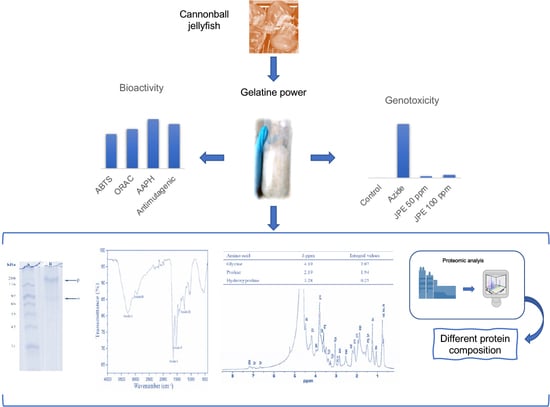
Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Protein Extraction and Gelatin Production
2.3. Biological Activity
2.3.1. Antioxidant Activity
2.3.2. Ames Assay
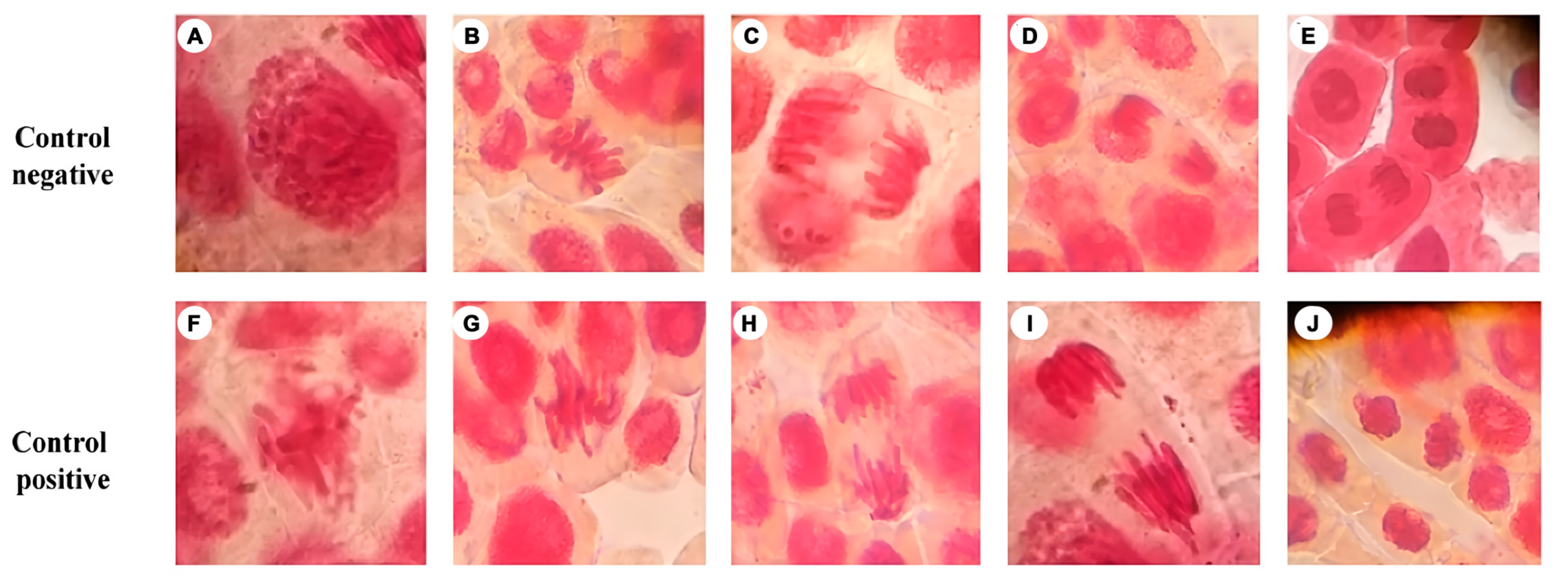
2.3.3. Genotoxicity Test
2.4. Protein Analysis
2.4.1. Chemical Analysis
2.4.2. Amino Acid Analysis
2.4.3. Electrophoretic Profile (SDS-PAGE)
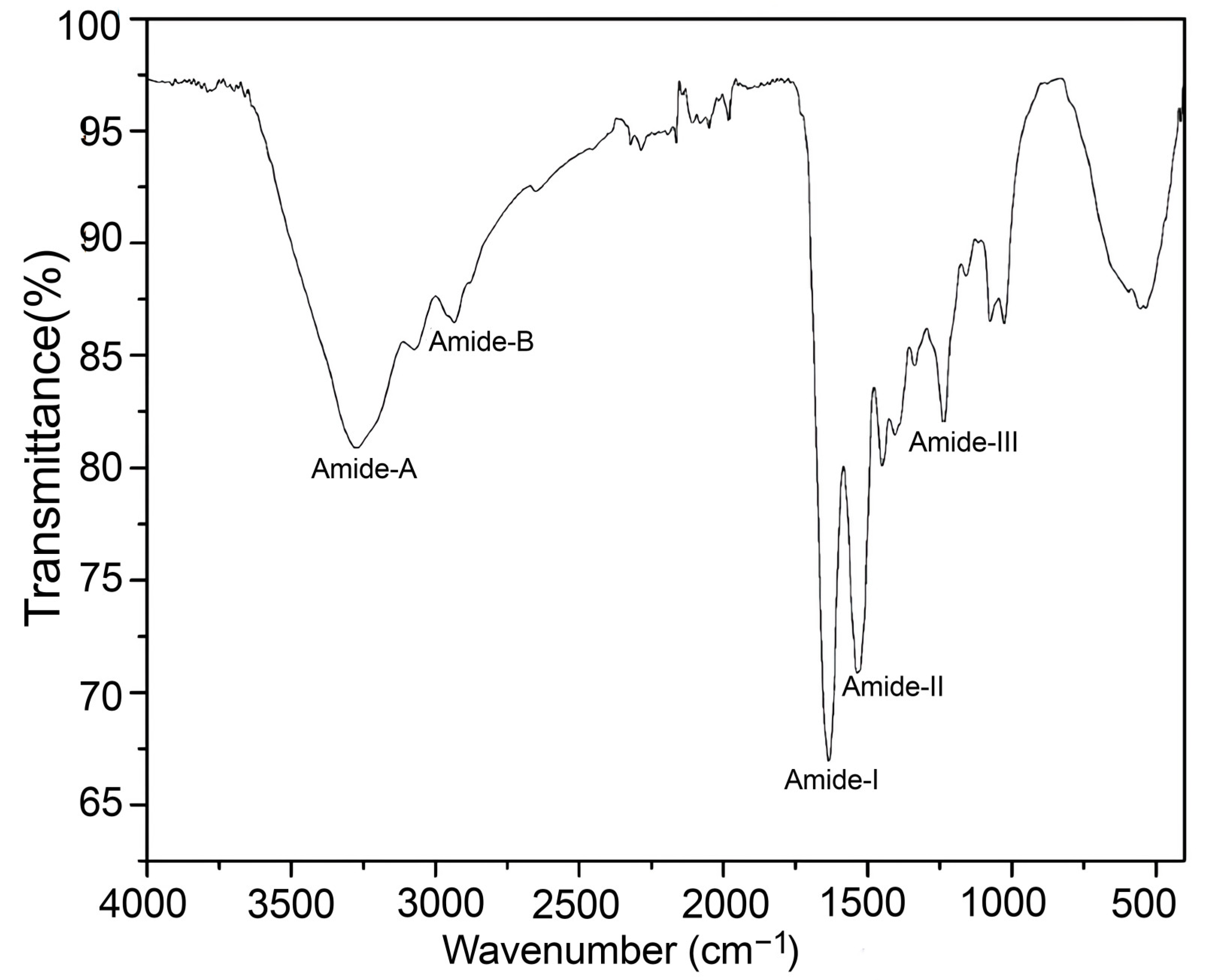
2.4.4. Fourier Transformed–Infrared Spectroscopy (FT-IR)
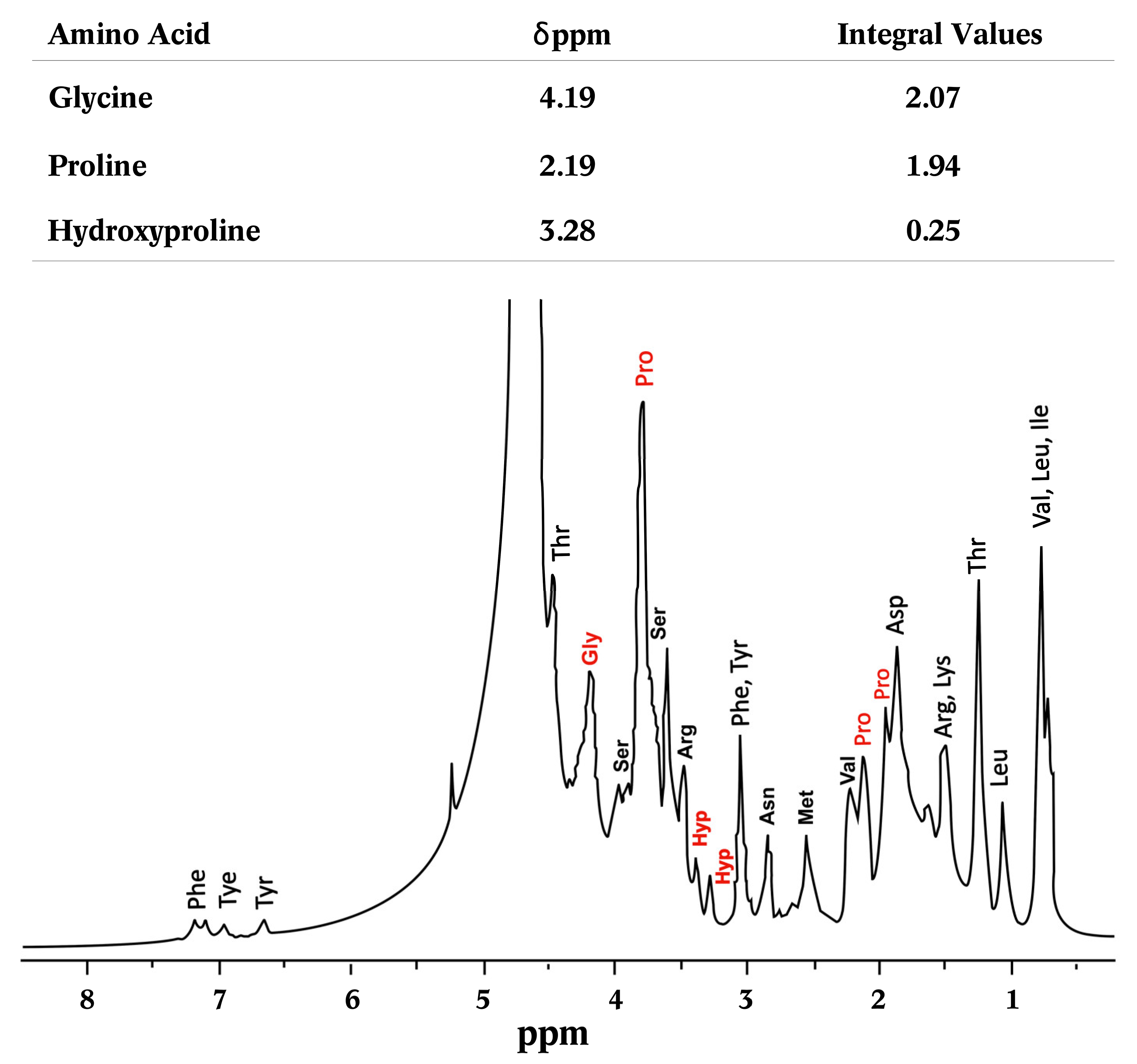
2.4.5. Nuclear Magnetic Resonance of Proton (1H-NMR)
2.4.6. Protein Identification
2.5. Statistical Analyses
3. Results and Discussion
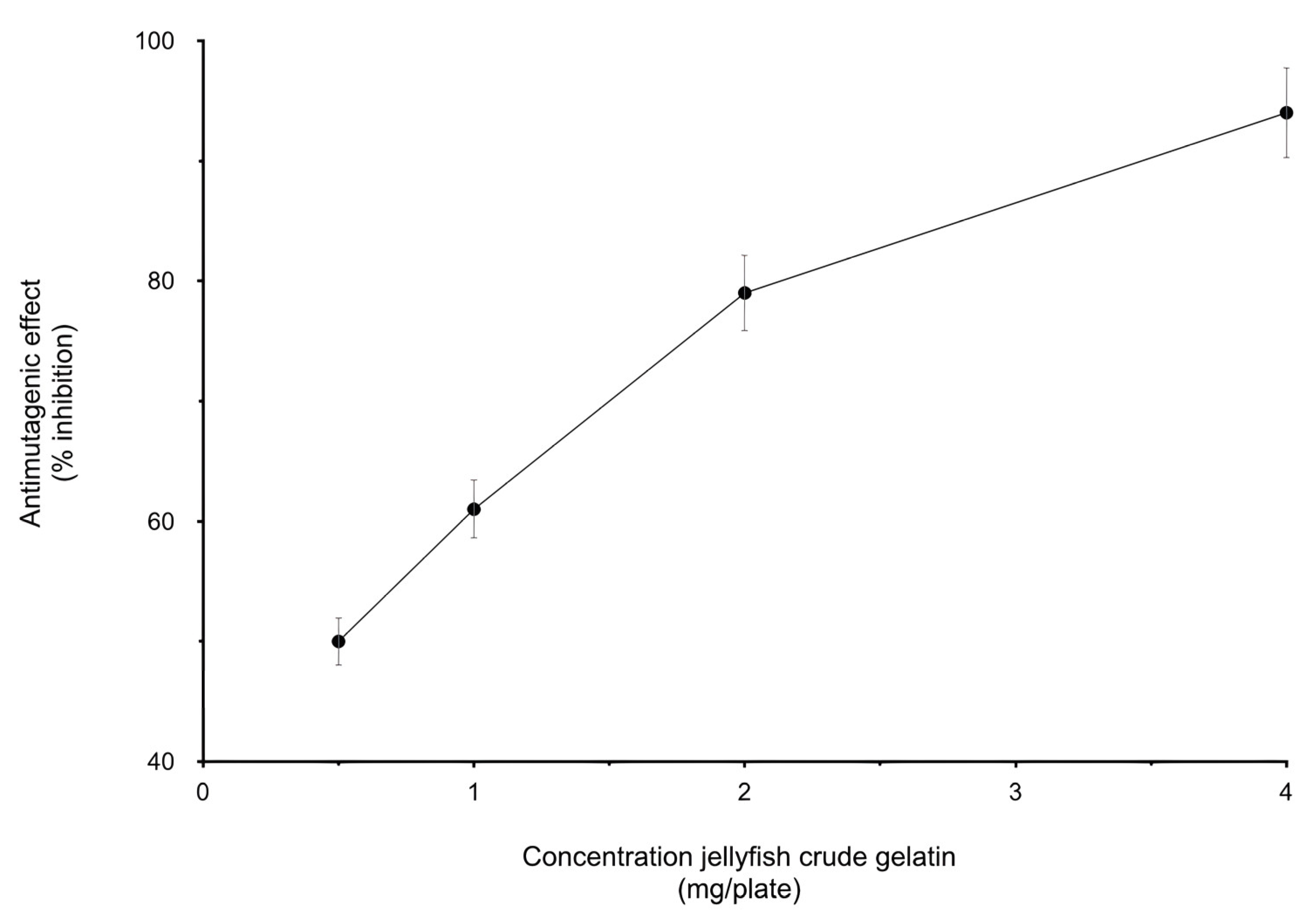
3.1. Biological Activity
3.2. Genotoxicity Test
3.3. Protein Analysis
