Influences of Aquaponics System on Growth Performance, Antioxidant Parameters, Stress Parameters and Gene Expression of Carassius auratus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Design and Installation
2.2. Treatment of Test Fish
2.3. Lettuce Growth Indices
2.4. Stress Parameters
2.5. Extraction and Quantitative PCR of Tissue RNA
2.6. Statistical Analysis
3. Results
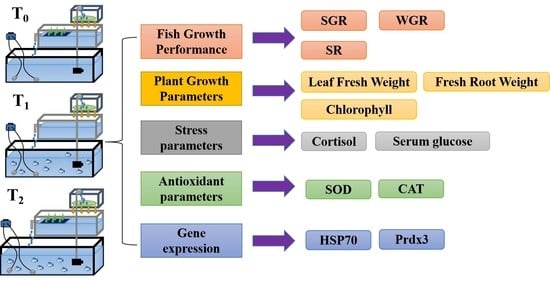
3.1. Fish Growth Performance
3.2. Plant Growth Parameters
3.3. Water Quality Parameters
3.4. Stress Parameters
3.5. Antioxidant Parameters
3.6. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, X.-L.; Rauan, A.; Xing, J.-X.; Sun, J.; Wu, W.-Y.; Ji, H. Influence of dietary Se supplementation on aquaponic system: Focusing on the growth performance, ornamental features and health status of Koi carp (Cyprinus carpio var. Koi), production of Lettuce (Lactuca sativa) and water quality. Aquac. Res. 2021, 52, 505–517. [Google Scholar] [CrossRef]
- Saseendran, S.; Dube, K.; Chandrakant, M.H.; Babitha Rani, A.M. Enhanced growth response and stress mitigation of genetically improved farmed Tilapia in a biofloc integrated aquaponic system with bell pepper. Aquaculture 2021, 533, 736200. [Google Scholar] [CrossRef]
- Milliken, S.; Ovca, A.; Antenen, N.; Villarroel, M.; Bulc, T.G.; Kotzen, B.; Junge, R. Aqu@teach—The First Aquaponics Curriculum to Be Developed Specifically for University Students. Horticulturae 2021, 7, 18. [Google Scholar] [CrossRef]
- Yamane, K.; Kimura, Y.; Takahashi, K.; Maeda, I.; Iigo, M.; Ikeguchi, A.; Kim, H.-J. The Growth of Leaf Lettuce and Bacterial Communities in a Closed Aquaponics System with Catfish. Horticulturae 2021, 7, 222. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Sandker, J.F.; van de Pol, I.L.E.; Urbina, M.A.; Wilson, R.W.; McKenzie, D.J.; Leiva, F.P. Body mass and cell size shape the tolerance of fishes to low oxygen in a temperature-dependent manner. Glob. Change Biol. 2022, 28, 5695–5707. [Google Scholar] [CrossRef]
- Blasco, F.R.; Taylor, E.W.; Leite, C.A.C.; Monteiro, D.A.; Rantin, F.T.; McKenzie, D.J. Tolerance of an acute warming challenge declines with body mass in Nile tilapia: Evidence of a link to capacity for oxygen uptake. J. Exp. Biol. 2022, 225, jeb244287. [Google Scholar] [CrossRef]
- Dinken, C.P.; Keretz, K.R.; Schramm, H.L.; Petrie-Hanson, L.; Schilling, M.W.; Allen, P.J. The Effects of Water Temperature and Simulated Angling on the Physiological Stress Response of Largemouth Bass. Trans. Am. Fish. Soc. 2022, 151, 487–506. [Google Scholar] [CrossRef]
- San, L.; Liu, B.; Liu, B.; Guo, H.; Guo, L.; Zhang, N.; Zhu, K.; Jiang, S.; Zhang, D. Transcriptome Analysis of Gills Provides Insights into Translation Changes Under Hypoxic Stress and Reoxygenation in Golden Pompano, Trachinotus ovatus (Linnaeus 1758). Front. Mar. Sci. 2021, 8, 763622. [Google Scholar] [CrossRef]
- Byrd, G.V.; Jha, B.R. Relative Growth of Lettuce (Lactuca sativa) and Common Carp (Cyprinus carpio) in Aquaponics with Different Types of Fish Food. Water 2022, 14, 3870. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, Q.; Peng, R.; Jiang, X.; Jiang, M.; Zeng, G.; Lin, J. Environmental factors of rearing water and growth performance of shrimp (Penaeus vannamei) in a microalgal monoculture system. Aquaculture 2022, 561, 738620. [Google Scholar] [CrossRef]
