Abstract
Complete mitochondrial genome (mitogenome) sequences can provide useful and varied information for evolutionary and phylogenetic studies. We report the first complete mitogenome sequence of the bumblebee shrimp (Gnathophyllum americanum). The mitogenome is 15,842 bp in length and contains 13 protein-encoding genes, 2 ribosomal RNA genes, and 22 transfer RNA genes. The nucleotide composition of the mitogenome was as follows: A,36.72%; T,29.62%; C,21.0%; and G,12.66%. The most common start and stop codons are ATG and TAA, respectively. The MGO (mitochondrial gene order) of G. americanum and Hymenocera picta is unique in Decapoda. Both the phylogeny based on mitochondrial genomic DNA data and the unique MGO pattern indicate that the genus Gnathophyllum might be a sister genus to the genus Hymenocera. The mitogenomic sequence data obtained in this study will provide more information on G. americanum for species identification, population genetics, and biogeography.
Keywords:
caridean shrimp; molecular phylogeny; relative synonymous codon usage; gene order rearrangement Key Contribution:
The first complete mitogenome sequence of the genus Gnathophyllum; the bumblebee shrimp (Gnathophyllum americanum).
1. Introduction
The bumblebee shrimp, Gnathophyllum americanum (Guérin-Méneville, 1855), is a caridean shrimp of the family Palaemonidae. Palaemonidae is the largest shrimp family within the infraorder Caridea, and includes more than 1200 species in 160 genera. Palaemonid shrimps are free-living or occur in association with a variety of different hosts to benefit from nutrient sources, shelter, and reproductive opportunities [1]. G. americanum can be considered a free-living species, although it remains in relative proximity to echinoderms [2]. The bumblebee shrimp is a popular marine ornamental species because of its unique and attractive color pattern. The special color pattern of the body is yellow or white with black stripes (Figure 1); thus, it is also referred to as striped bumblebee shrimp or striped harlequin shrimp. There is a black block with yellow ends on the propodus of the second pereiopod. Yellow stripes can be seen on the end of the tail.

Figure 1.
A bumblebee shrimp, G. americanum. (Photographed by Chen-Cheng Cheng).
The mitochondrial DNA of most metazoans is an extrachromosomal, double-stranded, closed-circular molecule that typically contains 37 genes, including 13 protein-encoding genes, two ribosomal RNA genes, and 22 transporter RNA genes [3]. The complete mitogenome DNA sequence is 14–20 kb in length [4]. The mitogenome exhibits maternal inheritance with a high evolution rate. Currently, high-throughput DNA sequencing technologies are used to obtain complete and accurate mitogenome sequences. A complete mitogenome is a powerful tool for assisting the analysis of taxonomy, evolution, and phylogeny [5]. Several structural genomic characteristics (e.g., genome size, gene content, gene order, nucleotide composition, nucleotide substitution rate, repeated sequences, non-coding sequences, and secondary structure of the encoded RNA) can be systematically and easily investigated in the small mitochondrial genome [3].
At present, little information is available on mtDNA molecules in the genus Gnathophyllum. Thus, this is the first report of complete mitochondrial sequences for the genus Gnathophyllum. Mitogenomic data from this study will provide useful molecular markers for further studies on the species identification, population genetics, and molecular biogeography of G. americanum.
2. Materials and Methods
For this study, G. americanum were collected by the Penghu Marine Biology Research Center (23°52′89″ N; 119°56′08″ E), Taiwan, and stored at the Fisheries Research Institute in Keelung, Taiwan (accession number: Gam_1110111-1; contact person: CH Sung, chsung@mail.tfrin.gov.tw). Total genomic DNA was extracted from the muscle using the QIAamp DNA Mini Kit (QIAGEN) and following the manufacturer’s instructions. The total DNA was sequenced using next-generation sequencing (Illumina MiSeq sequencing platform, Tri-I Biotech Inc., Taipei, Taiwan). After removing adapters and low-quality sequences, about 1.1 Gb of trimmed reads (average read length: 297.87 bp, number of reads: 3,921,418) were used for de novo assembling. The de novo assembly of the mitochondrial genome was conducted using CLC Genomics Workbench V20 (QIAGEN). The locations of the protein-coding genes, ribosomal RNAs (rRNAs), and transfer RNAs (tRNAs) were predicted by using MITOS Web Server (http://mitos.bioinf.uni-leipzig.de/, accessed on 19 December 2022) [6] and identified via alignment with other mitogenome sequences of Palaemonidae shrimps. Secondary structures of tRNAs were plotted using VARNA v3 [7] according to MITOS results. The open reading frames were also determined by ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/, accessed on 30 December 2022). The AT and GC skew were calculated according to the following formulas: AT skew = (A − T)/(A + T) and GC skew = (G − C)/(G + C) [8]. The genome map was drawn using OrganellarGenomeDRAW (OGDRAW) (https://chlorobox.mpimp-golm.mpg.de/OGDraw.html, accessed on 17 January 2023) [9]. The relative synonymous codon usage (RSCU) of the mitogenome was calculated and produced using DnaSP V6 [10]. Phylogenetic relationships were constructed based on the 13 protein-encoding 16S ribosomal RNA and 12S ribosomal RNA gene sequences of 22 Decapoda species from seven families. The BioEdit software was used for DNA sequence alignment. Model selection of the maximum likelihood (ML) method was performed, and the best model was determined through AIC and BIC scores. The general time-reversible (GTR) model with gamma distribution (+G) and invariant sites (+I) was selected as the best substitution model. The maximum likelihood tree was constructed using MEGA 11 [11] with 1000 bootstrap repeats under model GTR+G+I.
