Non-Linear Analyses of Fish Behaviours in Response to Aquatic Environmental Pollutants—A Review
Abstract
:1. Introduction
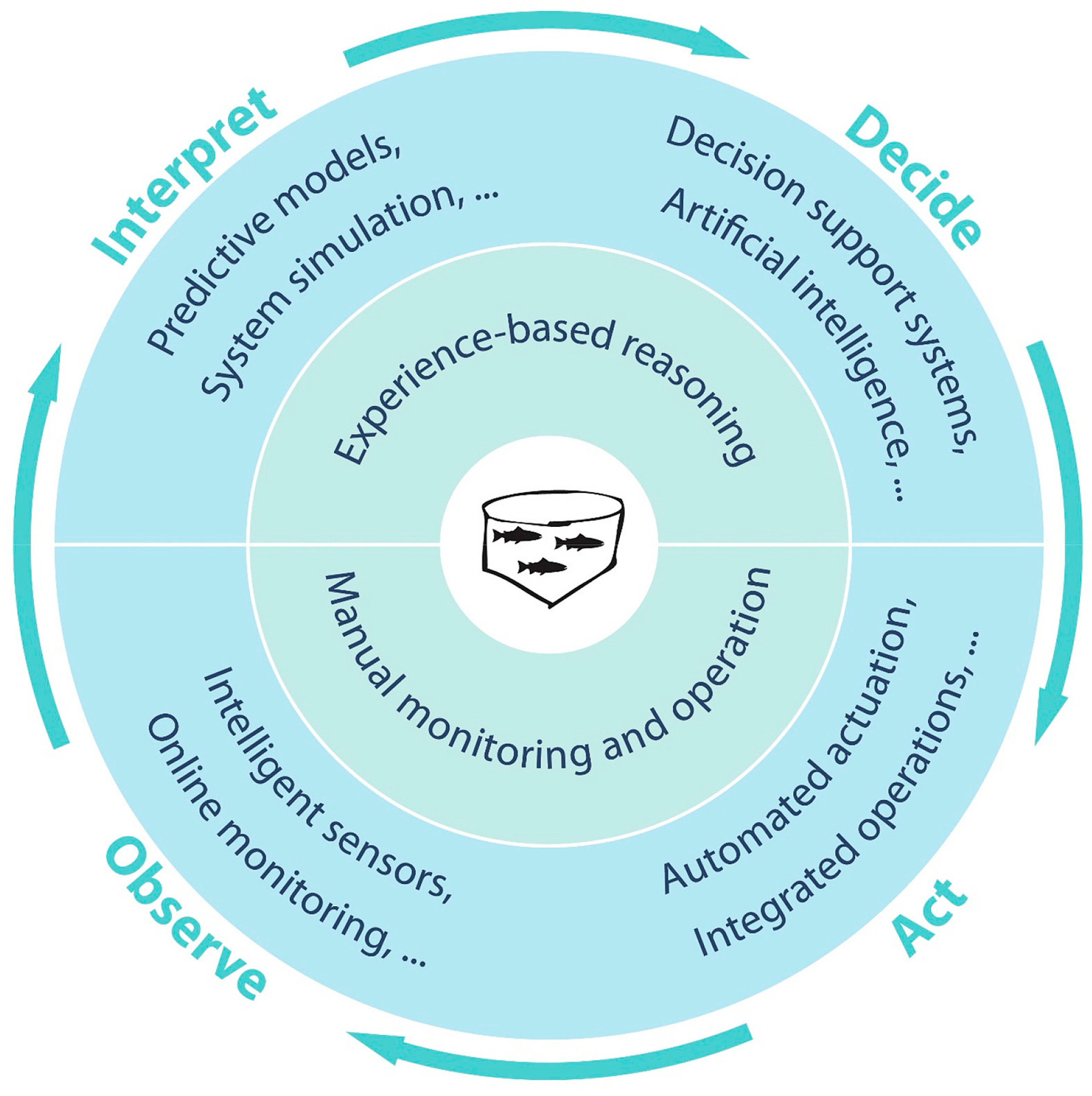
1.1. Precision Fish Farming
1.2. Contaminants as Stressors Influencing Fish Behaviour
1.3. Fractal Dimension (FD) and Entropy Properties in Biological Systems
1.4. Aim of the Work
2. Materials and Methods
3. Fractal Dimension and Entropy Analyses of Fish Behavioural Parameters
3.1. Effects of Exposure to Toxic Compounds and to Cleaning and Disinfecting Agents
3.2. Effects of Exposure to Stimulants, Anaesthetics and Antibiotics
3.3. Effects of Exposure to Heavy Metals
3.4. Effects of Exposure to Pesticides and to Persistent Organic Environmental Pollutants
4. General Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | Species | Method | Subject of the Study | Main Findings |
|---|---|---|---|---|
| Review Papers | ||||
| [43] | Review paper. Bacteria, algae, Daphnia, bivalves, fish and multispecies systems. | The methodology followed to perform the review is not stated. | Review of behavioural monitoring and computational methods as potential BEWSs to monitor drinking water. Continuous detection of many pollutants for effective water quality monitoring and management. | The authors review several computational and mathematical methods that have been widely used to study the structural characteristics of behavioural changes. The methods include algorithms for signal processing (e.g., permutation entropy, fractal dimension, Fourier transform and wavelet transformation) and for interpretation, i.e., machine learning (e.g., multilayer perceptron, self-organizing map, and hidden Markov model). |
| [77] | Review paper. Addresses examples with many different species. | The methodology followed to perform the review is not stated. | BEWS—Review paper of the technical issues involved in 2D and 3D video tracking of fish and the computational analyses (Fourier and wavelet transforms, fractal analysis and permutation entropy) and of machine learning techniques (multilayer perceptron, self-organizing map, hidden Markov model and deep learning) with clear potential to detect abnormal behavioural patterns and toxicological monitoring. | Video tracking methods need to be improved for reliable, real-life toxicity monitoring. All the computational and machine learning analyses considered have shown acceptable results to detect abnormal behaviours induced by toxic substances. |
| Toxic compounds, cleaning and disinfecting agents | ||||
| [78] | Japanese medaka (Oryzias latipes) | Three-dimensional (3D) video recording of the fish and calculation of their speed, vertical position in the test chamber and the Shannon–Weaver entropy [55] of both parameters. | Testing the entropy of swimming behavioural parameters of individual adult fish for the early detection of lethal doses of cyanide (KCN 10 mg/mL) and phenol (25 mg/mL) in water. | There were large individual differences. A significant decrease in the entropy of vertical position of the fish was noted after 10–30 min exposure to the toxicants and prior to any mortality. |
| [79] | Zebrafish (Danio rerio) | FD of the swimming trajectory of individual fish in a tank via 3D coordinate computation with perspective correction (3DCCPC), carried out using two video cameras. | Individual male behavioural responses of zebrafish to different sublethal concentrations of sodium hypochlorite. | The FD of the swimming trajectories trended to increase with increasing pH (i.e., increasing sodium hypochlorite concentration). |
| [80] | Zebrafish (D. rerio), Japanese medaka (Oryzias latipes) and red carp (Cyprinus carpio). | Quantification of swimming behavioural changes induced upon exposure to a common detergent (sodium dodecyl benzene sulfonate, SDBS) by three parameters: swimming trail, swimming speed, surface behaviour and their uniformity assessed by their Shannon–Weaver entropy. | Assessment of the uniformity of individual fish swimming behavioural responses to different concentrations of SDBS. | The entropy of the three species decreased (i.e., their behaviour became less “normal”) with increasing concentrations of the detergent, but with different sensitivities: zebrafish was the most sensitive species, responding at the lowest concentration, followed by red carp and medaka, which was the most resistant. |
| Stimulants, anaesthetics and antibiotics | ||||
| [81] | Zebrafish (D. rerio) | The interaction between the fish and a replica by transfer entropy. | Effect of caffeine on the interaction between individual zebrafish and a replica of a shoal of conspecifics. | The transfer entropy was always higher from the replica to the fish but only in fish exposed to at least 25–50 mg caffeine/mL the difference was significant. |