3.3.1. Yield
3.3.2. Chemical Composition
3.3.3. Amino Acid Analysis
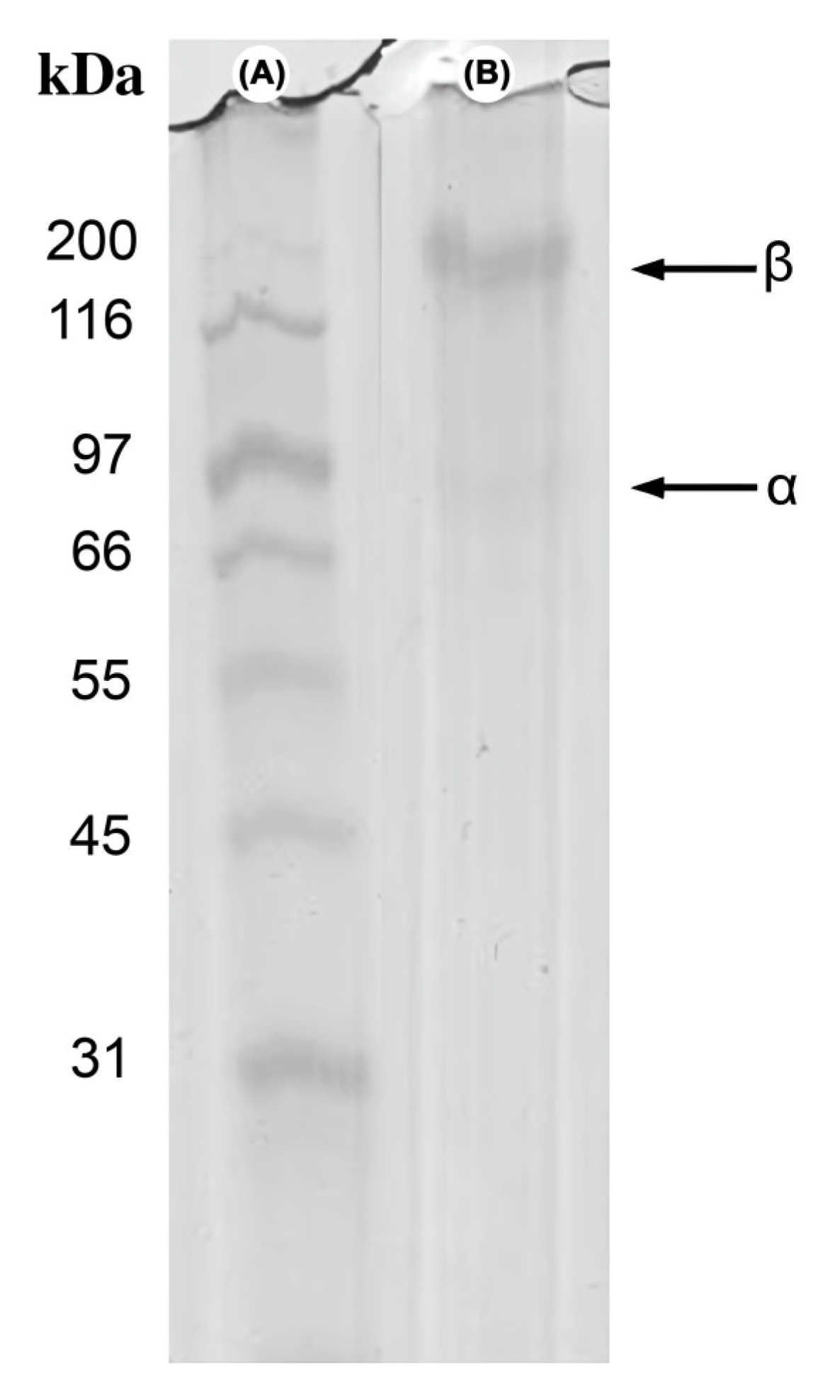
3.3.4. Electrophoretic Profile
3.3.5. Fourier Transformed–Infrared Spectroscopy (FT-IR)
3.3.6. Nuclear Magnetic Resonance of Proton (1H-NMR)
3.3.7. Proteomic Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riccio, G.; Martinez, A.K.; Martín, J.; Reyes, F.; D’Ambra, I.; Lauritiano, C. Jellyfish as an alternative source of bioactive antiproliferative compounds. Mar. Drugs 2022, 20, 350. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, J.; Álvarez-Tello, J. The jellyfish fishery in Mexico. Agri. Sci. 2013, 4, 57. [Google Scholar] [CrossRef]