3. Results and Discussion
3.1. Mitogenome Organization and Nucleotide Composition
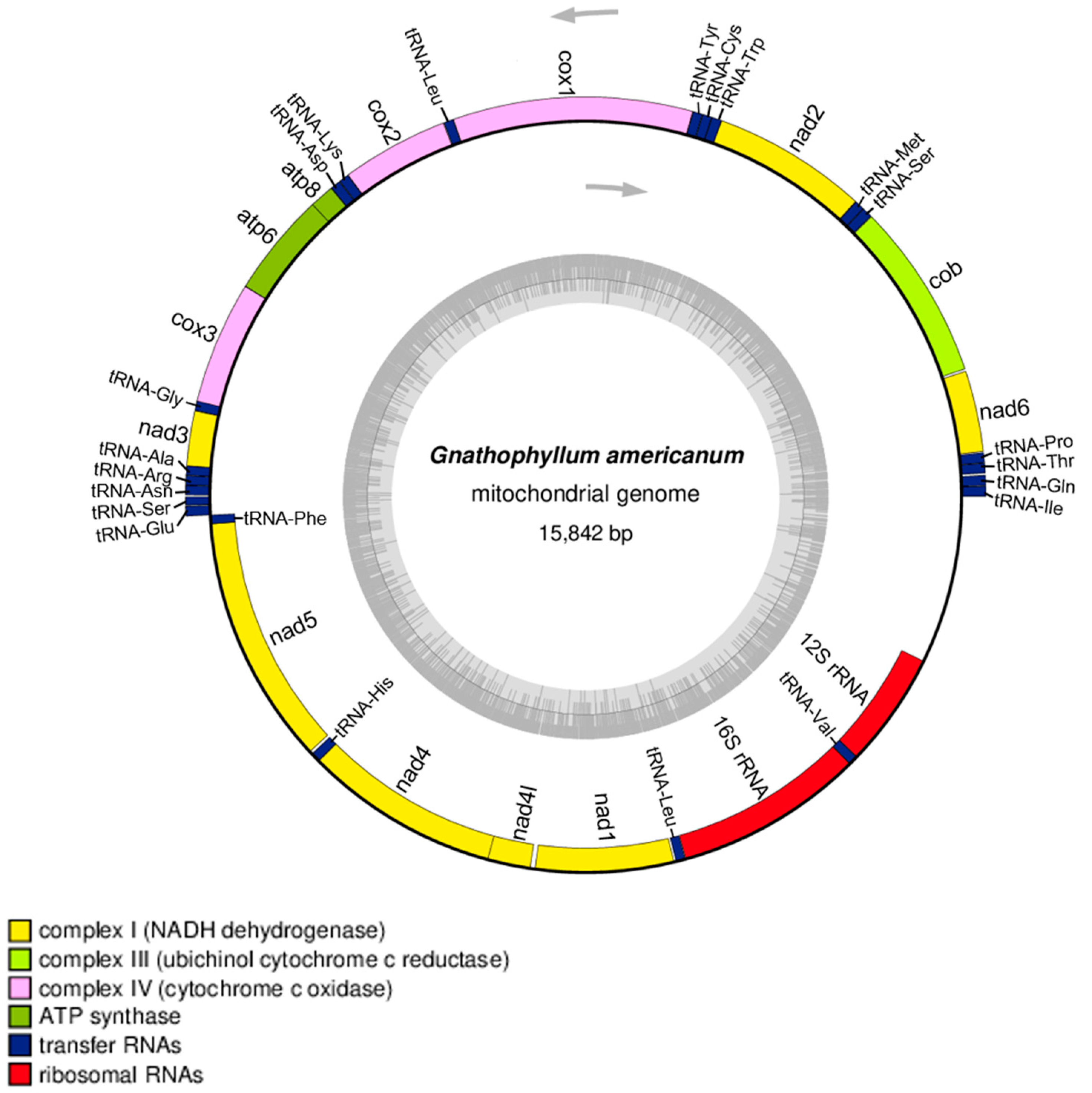
The complete mitogenome sequence of G. americanum is 15,842 bp in length (GenBank Accession No. OQ184740) and includes 13 protein-encoding genes, 2 rRNA genes, and 22 tRNA genes (Figure 2). A total of 24 genes (9 protein-coding genes and 15 tRNAs) are encoded on the lead strand, and 13 genes (4 protein-coding genes, 7 tRNAs, and 2 rRNAs) are encoded on the complementary strand. The overall nucleotide composition of the mitogenome was 36.72% A, 29.62% T, 21.0% C, and 12.66% G. The AT and GC skewness of the mitogenome sequence were 0.1070 and −0.2477 (representing the A-skew and C-skew, respectively). The total length of the 13 protein-encoding genes of G. americanum is 11,115 bp, accounting for 70.02% of the complete mitochondrial genome. The protein-coding genes include three cytochrome c oxidases (cox1–cox3), seven NADH dehydrogenases (nad1–nad6 and nad4l), two ATPases (atp6 and atp8), and one cytochrome b (cob).