| [82] | Mummichog killifish (Fundulus heteroclitus) | Velocity, total distance travelled, angular change, percent movement, space utilization, and path complexity (FD). | In-tank modelling of alterations in individual fish behaviour exposed to contaminants and stresses of the system, namely a reference toxicant (tricane methanesulfonae (m-aminobenzoic acid ethyl ester methansulfonate, MS222) | Development of a remotely controlled transportable system to detect sublethal stress- and contaminant-induced behaviour in fish. |
| [59] | Golden zebrafish (D. rerio) | 3D video recording, idTracking the trajectories and analysis of swimming, exploratory behaviour, fractal dimension and permutation entropy of the behavioural data, and PCA and hierarchical clustering of the FD and entropy data. | Analysis of the effects on the behaviour of fish in shoals of seven adult and mixed-gender fish after exposure to 20 antibiotics from eight classes. | Only azithromycin, cefuroxime, doxycycline and norfloxacin did not cause alterations in the fish behaviour at the tested 100 ppb. All other antibiotics did cause changes in behaviour. Amoxicillin, trimethoprim, and tylosin caused alterations even at 1ppb. |
| Heavy metals (Pb, Cu, Hg) and selenium (Se) | ||||
| [83] | Fathead minnows (Pimephales promelas) | Video recording their behaviour, calculating four individual specific reproductive behaviours and their fractal dimension. | Effect of the exposure of immature male–female pairs to sub-lethal concentrations of Pb on their individual specific reproductive behaviour. | Exposure induces less complexity (lower FD) in the fishes’ behavioural sequences, but only prior to secondary sexual characters being evident. |
| [94] | Medaka (O. latipes) | Video recording of individual and group (n = 4) responses (speed, Y-position, stop number, stop duration, turning rate and meandering) their multi-layer perception and FD. and their upon exposure to Cu 1 mg/L. | Effect of the exposure to sub-lethal concentrations of Cu on individual (MLP and FD) and collective (FD) behavioural parameters to assess their usefulness to detect the contaminant. | Exposure induces less complex behaviour and significantly decreases the FD in both groups and individual behaviours but the response is more consistent when analysing groups of fish. |
| [49] | European seabass (Dicentrarchus labrax). | The FD and SE of the trajectory following the response to a stochastic event of fish treated with MeH+ 4 μg m/L for 9 days. | Changes in the shoals’ behaviour in response to an event (FD and SE) upon exposure to MeHg+ in the water. | The Katz–Castiglioni FD and particularly the SE were the most sensitive algorithms to discriminate the responses of MeHg+-contaminated fish, indicating a potential value to develop a non-invasive method for the identification and quantification of behavioural differences. |
| [85] | European seabass (D. labrax) | SE of the schooling behaviour during recovery after exposure to MeHg+ 4 μg m/L for 9 days. | Quantification of the changes in the shoals’ behaviour by its SE, after exposure to MeHg+ in the water. | During the 11 days post-exposure period, the SE of the control fish trended to increase, while the SE of MeHg+-treated fish did not show a recovery trend. |
| [84] | European seabass (D. labrax) | SE of the shoaling and schooling behaviour after exposure to (Na2SeO3) 10 μg/L for 6 days. | Changes in the shoals’ behaviour (by SE) upon exposure to sodium selenite (Na2SeO3) in the water for 6 days. | The SE of the schooling response of the exposed group was only slightly lower than that of the control group. |
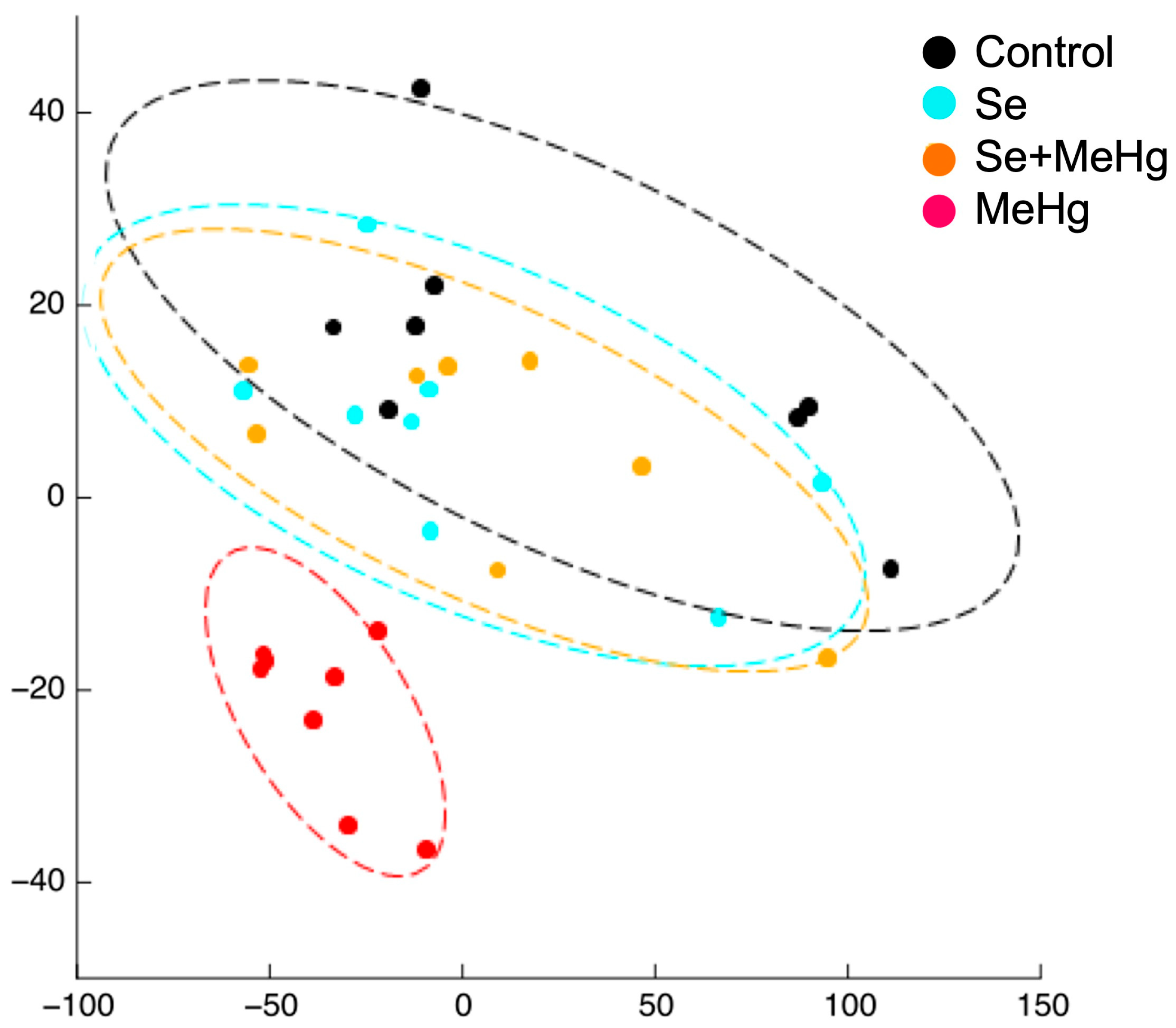
| [42] | European seabass (D. labrax) | SE of the video recorded trajectory of the shoal of fish fed with Se:Hg molar ratios of 29.5, 6.6, 0.8 and 0.4 for 14 days. | Testing the effect of feeding the fish different molar Se:Hg ratios on the SE of their trajectory. | The basal SE of fish fed with molar Se:Hg > 1 trended to increase. The basal SE of fish fed with molar Se:Hg < 1 tended to decrease. |
| [86] | Zebrafish (D. rerio) | Video recording of school behavioural responses and calculation of the Shannon–Weaver entropy of eight parameters after a 24 h exposure to a low dose (0.05 mg/L) of mercuric chloride (HgCl2). | Analyses of linear and non-linear measurements of the collective behaviour of exposed fish. | The use of eight parameters (the entropy of swimming speed, depth during each 3 min interval, and changes in the sum entropy of speed, depth, turning frequency, distance and dispersion) was optimal to detect low levels of the HgCl2 in 15–20 min. |
| [87] | Freshwater shrimps (Macrobrachium jelskii) | Video recording of individual shrimps’ behaviour in groups of three animals, exposed to HgCl2 10 μg/L. | Testing whether mathematical (linear parameters) and non-linear (fractal, information entropy and multifractal parameters) methods applied to video tracking of shrimps exposed to HgCl210 μg/L can adequately describe changes in their locomotion behaviour. | None of the methods detected the effect of a 96 h exposure to 10 μg/L mercuric chloride on either linear or non-linear locomotion parameters. |
