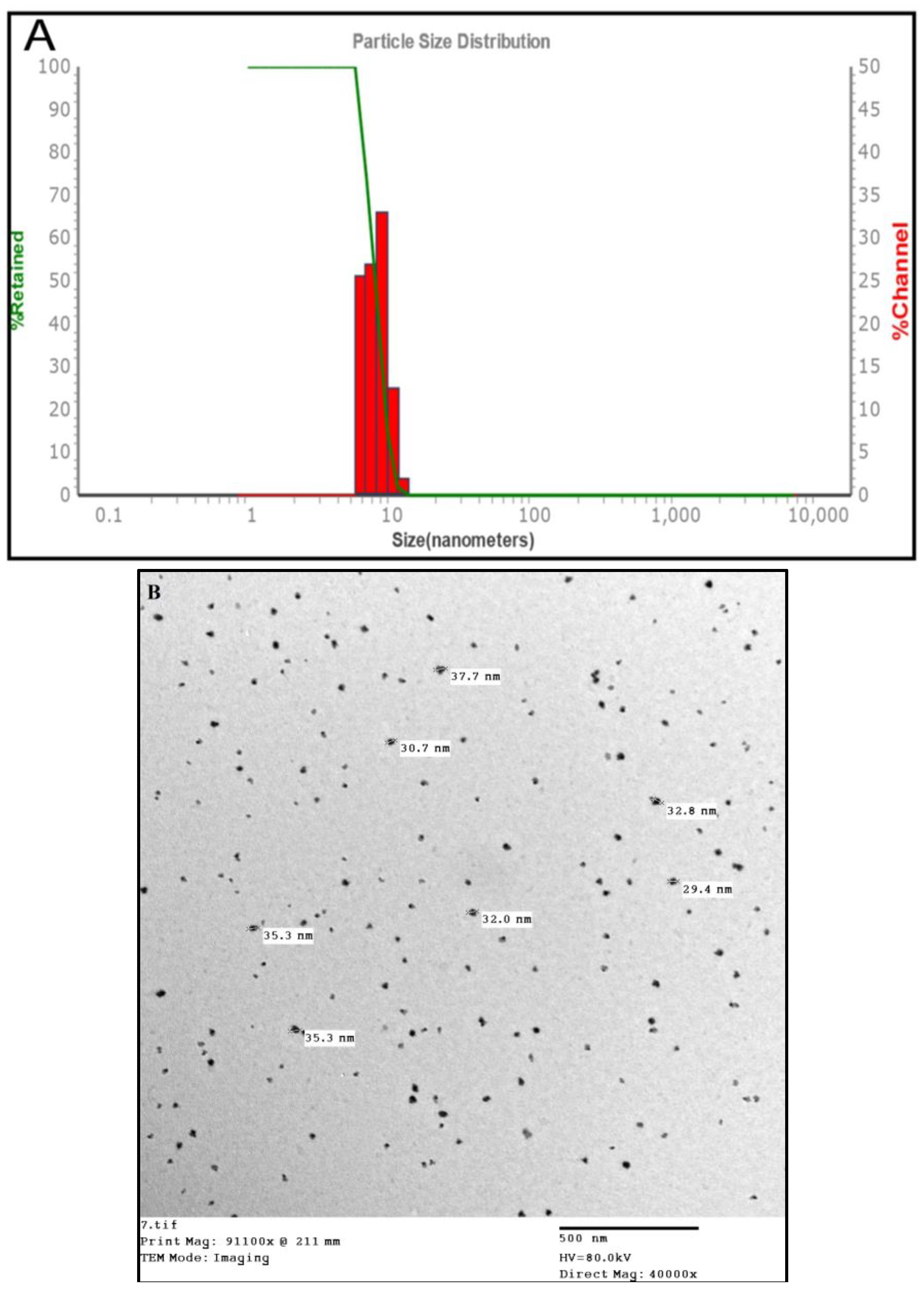
1. Introduction
The widespread use of chemotherapeutic agents to control bacterial diseases of farmed fish is controversial because these agents may have adverse effects on non-target animals, the environment, and human health. Hence, an alternative strategy is necessary to manage bacterial infections and protect fish from such hazards. One such strategy involves reducing reliance on chemotherapeutic agents by using herbal remedies and natural extracts of medicinal plants [
1,
2]. Medicinal plants are excellent sources of antioxidants that can improve fish growth, immunity, and disease control [
3,
4,
5].