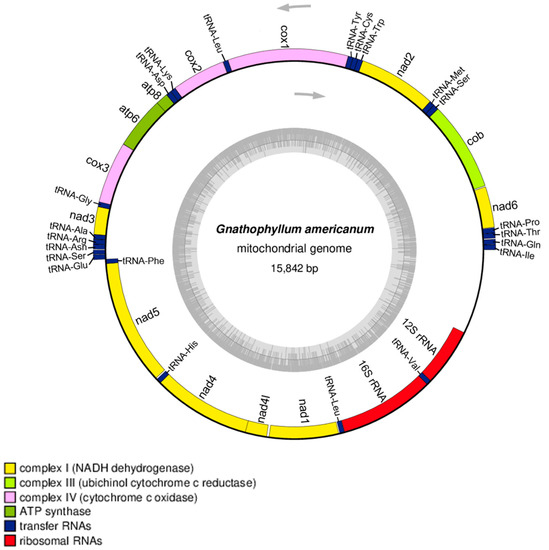
Figure 2.
Genome map of the mitochondrial genome of G. americanum. The outer blocks of the genome map represent the genes encoded on the lead strand, and the inside blocks are represented the genes encoded on the complementary strand. The map was drawn by OGDRAW.
The most common shared start codon between the protein-coding genes was ATG (atp6, cob, cox2, nad4, nad4l, nad5), followed by ATT (nad1, nad2), ATA (cox3, nad6), ATC (atp8), and GTG (nad3). The most common stop codon was TAA (atp6, cox3, nad1, nad2, nad3, nad4l), followed by TAG (atp8, nad6). There were five genes that used the incomplete (T) stop codon. There were 50 nucleotides found in 9 overlaps between adjacent genes in G. americanum, such as nad6/cob, atp8/atp6, atp6/cox3, cox3/tRNA-Gly, tRNA-Gly/nad3, nad3/tRNA-Ala, tRNA-Glu/tRNA-Phe, tRNA-Phe/nad5, and nad4/nad4l (Table 1). The longest overlap was 17 bp, between atp6 and cox3. In Decapoda, mitochondrial gene overlap was reported in many species [12,13,14]. The most common locations were atp6/atp8 and nad4l/nad4, but locations were also observed at different genes.

Table 1.
Characteristics of the mitochondrial genome of G. americanum.
