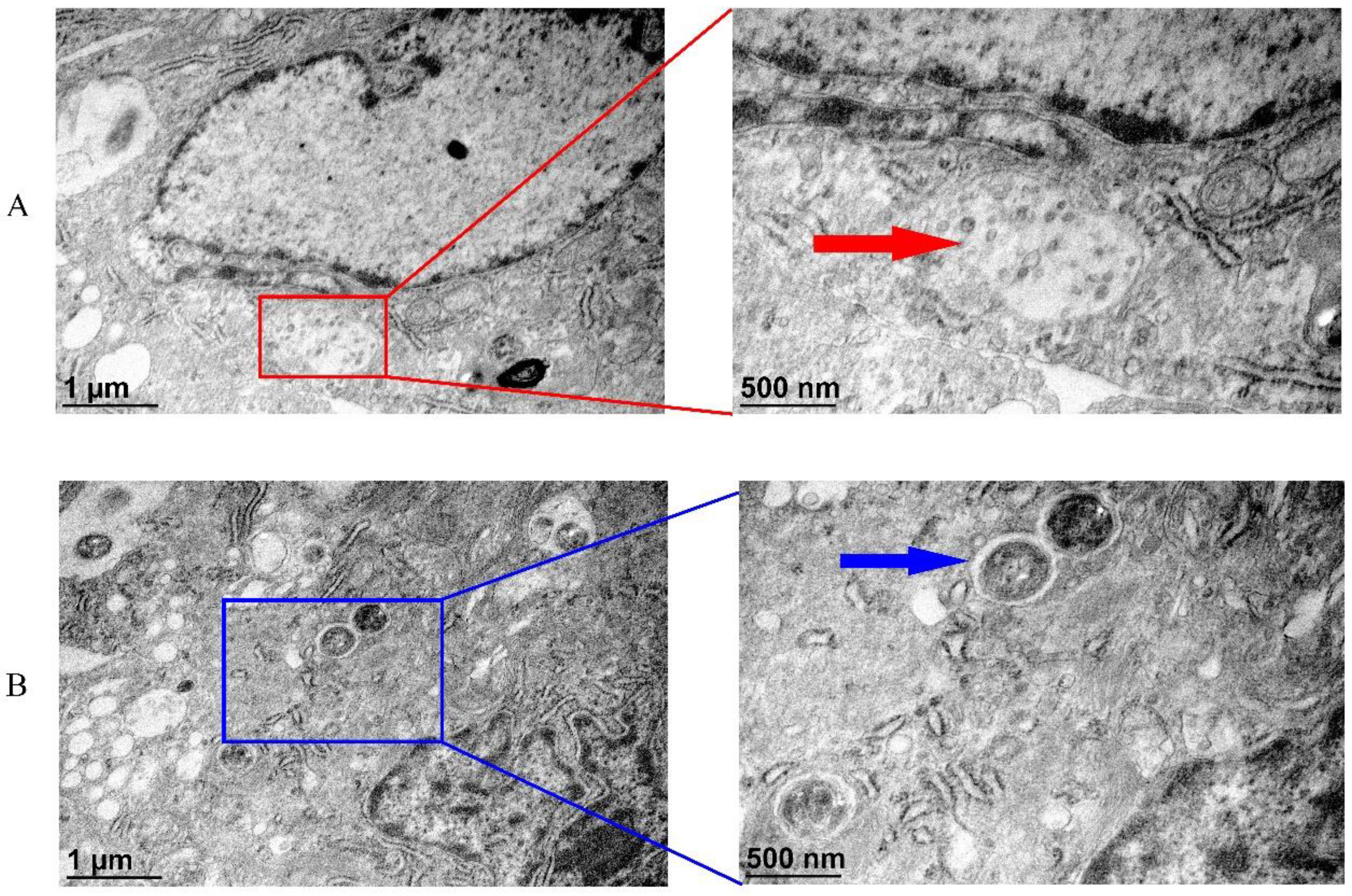
3.8. Transmission Electron Microscopic (TEM) Analysis of CGSIV and Streptococcus spp. to TAH-11
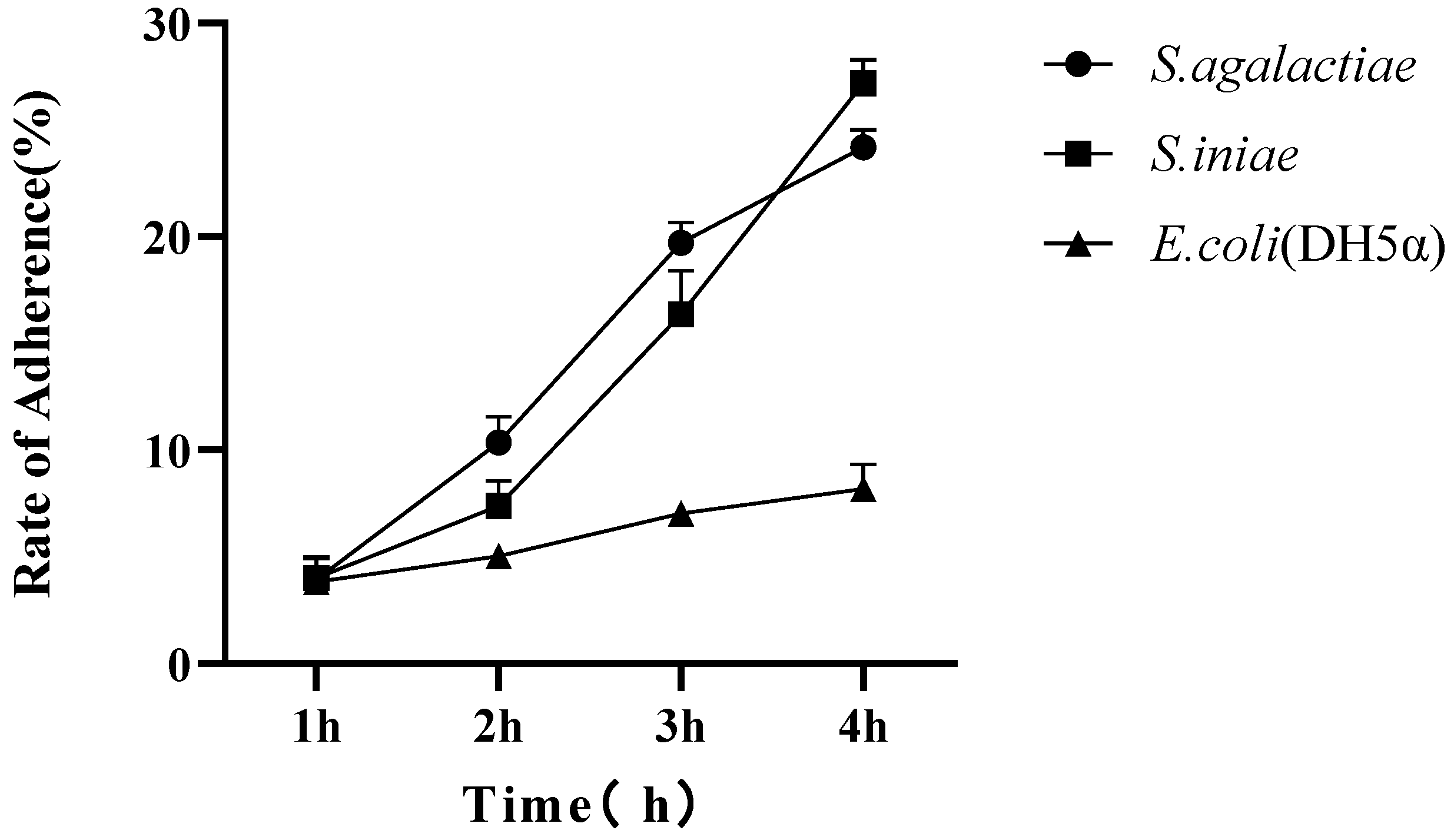
3.9. Adhesion Analysis of Streptococcus spp. to TAH-11
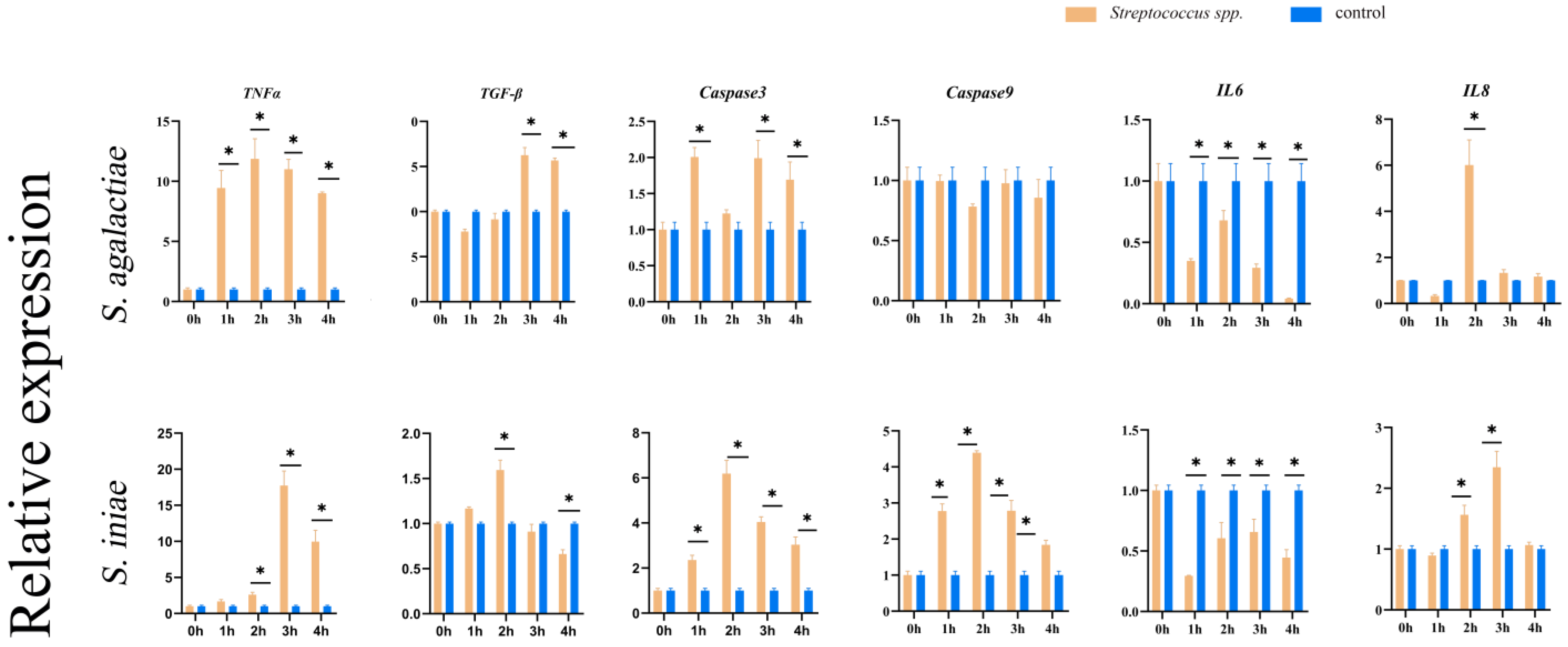
3.10. Analysis of Fish Immune-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Y.; Yuan, Y.; Dai, Y.; Gong, Y. Economic profitability of tilapia farming in China. Aquac. Int. 2017, 3, 1253–1264. [Google Scholar] [CrossRef]
- Jaemwimol, P.; Rawiwan, P.; Tattiyapong, P.; Saengnual, P.; Kamlangdee, A.; Surachetpon, W. Susceptibility of Important Warm Water Fish Species to Tilapia Lake Virus (Tilv) Infection. Aquaculture 2018, 497, 462–468. [Google Scholar] [CrossRef]
- Bekele, B.; Workagegn, K.B. Prevalence and Antimicrobial Susceptibility of Pathogenic Bacteria in Nile Tilapia, Oreochromis niloticus L. Int. J. Aquac. Fish. Sci. 2019, 5, 022–026. [Google Scholar] [CrossRef]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to Enhance Tilapia Immunity to Improve Their Health in Aquaculture. Rev. Aquac. 2023, 15, 41–56. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.P.; Wang, R.; Liang, W.W.; Huang, Y.; Li, J.; Lei, A.Y.; Huang, W.Y.; Gan, X. Pcr Detection and Pfge Genotype Analyses of Streptococcal Clinical Isolates from Tilapia in China. Vet. Microbiol. 2012, 159, 526–530. [Google Scholar] [CrossRef]
- Dönmez, A.E.; Cengizler, I. Pathomorphology of Experimental Streptococcus Iniae Infection in Tilapia (Oreochromis niloticus). Indian J. Anim. Res. 2020, 54, 234–238. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chao, C.B.; Bowser, P.R. Comparative Histopathology of Streptococcus Iniae and Streptococcus Agalactiae-Infected Tilapia. Bull.-Eur. Assoc. Fish Pathol. 2007, 27, 2–9. [Google Scholar]
