miR-155 Regulates Photoperiod Induced Gonadal Development in Atlantic Salmon (Salmo salar) by Targeting Brain-Derived Neurotrophic Factor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Synthesis of miRNA Mimics and Inhibitor
2.3. Plasma Melatonin Concentration Measurements
2.4. Luciferase Assay
2.5. Cell Culture and Transfection
2.6. qPCR
2.7. Western Immunoblotting
2.8. Statistical Analysis
3. Results
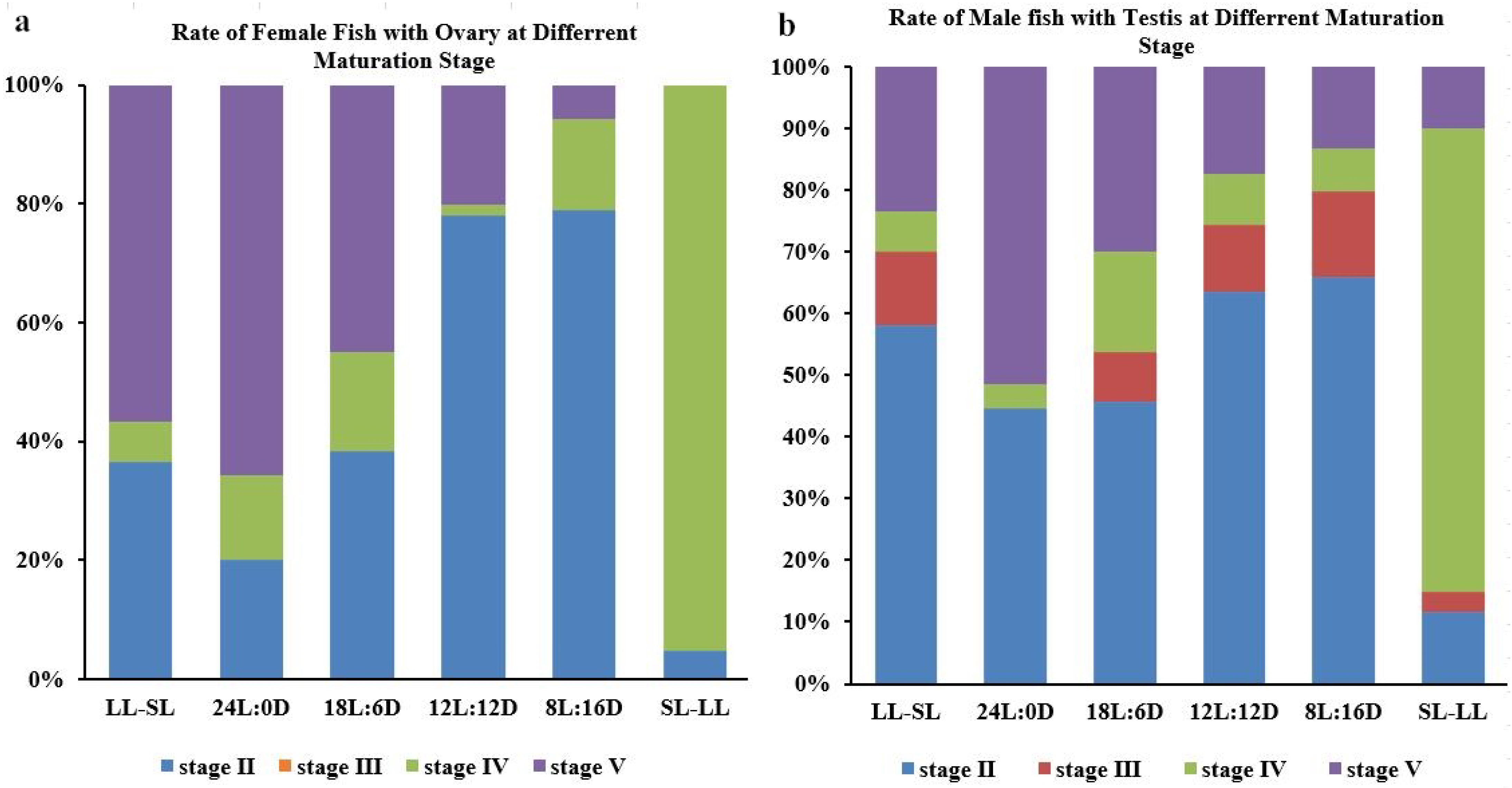
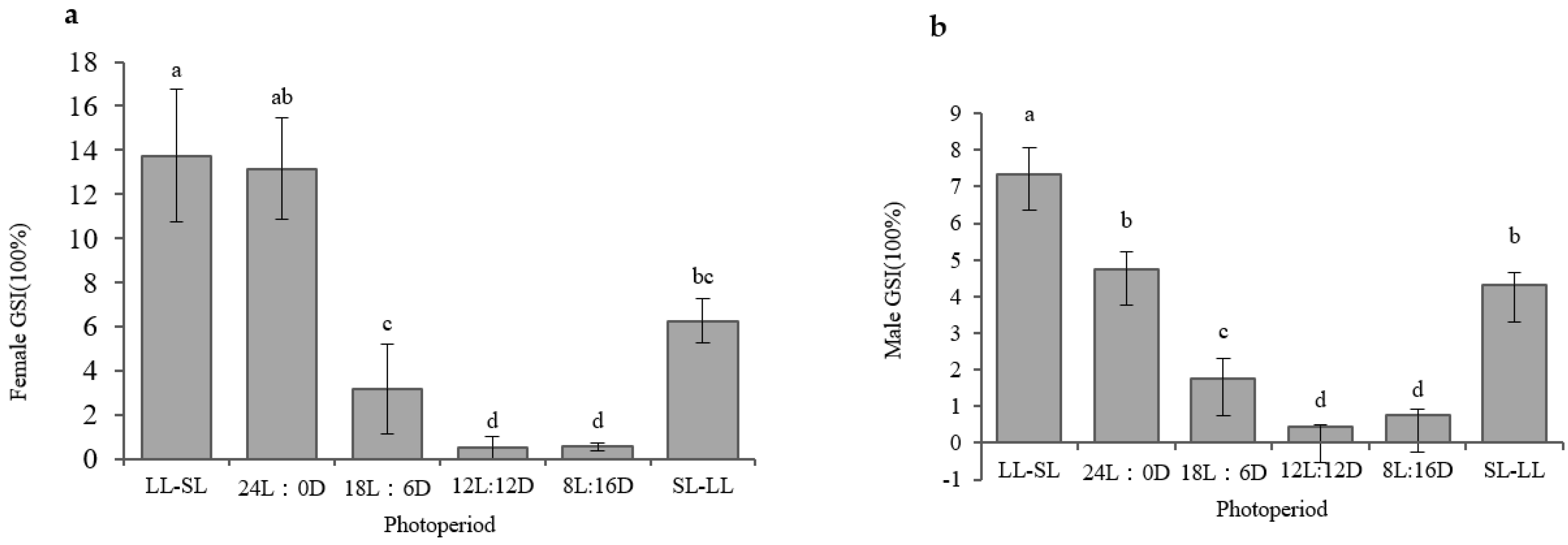
3.1. The Impact of the Photoperiod on Atlantic Salmon’s Gonadal Development in RAS Systems
3.2. Expression Pattern of miR-155 in the Hypothalamus of Atlantic Salmon under Different Photoperiods
3.3. miR-155 Suppresses Atlantic Salmon Photoperiodic Reproduction
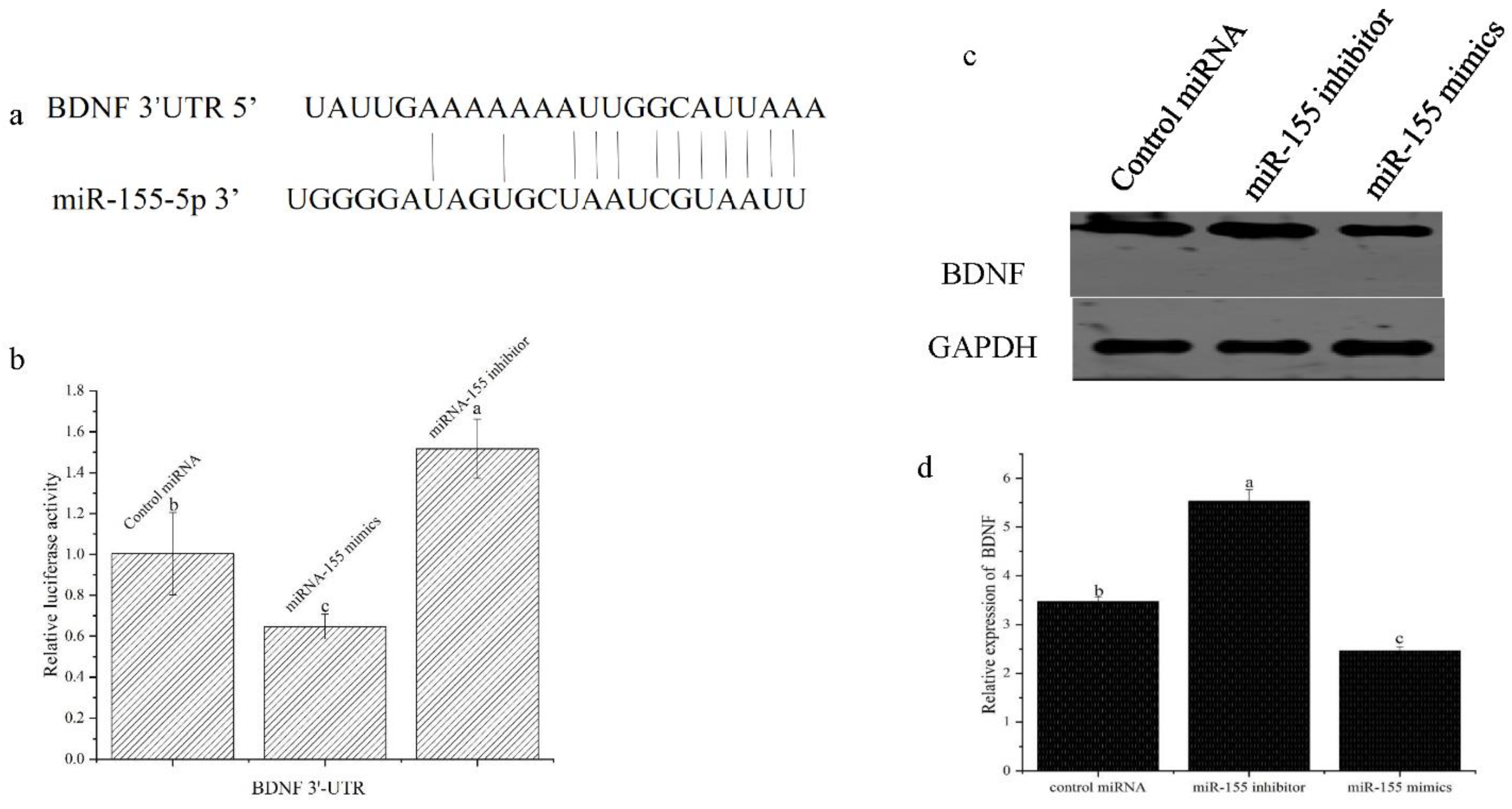
3.4. miR-155 Targets the BDNF mRNA in Atlantic Salmon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badruzzaman, M.; Bapary, M.A.J.; Takemura, A. Possible roles of photoperiod and melatonin in reproductive activity via changes in dopaminergic activity in the brain of a tropical damselfish, Chrysiptera cyanea. Gen. Comp. Endocrinol. 2013, 194, 240–247. [Google Scholar] [CrossRef]
- Carrillo, M.; Zanuy, S.; Felip, A.; Bayarri, M.J.; Moles, G.; Gomez, A. Hormonal and Environmental Control of Puberty in Perciform Fish The Case of Sea Bass. Ann. N. Y. Acad. Sci. 2009, 1163, 49–59. [Google Scholar] [CrossRef]
- Bromage, N.; Porter, M.; Randall, C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 2001, 197, 63–98. [Google Scholar] [CrossRef]
- Nilsen, T.O.; Ebbesson, L.O.; Kiilerich, P.; Björnsson, B.T.; Madsen, S.S.; McCormick, S.D.; Stefansson, S.O. Endocrine systems in juvenile anadromous and landlocked Atlantic salmon (Salmo salar): Seasonal development and seawater acclimation. Gen. Comp. Endocrinol. 