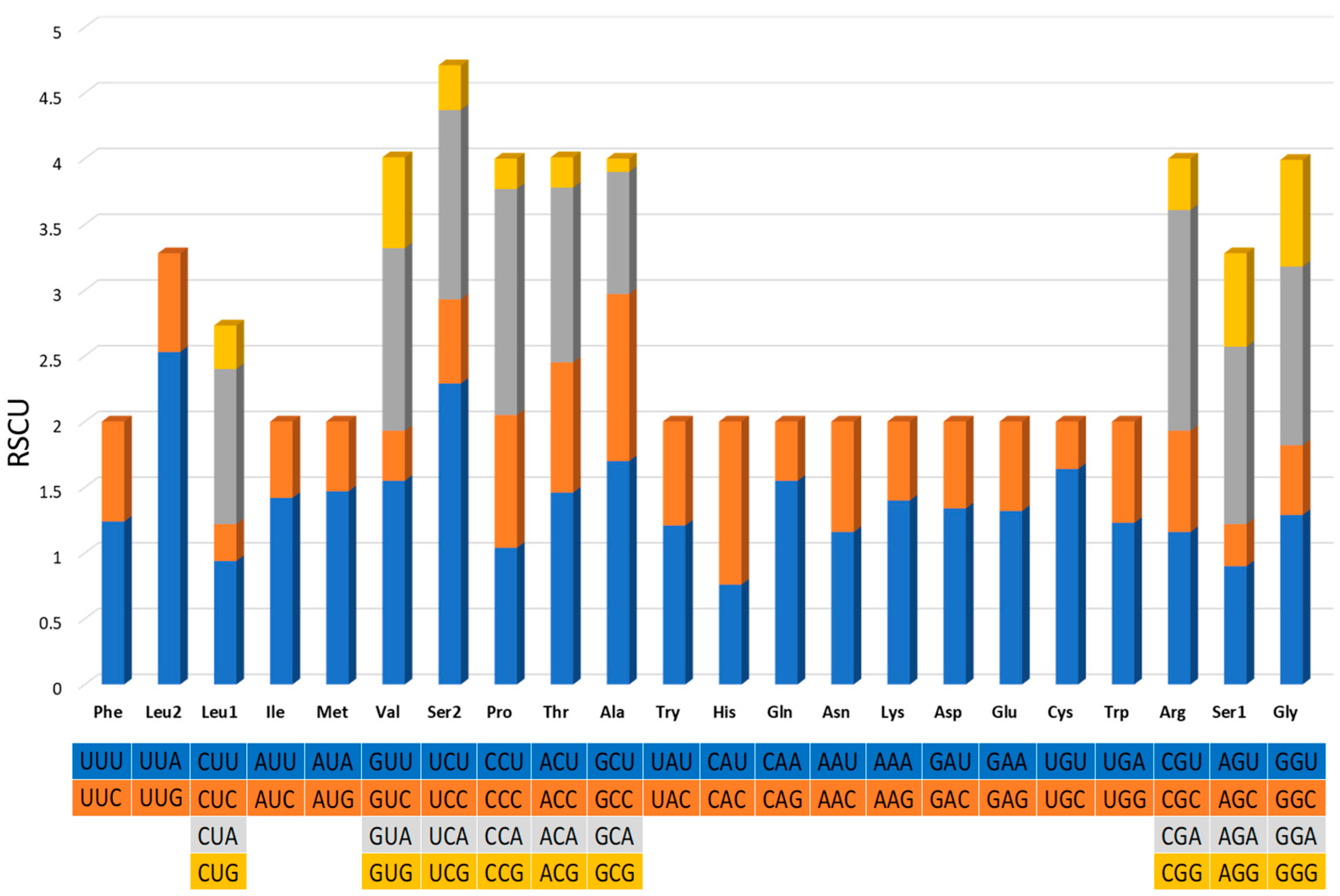
The 13 protein-encoding genes’ relative synonymous codon usage (RSCU) analysis of G. americanum is presented in Figure 3. The most commonly used codon was UUA-Leu, followed by UCU-Ser, CCA-Pro, and GCU-Ala. A total of 3705 amino acids were encoded, among which serine (10.07%) was the most used amino acid, followed by Phe (7.88%), Ile (7.64%), and Leu (7.45%). The least used codon and encoded amino acid were GCG-Ala and Cys (1.35%), respectively.
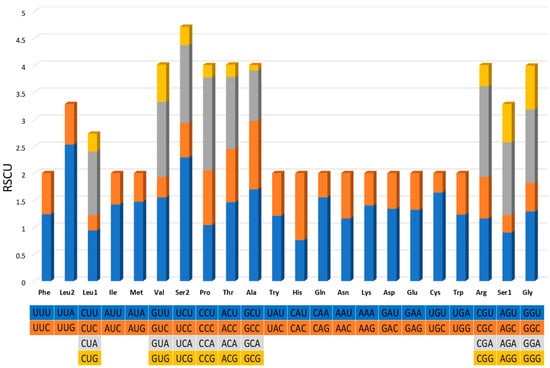
Figure 3.
Relative synonymous codon usage (RSCU) of the mitogenome of G. americanum. The stop codons are not shown.
