Abstract
An 8-week feeding trial was carried out to evaluate the effects of grapevine (Vitis vinifera) leaf extract (GLE) on the growth, oxidative enzymatic activities, immunity, and expression of antioxidant genes in zebrafish (Danio rerio). Three hundred and sixty zebrafish were supplied and fed with different levels of GLE: 0, 0.5, 1, and 2 g kg−1. The dietary administration of 1 g kg−1 of GLE significantly increased growth parameters in fish. Fish fed diets with GLE showed increased total protein. The total Ig and lysozyme activity significantly changed in the whole-body serum, but not in skin mucus. GLE significantly increased Catalase (CAT), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPx) activities compared to the control diet. GLE treatments caused a significant decrease in the malondialdehyde (MDA) content. In the skin mucus, only CAT and SOD activities significantly increased. The highest expression of Toll-like receptor-1 (TLR-1) and Tumor Necrosis Factor-α (TNFα) genes was achieved in fish fed 2 g kg−1 of GLE. CAT and SOD gene expressions were significantly higher in fish fed 1 and 2 g kg−1 of GLE. GPx gene expression was significantly higher in fish fed 1 g kg−1 of GLE. In conclusion, the results of the present study revealed that GLE affects growth performance and regulates antioxidant and immune gene expression. The determination of the optimum dosage merits further research.
Keywords:
antioxidant genes; growth; immunity; immunity genes; polyphenols; Vitis vinifera; zebrafish Key Contribution:
Grapevine leaf extract has the potential to be used as a feed additive in zebrafish to boost growth performance and regulate antioxidant and immune gene expression.
1. Introduction
Polyphenols are a group of phytochemicals, secondary metabolites, present in fruits and vegetables and synthesized to control pathogen infections and to dissuade herbivorous animals from eating them [1]. In recent years, polyphenols have stirred interest due to their antioxidant properties, which enable them to complement the functions of vitamins and enzymes in the defense against oxidative stress caused by reactive oxygen species or free radicals, generated during the cell metabolic processes [2]. Food and nutraceutical industries have largely exploited the antioxidant power of polyphenols and their protective effects on the cardiovascular and nervous system and against diseases such as cancer and diabetes, and pathological conditions such as obesity and inflammation [3,4]. The multifaceted polyphenol effects have been also exploited in terrestrial animal farming, due to their antimicrobial, growth-promoting, anti-inflammatory, and antioxidant properties, while acting as natural antibiotics [5,6,7]. However, the effects of polyphenols are not generalizable. Their chemical structure is highly variable and, with it, the type of activity. Polyphenols are highly diverse compounds that include more than 8000 different chemical types divided into several subtypes, ranging from fairly basic substances (such as phenolic acids) to complex molecules (such as tannins). Based on their chemical structure (in particular, on the number of aromatic rings contained and the structural elements that bind the aromatic rings together), several classes that include flavonoids, phenolic acids, stilbenes, and lignans are identified [8].
Interestingly, some polyphenols show pro-oxidant properties and other harmful effects depending on a variety of factors such as structure, concentration, presence of metal ions, microenvironment inside different tissues, and duration of administration [9]. Just to name a few, catechins from grape seeds show in vitro prooxidant activity on leukocytes [10]. Curcumin administered to humans behaves as a pro-oxidant depending on the body’s location, age, sex, genetic structure, and concentration [11]. Resveratrol shows cytotoxic and pro-oxidant effects on hepatic cells depending on its concentration and time of exposure [12]. Moreover, it shortens the lifespan of Saccharomyces cerevisiae by a pro-oxidant mechanism [13].
The application of polyphenols in aquaculture is still in its infancy; however, several investigations indicate that they can be considered as feed additives with useful applications as growth promoters, antioxidants, and immune boosters, as well as acting as natural antibiotics [14,15]. Indeed, polyphenols from the chestnut shell and olive mill wastewater (OMWW) improved weight gain and stimulated the immune response and the antioxidant properties in Nile tilapia (Oreochromis niloticus) [16], juvenile beluga sturgeon (Huso huso) [17], common carp (Cyprinus carpio) [18], and convict cichlid (Amatitlania nigrofasciata) [19]. However, diets supplemented with 10, 20, or 30 g kg−1 of tannic acid resulted in poor growth performance in juvenile European seabass (Dicentrarchus labrax L.) [20]. The administration of diets containing 10 and 50 g kg−1 of OMWW caused growth inhibition of gilthead sea bream (Sparus aurata) [21] and rainbow trout (Oncorhynchus mykiss) [22,23]. Further, studies carried out on zebrafish show that the efficacy of polyphenols in counteracting intestinal inflammation depends not only on the doses employed [24] but also on the timing of the treatment [25].
The emerging picture is a highly variable one, indicating that it is not possible to generalize the type of polyphenols and the actions performed and that each polyphenol or mixture of polyphenols must be characterized and evaluated for the effect exerted. In this frame, the zebrafish—a widely employed biomedicine and, more recently, aquaculture model [26,27]—may represent an extraordinary resource to evaluate the efficacy of polyphenols in aquaculture, by limiting the costs of experimentation and providing basic but crucial data such as suitable concentrations and biological effects, which can be defined and later adapted to the species of commercial interest. Moreover, this approach would comply with the principles of the 3R (replacement, reduction, and refinement) in animal experimentation, proposed by [28] and at the basis of the Directive on the protection of animals used for scientific purposes of the EU (2010/63/EU, law on 22 September 2010).
The use of phytochemicals in aquaculture to promote growth and control disease outbreaks and microbe pathogen infection is a promising tool with minimum environmental impact. Thus, we undertook this study to evaluate the role of polyphenols extracted from Vitis vinifera L. leaf on the growth performance, antioxidant status, and immunity of zebrafish (Danio rerio).
2. Materials and Methods
2.1. Ethics
The experiments were performed in compliance with the protocols (357; 8 November 2000) approved by the ethics committee of the faculty of sciences of the University of Tehran.