2008, 155, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.T.; Stefansson, S.O.; McCormick, S.D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 2011, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.K.; Chattoraj, A.; Mukherjee, S.; Moniruzzaman, M. Melatonin: A potent candidate in the regulation of fish oocyte growth and maturation. Gen. Comp. Endocrinol. 2013, 181, 215–222. [Google Scholar] [CrossRef]
- Falcón, J.; Migaud, H.; Muñoz-Cueto, J.; Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Imsland, A.K.; Handeland, S.O.; Stefansson, S.O. Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic salmon. Aquac. Int. 2014, 22, 1331–1345. [Google Scholar] [CrossRef]
- Martinez, E.P.; Balseiro, P.; Stefansson, S.O.; Kaneko, N.; Norberg, B.; Fleming, M.S.; Imsland, A.K.; Handeland, S.O. Interaction of temperature and feed ration on male postsmolt maturation of Atlantic salmon (Salmo salar L.). Aquaculture 2023, 562, 738877. [Google Scholar] [CrossRef]
- Handeland, S.O.; Berge, Å.; Björnsson, B.T.; Lie, Ø.; Stefansson, S.O. Seawater adaptation by out-of-season Atlantic salmon (Salmo salar L.) smolts at different temperatures. Aquaculture 2000, 181, 377–396. [Google Scholar] [CrossRef]
- McCormick, S.D.; O’Dea, M.F.; Moeckel, A.M.; Björnsson, B.T. Endocrine and physiological changes in Atlantic salmon smolts following hatchery release. Aquaculture 2003, 222, 45–57. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Palm, I.F.; La Fleur, S.E.; Scheer, F.A.J.L.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN Outputs and the Hypothalamic Balance of Life. J. Biol. Rhythm. 2006, 21, 458–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahjahan, M.; Al-Emran, M.; Islam, S.M.; Baten, S.A.; Rashid, H.; Haque, M.M. Prolonged photoperiod inhibits growth and reproductive functions of rohu Labeo rohita. Aquac. Rep. 2020, 16, 100272. [Google Scholar] [CrossRef]
- Migaud, H.; Davie, A.; Taylor, J.F. Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J. Fish Biol. 2010, 76, 27–68. [Google Scholar] [CrossRef]