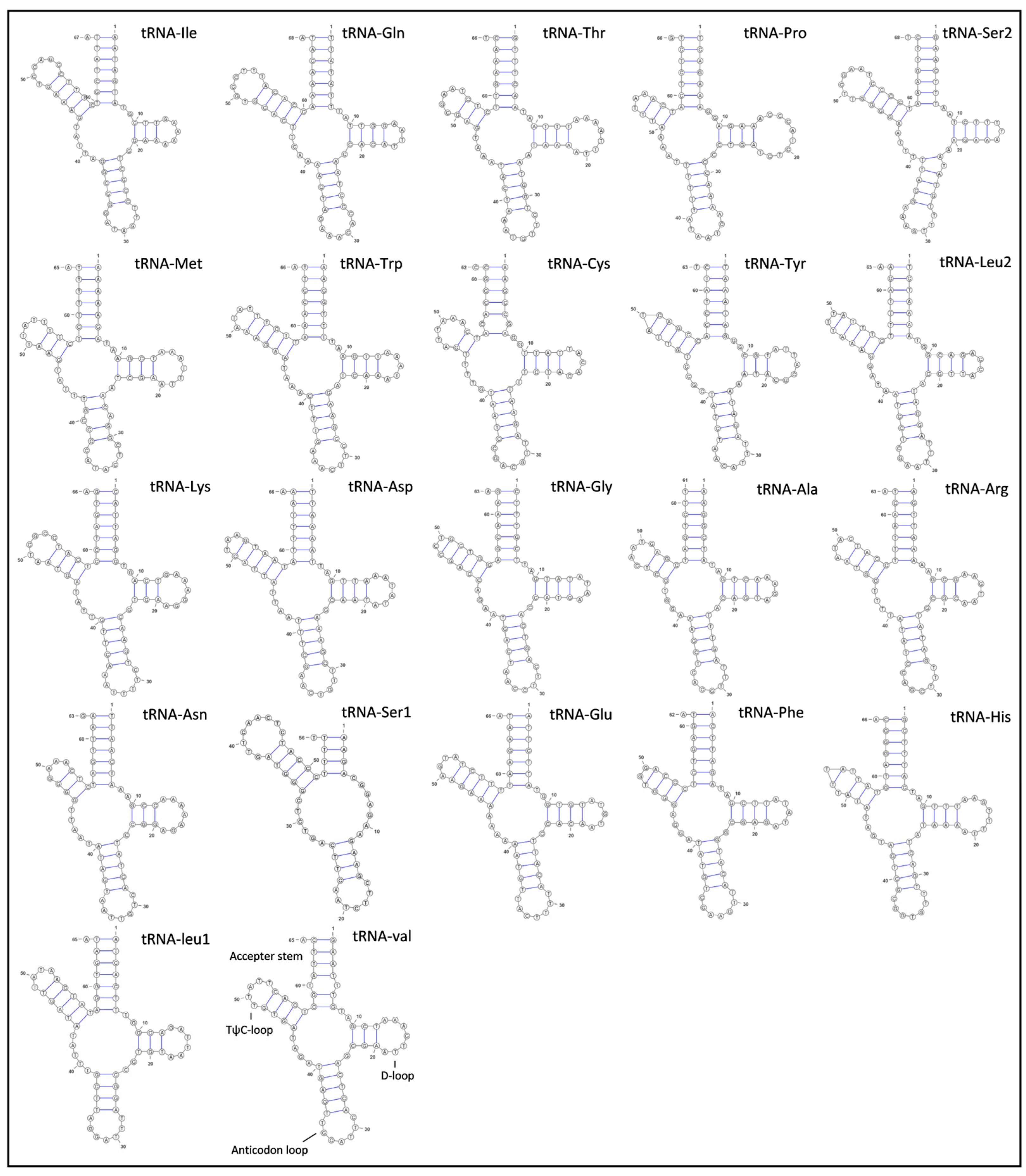
G. americanum’s 16S ribosomal RNA gene (1287 bp) was located between tRNA-Leu1 and tRNA-Val, while 12S ribosomal RNA gene (788 bp) was located between tRNA-Val and the putative control region, and both rRNA genes were encoded by the H-strand. The total length of rRNAs was 2075 bp, and the AT content of two rRNA genes was 70.51%. G. americanum had 22 typical tRNAs, varying from 56 bp (tRNA-Ser1) to 68 bp (tRNA-Gln and tRNA-Ser1) in length (Table 1). The total length of tRNAs was 416 bp, and the AT content was 67.37%. The predicted secondary structures of the 22 tRNAs are shown in Figure 4. Most tRNAs had the typical cloverleaf-shaped secondary structures, including an amino acid accepting arm, D-loop, anticodon loop, and TψC loop. However, the tRNA-Ser1 lacks the D-loop arm stem and D-loop, which is a common feature in many mitogenomes of metazoans [15]. The total length of the putative control region was 1143 bp, and the AT content was 78.48%. The putative control region was located between 12S ribosomal RNA gene and tRNA-Ile, which was similar to other caridean shrimps [5,13].
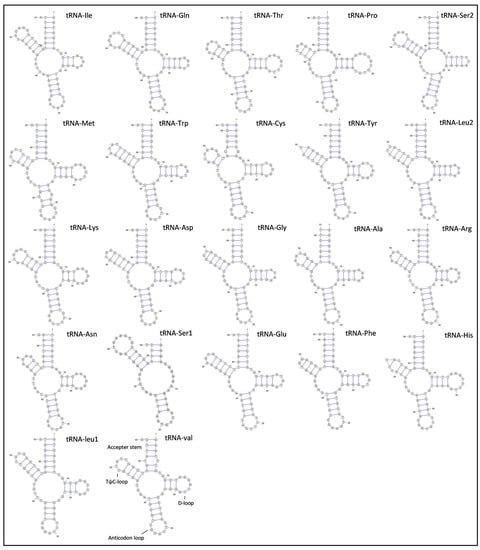
Figure 4.