2.2. Experimental Design and Feed Formulation
The proximate composition of the basal diet (control diet) is reported in Table 1. It was a commercial diet (Biomar, Nersac, France) used in previous studies with zebrafish. The basal diet was supplemented with Vitis vinifera leaf spray-dried extract (Grapevine leaf extract = GLE) supplied by a company specializing in the production of botanical extracts. According to the manufacturer’s technical sheet (EPO Istituto Farmochimico Fitoterapico S.r.l., Milan, Italy), the extract was obtained by using warm water as the extraction solvent. The composition of the extract was carbohydrates 95–97%, lipids 1–2%, proteins 0–1%, minerals 1–2%, and polyphenols 5%. Three hundred and sixty healthy zebrafish were purchased from a private company (Gorgan Mahi, Gorgan, Iran). Before the commencement of the feeding trial, zebrafish were adapted for 14 days to the lab conditions and fed with the control diet three times per day. Zebrafish were weighed (see Section 3.1 “Growth performance” for initial weights) and divided into 12 aquaria of 100 L with a capacity of 30 fish/aquaria and treated as follows: (1) control group fed with the basal diet; (2) group fed with the basal diet added with 0.5 g kg−1 of feed of Vitis vinifera leaf extract (GLE1); (3) group fed with the basal diet added with 1 g kg−1 of feed of Vitis vinifera leaf extract (GLE2); (4) group fed with the basal diet added with 2 g kg−1 of feed of Vitis vinifera leaf extract (GLE3). The preparation of the experimental diets was performed as described in our previous paper [29]. Briefly, the commercial diet was powdered and desired levels of GLP were added at the expense of cellulose (to keep the total energy of different diets identical). Then, they were re-pelleted and grounded to produce a suitable crumble (1 mm). All diets were maintained at 4 °C until use. The trial length was 8 weeks. During the feeding trial, fish were fed up to apparent satiation. Care was taken to minimize feed loss. The tank conditions were as follows: water DO (7.8 ± 0.2 mg L−1), temperature 26.50 ± 1.20 °C, and pH 7.02 ± 0.3.

Table 1.
Proximate composition of the basal diet.
2.3. Growth Performance
Growth performance parameters were determined following the formula below. To determine the weight of fish, all fish were anesthetized with clove powder (250 mg/L) and weighed.
where IW is the initial weight and FW is the final weight.
Weight Gain (WG) = final body weight (FW) − initial body weight (IW)
Specific growth rate (SGR) = 100 × [(ln FW − ln IW)/the length of feeding trial]
2.4. Determination of Innate Immune and Antioxidant Parameters
Given the small size of the fish at the end of the trial, it was not possible to collect blood and serum. Therefore, at the end of the feeding trial, nine fish were sampled per treatment, and a whole-body extract (WBE) was obtained as reported in [29,30]. Skin mucus (n = 9) was collected using a plastic zip pack as described in [31]. To determine whole-body serum lysozyme activity, we used a lysozyme-sensitive bacteria (Micrococcus luteus (PTTC)), which was determined following [32] by using a turbidimetric assay. The decrease in absorbance by each 0.001 min−1 was considered a unit of lysozyme activity. Total protein was determined according to the standard method of Lowry [33]. The WB serum Ig levels were determined according to [34] using polyethylene glycol (Sigma-Aldrich, Saint Louis, MO, USA). Briefly, the method is based on precipitation decreasing immunoglobulin molecules using polyethylene glycol and the determination of total Ig by re-measuring total protein. The activity of antioxidant enzymes including Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione Peroxidase (GPx) was determined by using commercial kits (ZellBio GmbH, Lonsee, Germany) according to the manufacturer’s instructions. Malondialdehyde (MDA) was evaluated by the calorimetric method after [31].
2.5. Sampling for Gene Expression Study
At the end of the study, nine fish from each treatment (3 fish per tank) were randomly sampled and sacrificed with a clove solution (1000 mg L−1). The intestine was dissected and instantly put in liquid nitrogen. Intestine samples were then transferred to a −80 °C refrigerator until further analysis.
2.6. RNA Isolation and Laboratory Methods of Gene Expression
The total RNA of samples was extracted and cDNA was synthesized as described in our previous paper [35]. Briefly, the isolation of total RNA was performed by using BIOZOL Reagent. Total RNA was treated with DNase I (Fermentas, Vilnius, Lithuania) to remove DNA. After checking and confirming the quality and quantity of isolated RNA with a Nanodrop Spectrophotometer and 1.5% Agarose gel, cDNA samples were prepared by using the DNA synthesis kit (Invitrogen, Waltham, MA, USA). To determine the expression levels of genes, primers were designed with reference to the Gene Bank sequences (Table 2) by Primer3 software. Then, the real-time PCR analysis and normalization of expression levels were performed as described in [36]. The glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was considered a housekeeping gene.. The IQ5 optical system software (Bio-Rad, Hercules, CA, USA) and ∆∆Ct method were used for data analysis.

Table 2.
Sequences of the primers used to study the expression of selected immune and antioxidant-related genes expression in Zebrafish. TLR-1 = Toll-like receptor-1, TNFα = Tumor Necrosis Factor-α, SOD = Superoxide Dismutase, CAT = Catalase, GPx = Glutathione Peroxidase, Gapdh = glyceraldehyde-3-phosphate dehydrogenase.
2.7. Data Analysis
The study was designed as a complete randomized design including four treatments in triplicates. Data are presented as mean ± S.D. First, the normality of the data was checked and confirmed using the Kolmogorov–Smirnov test. To determine statistically significant differences at p < 0.05, data were subjected to one-way ANOVA followed by Duncan’s multiple tests. The statistical analysis was performed using SPSS (version 16, SPSS, Armonk, NY, USA).
3. Results
3.1. Growth Performance
The results of GLE on growth performance are reported in Table 3. Zebrafish fed with 1 g kg−1 of GLE showed higher values of FW, WG, and SGR compared with GLE2, GLE3, and control groups (p < 0.05). Zebrafish fed with 0.5 and 2 g kg−1 of GLE showed growth performance parameters not statistically significant with respect to the control (p > 0.05). No mortality occurred during the trial. The survival rate was 100% in all groups.

Table 3.
Growth performance parameters.
3.2. Innate Immune Parameters
Table 4 displays the effects of different levels of GLE on immune parameters in zebrafish WBE and skin mucus. Zebrafish-treated groups showed the highest (p < 0.05) levels of WBE and skin mucus total protein. Total Ig and lysozyme activity significantly increased in WBE (p < 0.05) but not in the skin mucus (p > 0.05) with respect to the control group.

Table 4.