- Bleve, G.L.; Ramires, F.A.; Gallo, A.; Leone, A. Identification of safety and quality parameters for preparation of jellyfish based novel food products. Foods 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Balamurugan, E.; Menon, V. In Vitro radical scavanging activities of Chrysaora quinquecirrha nematocyst venom. Drug Discov. Ther. 2009, 3, 56–61. [Google Scholar] [PubMed]
- Harada, K.; Maeda, T.; Hasegawa, Y.; Tokunaga, T.; Ogawa, S.; Fukuda, K.; Nagatsuka, N.; Nagao, K.; Ueno, S. Antioxidant activity of the giant jellyfish Nemopilema nomurai measured by the oxygen radical absorbance capacity and hydroxyl radical averting capacity methods. Mol. Med. Rep. 2011, 4, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Isolation, identification and characterization of a novel antioxidant protein from the nematocyst of the jellyfish Stomolophus meleagris. Int. J. Biol. Macromol. 2012, 51, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Abedin, M.Z.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol. Biotechnol. 2014, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, C.; Zhao, J.; Shi, X.; Sun, J.; Liu, J.; Fu, Y.; Jin, W.; Zhu, B. Separation and characterization of antioxidative and angiotensin converting enzyme inhibitory peptide from jellyfish gonad hydrolysate. Molecules 2018, 23, 94. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [PubMed]
- Upata, M.; Siriwoharn, T.; Makkun, S.; Yarnpakdee, S.; Regenstein, J.M.; Wangtueai, S. Tyrosinase inhibitory and antioxidant activity of enzymatic protein hydrolysate from jellyfish (Lobonema smithii). Foods 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Urquidy, B.S.; Velazquez-Valdez, L.P.; Bracamontes-Picos, S.J.; Del Toro-Sánchez, C.L.; Chan-Higuera, J.E.; Ezquerra-Brauer, J.M. Conversion of dry-salted cannonball jellyfish (Stomolophus meleagris) umbrella and oral arms to cornmeal snacks and gelatin with antioxidant properties. Fishes 2022, 7, 277. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Cho, S.; Ahn, J.R.; Koo, J.S.; Kim, S.B. Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fish. Aquatic Sci. 2014, 17, 299–304. [Google Scholar] [CrossRef]
- Rodsuwan, U.; Thumthanaruk, B.; Kerdchoechuen, O.; Laohakunjit, N. Functional properties of type A gelatin from jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 507–514. Available online: http://www.ifrj.upm.edu.my/23%20(02)%202016/(8).pdf (accessed on 6 November 2022).