- Ze, Z. Research Advances on Tilapia Streptococcosis. Pathogens 2021, 10, 558. [Google Scholar] [CrossRef]
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef]
- Huang, J. Title of Study on Etiology, Pathology of Tilapia Streptococcus Agalactiaedisease and on the Prokaryotic Expression of Cpse Gene. Ph.D. Thesis, Sichuan Agricultural University, Chengdu, China, 2012. [Google Scholar]
- Zhu, J.L.; Zou, Z.Y.; Li, D.Y.; Xiao, W.; Han, J.; Yang, H. Pathological changes in tilapia (Oreochromis niloticus) naturally infected byStreptococcus agalactiae. J. Fish. China 2014, 38, 1937–1944. [Google Scholar]
- Perera, R.P.; Fiske, R.A.; Johnson, S.K. Histopathology of Hybrid Tilapias Infected Witha Biotype of Streptococcus Iniae. J. Aquat. Anim. Health 1998, 10, 294–299. [Google Scholar] [CrossRef]
- Nermeen, A.M.; Reham, A.M.; Sherif, M.; Mohamed, A.; Mohamed, M. Eutrophication, Ammonia Intoxication, and Infectious Diseases: Interdisciplinary Factors of Mass Mortalities in Cultured Nile Tilapia. J. Aquat. Anim. Health 2016, 28, 187–198. [Google Scholar] [CrossRef]
- Deng, M.L. Title of Isolation and Identification of Streptococcusiniae from Sturgeon and Analysis of Evolution of Capsular Genes. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2016. [Google Scholar]
- Thu, N.D.; David, M.; Esteban, S. Intracoelomic- and Intramuscular-Injection Challenge Model of Piscine Streptococcosis in White Sturgeon Fingerlings. J. Aquat. Anim. Health 2020, 32, 133–138. [Google Scholar] [CrossRef]
- Komuro, J.; Ueda, K.; Kaneko, M.; Nitta, S.; Kasao, M.; Yokoyama, M. Various Cardiac Abnormalities Caused by Bacterial Myocarditis. Int. Heart J. 2018, 59, 229–232. [Google Scholar] [CrossRef]