| Pesticides and persistent environmental pollutants | ||||
| [87] | Freshwater shrimps (Macrobrachium jelskii) | Video recording of individual shrimps’ behaviour in groups of three animals, exposed 0.15 μg/L of the pesticide deltamethrin. | Testing whether mathematical (linear parameters) and non-linear (fractal, information entropy and multifractal parameters) methods applied to video tracking of shrimps exposed to 0.15 μg/L of the pesticide deltamethrin can adequately describe changes in their locomotion behaviour. | 72 h of exposure to 0.15 μg/L of deltamethrin altered the values of some linear (e.g., the track length) and non-linear (fractal dimension (box counting or information entropy) and multifractal analysis) parameters of their locomotion behaviour. |
| [88] | Japanese medaka (O. latipes) | Resting, swimming (in a straight line and in circles) and SE of individual fish placed in pairs in the tanks and exposed to tributyltin (TBT), polychlorinated biphenyls (PCBs), or a mixture (at 1 µg/g bw/day) of each for 3 weeks. | Changes in the behaviour of individual fish reflected in the SE of the mean swimming velocity and the position of individual fish estimated from data on the resting, swimming in a straight line and swimming in circles. | PCBS induced swimming patterns consistent with hyperactivity and TBT increased the entropy of fish’s position. |
| [89] | Japanese medaka (O. Latipes) | Video recording of the swimming trajectories of medaka fed (at 3% of bw) the PCB Kanechlor-400 during 3 weeks in doses up to 125 µg/g feed. | Effects of Kanechlor-400 on the collective behaviour of groups of three sexually immature of fish and in groups of mixed-treated and untreated fish. Analysis of the fractal dimension of the trajectory and the fractal dimensions of swimming velocity and turning angle. | Kanechlor-400 induced a shortened schooling time, increased frequency of behavioural pattern change changed, disintegration of schools and increased the frequency of collisions (hyperactivity). When mixed in the same group, Kanechlor 400-exposed medaka influenced the behaviour of unexposed fish in the same school. FDs of the swimming trajectory and turning angle significantly increased but only in the highest PCB-exposed group. |
| Reference | Toxicant | FD/Entropy | Individual/Collective Behaviour | Modification upon Exposure |
|---|---|---|---|---|
| Fractal dimension analyses | ||||
| [79] | Sublethal NaClO and pH | FD of swimming trajectories and FD of swimming velocities. | Individual | The FD of the trajectories increased with pH while the FD of the velocities increased with NaClO. |
| [89] | 3 weeks exposure to the PCB Kanechlor-400 in the feed (3% of body wt and doses up to 125 µg/g feed). | Effects of Kanechlor-400 on the several parameters of collective behaviour of groups of three sexually immature of fish. The fractal dimension of the trajectory and the fractal dimensions of swimming velocity and turning angle. | Collective (n = 3 immature fish) | The FDs of the swimming trajectory and turning angle increased significantly, but only in the highest PCB-exposed group. |
| [87] | Up to 96 h exposure to HgCl2 (10 μg/L). | Track length, speed and D2P as well as the non-linear fractal dimension, box counting or information entropy and multifractal analysis methods. | Individual behaviour in groups (n = 3) | The multifractal nature of locomotion was initially significantly higher in HgCl2-treated fish. It decreased later to the same values as the control. Other parameters did not change. |
| [82] | MS222 | Individual | The FD decreased. | |
| [83] | Exposure to 0.5 ppm Pb | FD of specific reproductive behaviours. | Individual behaviour in groups (n = 2) | The FD decreased (decreased the complexity of the behavioural reproductive sequences) but only before secondary sexual characters were evident. |
| [94] | Cu, sublethal | FD (individual and group) of speed, Y-position, stop number, stop duration, turning rate and meandering. | Individual and collective (n = 4) | The FD decreased for both individual and collective responses. The FD of group responses were less variable than those of individual fish. |
| [87] | Up to 96 h exposure to deltamethrin (0.15 μg/L). | Track length, speed and D2P as well as the non-linear fractal dimension (estimated via box counting and information entropy) and multifractal analysis methods. | Individual behaviour in groups (n = 3) | The fractal and multifractal dimensions of the behaviour decreased after 72 h of exposure. |
| Fractal and Entropy analyses | ||||
| [59] | Acute exposure to 20 antibiotics from 8 families. | FD and entropy of the swimming trajectory of the collective response. | Collective (n = 7) | The FD decreased upon exposure to amoxicillin, penicillin and tylosin (the most effective antibiotics). Permutation entropy decreased with oxytetracycline and increased with amikacin. |