Effects of different levels of GLE on immune parameters in zebrafish WBE and skin mucus.
3.3. Antioxidant Enzyme Activity
Zebrafish fed different levels of GLE showed a significant (p < 0.05) increase in the activity of the antioxidant enzymes CAT, SOD, and GPx in the WBE and CAT in the skin mucus compared to the control (Table 5). No significant (p > 0.05) difference was recorded in the case of the skin mucus GPx activity between the treated groups and the control group. The MDA content in the WBE was significantly (p < 0.05) lower in the treated group than the control group, while no significant (p > 0.05) differences were detected in the case of the skin mucus MDA content between the treated groups and the control group (Table 5).

Table 5.
Antioxidant enzyme activities and MDA content in zebrafish whole-body extract (WBE) and skin mucus.
3.4. Immune and Oxidative Gene Expression
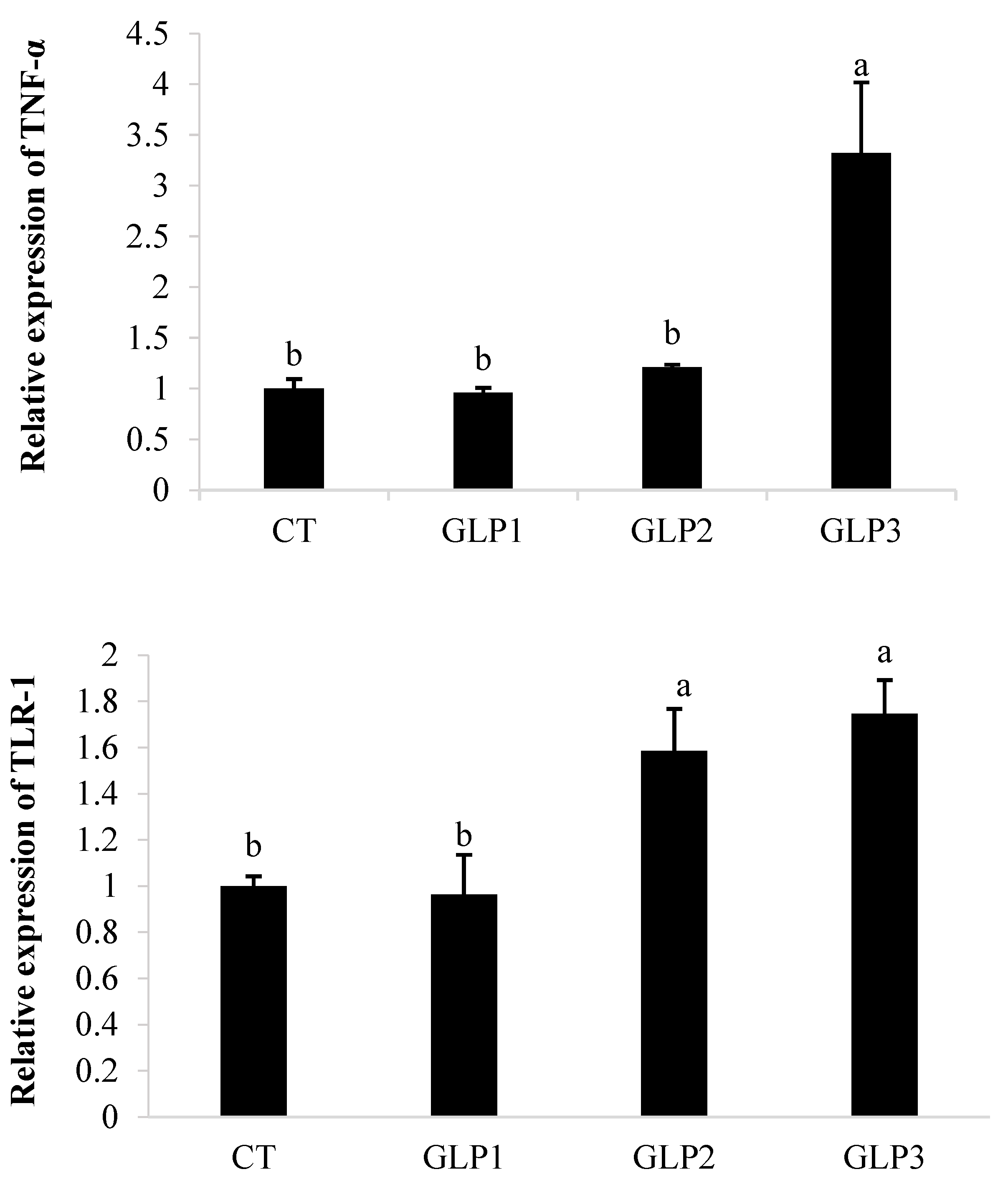
Figure 1 shows that GLE affected the expression of TNFα and TLR-1 in the gastrointestinal tract of zebrafish. In the group added with 2 g kg−1 of GLE, TNFα gene expression appeared to be significantly (p < 0.05) upregulated compared to the control group, as well as in the groups fed with 0.5 and 1 g kg−1 of GLE. Zebrafish fed with 1 or 2 g kg−1 of GLE showed a significant increase in TLR-1 gene expression (p < 0.05) compared to the group fed with 0.5 g kg−1 of GLE and the control group.
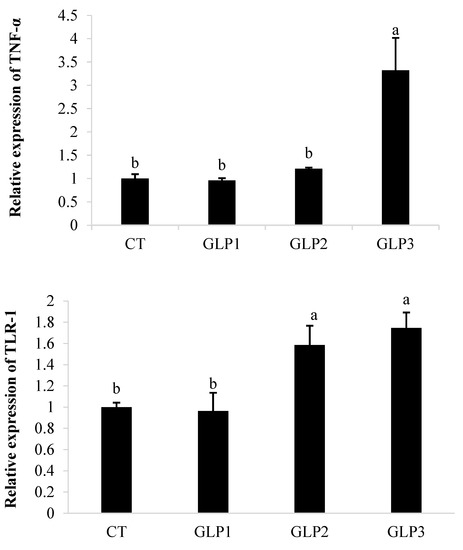
Figure 1.