- Chi, L.; Li, X.; Liu, Q.; Liu, Y. Photoperiod regulate gonad development via kisspeptin/kissr in hypothalamus and saccus vasculosus of Atlantic salmon (Salmo salar). PLoS ONE 2017, 12, e0169569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasuwa, H.; Ueda, J.; Ikawa, M.; Okabe, M. MiR-200b and miR-429 Function in Mouse Ovulation and Are Essential for Female Fertility. Science 2013, 341, 71–73. [Google Scholar] [CrossRef]
- Gay, S.; Bugeon, J.; Bouchareb, A.; Henry, L.; Delahaye, C.; Legeai, F.; Montfort, J.; Le Cam, A.; Siegel, A.; Bobe, J.; et al. MiR-202 controls female fecundity by regulating medaka oogenesis. PLoS Genet. 2018, 14, e1007593. [Google Scholar] [CrossRef] [PubMed]
- Cardona, E.; Guyomar, C.; Desvignes, T.; Montfort, J.; Guendouz, S.; Postlethwait, J.H.; Skiba-Cassy, S.; Bobe, J. Circulating miRNA repertoire as a biomarker of metabolic and reproductive states in rainbow trout. BMC Biol. 2021, 19, 235. [Google Scholar] [CrossRef]
- Leng, R.-X.; Pan, H.-F.; Qin, W.-Z.; Chen, G.-M.; Ye, D.-Q. Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev. 2011, 22, 141–147. [Google Scholar] [CrossRef]
- Seddiki, N.; Brezar, V.; Ruffin, N.; Lévy, Y.; Swaminathan, S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology 2014, 142, 32–38. [Google Scholar] [CrossRef]
- Surbhi; Schatz, K.C.; Kyne, R.F.; Nelson, R.J.; Paul, M.J. Photoperiod regulates hypothalamic miR-155 gene expression in female, but not male, Siberian hamsters (Phodopus sungorus). Behav. Neurosci. 2019, 133, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Langlet, F.; Chachlaki, K.; Roa, J.; Rasika, S.; Jouy, N.; Gallet, S.; Gaytan, F.; Parkash, J.; Tena-Sempere, M. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat. Neurosci. 2016, 19, 835–844. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Bliss, D.; Breau, C.; Damon-Randall, K.; Sundt-Hansen, L.E.; Hatfield, E.M.; Horsburgh, G.; Hansen, H.; Maoiléidigh, N.; Sheehan, T.; et al. Atlantic salmon in a rapidly changing environment—Facing the challenges of reduced marine survival and climate change. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2654–2665. [Google Scholar] [CrossRef]
- Martins, C.; Eding, E.H.; Verdegem, M.C.; Heinsbroek, L.T.; Schneider, O.; Blancheton, J.-P.; d’Orbcastel, E.R.; Verreth, J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Irachi, S.; Hall, D.J.; Fleming, M.S.; Maugars, G.; Björnsson, B.T.; Dufour, S.; Uchida, K.; McCormick, S.D. Photoperiodic regulation of pituitary thyroid-stimulating hormone and brain deiodinase in Atlantic salmon. Mol. Cell. Endocrinol. 2021, 519, 111056. [Google Scholar] [CrossRef] [PubMed]
- Ytrestøyl, T.; Hjelle, E.; Kolarevic, J.; Takle, H.; Rebl, A.; Afanasyev, S.; Krasnov, A.; Brunsvik, P.; Terjesen, B.F. Photoperiod in recirculation aquaculture systems and timing of seawater transfer affect seawater growth performance of Atlantic salmon (Salmo salar). J. World Aquac. Soc. 2023, 54, 73–95. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Mishra, A.; Bohra, A. Non-coding RNAs and plant male sterility: Current knowledge and future prospects. Plant Cell Rep. 2018, 37, 177–191. [Google Scholar] [CrossRef]
- Di, R.; He, J.; Song, S.; Tian, D.; Liu, Q.; Liang, X.; Ma, Q.; Sun, M.; Wang, J.; Zhao, W.; et al. Characterization and comparative profiling of ovarian microRNAs during ovine anestrus and the breeding season. BMC Genom. 2014, 15, 899. [Google Scholar] [CrossRef] [Green Version]
- Larson, T.A.; Lent, K.L.; Bammler, T.K.; MacDonald, J.W.; Wood, W.E.; Caras, M.L.; Thatra, N.M.; Budzillo, A.; Perkel, D.J.; Brenowitz, E.A. Network analysis of microRNA and mRNA seasonal dynamics in a highly plastic sensorimotor neural circuit. BMC Genom. 2015, 16, 905. [Google Scholar] [CrossRef] [Green Version]