| [49] | MeHg+ 4 μg /L | The Higuchi FD [96], Katz FD [97] Katz- Castiglioni FD [98] and SE [54] and Permutation entropy [99] of the trajectory following the response to a stochastic event of fish intoxicated with MeHg+ in the water. | Collective (n = 81, 41) | Upon exposure to MeHg+, the Higuchi FD suffered a small decrease.; Katz FD no change and Katz–Castiglioni FD trended to increase; the SE clearly decreased and permutation entropy showed only a small decrease. |
| Entropy analyses | ||||
| [81] | Caffeine up to 50 mg/mL | Transfer entropy between a zebrafish to a replica shoal of zebrafish. | Individual | The transfer entropy from the replica to the alive fish increased. |
| [88] | 3 weeks exposure to tributyltin (TBT), polychlorinated biphenyls (PCBs), or a mixture (at 1 µg/g bw/day of each chemical). | The SE of the mean swimming velocity and the position of individual fish estimated from data on the resting, swimming in a straight line and swimming in circles behaviours of individual fish. | Individual behaviour in groups (n = 2) | The SE of TBT- and PCB-treated fish increased, only the former did so significantly. |
| [85] | 11 days recuperation after exposure to MeHg+ 4 μg /L | SE of the control and MeHg+ exposed fish groups, stressed by halting the water flow during the experiment in both tanks. | Collective (n = 26 and n = 19) | The SE trended to increase in control fish group and to decrease slightly in the treated group. |
| [42] | 14 days exposure to feeds containing Se:Hg molar ratios > 1 (29.6 and 6.6) and <1 (0.8 and 0.4). | SE of the shoaling (basal) and schooling (response) behaviours of the group of fish. | Collective (n = 7) | The basal SE of fish fed with molar Se:Hg > 1 trended to increase. The basal SE of fish fed with molar Se:Hg < 1 trended to decrease. |
| [86] | 24 h exposure to HgCl2 (50 μg/L mg/L). | Eight parameters (the entropy of swimming speed, depth during each 3 min interval, and changes in the sum entropy of speed, depth, turning frequency, distance and dispersion) was optimal to detect low levels of the contaminant in 15–20 min. | Collective (n = 5) | Shannon–Weaver entropy displayed stable values over the pre-exposure period, a sudden significant increase upon the addition of HgCl2 for 15 min and then a fast decrease to low values. |
| [78] | Lethal concentrations of phenol and KCN. | SE of the vertical position of the fish. | Individual | The SE decreased with increasing amounts of toxicant. |
| [80] | Detergent SDBS | Shannon–Weaver entropy of swimming trail and speed and their surface behaviour. | Individual | The Shannon–Weaver entropy decreased with increasing concentrations of the detergent with species-specific sensitivities: zebrafish was the most sensitive of the three, followed by red carp and medaka. |
| [84] | Na2SeO3, 10 μg/L, 6 days | SE of the schooling responses of control and treated groups. | Collective (n = 76) | The SE of the treated group was only slightly lower than the SE of the control. |
References
- FAO. The State of World Fisheries and Aquaculture 2022. SOFIA. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Stentiford, G.D.; Sritunyalucksana, K.; Flegel, T.W.; Williams, B.; Withyachumnarnkul, B.; Itsathitphaisarn, O.; Bass, D. New Paradigms to Help Solve the Global Aquaculture Disease Crisis. PLoS Pathog. 2017, 13, e1006160. [Google Scholar] [CrossRef] [PubMed]
- Suryan, R.M.; Arimitsu, M.L.; Coletti, H.A.; Hopcroft, R.R.; Lindeberg, M.R.; Barbeaux, S.J.; Batten, S.D.; Burt, W.J.; Bishop, M.A.; Bodkin, J.L.; et al. Ecosystem response persists after a prolonged marine heatwave. Sci. Rep. 2021, 11, 6235. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Anti-anxiety drug makes river fish more aggressive. Nature 2013, 815, 814–815. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- EATiP. A Review of the Strategic Research and Innovation Agenda. Our Vision for the Future of European Aquaculture. European Aquaculture Technology and Innovation Platform 2017. Available online: https://eatip.eu/wp-content/uploads/2018/02/EATIP-SRIA-2017.pdf (accessed on 11 May 2023).
- Science Advice for Policy by European Academies (SAPEA). A Sustainable Food System for the European Union; SAPEA: Berlin, Germany, 2020; Available online: https://www.sapea.info/wp-content/uploads/sustainable-food-system-report.pdf (accessed on 11 May 2023)ISBN 978-3-9820301-7-3.
- European Commission; European Innovation Council and SMEs Executive Agency. Identification of Emerging Technologies and Breakthrough Innovations. Lopatka, M., Pólvora, A., Manimaaran, S., Eds.; Publications Office of the European Union: Luxenbourgh, 2022. [Google Scholar]
- European Commission 2020 Strategic Foresight Report. Charting the Course Towards a More Resilient Europe; 2020. Available online: https://commission.europa.eu/system/files/2021-04/strategic_foresight_report_2020_1_0.pdf (accessed on 11 May 2023).