Ginger (
Zingiber officinale), a member of the
Zingiberaceae family, is an edible plant, spice, and medicinal herb [
6,
7]. Ginger dry powder contains 3–6% lipid, 9% protein, 60–70% carbohydrates, 3–8% fiber, 2–6% proteases, 1–3% volatile compounds such as gingerol, zingiberol, shogaol, and zingiberene, as well as vitamins A, C, and B3 [
8]. Gingerol is responsible for ginger’s intense aroma and taste, which increases food’s palatability and promotes digestive enzyme secretion [
9,
10]. Ginger contains various bioactive compounds, such as terpenes, oleoresin known as ginger oil, flavonoids, alkaloids, gingerol, paradols, shogaol, zingiberene, and zingerone, which have anti-inflammatory, anticancer, antidiabetic, anticlotting, antiarthritic, and antioxidative properties [
11]. Ginger also has antifungal and antibacterial properties [
12,
13,
14].
Nanotechnology is a rapidly evolving science of small bodies with increased reactivity and solubility and represents a new enabling technology suitable for biomedical, pharmaceutical, and food system applications [
15]. It provides rapid disease detection and improved fish drug absorption. In aquaculture, it is used in fish biotechnology, genetics, and reproduction. Additionally, nanomaterials may inhibit and treat fish diseases [
16,
17]. Numerous studies have shown that ginger nanoparticles enhance antioxidant activity by raising antioxidant enzyme levels and reducing oxidative stress [
18]. Ginger nanoparticles (GNPs) have been readily developed for large-scale manufacture. They are believed to be effective natural therapeutic products for preventing certain bacterial infections such as motile
Aeromonas septicemia and
Edwardsiellosis [
17,
19].
Nile tilapia (
Oreochromis niloticus) is among the most important farmed freshwater fish species due to its nutritional value, rapid growth, and resistance to unfavorable environmental conditions [
20,
21]. Intensification of fish farming leads to outbreaks of bacterial diseases that continue to cause many deaths and significant economic losses [
22].
Aeromonas and
Pseudomonas species are among the bacteria identified as potential disease-causing agents in fish [
23].
Aeromonas hydrophila is one of the most pathogenic bacteria related to diseases in freshwater fishes, especially for
O. niloticus [
24]. This bacterium becomes pathogenic during stressful conditions [
25]. It produces disease signs such as extensive hemorrhages, pale gill color, caudal fin erosion, and ulceration, resulting in high mortality rates and significant economic losses for global aquaculture [
26].
Pseudomonas spp. is another major aquaculture pathogen after
Aeromonas spp. in terms of importance and economic impact [
27].
Pseudomonas putida is a genetically related fluorescent pseudomonad from the
Pseudomonadaceae family of the genus
Pseudomonas. The most severe lesions associated with
P. putida infection in fish are hemorrhagic areas on the skin and fins, exophthalmia (either unilateral or bilateral), ascites, and fin rot [
28]. After
P. putida infection, superficial or deep skin ulcerations were also detected. A post-mortem examination showed a slight enlargement of the liver, spleen, and kidney and engorgement of the intestine with a yellowish fluid [
29,
30].
The current study aimed to compare diets supplemented with conventional GP or GNPs by studying the effects of two doses, 0.5 and 1%, of those ginger derivatives on different growth, immune, and health aspects. The parameters evaluated were growth performance, digestive enzyme (lipase and amylase) activity, blood hematology, blood biochemical indices, immune indices (interleukin 10, immunoglobulin M, nitric oxide, and lysozymes), antioxidant activity, histological characteristics of kidney, spleen, liver, and intestine, and resistance to A. hydrophila or P. putida infection.