- Sajini, V.; Preethi, V.; Saravanan, S.; Visaga, A.S.; Shibi, M.; Vimalraj, S.; Balamurugan, P.; Diraviyam, T. Inflammation in Myocardial Injury- Stem Cells as Potential Immunomodulators for Myocardial Regeneration and Restoration. Life Sci. 2020, 250, 117582. [Google Scholar] [CrossRef]
- Fryer, J.L.; Lannan, C.N. Three Decades of Fish Cell Culture: A Current Listing of Cell Lines Derived from Fishes. J. Tissue Cult. Methods 1994, 16, 87–94. [Google Scholar] [CrossRef]
- Xu, R.; Karrow, N.A.; Shandilya, U.K.; Sun, L.-H.; Kitazawa, H. In-Vitro Cell Culture for Efficient Assessment of Mycotoxin Exposure, Toxicity and Risk Mitigation. Toxins 2020, 12, 146. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Mulukutla, B.C.; Mashek, D.G.; Hu, W.-S. Regulation of Metabolic Homeostasis in Cell Culture Bioprocesses. Trends Biotechnol. 2020, 38, 1113–1127. [Google Scholar] [CrossRef]
- Le, H.; Vishwanathan, N.; Jacob, N.M.; Gadgil, M.; Hu, W.-S. Cytotoxicity and Genotoxicity Assays with Cultured Fish Cells: A Review. Toxicol. Vitr. 1991, 5, 1553–1564. [Google Scholar] [CrossRef]
- Wolf, K.; Quimby, M.C. Established Eurythermic Line of Fish Cells in Vitro. Science 1962, 135, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.S.; Kumar, M.P. Isolation, Characterization and Differentiation of Mouse Cardiac Progenitor Cells. Methods Mol. Biol. 2018, 1842, 183–191. [Google Scholar] [CrossRef]
- Kaarbø, M.; Crane, D.I.; Murrell, W.G. Rhoa Regulation of Cardiomyocyte Differentiation. Sci. World J. 2013, 2013, 491546. Available online: https://www.hindawi.com/journals/tswj/2013/491546/ (accessed on 14 March 2023). [CrossRef]
- Pablo, C.; Enrique, O.; Karime, S.; Raúl, C.; Enrique, J. Calcium Fluxes, Ion Currents and Dihydropyridine Receptors in a New Immortal Cell Line from Rat Heart Muscle. J. Mol. Cell. Cardiol. 1993, 25, 829–845. [Google Scholar] [CrossRef]