Tumor necrosis factor-α (TNF-α) and Toll-like receptor-1 (TLR-1) gene expression in zebrafish gastrointestinal tract. CT = control group; GLE1 = fish fed with 0.5 g kg−1 of Vitis vinifera leaf extract; GLE2 = fish fed with 1 g kg−1 of Vitis vinifera leaf extract; GLE3: fish fed with 2 g kg−1 of Vitis vinifera leaf extract. Bars assigned with different letters denote significant differences (p < 0.05).
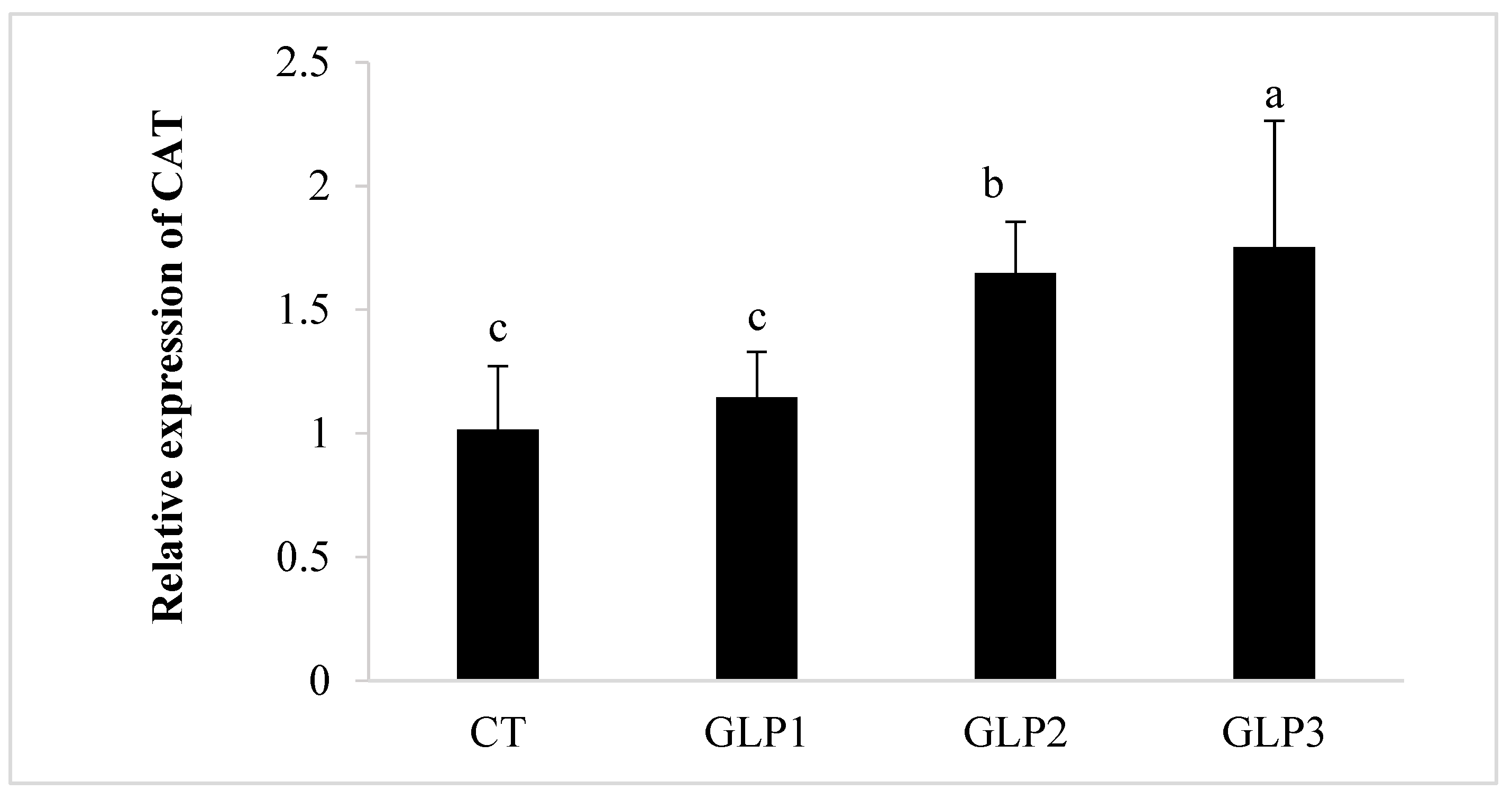
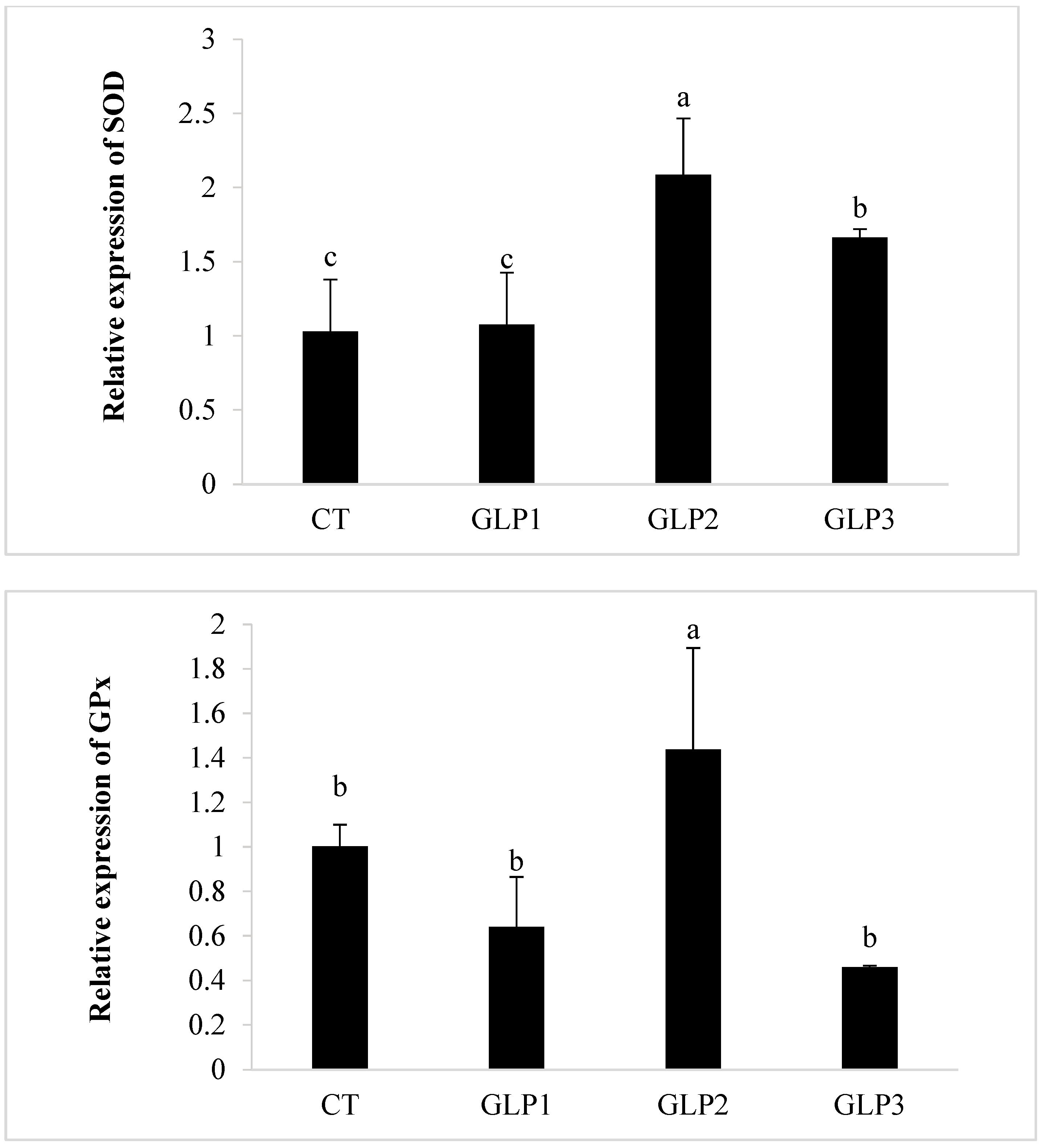
Zebrafish added with 1 and 2 g kg−1 of GLE had significantly (p < 0.05) higher CAT and SOD gene expressions in the gastrointestinal of zebrafish with respect to the control and the 0.5 g kg−1 GLE-treated groups (Figure 2). Only 1 g kg−1 of GLE-added diet significantly increased the expression of the GPx gene (p < 0.05), with respect to the control group and the other treated groups (Figure 2).
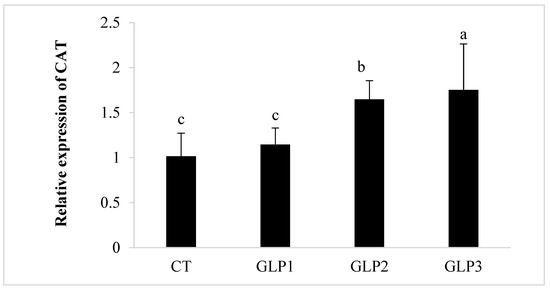
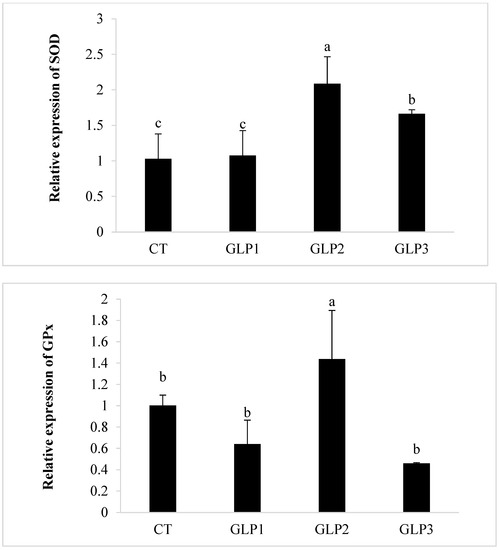
Figure 2.
Catalase (CAT), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPx) gene expression in zebrafish gastrointestinal tract. CT = control group; GLE1 = fish fed with 0.5 g kg−1 of Vitis vinifera leaf extract; GLE2 = fish fed with 1 g kg−1 of Vitis vinifera leaf extract; GLE3 = fish fed with 2 g kg−1 of Vitis vinifera leaf extract. Bars assigned with different letters denote significant differences (p < 0.05).
4. Discussion
In this study, we report the effects of grapevine (Vitis vinifera L.) leaf extract on the growth performance, antioxidant status, and immunity of zebrafish (Danio rerio).
Natural products such as plant extracts perform many pro-health functions by virtue of the polyphenol content and are widely used in aquaculture [14,37]. However, standardization is still far away and critical issues remain due to the natural variability in the polyphenol content, different extraction techniques, and lack of chemical characterization of polyphenols, which