- Atique, F.; Lindholm-Lehto, P.; Pirhonen, J. Is Aquaponics Benefificial in Terms of Fish and Plant Growth and Water Quality in Comparison to Separate Recirculating Aquaculture and Hydroponic Systems? Water 2022, 14, 1447. [Google Scholar] [CrossRef]
- Kralik, B.; Weisstein, F.; Meyer, J.; Neves, K.; Anderson, D.; Kershaw, J. From water to table: A multidisciplinary approach comparing fish from aquaponics with traditional production methods. Aquaculture 2022, 552, 737953. [Google Scholar] [CrossRef]
- Wang, S.-X.; Zhang, J.-Y.; Du, X.-K.; Liu, D.-J.; Liu, L.-X.; Shen, X.-H. Comparative analysis of the intestinal microbiota in goldfish and crucian carps between different aquaponics and traditional farming. Aquac. Rep. 2022, 25, 101240. [Google Scholar] [CrossRef]
- Mishina, T.; Takeshima, H.; Takada, M.; Iguchi, K.I.; Zhang, C.; Zhao, Y.; Kawahara-Miki, R.; Hashiguchi, Y.; Tabata, R.; Sasaki, T.; et al. Interploidy gene fow involving the sexual-asexual cycle facilitates the diversifcation of gynogenetic triploid Carassius fish. Sci. Rep. 2021, 11, 22458. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, B.; Lai, Q.; Lu, Y.; Li, L.; Li, Y.; Liu, S. Immunity, antioxidant capacity and disease resistance of crucian carp (Carassius auratus) by single or in combination dietary Bacillus subtilis and xylo-oligosaccharides. Comp. Biochem. Physiol. Part C 2022, 256, 109296. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, G.; Tian, Y.; Li, K.; Zhao, Y.; Liang, J.; Gu, Q.; Li, X. Blood biochemistry profile of Qihe crucian carp Carassius auratus in different aquaponic systems. Environ. Sci. Pollut. Res. 2022, 27, 42898–42907. [Google Scholar] [CrossRef]
- Albadwawi, M.A.O.K.; Ahmed, Z.F.R.; Kurup, S.S.; Alyafei, M.A.; Jaleel, A. A Comparative Evaluation of Aquaponic and Soil Systems on Yield and Antioxidant Levels in Basil, an Important Food Plant in Lamiaceae. Agronomy 2022, 27, 3007. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, Z.; Zhang, R.; Guo, K.; Wang, S.; Xu, W.; Wang, C. The effects of temperature changes on the isozyme and Hsp70 levels of the Amur sturgeon, Acipenser schrenckii, at two acclimation temperatures. Aquaculture 2022, 551, 737743. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.-P.; Shaheen, A.; Matter, A. Nano spirulina dietary supplementation augments growth, antioxidative and immunological reactions, digestion, and protection of Nile tilapia, Oreochromis niloticus, against Aeromonas veronii and some physical stressors. Fish Physiol. Biochem. 2020, 46, 2143–2155. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Simó-Mirabet, P.; de Las Heras, V.; Calduch-Giner, J.À.; Pérez-Sánchez, J. Tissue-Specific Orchestration of Gilthead Sea Bream Resilience to Hypoxia and High Stocking Density. Front. Physiol. 2019, 10, 840. [Google Scholar] [CrossRef] [Green Version]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise Monitoring of Lettuce Functional Responses to Minimal Nutrient Supplementation Identifies Aquaponic System’s Nutrient Limitations and Their Time-Course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Villarroel, M.; Miranda-de la Lama, G.C.; Escobar-Álvarez, R.; Moratiel, R. Fish Welfare in Urban Aquaponics: Effects of Fertilizer for Lettuce (Lactuca sativa L.) on Some Physiological Stress Indicators in Nile Tilapia (Oreochromis niloticus L.). Water 2022, 14, 935. [Google Scholar] [CrossRef]