- Nordgarden, U.; Oppedal, F.; Taranger, G.; Hemre, G.I.; Hansen, T. Seasonally changing metabolism in Atlantic salmon (Salmo salar L.) I–Growth and feed conversion ratio. Aquac. Nutr. 2003, 9, 287–293. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.-A.; Dufour, S.; Karlsen, Ø.; Norberg, B. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kråkenes, R.; Hansen, T.; Stefansson, S.O.; Taranger, G.L. Continuous light increases growth rate of Atlantic salmon (Salmo salar L.) postsmolts in sea cages. Aquaculture 1991, 95, 281–287. [Google Scholar] [CrossRef]
- Andersson, E.; Schulz, R.W.; Male, R.; Bogerd, J.; Patiña, D.; Benedet, S.; Norberg, B.; Taranger, G.L. Pituitary gonadotropin and ovarian gonadotropin receptor transcript levels: Seasonal and photoperiod-induced changes in the reproductive physiology of female Atlantic salmon (Salmo salar). Gen. Comp. Endocrinol. 2013, 191, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Mørkøre, T.; Rørvik, K.-A. Seasonal variations in growth, feed utilisation and product quality of farmed Atlantic salmon (Salmo salar) transferred to seawater as 0+ smolts or 1+ smolts. Aquaculture 2001, 199, 145–157. [Google Scholar] [CrossRef]
- Davie, A.; Porter, M.J.; Bromage, N.R.; Migaud, H. The role of seasonally altering photoperiod in regulating physiology in Atlantic cod (Gadus morhua). Part I. Sexual maturation. Can. J. Fish. Aquat. Sci. 2007, 64, 84–97. [Google Scholar] [CrossRef]
- Peiris, T.S.; Machaalani, R.; Waters, K.A. Brain-derived neurotrophic factor mRNA and protein in the piglet brainstem and effects of Intermittent Hypercapnic Hypoxia. Brain Res. 2004, 1029, 11–23. [Google Scholar] [CrossRef]
- Lalo, U.; Bogdanov, A.; Moss, G.W.; Pankratov, Y. Astroglia-Derived BDNF and MSK-1 Mediate Experience- and Diet-Dependent Synaptic Plasticity. Brain Sci. 2020, 10, 462. [Google Scholar] [CrossRef]