- Yu, D.P.; Cheng, J.; Xia, H.L.; Xia, L.Q.; Cai, J.; Lu, Y.S. Characterization of one strain of iridovirus isolate from Andrias davidianus. Freshw. Fish. 2022, 52, 11–17. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ren, Y.; Zhang, Y.; Yang, Y.; Wang, G.; He, Z.; Liu, Y.; Cao, W.; Wang, Y.; Fu, Y.; et al. Establishment and Characterization of a Cell Line from the Brain of the Japanese Flounder (Paralichthys olivaceus) and Its Application in the Study of Viral Infection. Aquaculture 2023, 562, 738825. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, X.; White, J.C.; Liu, L.; Sun, W.; Zhang, S.; Zeng, J.; Deng, S.; Liu, D.; Zhao, X.; et al. Transcriptome Analysis Reveals the Growth Promotion Mechanism of Enteropathogenic Escherichia coli Induced by Black Phosphorus Nanosheets. ACS Nano 2023, 17, 3574–3586. [Google Scholar] [CrossRef]
- Bairoch, A. The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. 2018, 29, 25–38. [Google Scholar] [CrossRef]
- Lakra, W.S.; Swaminathan, T.R.; Joy, K.P. Development, Characterization, Conservation and Storage of Fish Cell Lines: A Review. Fish Physiol. Biochem. 2011, 37, 1–20. [Google Scholar] [CrossRef]
- Thangaraj, R.S.; Ravi, C.; Kumar, R.; Dharmaratnam, A.; Saidmuhammed, B.V.; Pradhan, P.K.; Sood, N. Derivation of Two Tilapia (Oreochromis niloticus) Cell Lines for Efficient Propagation of Tilapia Lake Virus (Tilv). Aquaculture 2018, 492, 206–214. [Google Scholar] [CrossRef]
- Minghetti, M.; Leaver, M.J.; Tocher, D.R. Transcriptional Control Mechanisms of Genes of Lipid and Fatty Acid Metabolism in the Atlantic Salmon (Salmo salar L.) Established Cell Line, Shk-1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Lin, X.; Xu, L.; Gao, L.; Zhang, M.; Wang, N.; Wu, S. Establishment and Characterization of a Heart-Derived Cell Line from Goldfish (Carassius auratus). Fish Physiol. Biochem. 2017, 43, 977–986. [Google Scholar] [CrossRef]