prevent reaching general conclusions about the type and optimal doses of the extracts. In this study, we used a commercially available GLE with a claimed polyphenol content of 5%. Caftaric acid, quercetin, kaempferol, catechins, and rutin are the major phenolic compounds. reported in vine leaves [38,39,40].
When fish live in suboptimal conditions, they stop growing [41]. The evaluation of the growth performance is therefore crucial to establish the goodness of treatment and is a common welfare marker in aquaculture. The outcome of the present study indicates that zebrafish fed with GLE had improved growth performance. It has been reported in the literature that plant extract inclusion in the diet has positive effects on aquatic species’ growth performance. Olive mill wastewater (OMWW) extract, rich in hydroxytyrosol, one of the most powerful natural antioxidants, brought about the growth improvement of the crayfish (Astacus leptodactylus) [42]. Chestnut wood extract rich in tannins and OMWW extract significantly increased the growth and feed efficiency in Nile tilapia [16], beluga sturgeon [17], and common carp [18]. On the contrary, in the study by Omnes et al. [20], the diet added with different doses of tannins had a negative impact on the growth and feed conversion ratio in the sea bass. In another study, Sicuro et al. [22,23] found that a diet containing different levels of OMWW extract negatively affected the growth parameters in rainbow trout. Such inconsistencies are likely caused by the different doses of polyphenols present in the extracts employed in the studies, further sustaining that the amount of polyphenols is crucial to the achievement of the expected benefits. The polyphenol doses employed in the present study are indeed lower than those employed by Omnes et al. [20] and Sicuro et al. [21,22,23], and in agreement with those usually employed as fish feed integrators reported in the literature [37].