Secondary structures of 22 tRNAs in the mitogenome of G. americanum.
3.2. Phylogenetic Relationships
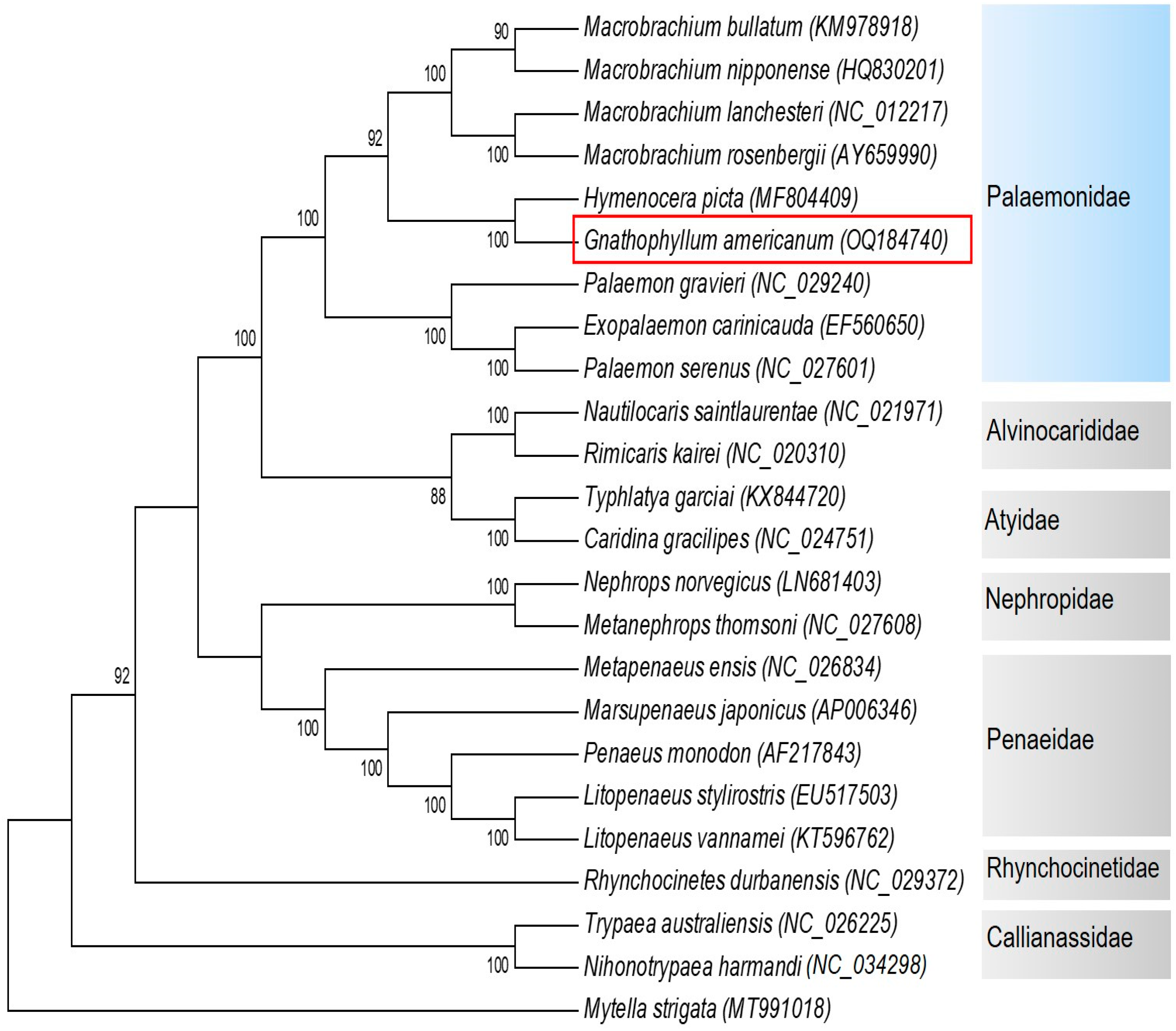
We reconstructed the phylogenetic relationships of 23 Decapoda species based on 13 protein-encoding 16S ribosomal RNA and 12S ribosomal RNA genes’ DNA sequences with Mytella strigata as the outgroup, using the maximum likelihood (ML) method (Figure 5). Among 21 nodes in total, bootstrap supports at 14 nodes were 100%, and the other 5 nodes were high (>70%). The clade including Palaemonidae species was highly supported (100%). The families Palaemonidae, Alvinocarididae, Atyidae, Nephropidae, Penaeidae, Rhynchocinetidae, and Callianassidae were monophyletic. The phylogenetic position of G. americanum was close to Hymenocera picta in our study, which was consistent with results of a previous study [2]. The result may indicate that the genus Gnathophyllum is a sister genus to the genus Hymenocera. The clade containing G. americanum and H. picta was a sister to the genus Macrobrachium. Exopalaemon carinicauda was grouped in the genus Palaemon in this study, which is consistent with the results of previous studies [5,16]. The generic position of Exopalaemon carinicauda should be reconsidered.