4. Discussion
Aquaculture has witnessed a significant increase in phytochemical agents (herbal components) used for various purposes, including growth promotion and disease control. These agents also help to reduce the utilization of hazardous antibiotics [
59,
60]. Furthermore, nanotechnology applications for aquaculture, although a relatively new approach, may, with improved technical innovation, provide tools to deal with most fish management and disease problems [
61,
62,
63].
In the current study, diet enrichment with GP or GNPs improved fish growth, with the highest growth reported in the GNPs1 treatment. These results may indicate greater availability of nutrients for absorption, especially in GNPs TRTs. The growth-promoting effect of GP and GNPs can be attributed to their physicochemical properties and appetizer effect, which promote digestion, fat, and protein metabolism [
12]. In addition, the increased growth hormone level and activity of digestive enzymes (amylase and lipase) reported in the supplemented TRTs would also promote fish growth. In addition to this, GP content of carbohydrates, minerals, vitamins, and other phytochemical compounds enhances animal growth and health [
59]. Ginger rhizomes contain proteinase, which improves protein digestion and amino acid absorption [
64]. Ginger also enhances the growth of microbial flora in the intestine [
65]. The most significant growth was reported in the GNPs1 TRT, which can be explained by the enhanced bioavailability of nutrient nanoparticles and their high absorption, given their efficient entry into cells [
66]. It was reported that growth rates of common carp (
Cyprinus carpio) fingerlings were significantly increased after consuming diets containing ginger nanoparticles [
17]. Moreover, Ude et al. [
59] proposed that dietary supplementation with 1% ginger boosted the growth performance of African catfish (
Clarias gariepinus). Accordingly, Korni and Khalil [
17] hypothesized that adding GP and GNPs supplements to the diet of
Cyprinus carpio fingerlings improved growth and FCR. Mohammadi et al. [
67] reported improved weight gain, FCR, and RGR in common carp that were fed diets supplemented with ginger extract (0.1, 0.2, 0.4%), especially those that received 0.2% ginger extract. The improvement of fish growth by ginger has also been reported for
Lates calcarifer fed 1% dried ginger plant for 15 days [
12],
Oreochromis niloticus fed 0.5% ginger essential oil for 55 days [
68], and
Labeo rohita fed 0.6–1% dried ginger plant for 60 days [
69].
The present study showed increased total protein, growth hormone, and digestive enzyme activities in the supplemented groups. El-Sebai et al. [
31] showed that ginger enhances protein and fat metabolism by increasing digestive enzyme secretion, improving digestion and absorption rates, and reducing pathogenic bacteria’s hazardous effects. Moreover, the phenolic compounds present in ginger, such as gingerols, paradols, and shogaols, enhance the secretion of pancreatic and intestinal lipase enzymes. This action facilitates fat digestion and absorption and stimulates protein synthesis [
70]. Korni and Khalil [
17] indicated improved growth and immunity by adding GP and GNPs to
Cyprinus carpio fingerlings diets. Swain et al. [
71] demonstrated that supplementing striped catfish (
Pangasianodon hypophthalmus) diets with ginger promotes their growth performance.