Free radicals or ROS (reactive oxygen species) are produced by cells in response to numerous metabolic activities and are normally neutralized by endogenous antioxidants such as glutathione [43]. The overproduction of ROS within cells is a negative but possible event if the cells are exposed to stressful or toxic agents and lead to an increase in oxidative stress [44], an important biomarker in modern aquaculture. The present study reveals that GLE increased the serum and mucus activity of CAT, SOD, and GPx, crucial enzymes responsible for removing excessive free radicals [45]. Such an outcome could be explained by the antioxidant properties able to neutralize ROS and stimulate oxidative stress enzymes of polyphenols [46]. Our findings are in line with the literature indicating that polyphenols administered to farmed fish improve antioxidant defenses [15].
Polyphenols extracted from grapes modulated the antioxidant-relevant gene expression in trout [47]. Previous studies showed that GLE’s antioxidant activity is due to the high amount of quercetin [48]. In zebrafish exposed to different levels of quercetin, CAT, SOD, and GPx activity and gene expression increased, reaching the highest value with 1 µg/L of quercetin [49]. In zebrafish exposed to the pro-oxidant triphenyltin, a slight increase in SOD but not in CAT and GPX activity was detected in quercetin-pretreated zebrafish [50]. In silver catfish (Rhamdia quelen), the activity of CAT, SOD, and GPX was significantly higher in tissues of fish fed with diets containing quercetin [51].
Parallel to the increase in the antioxidant enzymes, a noticeable decrease in MDA took place. MDS is a chemical compound produced as a consequence of lipid peroxidation and widely employed as a mark of oxidative stress and indicator of peroxidative tissue damage [52]. This is not surprising, since the antioxidant enzymes CAT, SOD, and GPx not only remove excessive ROS but also act on the reduction in lipid peroxidation damage [53]. Indeed, according to our data, quercetin decreased the MDA content in zebrafish [49]. Moreover, tea polyphenols improved the antioxidant enzyme activity and decreased MDA levels in Wuchang bream (Megalobrama amblycephala) [54], and apple polyphenols decreased the MDA content while increasing CAT gene expression in grass carp (Ctenopharyngodon idellus) [55].
Some of the most appropriate innate immune indicators of fish health status and immune response are lysozyme activity and immunoglobulins levels [56,57]. In the present study, zebrafish-fed diets added with GLE showed increased total proteins, total Ig levels, and lysozyme activity. These results are in agreement with the literature data reporting that polyphenols improve innate immune parameters in Nile tilapia [16], Beluga sturgeon [17], common carp [18], and convict cichlid (Amatitlamia nigrofasciata) [19].
Another important class of proteins that play a key role in innate immunity is represented by Toll-like receptors, belonging to the pattern recognition receptors (PRRs), essential proteins capable of recognizing conserved molecules collectively known as microbial/pathogen/danger-associated molecular patterns [58]. PRRs trigger the signaling pathways, including the Nf-Kb pathway, leading to signaling molecule activation of the innate immune response via chemokines, cytokines, antimicrobial peptides, and interferons [59]. In zebrafish (present study), the TLR-1 gene was upregulated by polyphenols, which could partially explain the increase in TNFα as a result of the Nf-Kb pathway activation. This line of reasoning is sustained by Zhang et al. [50], reporting that in zebrafish treated with quercetin-enriched diets, the inflammation decreased by Nf-kB signaling pathway regulation. Altogether, the increase in TNFα and TLR-1 can be regarded as an improvement of the zebrafish’s innate immune system to resist pathogen attacks. In the present study, the zebrafish treated with 2 g kg−1 of GLE showed a significant increase in the gene expression of TNFα—a pro-inflammatory cytokine—as well as TLR-1. Phytochemicals have been found to increase pro-inflammatory cytokines (TNFα, IL-1, and IL-8) in fish [14,18]. TNFα in fish is upregulated during the early stages of infection when its intervention is crucial to promote phagocytosis and anti-bactericidal activities [60]. Thus, the transcription of pro-inflammatory cytokines may be considered advantageous to ameliorate fish resistance against pathogens and maintain immunological response. Similarly, a higher relative expression of the TNFα gene was noticed in the Nile tilapia fed with fenugreek (Trigonella foenum-graecum L.) [61], and Leucas aspera-added diets [62]. Lemon verbena (Aloysia citrodora) added 2% upregulated TNFα and IL- 8 in rainbow trout, while Apple (Malus pomila) upregulated TNFα, IL-1, and IL-8 [63]. Dried lemon peel administered at 2.5 and 5% upregulated TNFα, IL-1, and IL-8 in the head kidney of Labeo rohita [64].
5. Conclusions
In conclusion, considering the data obtained, it is thought that adding 0.5–1 g kg of Vitis vinifera extract polyphenols to the feeds affects the growth performance of fish, improves the immune system, and fortifies the antioxidant defense system. Investigating the effect of supplements under normal environmental conditions might seem superfluous, as many studies evaluate its effects in suboptimal conditions (stress, temperature, hypoxia, etc.), and/or in the presence of infections. However, it is extremely important to understand the effects of integrators under normal environmental conditions to evaluate the dose and duration of therapy that provides the best protection. In future studies, it will be useful to investigate the effectiveness of Vitis vinifera extracts on aquatic organisms exposed to biotic and abiotic stress factors.
Author Contributions
Conceptualization, S.H.H. and M.P.; methodology, S.H.H., M.Y. (Morteza Yousefi) and H.V.D.; formal analysis, M.Y. (Morteza Yousefi); investigation, Z.F.; resources, S.H.H., H.V.D., M.Y. (Morteza Yousefi) and M.P.; data curation, M.Y. (Metin Yazici); writing—original draft preparation, E.E.-H.; writing—review and editing, S.H.H. and M.P.; supervision, M.P.; funding acquisition, S.H.H., H.V.D., M.Y. (Morteza Yousefi) and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the University of Sannio (funds FRA number 2022 to Marina Paolucci), Chiang Mai university, and GUASNR.