- Amit, K.; Neha, S.; Mukunda, G.; Srivastava, J.K.; Akhilesh, M.K.; Lakra, W.S. Establishment and Characterization of a New Muscle Cell Line of Zebrafish (Danio rerio) as an in Vitro Model for Gene Expression Studies. Anim. Biotechnol. 2016, 27, 166–173. [Google Scholar] [CrossRef]
- Nagpure, N.S.; Mishra, A.K.; Ninawe, A.S.; Rasal, A.; Dubey, A.; Kumar, A.; Goswami, M.; Kumar, R.; Jena, J.K. Molecular and Cytogenetic Characterization of Fish Cell Lines and Its Application in Aquatic Research. Natl. Acad. Sci. Lett. 2016, 39, 11–16. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Z.; Wang, L.; Sun, F.; Lee, M.; Wen, Y.; Qin, Q.; Yue, G.H. LAMP for the rapid diagnosis of iridovirus in aquaculture. Aquac. Fish. 2022, 7, 158–165. [Google Scholar] [CrossRef]
- Ditlecadet, D.; Gautreau, C.; Boston, L.; Liston, R.; Johnsen, E.; Gagné, N. First report of successful isolation of a HPR0-like variant of the infectious salmon anaemia virus (ISAV) using cell culture. J. Fish Dis. 2021, 45, 479–483. [Google Scholar] [CrossRef]
- Wang, C.; Bao, Q.; Hou, C.; Sun, M.; Song, X.; Cao, S.; Wang, X.; Shen, Q.; Zhao, Y.; Wang, D. Mono-Macrophage-Derived Manf Alleviates Bacterial Myocarditis by Inhibiting Nf-Kappab Activation and Myocardial Inflammation. Inflammation 2021, 44, 1916–1926. [Google Scholar] [CrossRef]
- Elamm, C.; Fairweather, D.; Cooper, L.T. Pathogenesis and Diagnosis of Myocarditis. Heart 2012, 98, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Landwehr-Kenzel, S.; Henneke, P. Interaction of Streptococcus Agalactiae and Cellular Innate Immunity in Colonization and Disease. Front. Immunol. 2014, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial Ischemia-Reperfusion Injury: A Neglected Therapeutic Target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [PubMed]