Figure 5.
Bootstrap consensus tree of the 23 Decapoda species based on the DNA sequence of 13 protein-encoding 16S ribosomal RNA and 12S ribosomal RNA genes. The tree was reconstructed with the maximum likelihood (ML) method using MEGA v.11 based on the GTR+I+G model. Bootstrap values (1000 replications) greater than 70% are shown at the branch nodes.
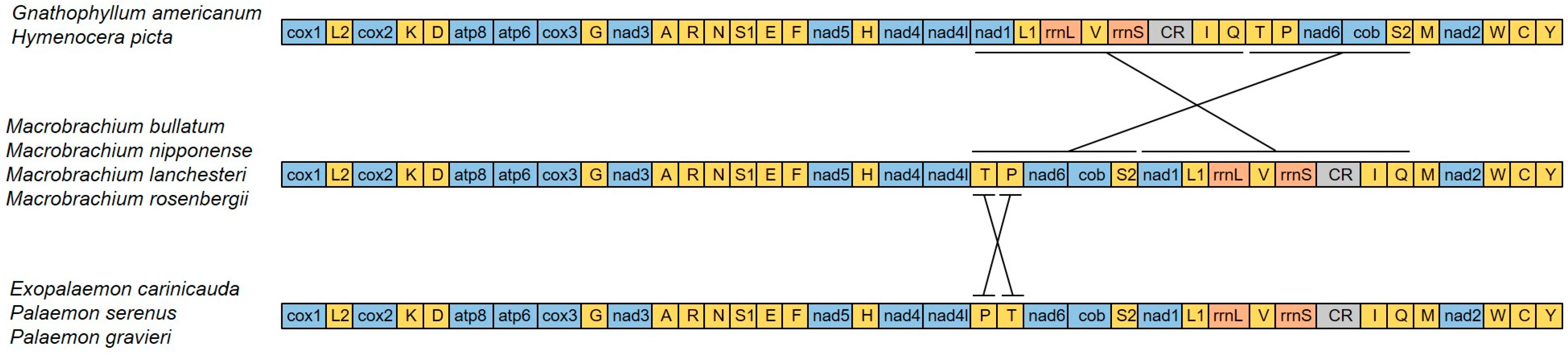
3.3. Gene Rearrangement
Gene rearrangement in the Decapoda mitogenome commonly occurs and can be a tool to study phylogenetic relationships [13,14]. We compared the mitochondrial gene orders (MGOs) of nine species in Palaemonidae (including genera Macrobrachium, Gnathophyllum, Hymenocera, Exopalaemon, and Palaemon) (Figure 6). The genus Macrobrachium (including M. bullatum, M. nipponense, M. lanchesteri, and M. rosenbergii) shares the ancestral pattern of Decapoda [17]. Exopalaemon carinicauda, Palaemon serenus, and P. graviera have an identical pattern of the mitochondrial gene order. Compared to the ancestral type of Decapoda, this type has the positions of trnP and trnT translocated. The mitochondrial gene order was identical between G. americanum and Hymenocera picta. Compared to the ancestral pattern of Decapoda, the gene region from nad1 to trnQ was rearranged with the gene region from trnT to trnS2. This MGO pattern is unique in Decapoda. Both the phylogeny based on mitochondrial genomic DNA data and the unique MGO pattern indicate that the genera Gnathophyllum and Hymenocera have a common ancestor.
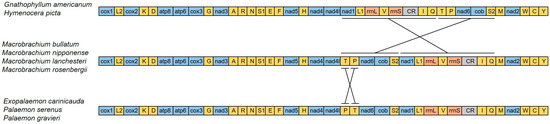
Figure 6.
Linearized mitochondrial gene orders of Palaemonidae. All genes are transcribed from left to right. tRNA genes are marked by the single-letter codes of the encoded amino acids. The 16S rRNA and 12S rRNA are the large and small ribosomal RNA subunits.