- Flores, R.M.V.; Preckel, P.V.; Quagrainie, K.; Widmar, N.O.; Silva, L.; da Costa, J.I.; Pinho, S.M.; Portella, M.C.; Branco, T.C.; Filho, M.X.P. Efciency tests for screening production strategies in a lettuce-juvenile tilapia aquaponics system in Brazil. Aquac. Int. 2022, 30, 2403–2424. [Google Scholar] [CrossRef]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The Effect of Anaerobic and Aerobic Fish Sludge Supernatant on Hydroponic Lettuce. Agronomy 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Adel, M.; Omidi, A.H.; Dawood, M.A.; Karimi, B.; Shekarabi, S.P.H. Dietary Gracilaria persica mediated the growth performance, fillet colouration, and immune response of Persian sturgeon (Acipenser persicus). Aquaculture 2020, 530, 735950. [Google Scholar] [CrossRef]
- Agusti, C.; Carbajal, A.; Olvera-Maneu, S.; Domingo, M.; Lopez-Bejar, M. Blubber and serum cortisol concentrations as indicators of the stress response and overall health status in striped dolphins. Comp. Biochem. Physiol. Part A 2022, 272, 111268. [Google Scholar] [CrossRef]
- Sundar, L.S.; Chen, G.S. Study on the Growth Performance of Lettuce (Lactuca sativa) and Pak Choi (Brassica chinensis) in Different Aquaponic Growing Systems. Horticulturae 2020, 6, 69. [Google Scholar] [CrossRef]
- Maitland, D.M.; Baker, J.; Chambers, G.; Ross, N.W.; Colombo, S.M. Population growth dynamics and their implications for fish welfare in mixed-size cohorts of Cyprinus Carpio var koi grown in a commercial-scale aquaponics system. Aquac. Int. 2022, 30, 187–210. [Google Scholar] [CrossRef]
- Vlahos, N.; Levizou, E.; Stathopoulou, P.; Berillis, P.; Antonopoulou, E.; Bekiari, V.; Krigas, N.; Kormas, K.; Mente, E. An Experimental Brackish Aquaponic System Using Juvenile Gilthead Sea Bream (Sparus aurata) and Rock Samphire (Crithmum maritimum). Sustainability 2019, 11, 4820. [Google Scholar] [CrossRef] [Green Version]
- Zarantoniello, M.; Chemello, G.; Ratti, S.; Pulido-Rodríguez, L.F.; Daniso, E.; Freddi, L.; Salinetti, P.; Nartea, A.; Bruni, L.; Parisi, G.; et al. Growth and Welfare Status of Giant Freshwater Prawn (Macrobrachium rosenbergii) Post-Larvae Reared in Aquaponic Systems and Fed Diets including Enriched Black Soldier Fly (Hermetia illucens) Prepupae Meal. Animals 2023, 13, 715. [Google Scholar] [CrossRef]
- Danner, R.I.; Mankasingh, U.; Anamthawat-Jonsson, K.; Thorarinsdottir, R.I. Designing Aquaponic Production Systems towards Integration into Greenhouse Farming. Water 2019, 11, 2123. [Google Scholar] [CrossRef] [Green Version]
- Supajaruwong, S.; Satanwat, P.; Pungrasmi, W.; Powtongsook, S. Design and function of a nitrogen and sediment removal system in a recirculating aquaculture system optimized for aquaponics. Environ. Eng. Res. 2021, 26, 190494. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Goddek, S.; Keesman, K.J. Improving nutrient and water use efficiencies in multi-loop aquaponics systems. Aquac. Int. 2020, 28, 2481–2490. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Deng, X.; Zhang, Z.; Yin, L. Comprehensive evaluation of physiological traits under nitrogen stress and participation of linolenic acid in nitrogendeficiency response in wheat seedlings. BMC Plant Biol. 2020, 20, 501. [Google Scholar] [CrossRef]