Hematological parameters provide information on the animal’s health status [
72]. In addition, leukocytes are essential for the innate immune system, and their count allows for the assessment of fish health and immunity [
59]. The present study showed an improvement in the erythrogram and leukogram of fish fed diets enriched with GP or GNPs, particularly for GP1 and GNPs1 groups, indicating the immunostimulant properties of these ginger derivatives. Low levels of erythrocytes and hemoglobin are closely associated with impairment of the antioxidant system [
73,
74]. Hoseini et al. [
75] established that exposure to ammonia causes oxidative stress by reducing the activities of antioxidant enzymes, causing anemia in
Cyprinus carpio. Ginger is a powerful natural antioxidant [
76] whose supplementation in the diet may protect RBCs from hemolysis by free radicals, thereby extending their lifespan. The increase in RBC count found in the current study is consistent with the reported antioxidant properties of GP and GNPs that protect RBC membranes from degradation [
77]. Ginger is a source of phytochemical constituents such as phytate, oxalate, saponin, and tannin, as well as aromatic components which enhance the antioxidant and immune responses of fish [
59]. Both GP and GNPs supplementation increases circulating hemoglobin levels in fish, consequently enhancing oxygen transport and improving health by stimulating growth and immune response [
63,
72]. In addition to this, the elevation of erythrocytes and hemoglobin could be due to the effect of ginger on hematopoiesis [
78]. Positive effects of ginger supplementation on erythrocytes and hemoglobin have also been conveyed in earlier studies on
Huso huso [
79],
Oncorhynchus mykiss [
80], and
Lates calcarifer [
12]. The study by Mohammadi et al. [
67] showed an increase in the erythrocyte and leucocyte count, as well as hematocrit and hemoglobin values, in common carp that received diets supplemented with ginger extract (0.1, 0.2, 0.4%). Udoh et al. [
81] proved that high WBC counts, mainly due to increased lymphocytes and other phagocytes, enable fish to resist stressful conditions and infections. The improvement in hematological parameters can be explained by the presence of bioactive compounds in ginger, such as gingerols, zingerone, quercetin, paradols, shogaols, gingerenone-A, 6-dehydrogingerdione, β-bisabolene, zingiberene, α-farnesene, and β-sesquiphellandrene [
6]. Furthermore, GNPs stimulate the production of anti-inflammatory cytokines while reducing that of pro-inflammatory cytokines [
6]. GNPs are more stable than GP, preventing active component degradation and improving their effectiveness [
82]. Korni and Khalil [
17] indicated that ginger nanoparticles produced greater stimulation of
Cyprinus carpio fingerlings’ immune response than ginger particles. Sharif Rohani et al. [
83] stated that
aloe vera extract nanoparticles promote and boost the immune response of Siberian sturgeon (
Acipenser baerii).
Evidence demonstrated that ginger has strong antioxidant properties by boosting the action of several antioxidant enzymes and lowering reactive oxygen species (ROS) levels, which are responsible for the onset of many chronic diseases. It also reduces the number of free radicals, the precursors for lipid peroxidation [
84]. This study revealed that fish fed diets supplemented with GP and GNPs presented a significant improvement in SOD, CAT, and GSH values, confirming the powerful antioxidant effect of the supplements. This effect can be attributed to the high content of polyphenolic compounds in ginger, including [
6]-gingerol, paradols, shogaols, and diarylheptanoids, which have potent antioxidant properties [
85]. GNPs are believed to have more powerful antioxidant properties than ginger extract. In particular, shogaols regulate numerous antioxidants and the detoxication of enzymes’ genetic expression [
84,
86]. The antioxidant activity of ginger have been reported by Ahmadifar et al. [
9] in zebrafish, Sukumaran et al. [
69] in
Labeo rohita fingerlings, and Fazelan et al. [
87] in
Cyprinus carpio.
Lysozyme plays a crucial role in the immune system of fish, as it effectively lyses peptidoglycans present in bacterial cell walls. Furthermore, it stimulates phagocytosis and the complement system [
88]. Nitric oxide has been shown to have powerful bactericidal properties [
89]. The soluble IgM fraction has potent immunostimulatory activity [
31]. IL-10, a cytokine associated with the adaptive immune system, protects tissues by decreasing the production of pro-inflammatory cytokines and eliminating excessive inflammation [
70]. The present study has observed significant improvements in lysozyme, nitric oxide, IL-10, and IgM levels in the groups that received diets enriched with GP or GNPs. This finding indicates an enhancement of the fish immune system. El-Sebai et al. [
31] showed that ginger supplementation in the
O. niloticus diet boosted its immunity. Hence, the enhanced immune response may be attributed to phytochemical components (gingerol, paradols, and shogaols) and aromatic compounds present in ginger that stimulate antioxidant activity and immune functions in fish [
6]. Mohammadi et al. [
67] also reported the immunostimulant effect of ginger extract at 0.2 and 0.4% in
Cyprinus carpio, indicated by increased lysozyme activity and immunoglobulin levels. Fazelan et al. [
87] exhibited that ginger supplementation at 10 g kg
−1 diet led to a significant increase in serum Ig in
Cyprinus carpio exposed to high stocking-density stress, suggesting that dietary ginger may stimulate immune cells leading to enhanced immunity against harmful agents and stress.