- Chiarelli, P.G.; Pegg, R.B.; Kumar, D.; Sloval, K.M. Exploring the feasibility of developing novel gelatine powders from salted, dried cannonball jellyfish (Stomolophus meleagris). Food Biosci. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Carrera, M.; Cañas, B.; Gallardo, J.M. Proteomics for the assessment of quality and safety of fishery products. Food Res. Int. 2013, 54, 972–979. [Google Scholar] [CrossRef]
- Cuevas-Acuña, D.A.; Placencia-Jatomea, M.; Santacruz-Ortega, H.C.; Torres-Arreola, W.; Ezquerra-Brauer, J.M. Development of chitosan/squid skin gelatin hydrolysate films: Structural, physical, antioxidant, and antifungal properties. Coatings 2021, 11, 1088. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Protoggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 5, 3273–3327. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, Y.; Liu, G. Flavonoids from the leaves of Actinidia kolomikta. Chem. Nat. Compd. 2010, 46, 205–208. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mut. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, S. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mut. Res. 2007, 626, 4–14. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Vazquez-Ortiz, F.; Moron-Fuenmayor, O.; Gonzalez-Mendez, N. Hydroxyproline measurement by HPLC: Improved method of total collagen determination in meat samples. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2771–2780. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sarabia-Sainz, H.M.; Torres-Arreola, W.; Márquez-Ríos, E.; Santacruz-Ortega, H.C.; Rouzaud-Sández, O.; Valenzuela-Soto, E.M.; Burgara-Estrella, A.J.; Ezquerra-Brauer, J.M. Interrelation of collagen chemical structure and nanostructure with firmness of three body regions of jumbo squid (Dosidicus gigas). Food Biophys. 2017, 12, 491–499. [Google Scholar] [CrossRef]
- Orsini, D.; Valeria, A.; Tironi, M.; Añón, C. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT-Food Sci. Technol. 2011, 44, 1752–1760. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Wang, Q.; Dong, J.; Lu, S.; Lyu, B.; Ma, X. Antioxidant activities of peptides derived from mutton ham, Xuanwei ham and Jinhua ham. Int. Food Res. J. 2021, 142, 110195. [Google Scholar] [CrossRef]
- Mohammadi, M.; Soltanzadeh, M.; Ebrahimi, A.R.; Hamishehkar, H. Spirulina platensis protein hydrolysates: Techno-functional, nutritional and antioxidant properties. Algal Res. 2022, 65, 2211–9264. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Mildenberger, J.; Bruheim, I.; Solibakke, P.; Atanassova, M. Development of a protein concentrate for human consumption by direct enzymatic hydrolysis of antarctic krill (Euphausia superba). LWT-Food Sci. Technol. 2023, 173, 114254. [Google Scholar] [CrossRef]
- Kindleysides, S.; Quek, S.; Miller, M.R. Inhibition of fish oil oxidation and the radical scavenging activity of New Zealand seaweed extracts. Food Chem. 2012, 133, 1624–1631. [Google Scholar] [CrossRef]
- Tenore, G.C.; Ritieni, A.; Campiglia, P.; Stiuso, P.; Di-Maro, S.; Sommella, E.; Pepe, G.; D’Urso, E.; Novellino, E. Antioxidant peptides from “Mozzarella di Bufala Campana DOP” after simulated gastrointestinal digestion: In Vitro intestinal protection, bioavailability, and anti-haemolytic capacity. JFF 2015, 15, 365–375. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Heydari, S.; Hosseini, S.; Mortazavian, A.M.; Taheri, S. Extraction of bioactive peptides produced in probiotic yoghurt and determination of their biological activities. Int. Dairy J. 2023, 139, 105544. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Lin, S.; Cheng, S. Contribution of specific amino acid and secondary structure to the antioxidant property of corn gluten proteins. Int. Food Res. J. 2018, 105, 836–844. [Google Scholar] [CrossRef]