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Føre, M.; Frank, K.; Norton, T.; Svendsen, E.; Alfredsen, J.A.; Dempster, T.; Eguiraun, H.; Watson, W.; Stahl, A.; Sunde, L.M.; et al. Precision fish farming: A new framework to improve production in aquaculture. Biosyst. Eng. 2018, 173, 176–193. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabrera-Álvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Rev. Aquac. 2021, 14, 704–728. [Google Scholar] [CrossRef]
- Barreto, M.O.; Planellas, S.R.; Yang, Y.; Phillips, C.; Descovich, K. Emerging indicators of fish welfare in aquaculture. Rev. Aquac. 2021, 14, 343–361. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Du, L.; Zheng, Y.; Wang, Z. Recent advances in intelligent recognition methods for fish stress behavior. Aquac. Eng. 2021, 96, 102222. [Google Scholar] [CrossRef]
- Marques, T.A.; Thomas, L.; Martin, S.W.; Mellinger, D.K.; Ward, J.A.; Moretti, D.J.; Harris, D.; Tyack, P.L. Estimating animal population density using passive acoustics. Biol. Rev. 2012, 88, 287–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Føre, M.; Svendsen, E.; Alfredsen, J.; Uglem, I.; Bloecher, N.; Sveier, H.; Sunde, L.; Frank, K. Using acoustic telemetry to monitor the effects of crowding and delousing procedures on farmed Atlantic salmon (Salmo salar). Aquaculture 2018, 495, 757–765. [Google Scholar] [CrossRef]
- Rose, C.S.; Stoner, A.W.; Matteson, K. Use of high-frequency imaging sonar to observe fish behaviour near baited fishing gears. Fish. Res. 2005, 76, 291–304. [Google Scholar] [CrossRef]
- Gesto, M.; Zupa, W.; Alfonso, S.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Using acoustic telemetry to assess behavioral responses to acute hypoxia and ammonia exposure in farmed rainbow trout of different competitive ability. Appl. Anim. Behav. Sci. 2020, 230, 105084. [Google Scholar] [CrossRef]
- Halvorsen, M.B.; Zeddies, D.G.; Ellison, W.T.; Chicoine, D.R.; Popper, A.N. Effects of mid-frequency active sonar on hearing in fish. J. Acoust. Soc. Am. 2012, 131, 599–607. [Google Scholar] [CrossRef]
- Kruusmaa, M.; Gkliva, R.; Tuhtan, J.A.; Tuvikene, A.; Alfredsen, J.A. Salmon behavioural response to robots in an aquaculture sea cage. R. Soc. Open Sci. 2020, 7, 191220. [Google Scholar] [CrossRef] [Green Version]
- Kruusmaa, M.; Rieucau, G.; Montoya, J.C.C.; Markna, R.; Handegard, N.O. Collective responses of a large mackerel school depend on the size and speed of a robotic fish but not on tail motion. Bioinspiration Biomim. 2016, 11, 056020. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Liu, J.; Wang, H.; Zhu, J.; Li, D.; Zhao, R. Application of machine learning in intelligent fish aquaculture: A review. Aquaculture 2021, 540, 736724. [Google Scholar] [CrossRef]
- Gladju, J.; Kamalam, B.S.; Kanagaraj, A. Applications of data mining and machine learning framework in aquaculture and fisheries: A review. Smart Agric. Technol. 2022, 2, 100061. [Google Scholar] [CrossRef]
- Saberioon, M.; Gholizadeh, A.; Cisar, P.; Pautsina, A.; Urban, J. Application of machine vision systems in aquaculture with emphasis on fish: State-of-the-art and key issues. Rev. Aquac. 2016, 9, 369–387. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Yu, H.; Fang, X.; Song, L.; Li, D.; Chen, Y. Computer Vision Models in Intelligent Aquaculture with Emphasis on Fish Detection and Behavior Analysis: A Review. Arch. Comput. Methods Eng. 2021, 28, 2785–2816. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Q.; An, D. Intelligent monitoring and control technologies of open sea cage culture: A review. Comput. Electron. Agric. 2020, 169, 105119. [Google Scholar] [CrossRef]
- Sadoul, B.; Mengues, P.E.; Friggens, N.; Prunet, P.; Colson, V. A new method for measuring group behaviours of fish shoals from recorded videos taken in near aquaculture conditions. Aquaculture 2014, 430, 179–187. [Google Scholar] [CrossRef]
- Deakin, A.G.; Spencer, J.W.; Cossins, A.R.; Young, I.S.; Sneddon, L.U. Welfare Challenges Influence the Complexity of Movement: Fractal Analysis of Behaviour in Zebrafish. Fishes 2019, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Bao, W.; Zhang, F.; Zhu, S.; Liu, Y.; Lu, H.; Shen, M.; Ye, Z. Modified motion influence map and recurrent neural network-based monitoring of the local unusual behaviors for fish school in intensive aquaculture. Aquaculture 2018, 493, 165–175. [Google Scholar] [CrossRef]
- Van De Vis, J.W.; Poelman, M.; Lambooij, E.; Begout, M.-L.; Pilarczyk, M. Fish welfare assurance system: Initial steps to set up an effective tool to safeguard and monitor farmed fish welfare at a company level. Fish Physiol. Biochem. 2012, 38, 243–257. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007, 92, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Sterling, P.; Eyer, J. Allostasis: A New Paradigm to Explain Arousal Pathology. In Handbook of Life Stress, Cognition and Health; Fisher, S., Reason, J., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1988; pp. 629–639. ISBN 0471912697. [Google Scholar]
- Kristiansen, T.S.; Madaro, A.; Stien, L.H.; Bracke, M.B.M.; Noble, C. Theoretical Basis and Principles for Welfare Assessment of Farmed Fish, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 38. [Google Scholar]
- Bonga, S.E.W. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Rapini, R.; Marrazza, G. Electrochemical aptasensors for contaminants detection in food and environment: Recent advances. Bioelectrochemistry 2017, 118, 47–61. [Google Scholar] [CrossRef]
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Soozanipour, A.; Low, Z.-X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for wastewater monitoring: A review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Baca, C.P.; Spirito, C.M.; Bae, J.S.; Szegletes, Z.M.; Barott, N.; Sausele, D.J.; Brooks, Y.M.; Weller, D.L.; Richardson, R.E. Rapid qPCR-Based Water Quality Monitoring in New York State Recreational Waters. Front. Water 2021, 3, 711477. [Google Scholar] [CrossRef]