- Przybył, B.J.; Szlis, M.; Wójcik-Gładysz, A. Brain-derived neurotrophic factor (BDNF) affects the activity of the gonadotrophic axis in sheep. Horm. Behav. 2021, 131, 104980. [Google Scholar] [CrossRef]
- Xia, H.; Mao, Q.; Paulson, H.L.; Davidson, B.L. siRNA-mediated gene silence in vitro and in vivo. Nat. Biotechnol. 2002, 20, 1006–1010. [Google Scholar] [CrossRef]



| Genes | Sequences of Primers | Products (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| GnRH | F: GTGGTGGTGTTGGCGTTGGTA R: GTGATGCTGAATGTCTGCTTG | 285 | 60 °C |
| LhR | F: 5′-CGCCCATCTCGTTCTTCGCTATATCC-3′ | 306 | 58 °C |
| R:5′-GCAATGGCAGAGGGTCCATCATTTGTG-3′ | |||
| fshR | F:5′-GGGGTAAGCAGCTACAGCAAGGTGAG-3′ | 265 | 58 °C |
| R: 5′-CAGAGAGGGCGAAGAAGGAAATAGGC-3′ | |||
| β-actin | F: 5′-GACGCGACCTCACAGACTACCT-3′ | 282 | 58 °C |
| R: 5′-CGTGGATACCGCAAGACTCCATAC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; Wang, Y.; Jiang, P.; Li, J.; Liu, Q.; Chi, L. miR-155 Regulates Photoperiod Induced Gonadal Development in Atlantic Salmon (Salmo salar) by Targeting Brain-Derived Neurotrophic Factor. Fishes 2023, 8, 345. https://doi.org/10.3390/fishes8070345
Guo T, Wang Y, Jiang P, Li J, Liu Q, Chi L. miR-155 Regulates Photoperiod Induced Gonadal Development in Atlantic Salmon (Salmo salar) by Targeting Brain-Derived Neurotrophic Factor. Fishes. 2023; 8(7):345. https://doi.org/10.3390/fishes8070345
Chicago/Turabian StyleGuo, Teng, Yanfeng Wang, Ping Jiang, Jun Li, Qinghua Liu, and Liang Chi. 2023. "miR-155 Regulates Photoperiod Induced Gonadal Development in Atlantic Salmon (Salmo salar) by Targeting Brain-Derived Neurotrophic Factor" Fishes 8, no. 7: 345. https://doi.org/10.3390/fishes8070345
APA StyleGuo, T., Wang, Y., Jiang, P., Li, J., Liu, Q., & Chi, L. (2023). miR-155 Regulates Photoperiod Induced Gonadal Development in Atlantic Salmon (Salmo salar) by Targeting Brain-Derived Neurotrophic Factor. Fishes, 8(7), 345. https://doi.org/10.3390/fishes8070345