- Mudd, N.; Martin-Gonzalez, F.; Ferruzzi, M.; Liceaga, A.M. In vivo antioxidant effect of edible cricket (Gryllodes sigillatus) peptides using a Caenorhabditis elegans model. FHFH 2022, 2, 100083. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, H.; Su, G.; Zhao, Q.; Zhao, M. Radical scavenging activities of Tyr-, Trp-, Cys-and Met-Gly and their protective effects against AAPH-induced oxidative damage in human erythrocytes. Food Chem. 2016, 197, 807–813. [Google Scholar] [CrossRef]
- Ikken, Y.; Morales, P.; Martinez, A.; Marin, M.L.; Haza, A.I.; Cambero, M.I. Antimutagenic effect of fruit and vegetable ethanolic extracts against N-nitrosamines evaluated by the Ames test. J. Agric. Food Chem. 1999, 47, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Hyun-Taek, O.; Soo-Hyun, K.; Hyun-Jin, C.; Mi-Ja, C.; Seung-Shi, H. Antioxidative and antimutagenic activities of 70% ethanol extract from masou salmon (Oncorhynchus masou). Toxicol. In Vitro 2008, 22, 1484–1488. [Google Scholar] [CrossRef]
- Suárez-Jiménez, G.M.; Burgos-Hernández, A.; Torres-Arreola, W.; López-Saiz, C.M.; Velázquez Contreras, C.A.; Ezquerra-Brauer, J.M. Bioactive peptides from collagen hydrolysates from squid (Dosidicus gigas) by-products fractionated by ultrafiltration. Int. J. Food Sci. Technol. 2019, 54, 1054–1061. [Google Scholar] [CrossRef]
- Pandey, H.; Kumar, V.; Roy, B.K. Assessment of genotoxicity of some common food preservatives using Allium cepa L. as a test plant. Toxicol. Rep. 2014, 1, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Nurilmala, M.; Suryamarevita, H.; Hizbullah, H.; Jacoeb, M.; Ochiai, Y. Fish skin as a biomaterial for halal collagen and gelatin. Saudi J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Saidi, G.S.; Guizani, N. Thermal characterisation of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin. Food Chem. 2008, 108, 472–481. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Rusanova, P.; Bono, G.; Dara, M.; Falco, F.; Gancitano, V.; Lo Brutto, S.; Okpala, C.O.R.; Nirmal, N.P.; Quattrocchi, F.; Sardo, G.; et al. Effect of different packaging methods on the free amino acid profiles of the deep-water rose shrimp (Parapenaeus longirostris) during frozen storage. Front. Nutr. 2022, 9, 955216. [Google Scholar] [CrossRef]
- Derkus, B.; Arslan, Y.; Tahir, A.; Kantarcioglu, I.; Emregul, K.; Emregul, E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sens. Actuators B Chem. 2016, 228, 725–736. [Google Scholar] [CrossRef]
- Giacomelli, C.; Rodrigues, A.; Lima, A.; Mello, R.; Morisso, F.; Prestes-Dornelles, R.C.; Kubota, H.E. Gelatin extracted from jundiá skin (Rhamdia quelen): An alternative to the discarded by-product. Int. Food Res. J. 2022, 161, 111829. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhao, Y.Q.; Wang, Y.M.; Yang, X.R.; Chi, C.F.; Wang, B. Gelatins and antioxidant peptides from Skipjack tuna (Katsuwonus pelamis) skins: Purification, characterization, and cytoprotection on ultraviolet-A injured human skin fibroblasts. Food Biosci. 2022, 50 Pt B, 102138. [Google Scholar] [CrossRef]
- Yang, L.; Yang, m.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Structural and emulsion stabilization comparison of four gelatins from two freshwater and two marine fish skins. Food Chem. 2022, 371, 131129. [Google Scholar] [CrossRef] [PubMed]