- Cihakova, D.; Rose, N.R. Chapter 4 Pathogenesis of Myocarditis and Dilated Cardiomyopathy. Adv. Immunol. 2008, 99, 95–114. [Google Scholar] [CrossRef]
- Wu, B.; Ni, H.; Li, J.; Zhuang, X.; Zhang, J.; Qi, Z.; Chen, Q.; Wen, Z.; Shi, H.; Luo, X.; et al. The Impact of Circulating Mitochondrial DNA on Cardiomyocyte Apoptosis and Myocardial Injury after Tlr4 Activation in Experimental Autoimmune Myocarditis. Cell. Physiol. Biochem. 2017, 42, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, B.; Feng, J.; Li, W.; Jiang, B.; Liu, C.; Huang, Y.; Su, Y. The Influence of Enzyme Eii of the Cellobiose-Phosphotransferase System on the Virulence of Streptococcus Agalactiae in Nile Tilapia (Oreochromis niloticus). Aquaculture 2021, 535, 736340. [Google Scholar] [CrossRef]
- Su, Y.-L.; Feng, J.; Li, Y.-W.; Bai, J.-S.; Li, A.-X. Development of a quantitative PCR assay for monitoring Streptococcus agalactiae colonization and tissue tropism in experimentally infected tilapia. J. Fish Dis. 2016, 39, 229–238. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Sequence (5′–3′) | GenBank Accession | Primer Efficiency |
|---|---|---|---|
| q-Caspase3-F | CGAAACGGTACTGACGTGGA | NM_001282894.1 | 96% |
| q-Caspase3-R | GAGCCGTCCGTACCAAAGAA | ||
| q-Caspase9-F | GTTGTCCGCCCTGTAATCCA | XM_025901776.1 | 93% |
| q-Caspase9-R | GTCTTAACTGCCACCCGTCA | ||
| q-TNF-α-F | CTCAGAGTCTATGGGAAGCAG | XM_025902124.1 | 97% |
| q-TNF-α-R | GCAAACACGCCAAAGAAGGT | ||
| q-TGF-β-F | TGGGACTATGAGCAGGAGGG | NM_001311325.1 | 96% |
| q-TGF-β-R | AACAGCAGTTGTGTGATTGGGT | ||
| q-IL-8-F | GATAAGCAACAGAATCATTGTCAGC | XM_019359413.2 | 97% |
| q-IL-8-R | CCTCGCAGTGGGAGTTGG | ||
| q-β-actin-F | AACAACCACACACCACACATTTC | XM_003455949.5 | 98% |
| q-β-actin-R | TGTCTCCTTCATCGTTCCAGTTT | ||