- Eguiraun, H.; Casquero, O.; Martinez, I. The Shannon Entropy Trend of a Fish System Estimated by a Machine Vision Approach Seems to Reflect the Molar Se:Hg Ratio of Its Feed. Entropy 2018, 20, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, M.-J.; Park, Y.-S. Biological early warning system based on the responses of aquatic organisms to disturbances: A review. Sci. Total. Environ. 2014, 466–467, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems—Impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhu, J.; Wang, A.; Wang, J.; Wu, Y.; Yao, W. Early detection of cyanide, organophosphate and rodenticide pollution based on locomotor activity of zebrafish larvae. PeerJ 2021, 9, e12703. [Google Scholar] [CrossRef]
- Eguiraun, H.; Martinez, I. Entropy and Fractal Techniques for Monitoring Fish Behaviour and Welfare in Aquacultural Precision Fish Farming—A Review. Entropy 2023, 25, 559. [Google Scholar] [CrossRef]
- Eguiraun, H.; Izagirre, U.; Martinez, I. A paradigm shift in safe seafood production: From contaminant detection to fish monitoring—Application of biological warning systems to aquaculture. Trends Food Sci. Technol. 2015, 43, 104–113. [Google Scholar] [CrossRef]
- Eguiraun, H.; Casquero, O.; Sørensen, A.J.; Martinez, I. Reducing the Number of Individuals to Monitor Shoaling Fish Systems—Application of the Shannon Entropy to Construct a Biological Warning System Model. Front. Physiol. 2018, 9, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguiraun, H.; López-De-Ipiña, K.; Martinez, I. Application of Entropy and Fractal Dimension Analyses to the Pattern Recognition of Contaminated Fish Responses in Aquaculture. Entropy 2014, 16, 6133–6151. [Google Scholar] [CrossRef] [Green Version]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Brausch, J.M.; Connors, K.A.; Brooks, B.W.; Rand, G.M. Human Pharmaceuticals in the Aquatic Environment: A Review of Recent Toxicological Studies and Considerations for Toxicity Testing. Rev. Environ. Contam. Toxicol. 2012, 218, 1–99. [Google Scholar] [CrossRef]
- Steele, C.W. Open field exploratory behaviour of fish: An underutilized tool for behavioural toxicology. Mar. Pollut. Bull. 1983, 14, 124–125. [Google Scholar] [CrossRef]
- Kleerekoper, H. Effects of Sublethal Concentrations of Pollutants on the Behavior of Fish. J. Fish. Res. Board Can. 1976, 33, 2036–2039. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Shannon, C.E.; Weaver, W.W. The Mathematical Theory of Communications; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Espino, M.; Eguiraun, H.; de Cerio, O.D.; Carrero, J.A.; Etxebarria, N.; Martinez, I. Antioxidant Activities and Selenogene Transcription in the European Sea Bass (Dicentrarchus labrax) Liver Depend, in a Non-linear Manner, on the Se/Hg Molar Ratio of the Feeds. Biol. Trace Element Res. 2021, 200, 2365–2379. [Google Scholar] [CrossRef]
- Døving, K.B. Assessment of animal behaviour as a method to indicate environmental toxicity. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 100, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, L.; Petitjean, Q.; Côte, J.; Laffaille, P.; Jean, S. Effects of Pollution on Fish Behavior, Personality, and Cognition: Some Research Perspectives. Front. Ecol. Evol. 2020, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Suryanto, M.E.; Yang, C.-C.; Audira, G.; Vasquez, R.D.; Roldan, M.J.M.; Ger, T.-R.; Hsiao, C.-D. Evaluation of Locomotion Complexity in Zebrafish after Exposure to Twenty Antibiotics by Fractal Dimension and Entropy Analysis. Antibiotics 2022, 11, 1059. [Google Scholar] [CrossRef]
- Okoye, C.O.; Addey, C.I.; Oderinde, O.; Okoro, J.O.; Uwamungu, J.Y.; Ikechukwu, C.K.; Okeke, E.S.; Ejeromedoghene, O.; Odii, E.C. Toxic Chemicals and Persistent Organic Pollutants Associated with Micro-and Nanoplastics Pollution. Chem. Eng. J. Adv. 2022, 11, 100310. [Google Scholar] [CrossRef]
- Llorca, M.; Farré, M. Current Insights into Potential Effects of Micro-Nanoplastics on Human Health by in-vitro Tests. Front. Toxicol. 2021, 3, 752140. [Google Scholar] [CrossRef]
- Weilgart, L. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007, 85, 1091–1116. [Google Scholar] [CrossRef]
- Herbert-Read, J.E.; Kremer, L.; Bruintjes, R.; Radford, A.N.; Ioannou, C.C. Anthropogenic Noise Pollution from Pile-Driving Disrupts the Structure and Dynamics of Fish Shoals. Proceeding R. Soc. B 2017, 84, 20171627I. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitano, H. Computational Systems Biology. Nature 2002, 420, 206–210. [Google Scholar] [CrossRef]
- Spasic, S.; Savic, A.; Nikolic, L.; Budimir, S.; Janosevic, D.; Mitrovic, A. Applications of Higuchi’s Fractal Dimension in the Analysis of Biological Signals. In Proceedings of the 2012 20th Telecommunications Forum, TELFOR, Belgrade, Serbia, 20–22 November 2012; pp. 639–641. [Google Scholar] [CrossRef]
- Mandelbrot, B. How Long Is the Coast of Britain? Statistical Self-Similarity and Fractional Dimension. Science 1967, 156, 636–638. [Google Scholar] [CrossRef] [Green Version]
- Zmeskal, O.; Dzik, P.; Vesely, M. Entropy of fractal systems. Comput. Math. Appl. 2013, 66, 135–146. [Google Scholar] [CrossRef]
- Kith, K.; Sourina, O.; Kulish, V.; Khoa, N.M. An Algorithm for Fractal Dimension Calculation Based on Renyi Entropy for Short Time Signal Analysis. In Proceedings of the 2009 7th International Conference on Information, Communications and Signal Processing, ICICS 2009, Macau, China, 8–10 December 2009; pp. 1–5. [Google Scholar] [CrossRef]
- Ezeiza, A.; de Ipiña, K.L.; Hernández, C.; Barroso, N. Enhancing the Feature Extraction Process for Automatic Speech Recognition with Fractal Dimensions. Cogn. Comput. 2012, 5, 545–550. [Google Scholar] [CrossRef]
- Sekine, M.; Tamura, T.; Akay, M.; Fujimoto, T.; Togawa, T.; Fukui, Y. Discrimination of walking patterns using wavelet-based fractal analysis. IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 188–196. [Google Scholar] [CrossRef]
- Inada, Y.; Kawachi, K. Order and Flexibility in the Motion of Fish Schools. J. Theor. Biol. 2002, 214, 371–387. [Google Scholar] [CrossRef] [Green Version]