Furthermore, our results indicated that GNPs have a more potent immunostimulatory effect than GP, which may be due to a smaller nanoparticle size and, therefore, a large exposed surface area, promoting greater solubility and bioavailability of active constituents [
62,
63]. Korni and Khalil [
17] suggested that supplementing
Cyprinus carpio fingerling diets with GNPs boosts their growth and immunity more than GP. This can be attributed to the nanoextraction of ginger, causing alterations in its characteristics. Zhang et al. [
90] revealed that GNPs have a potent anti-inflammatory action by stimulating anti-inflammatory cytokines and reducing the concentrations of pro-inflammatory cytokines.
The present study showed that GP and GNPs supplementation increased resistance against
A. hydrophila and
P. putida infections. Ginger was reported to have potent antibacterial properties since it contains several bioactive substances such as gingerenone A, 6-shogaol, tannins, and saponins. These substances inhibit the growth of various Gram-positive and Gram-negative bacteria by suppressing bacterial biofilm formation and causing lysis of the bacterial cell wall [
6]. Payung et al. [
13] showed that dietary ginger supplementation promotes bacterial resistance of Nile tilapia to
Aeromonas hydrophila. These findings matched those of Bakr et al. [
86], who stated that ginger nanoparticles suppress the growth of a wide range of bacteria, including
Salmonella typhimurium,
Escherichia coli,
Staphylococcus aureus, and
Pseudomonas aeruginosa.Enrichment of Nile tilapia diets with GP or GNPs improves liver function as indicated by reduced ALT and AST levels. The hepatoprotective effect of medicinal plants is primarily associated with their antioxidant activity, which prevents hepatic tissue damage [
37]. This protective effect may be due to the bioactive ingredients in ginger, such as shogaols and gingerols, which act as free-radical scavengers preventing lipid peroxidation of hepatic cell membranes and, consequently, hepatic damage [
85]. Abd-Elrhman et al. [
84] stated that GNPs have a potent hepatoprotective effect against carbon tetrachloride (CCL
4), which cause liver fibrosis in rats by decreasing the levels of ALT and AST enzymes. El-Sebai et al. [
31] proved that supplementing the diet of Nile tilapia with ginger has a potent antioxidant effect and hepatoprotective function by preventing lipid peroxidation of cell membranes. Furthermore, dietary supplementation with GP or GNPs reduced serum urea and creatinine levels in the current study.
Regarding the histological examination of the spleen, liver, and kidney, fish fed the experimental diets showed the same typical histoarchitecture and morphology as the control group, indicating that dietary enrichment with GP or GNPs had no harmful effects on the internal organs of fish. These results confirmed the results of blood biochemistry (tests for liver and kidney function evaluation). It is well known that gut functions are crucial for nutrient digestion and absorption. Therefore, good and adequate growth depends mainly on the intestine’s condition. The villus of the digestive tract constitutes a vital sign of how effectively fish absorb nutrients [
91]. Our results revealed that both ginger supplements, especially GNPs, enhance the mucosal-associated lymphoid tissue and the count of wandering eosinophilic granular cells. They also increase the values of all measured morphometric parameters (villus height, width, and surface area). The increase in villus height and width leads to a greater intestinal absorption area in fish fed the different experimental diets, consequently improving growth [
92].