Institutional Review Board Statement
Committee of ethics of the faculty of sciences of the University of Tehran; Approval code 357.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2013, 3, 232–249. [Google Scholar]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs. In Plant Based “Green Chemistry 2.0”; Li, Y., Chemat, F., Eds.; Green Chemistry and Sustainable Technology; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2016, 101, 605–628. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in Monogastric Nutrition—A Review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence; InTech: London, UK, 2012; Volume 2, pp. 23–48. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef]
- Martins, L.A.M.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.F.; Souza, I.C.C.; Moreira, J.C.F.; Gottfried, C.; Guma, F.C.R. Resveratrol Induces Pro-oxidant Effects and Time-Dependent Resistance to Cytotoxicity in Activated Hepatic Stellate Cells. Cell Biochem. Biophys. 2014, 68, 247–257. [Google Scholar] [CrossRef]
- Ramos-Gomez, M.; Olivares-Marin, I.K.; Canizal-García, M.; González-Hernández, J.C.; Nava, G.M.; Madrigal-Perez, L.A. Resveratrol induces mitochondrial dysfunction and decreases chronological life span of Saccharomyces cerevisiae in a glucose-dependent manner. J. Bioenerg. Biomembr. 2017, 49, 241–251. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Pourmohammadi Fallah, H.; Yousefi, M.; Dawood, M.A.O.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Doan, H. The Gene Regulatory Roles of Herbal Extracts on the Growth, Immune System, and Reproduction of Fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Effects of chestnut (Castanea sativa) polyphenols on growth, immune response, and disease resistance of Nile tilapia (Oreochromis niloticus) culture under biofloc system. Fish Shellfish Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Imanpour, M.R.; Mazandarani, M.; Sanchouli, H.; Volpe, M.G.; Paolucci, M. Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture 2020, 528, 735494. [Google Scholar] [CrossRef]
- Jahazi, A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; Van Doan, H.; Paolucci, M. Dietary supplementation of polyphenols positively affects the innate immune response, oxidative status, and growth performance of common carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Hoseinifar, H.; Jahazi, A.; Nikdehghan, N.; Van Doan, H.; Volpe, M.G.; Paolucci, M. Effects of dietary polyphenols from agricultural by-products on mucosal and humoral immune and antioxidant responses of convict cichlid (Amatitlania nigrofasciata). Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Omnes, M.-H.; Le Goasduff, J.; Le Delliou, H.; Le Bayon, N.; Quazuguel, P.; Robin, J.H. Effects of dietary tannin on growth, feed utilization and digestibility, and carcass composition in juvenile European seabass (Dicentrarchus labrax L.). Aquac. Rep. 2017, 6, 21–27. [Google Scholar] [CrossRef]
- Sicuro, B.; Daprà, F.; Gai, F.; Palmegiano, G.B.; Schiavone, R.; Zilli, L.; Vilella, S. Olive oil by-product as a natural antioxidant in gilthead sea bream (Sparus aurata) nutrition. Aquac. Int. 2010, 18, 511–522. [Google Scholar] [CrossRef]
- Sicuro, B.; Barbera, S.; Daprà, F.; Gai, F.; Gasco, L.; Paglialonga, G.; Palmegiano, G.B.; Vilella, S. The olive oil by-product in ’Rainbow trout Onchorynchus mykiss (Walbaum)’ farming: Productive results and quality of the product. Aquac. Res. 2010, 41, e475–e486. [Google Scholar] [CrossRef]
- Sicuro, B.; Badino, P.; Daprà, F.; Gai, F.; Galloni, M.; Odore, R.; Palmegiano, G.B.; Macchi, E. Physiological effects of natural olive oil antioxidants utilization in rainbow trout (Onchorynchus mykiss) feeding. Aquacult. Int. 2010, 18, 415–431. [Google Scholar] [CrossRef]
- Orso, G.; Solovyev, M.M.; Facchiano, S.; Tyrikova, E.; Sateriale, D.; Kashinskaya, E.; Pagliarulo, C.; Hoseinifar, H.S.; Simonov, E.; Varricchio, E.; et al. Chestnut shell tannins: Effects on intestinal inflammation and dysbiosis in Zebrafish. Animals 2021, 11, 1538. [Google Scholar] [CrossRef]
- Imperatore, R.; Orso, G.; Facchiano, S.; Scarano, P.P.; Hoseinifar, S.H.; Ashouri, G.; Guarino, C.; Paolucci, M. Antiinflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture 2023, 563, 738878. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef] [PubMed]
- Aleström, P.; Winther-Larsen, H.C. Zebrafish offer aquaculture research their services. In Genomics in Aquaculture, 1st ed.; MacKenzie, S., Jentoft, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 165–194. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Johns Hopkins University: Methuen, MA, USA, 1959. [Google Scholar]
- Holbech, H.; Andersen, L.; Petersen, G.I.; Korsgaard, B.; Pedersen, K.L.; Bjerregaard, P. Development of an ELISA for vitellogenin in whole body homogenate of zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. 2001, 130, 119–131. [Google Scholar] [CrossRef]
- Yousefi, S.; Hoseinifar, S.H.; Paknejad, H.; Hajimoradloo, A. The effects of dietary supplement of galactooligosaccharide on innate immunity, immune related genes expression and growth performance in zebrafish (Danio rerio). Fish Shellfish Immunol. 2018, 73, 192–196. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar] [CrossRef]
- Clerton, P.; Troutaud, D.; Verlhac, V.; Gabaudan, J.; Deschaux, P. Dietary vitamin E and rainbow trout (Oncorhynchus mykiss) phagocyte functions: Effect on gut and on head kidney leucocytes. Fish Shellfish Immunol. 2001, 11, 1–13. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D.P. Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs, and total immunoglobulin level in serum. In Fish Disease Diagnosis and Prevention Methods; Siwicki, A.K., Anderson, D.P., Waluga, J., Eds.; Wydawnictwo Instytutu Rybactwa Strodladowego: Olsztyn, Poland, 1993; pp. 105–112. [Google Scholar]
- Zou, H.K.; Hoseinifar, S.H.; Kolangi Miandare, H.; Hajimoradloo, A. Agaricus bisporus powder improved cutaneous mucosal and serum immune parameters and up-regulated intestinal cytokines gene expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 2016, 58, 380–386. [Google Scholar]