- Felix-Felician, F.; Rui-He, Y.; Meng-Zhen, L.; Chun-Jie, L.; Hui-Qin, C.; Ying, J.; Tao, T.; Wei-Yan, Q.; Han-Mei, X. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. CJT 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–333. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Pamungkas, B.F.; Murdiati, A.S.; Indrati, R. Characterization of the acid- and pepsin-soluble collagens from haruan (Channa striatus) scales. Pak. J. Nutr. 2019, 18, 324–332. [Google Scholar] [CrossRef][Green Version]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L.; Fanizzi, F.P. 1H NMR Metabolic Profile of scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in female gonads and somatic tissues: Preliminary results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef]
- Binarová, P.; Tuszynski, J. Tubulin: Structure, functions and roles in disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Application of nanoLC–MS/MS to the shotgun proteomic analysis of the nematocyst proteins from jellyfish Stomolophus meleagris. J. Chromatogr. B 2012, 899, 86–95. [Google Scholar] [CrossRef]
- Hoeksema, M.; Van-Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 2016, 11, 441–453. [Google Scholar] [CrossRef]
| Treatment | Genotoxicity Percentage |
|---|---|
| Negative control | 0 c |
| Sodium azide (10 ng) | 88 a |
| JCG 50 ppm | 3.44 b |
| JCG 100 ppm | 5.17 b |
| Amino Acid | mg Amino Acid/100 g Protein |
|---|---|
| Alanine | 269 |
| Arginine | 359 |
| Aspartic acid | 440 |
| Glutamic acid | 508 |
| Glycine | 1336 |
| Histidine | n.d. |
| Isoleucine | 126 |
| Lysine | 175 |
| Isoleucine | 126 |
| Leucine | 175 |
| Lysine | 224 |
| Phenylalanine | 85 |
| Proline | 345 |
| Serine | 149 |
| Threonine | 190 |
| Tyrosine | 95 |
| Valine | 134 |
| Accession Number | Description | Uni. Pep. | PSM | Cov (%) | Score |
|---|---|---|---|---|---|
| tr|Q7YZL5|Q7YZL5 _AURAU | Tubulin alpha chain (Fragment) OS = Aurelia aurita OX = 6145 PE = 2 SV = 1 | 24 | 28 | 38 | 212.94 |
| tr|U3PBW0|U3PBW0 _AURAU | Beta actin (Fragment) OS = Aurelia aurita OX = 6145 PE = 2 SV = 1 | 14 | 17 | 37 | 187.05 |
| tr|A0A2Z5WH75|A0A2Z5WH75 _9CNID | Actin-2 OS = Aurelia sp. 2017-HT OX = 2136148 PE = 2 SV = 1 | 16 | 19 | 34 | 187.05 |
| tr|Q5VJP9|Q5VJP9 _AURAU | Tubulin beta chain (Fragment) OS = Aurelia aurita OX = 6145 PE = 2 SV = 1 | 20 | 25 | 46 | 113.72 |
| tr|Q5VJP9|Q5VJP9 _AURAU | Histone H3 (Fragment) OS = Aurelia aurita OX = 6145 PE = 3 SV = 1 | 2 | 3 | 9 | 56.75 |
| tr|G9IBZ5|G9IBZ5 _CARAL | Cytochrome c oxidase subunit 1 OS = Carybdea alata OX = 1193083 GN = cox1 PE = 3 SV = 1 | 16 | 44 | 40 | 50.35 |
| tr|V9GWB0|V9GWB0 _CRASO | Collagen type IV OS = Craspedacusta sowerbii OX = 128124 PE = 2 SV = 1 | 15 | 17 | 14 | 38.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esparza-Espinoza, D.M.; del Carmen Santacruz-Ortega, H.; Plascencia-Jatomea, M.; Aubourg, S.P.; Salazar-Leyva, J.A.; Rodríguez-Felix, F.; Ezquerra-Brauer, J.M. Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity. Fishes 2023, 8, 246. https://doi.org/10.3390/fishes8050246
Esparza-Espinoza DM, del Carmen Santacruz-Ortega H, Plascencia-Jatomea M, Aubourg SP, Salazar-Leyva JA, Rodríguez-Felix F, Ezquerra-Brauer JM. Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity. Fishes. 2023; 8(5):246. https://doi.org/10.3390/fishes8050246
Chicago/Turabian StyleEsparza-Espinoza, Dania Marisol, Hisila del Carmen Santacruz-Ortega, Maribel Plascencia-Jatomea, Santiago P. Aubourg, Jesús Aarón Salazar-Leyva, Francisco Rodríguez-Felix, and Josafat Marina Ezquerra-Brauer. 2023. "Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity" Fishes 8, no. 5: 246. https://doi.org/10.3390/fishes8050246
APA StyleEsparza-Espinoza, D. M., del Carmen Santacruz-Ortega, H., Plascencia-Jatomea, M., Aubourg, S. P., Salazar-Leyva, J. A., Rodríguez-Felix, F., & Ezquerra-Brauer, J. M. (2023). Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity. Fishes, 8(5), 246. https://doi.org/10.3390/fishes8050246