| 18S rRNA-F | TTCATTGATGCACGAGCCGA | MF460356.1 | |
| 18S rRNA-R | CGCCGAGAAGACGATCAAAC | ||
| q-myoglobin-F | CCATGGAGCCACTGTGCTAA | NP_001266612.1 | 94% |
| q-myoglobin-R | GGGATCTTGTGCTTTGTCGC | ||
| q-troponin I-F | TCGAGGTACGACACCGAGAT | XM_025909096.1 | 98% |
| q-troponin I-R | TCTTCAGGGCGGGTTTCTTC | ||
| q-α-actin-F | TTGAGGCCAGATGAGAAGGC | XM_025901239.1 | 95% |
| q-α-actin-R | GACGGATCCACTCCAGCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, Y.; Jiang, D.; Zhang, D.; Huang, Y.; Cai, J.; Jian, J.; Wang, B. A New Conditionally Immortalized Nile Tilapia (Oreochromis niloticus) Heart Cell Line: Establishment and Functional Characterization as a Promising Tool for Tilapia Myocarditis Studies. Fishes 2023, 8, 167. https://doi.org/10.3390/fishes8030167
Chen Y, Li Y, Jiang D, Zhang D, Huang Y, Cai J, Jian J, Wang B. A New Conditionally Immortalized Nile Tilapia (Oreochromis niloticus) Heart Cell Line: Establishment and Functional Characterization as a Promising Tool for Tilapia Myocarditis Studies. Fishes. 2023; 8(3):167. https://doi.org/10.3390/fishes8030167
Chicago/Turabian StyleChen, Yanghui, Yuan Li, Dongneng Jiang, Defeng Zhang, Yu Huang, Jia Cai, Jichang Jian, and Bei Wang. 2023. "A New Conditionally Immortalized Nile Tilapia (Oreochromis niloticus) Heart Cell Line: Establishment and Functional Characterization as a Promising Tool for Tilapia Myocarditis Studies" Fishes 8, no. 3: 167. https://doi.org/10.3390/fishes8030167
APA StyleChen, Y., Li, Y., Jiang, D., Zhang, D., Huang, Y., Cai, J., Jian, J., & Wang, B. (2023). A New Conditionally Immortalized Nile Tilapia (Oreochromis niloticus) Heart Cell Line: Establishment and Functional Characterization as a Promising Tool for Tilapia Myocarditis Studies. Fishes, 8(3), 167. https://doi.org/10.3390/fishes8030167