- Hoseinifar, S.H.; Khalili, M.; Rufchaei, R.; Raeisi, M.; Attar, M.; Cordero, H.; Esteban, M.Á. Effects of date palm fruit extracts on skin mucosal immunity, immune related genes expression and growth performance of common carp (Cyprinus carpio) fry. Fish Shellfish Immunol. 2015, 47, 706–711. [Google Scholar] [CrossRef]
- Orso, G.; Imperatore, R.; Coccia, E.; Ashouri, G.; Paolucci, M. Lamiaceae as Feed Additives in Fish Aquaculture. Fishes 2022, 7, 349. [Google Scholar] [CrossRef]
- Dresch, R.R.; Dresch, M.K.; Guerreiro, A.F.; Biegelmeyer, R.; Holzschuh, M.H.; Rambo, D.F.; Henriques, A.T. Phenolic Compounds from the Leaves of Vitis labrusca and Vitis vinifera L. as a Source of Waste Byproducts: Development and Validation of LC Method and Antichemotactic Activity. Food Anal. Methods 2013, 7, 527–539. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Volpe, M.G.; Paolucci, M.; Pacifico, S. UHPLC-HR-MS/MS-guided recovery of bioactive flavonol compounds from Greco di Tufo vine leaves. Molecules 2019, 24, 3630–3640. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Paolucci, M.; Sansone, F.; Mencherini, T.; Pacifico, S.; Volpe, M.G. Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form. Appl. Sci. 2021, 11, 5827. [Google Scholar] [CrossRef]
- Segner, H.; Reiser, S.; Ruane, N.; Rösch, R.; Steinhagen, D.; Vehanen, T. Welfare of Fishes in Aquaculture; FAO Fisheries and Aquaculture Circular No. 1189; FAO: Budapest, Hungary, 2019. [Google Scholar]
- Parrillo, L.; Coccia, E.; Volpe, M.G.; Siano, F.; Pagliarulo, C.; Scioscia, E.; Varricchio, E.; Safari, O.; Eroldogan, T.; Paolucci, M. Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 2017, 473, 161–168. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. Reactive Oxygen Species (ROS) in Living Cells; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and Activators of SOD, GSH-Px, and CAT, Enzyme Inhibitors and Activators, Murat Senturk; IntechOpen: 2017. Available online: https://www.intechopen.com/chapters/52877 (accessed on 2 April 2023).
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mousavi, S.; Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Alizadeh-Salteh, S.; Khani Oushani, A.; Firouzamandi, M.; Mardani, K. Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol. Biochem. 2020, 46, 777–786. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhang, J.; Xie, J.; Yang, L.; Xing, Y.; Li, Z. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2020, 46, 759–770. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, D.; Wang, J.; Qi, Q. The effects of TPT and dietary quercetin on growth, hepatic oxidative damage and apoptosis in zebrafish. Ecotoxicol. Environ. Saf. 2021, 224, 112697. [Google Scholar] [CrossRef] [PubMed]
- Pês, T.S.; Saccol, E.M.H.; Ourique, G.M.; Londero, É.P.; Gressler, L.T.; Golombieski, J.I.; Glanzner, W.G.; Llesuy, S.F.; Gonçalves, P.B.D.; Neto, J.R.; et al. Quercetin in the diet of silver catfish: Effects on antioxidant status, blood parameters and pituitary hormone expression. Aquaculture 2016, 458, 100–106. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuroendocrinol. Lett. 2009, 30, 2–12. [Google Scholar]
- Long, M.; Lin, W.; Hou, J.; Guo, H.; Li, L.; Li, D.; Tang, R.; Yang, F. Dietary supplementation with selenium yeast and tea polyphenols improve growth performance and nitrite tolerance of Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2017, 68, 74–83. [Google Scholar] [CrossRef]
- Yang, G.; Yu, R.; Geng, S.; Xiong, L.; Yan, Q.; Kumar, V.; Wen, C.; Peng, M. Apple polyphenols modulates the antioxidant defense response and attenuates inflammatory response concurrent with hepatoprotective effect on grass carp (Ctenopharyngodon idellus) fed low fish meal diet. Aquaculture 2021, 534, 736284. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Adeshina, I.; Jenyo-Oni, A.; Ajani, E.K.; Emikpe, B.O. Growth, physiological, antioxidants, and immune response of African catfish, Clarias gariepinus (B.), to dietary clove basil, Ocimum gratissimum, leaf extract and its susceptibility to Listeria monocytogenes infection. Fish Shellfish Immunol. 2018, 78, 346–354. [Google Scholar] [CrossRef]
- Adeshina, I.; Jenyo-Oni, A.; Emikpe, B.O.; Ajani, E.K.; Abdel-Tawwab, M. Stimulatory effect of dietary clove, Eugenia caryophyllata, bud extract on growth performance, nutrient utilization, antioxidant capacity, and tolerance of African catfish, Clarias gariepinus (B.), to Aeromonas hydrophila infection. J. World Aquac. Soc. 2019, 50, 390–405. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Itav, S.; Elinav, E. Integration of innate immune signaling. Trends Immunol. 2016, 37, 84–101. [Google Scholar] [CrossRef]
- Grayfer, L.; Walsh, J.G.; Belosevic, M. Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-alpha. Dev. Comp. Immunol. 2008, 32, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.M.; Dawood, M.A.; Assar, D.H.; Omar, A.A.; Elbialy, Z.I.; Farrag, F.A.; Shukry, M.; Zayed, M.M. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 2020, 515, 734589. [Google Scholar] [CrossRef]
- Kurian, A.; Van Doan, H.; Tapingkae, W.; Elumalai, P. Modulation of mucosal parameters, innate immunity, growth and resistance against Streptococcus agalactiae by enrichment of Nile tilapia (Oreochromis niloticus) diet with Leucas aspera. Fish Shellfish Immunol. 2019, 97, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Rashidian, G.; Ghafarifarsani, H.; Jahazi, M.A.; Soltani, M.; Van Doan, H.; El-Haroun, E.; Paolucci, M. Effects of Apple (Malus pomila) Pomace-Derived Pectin on the Innate Immune Responses, Expressions of Key Immune-Related Genes, Growth Performance, and Digestive Enzyme Activity of Rainbow Trout (Oncorhynchus mykiss). Animals 2021, 11, 2117. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Thamizharasan, S.; Devi, G.; Van Doan, H.; Ajith Kumar, T.T.; Hoseinifar, S.H.; Balasundaram, C. Dried lemon peel enriched diet improves antioxidant activity, immune response and modulates immuno-antioxidant genes in Labeo rohita against Aeromonas sorbia. Fish Shellfish Immunol. 2020, 106, 675–684. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).