- Tikhonov, D.; Enderlein, J.; Malchow, H.; Medvinsky, A.B. Chaos and fractals in fish school motion. Chaos Solitons Fractals 2001, 12, 277–288. [Google Scholar] [CrossRef]
- Tikhonov, D.; Malchow, H. Chaos and fractals in fish school motion, II. Chaos Solitons Fractals 2003, 16, 287–289. [Google Scholar] [CrossRef]
- Alados, C.L.; Escós, J.M.; Emlen, J.M. Fractal structure of sequential behaviour patterns: An indicator of stress. Anim. Behav. 1996, 51, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.P.; Garnett, R. The entropic basis of collective behaviour. J. R. Soc. Interface 2015, 12, 20150037. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Fu, L.; Liu, Z.; Liu, H.; Chen, L.; Liu, Y. Aquatic Toxic Analysis by Monitoring Fish Behavior Using Computer Vision: A Recent Progress. J. Toxicol. 2018, 2018, 2591924. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Kang, I.J.; Moroishi, J.; Nakamura, A. The application of entropy for detecting behavioral responses in Japanese medaka (Oryzias latipes) exposed to different toxicants. Environ. Toxicol. 2010, 25, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Nimkerdphol, K.; Nakagawa, M. Effect of sodium hypochlorite on zebrafish swimming behavior estimated by fractal dimension analysis. J. Biosci. Bioeng. 2008, 105, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ma, J.; Zhou, S.; Ma, F. Concentration-dependent toxicity effect of SDBS on swimming behavior of freshwater fishes. Environ. Toxicol. Pharmacol. 2015, 40, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Ladu, F.; Mwaffo, V.; Li, J.; Macrì, S.; Porfiri, M. Acute caffeine administration affects zebrafish response to a robotic stimulus. Behav. Brain Res. 2015, 289, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, A.S.; Salierno, J.D.; Gipson, G.T.; Molteno, T.C.; Hunter, C. A video-based movement analysis system to quantify behavioral stress responses of fish. Water Res. 2004, 38, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Alados, C.L.; Weber, D.N. Lead effects on the predictability of reproductive behavior in fathead minnows (pimephales promelas): A mathematical model. Environ. Toxicol. Chem. 1999, 18, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Eguiraun, H.; Lopez-De-Ipiña, K.; Martinez, I. Evolution of Shannon Entropy in a Fish System (European Seabass, Dicentrarchus labrax) during Exposure to Sodium Selenite (Na2SeO3). In Proceedings of the 2nd International Electronic Conference on Entropy and its Applications, Online, 15–30 November 2015; MDPI: Basel, Switzerland, 2015. Sciforum Electronic Conference Series, Session Complex Systems (C006). Volume 2. [Google Scholar] [CrossRef] [Green Version]
- Eguiraun, H.; López-De-Ipiña, K.; Martinez, I. Shannon Entropy in a European Seabass (Dicentrarchus labrax) System during the Initial Recovery Period after a Short-Term Exposure to Methylmercury. Entropy 2016, 18, 209. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, J.S.; Mi, F.J.; Zhang, G.H.; Sun, J. Monitoring low-level mercury contamination by zebrafish school behavioral responses. IOP Conf. Series Earth Environ. Sci. 2020, 612, 012077. [Google Scholar] [CrossRef]
- Tenorio, B.M.; Filho, E.A.D.S.; Neiva, G.S.M.; da Silva, V.A.; Tenorio, F.D.C.A.M.; Silva, T.D.J.D.; e Silva, E.C.S.; Nogueira, R.D.A. Can fractal methods applied to video tracking detect the effects of deltamethrin pesticide or mercury on the locomotion behavior of shrimps? Ecotoxicol. Environ. Saf. 2017, 142, 243–249. [Google Scholar] [CrossRef]
- Nakayama, K.; Oshima, Y.; Hiramatsu, K.; Honjo, T. Alteration of General Behavior of Male Medaka, oryzias latipes, Exposed to Tributyltin and/or Polychlorinated Biphenyls. J. Fac. Agric. Kyushu Univ. 2004, 49, 85–92. [Google Scholar] [CrossRef]
- Nakayama, K.; Oshima, Y.; Hiramatsu, K.; Shimasaki, Y.; Honjo, T. Effects of polychlorinated biphenyls on the schooling behavior of japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2005, 24, 2588–2593. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Chen, J.; Ren, Z.; Wang, Z.; Chon, T.-S. Evidence for the Stepwise Behavioral Response Model (SBRM): The effects of Carbamate Pesticides on medaka (Oryzias latipes) in an online monitoring system. Chemosphere 2012, 87, 734–741. [Google Scholar] [CrossRef]
- Nakagawa, M. A Critical Exponent Method to Evaluate Fractal Dimensions of Self-Affine Data. J. Phys. Soc. Jpn. 1993, 62, 4233–4239. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; de Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef]
- Audira, G.; Suryanto, M.E.; Chen, K.H.-C.; Vasquez, R.D.; Roldan, M.J.M.; Yang, C.-C.; Hsiao, C.-D.; Huang, J.-C. Acute and Chronic Effects of Fin Amputation on Behavior Performance of Adult Zebrafish in 3D Locomotion Test Assessed with Fractal Dimension and Entropy Analyses and Their Relationship to Fin Regeneration. Biology 2022, 11, 969. [Google Scholar] [CrossRef]
- Ji, C.W.; Lee, S.K.H.; Kwak, I.S.; Cha, E.Y.; Lee, S.K.H.; Chon, T.S. Computational Analysis of Movement Behaviors of Medaka (Oryzias latipes) after the Treatments of Copper by Using Fractal Dimension and Artificial Neural Networks. WIT Trans. Biomed. Health 2006, 10, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T. Approach to an irregular time series on the basis of the fractal theory. Phys. D Nonlinear Phenom. 1988, 31, 277–283. [Google Scholar] [CrossRef]
- Katz, M.J. Fractals and the analysis of waveforms. Comput. Biol. Med. 1988, 18, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, P. What is wrong in Katz’s method? Comments on: “A note on fractal dimensions of biomedical waveforms”. Comput. Biol. Med. 2010, 40, 950–952. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation Entropy: A Natural Complexity Measure for Time Series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E.; Goudie, C.; Sunde, M.L.; Kopecky, M.J.; Wagner, P.; Oh, S.-H.; Hoekstra, W.G. Selenium: Relation to Decreased Toxicity of Methylmercury Added to Diets Containing Tuna. Science 1972, 175, 1122–1124. [Google Scholar] [CrossRef] [Green Version]