4. Conclusions
The first complete mitogenome sequence of G. americanum is 15,842 bp in length and contains 13 protein-coding genes, 2 ribosomal RNA genes, and 22 transfer RNA genes. The most common shared start codon between the protein-coding genes was ATG. The most common termination codon was TAA. The mitochondrial gene order (MGO) of G. americanum and H. picta is a unique pattern in Decapoda. Both the phylogeny based on mitochondrial genomic DNA data and the unique MGO pattern indicate that the genus Gnathophyllum might be a sister genus to the genus Hymenocera. The results provide a better understanding of the evolutionary histories of Palaemonidae and related species. Moreover, the mitogenomic sequence data presented here will provide useful information for future studies related to the identification, population genetics, and biogeography of G. americanum.
Author Contributions
Conceptualization: C.-H.S., C.-W.H. and L.-J.W.; Original Draft: C.-H.S., C.-W.H. and L.-J.W.; Resources: C.-C.C.; Visualization: C.-H.S. and C.-C.C.; Writing—Review and Editing: C.-H.S. and L.-J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Council of Agriculture (COA) under Grant 110AS-6.2.5-AI-A1 and 111AS-6.1.5-AI-A1.
Data Availability Statement
The genome sequence data supporting the findings of this study are openly available from the GenBank of the National Center for Biotechnology Information (NCBI) at https://www.ncbi.nlm.nih.gov (accession number: ON942257).
Acknowledgments
We are grateful to H.C. Ho for assistance of DNA isolation.
Conflicts of Interest
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Frolová, P.; Horká, I.; Ďuriš, Z. Molecular phylogeny and historical biogeography of marine Palaemonid shrimps (Palaemonidae: Palaemonella–Cuapetes Group). Sci. Rep. 2022, 12, 15237. [Google Scholar] [CrossRef] [PubMed]
- Horká, I.; De Grave, S.; Fransen, C.H.J.M.; Petrusek, A.; Ďuriš, Z. Multiple host switching events shape the evolution of symbiotic Palaemonid shrimps (Crustacea: Decapoda). Sci. Rep. 2016, 6, 26486. [Google Scholar] [CrossRef] [PubMed]
- Gissi, C.; Iannelli, F.; Pesole, G. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity 2008, 101, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gong, L.; Zhang, Y.; Chen, J.; Liu, L.; Jiang, L.; Lü, Z.; Liu, B.; Tong, G.; Wei, X. The complete mitochondrial genome of Calappa bilineata: The first representative from the family Calappidae and its phylogenetic position within Brachyura. Genomics 2020, 112, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Miao, J.; Guo, Y.; Gong, L.; Jiang, L.; Lü, Z.; Xu, K.; Guo, B. The first mitochondrial genome of the genus Exhippolysmata (Decapoda: Caridea: Lysmatidae), with gene rearrangements and phylogenetic associations in Caridea. Sci. Rep. 2021, 11, 14446. [Google Scholar] [CrossRef] [PubMed]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo Metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Darty, K.; Denise, A.; Ponty, Y. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics 2009, 25, 1974–1975. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) Version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Han, C.; Guo, B.; Liu, K.; Lin, X.; Lu, X. The First complete mitochondrial genome of Eucrate crenata (Decapoda: Brachyura: Goneplacidae) and phylogenetic relationships within infraorder Brachyura. Genes 2022, 13, 1127. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, M.; Wu, Z.; Tian, M.; Cheng, H.; Zhao, F.; Meng, X. The complete mitochondrial genome of the ridgetail white prawn Exopalaemon carinicauda Holthuis, 1950 (Crustacean: Decapoda: Palaemonidae) revealed a novel rearrangement of tRNA genes. Gene 2009, 437, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hui, M.; Wang, M.; Sha, Z. The complete mitochondrial genome of the Alvinocaridid shrimp Shinkaicaris leurokolos (Decapoda, Caridea): Insight into the mitochondrial genetic basis of deep-sea hydrothermal vent adaptation in the shrimp. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Suematsu, T.; Ohtsuki, T. Losing the stem-loop structure from metazoan mitochondrial tRNAs and co-evolution of interacting factors. Front. Genet. 2014, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Kou, Q.; Li, X.; Chan, T.Y.; Chu, K.H.; Gan, Z.; Kou, Q. Molecular phylogeny of the superfamily Palaemonoidea (Crustacea: Decapoda: Caridea) based on mitochondrial and nuclear DNA reveals discrepancies with the current classification. Invertebr. Syst. 2013, 27, 502–514. [Google Scholar] [CrossRef]
- Tan, M.H.; Gan, H.M.; Lee, Y.P.; Bracken-Grissom, H.; Chan, T.-Y.; Miller, A.D.; Austin, C.M. Comparative mitogenomics of the Decapoda reveals evolutionary heterogeneity in architecture and composition. Sci. Rep. 2019, 9, 10756–10772. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).