- Ralston, N.V.C.; Ralston, C.R.; Raymond, L.J. Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments. Biol. Trace Element Res. 2015, 171, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralston, N.V.; Raymond, L.J. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2405–2416. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Blackwell, J.L.; Raymond, L.J. Importance of Molar Ratios in Selenium-Dependent Protection Against Methylmercury Toxicity. Biol. Trace Element Res. 2007, 119, 255–268. [Google Scholar] [CrossRef]
- Yamashita, M.; Imamura, S.; Yamashita, Y. Methylmercury and Selenium in Seafood. Kagaku Seibutsu 2012, 50, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, M.; Yamashita, Y.; Suzuki, T.; Kani, Y.; Mizusawa, N.; Imamura, S.; Takemoto, K.; Hara, T.; Hossain, A.; Yabu, T.; et al. Selenoneine, a Novel Selenium-Containing Compound, Mediates Detoxification Mechanisms against Methylmercury Accumulation and Toxicity in Zebrafish Embryo. Mar. Biotechnol. 2013, 15, 559–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, Y.; Yabu, T.; Yamashita, M. Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J. Biol. Chem. 2010, 1, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.L.; Nogueira, R.D.A. Multifractal dimension and lacunarity of yolk sac vasculature after exposure to magnetic field. Microvasc. Res. 2015, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Lombana, D.A.B.; Macrì, S.; Porfiri, M. Collective Emotional Contagion in Zebrafish. Front. Behav. Neurosci. 2021, 15, 730372. [Google Scholar] [CrossRef]
- Millot, S.; Bégout, M.-L.; Chatain, B. Exploration behaviour and flight response toward a stimulus in three sea bass strains (Dicentrarchus labrax L.). Appl. Anim. Behav. Sci. 2009, 119, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Millot, S.; Cerqueira, M.; Castanheira, M.-F.; Øverli, Ø.; Oliveira, R.F.; Martins, C.I.M. Behavioural Stress Responses Predict Environmental Perception in European Sea Bass (Dicentrarchus labrax). PLoS ONE 2014, 9, e108800. [Google Scholar] [CrossRef] [Green Version]
- Millot, S.; Péan, S.; Labbé, L.; Kerneis, T.; Quillet, E.; Dupont-Nivet, M.; Begout, M.-L. Assessment of Genetic Variability of Fish Personality Traits using Rainbow Trout Isogenic Lines. Behav. Genet. 2014, 44, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Kortet, R.; Vainikka, A.; Janhunen, M.; Piironen, J.; Hyvärinen, P. Behavioral variation shows heritability in juvenile brown trout Salmo trutta. Behav. Ecol. Sociobiol. 2014, 68, 927–934. [Google Scholar] [CrossRef]
- Giacomini, A.C.V.V.; de Abreu, M.S.; Koakoski, G.; Idalêncio, R.; Kalichak, F.; Oliveira, T.A.; da Rosa, J.G.S.; Gusso, D.; Piato, A.L.; Gil Barcellos, L.J. My stress, our stress: Blunted cortisol response to stress in isolated housed zebrafish. Physiol. Behav. 2015, 139, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Ríos, D.; Réale, D.; Freitas, C.; Moland, E.; Olsen, E.M. Individual level consistency and correlations of fish spatial behaviour assessed from aquatic animal telemetry. Anim. Behav. 2017, 124, 83–94. [Google Scholar] [CrossRef]
- Mikheev, V.N.; Pasternak, A.F.; Taskinen, J. Personality Influences Risk of Parasitism in Fish. Dokl. Biol. Sci. 2019, 488, 141–144. [Google Scholar] [CrossRef]
| Sensor Type | Sensor Implementation | Animal Behavioural Variables | Information Level |
|---|---|---|---|
| Sonar | Single-beam sonar | Biomass depth distribution within beam | Group |
| Sonar | Split-beam sonar | Biomass depth distribution | Individual-based group |
| Sonar | Multi-beam sonar | Movement dynamics (position, and speed) within beam | Group |
| Hydroacoustic Telemetry | Individual fish tags | Biomass depth distribution | Individual |
| Passive hydroacoustic sensing | Hydrophone | Movement dynamics (position, and speed) within entire cage volume | Group |
| Camera | Surface camera | e.g., depth, position, acceleration and spatial orientation | Group |
| Camera | Feeding camera (submerged) | Sound emitted from fish population, general soundscape | Individual-based group |
| Camera | Stereo camera (submerged) | Surface activity (jumping/splashing) | Individual-based group |
| Query | Documents |
|---|---|
| (TITLE-ABS-KEY (entropy) AND TITLE-ABS-KEY (fish) AND TITLE-ABS-KEY (“biological warning system” OR bws)) | 5 |
| (TITLE-ABS-KEY (entropy) AND TITLE-ABS-KEY (fish) AND TITLE-ABS-KEY (“biological early warning system” OR bews)) | 3 |
| (TITLE-ABS-KEY (fish AND behav*) AND TITLE-ABS-KEY (fractal* OR entropy)) | 147 |
| (TITLE-ABS-KEY (aquacult*) AND TITLE-ABS-KEY (fractal* OR entropy)) | 94 |
| (TITLE-ABS-KEY (“Fish behavio*”) AND TITLE-ABS-KEY (entropy)) | 12 |
| (TITLE-ABS-KEY (“Fish behavio*”) AND TITLE-ABS-KEY (fractal)) | 10 |
| (TITLE-ABS-KEY (“collective behaviour” OR “collective behavior”) AND TITLE-ABS-KEY (fish) AND TITLE-ABS-KEY (welfare OR stress* OR health OR disease)) | 25 |
| (TITLE-ABS-KEY ((collective AND behavio*) AND fish) AND TITLE-ABS-KEY (contaminant* OR welfare OR stress* OR health OR disease)) | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguiraun, H.; Martinez, I. Non-Linear Analyses of Fish Behaviours in Response to Aquatic Environmental Pollutants—A Review. Fishes 2023, 8, 311. https://doi.org/10.3390/fishes8060311
Eguiraun H, Martinez I. Non-Linear Analyses of Fish Behaviours in Response to Aquatic Environmental Pollutants—A Review. Fishes. 2023; 8(6):311. https://doi.org/10.3390/fishes8060311
Chicago/Turabian StyleEguiraun, Harkaitz, and Iciar Martinez. 2023. "Non-Linear Analyses of Fish Behaviours in Response to Aquatic Environmental Pollutants—A Review" Fishes 8, no. 6: 311. https://doi.org/10.3390/fishes8060311
APA StyleEguiraun, H., & Martinez, I. (2023). Non-Linear Analyses of Fish Behaviours in Response to Aquatic Environmental Pollutants—A Review. Fishes, 8(6), 311. https://doi.org/10.3390/fishes8060311