- Anderson, T.S.; Goldstein, L.T.; Timmons, M.B. Root nitrification capacity of lettuce plants with application to aquaponics. Aquac. Eng. 2019, 86, 101997. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Grafting Enhances the Photosynthesis and Nitrogen Absorption of Tomato Plants Under Low-Nitrogen Stress. J. Plant Growth Regul. 2022, 41, 1714–1725. [Google Scholar] [CrossRef]
- Gillespie, D.P.; Papio, G.; Kubota, C. High Nutrient Concentrations of Hydroponic Solution Can Improve Growth and Nutrient Uptake of Spinach (Spinacia oleracea L.) Grown in Acidic Nutrient Solution. Hortscience 2021, 56, 687–694. [Google Scholar] [CrossRef]
- Concepcion, R.; Dadios, E.; Cuello, J.; Duarte, B. Thermo-gas dynamics affect the leaf canopy shape and moisture content of aquaponic lettuce in a modified partially diffused microclimatic chamber. Sci. Hortic. 2022, 292, 110649. [Google Scholar] [CrossRef]
- Ni, M.; Yuan, J.; Zhang, L.; Hua, J.; Rong, H.; Gu, Z. In-situ and ex-situ purification effect of ecological ponds of Euryale ferox Salisb on shrimp aquaculture. Aquaculture 2021, 540, 736678. [Google Scholar] [CrossRef]
- Baßmann, B.; Harbach, H.; Weißbach, S.; Palm, H.W. Effect of plant density in coupled aquaponics on the welfare status of African catfish, Clarias gariepinus. J. World Aquac. Soc. 2020, 51, 183–199. [Google Scholar] [CrossRef]
- Ajima, M.N.O.; Kumar, K.; Poojary, N.; Pandey, P.K. Sublethal diclofenac induced oxidative stress, neurotoxicity, molecular responses and alters energy metabolism proteins in Nile tilapia, Oreochromis niloticus. Environ. Sci. Pollut. Res. 2021, 28, 44494–44504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kuang, S.; Sun, Y.; Sun, J.; Tian, X.; Hu, Y.; Hu, J.; Wang, Y.; Xu, S.L.; Xu, W.; et al. Effects of dietary vitamin C on growth, antioxidant enzyme activity and immune-related gene expression of Pampus argenteus. Aquac. Res. 2020, 53, 5342–5353. [Google Scholar] [CrossRef]
- González-Silvera, D.; Cuesta, A.; Esteban, M. Immune defence mechanisms presented in liver homogenates and bile of gilthead seabream (Sparus aurata). J. Fish Biol. 2021, 99, 1958–1967. [Google Scholar] [CrossRef]
- Senavirathna, M.D.H.J.; Zhaozhi, L.; Fujino, T. Root Adsorption of Microplastic Particles Affects the Submerged Freshwater Macrophyte Egeria densa. Water Air Soil. Pollut. 2022, 233, 80. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, C.; Zhu, D.; Li, S.; Wen, X. Effects of dietary Sargassum horneri on resisting hypoxia stress, which changes blood biochemistry, antioxidant status, and hepatic HSP mRNA expressions of juvenile black sea bream Acanthopagrus schlegelii. J. Appl. Phycol. 2020, 32, 3457–3466. [Google Scholar] [CrossRef]





| Parameter | Parameter Abbreviation |
|---|---|
| SGR | Specific growth rate |
| WGR | Relative growth rate |
| SR | Survival rate |
| SOD | Total superoxide dismutase |
| CAT | Catalase |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| HSP70 | 5′–ACTGAACTCGGTCATTGGCT–3′ | 5′–AGAGGCCAATTGCAGTTCAT–3′ |
| Prdx3 | 5′–TCGCAGTCTCAGTGGATTCC–3′ | 5′–CAGGAGGCATTGCTGATGAT–3′ |
| GAPDH | 5′–CAGGAGGCATTGCTGATGAT–3′ | 5′–GAAGGCTGGGGCTCATTT–3′ |
| Parameter | W1 (g) | W2 (g) | SGR (%) | WGR (%) | SR (%) |
|---|---|---|---|---|---|
| T1 | 7.00 ± 0.40 a | 9.12 ± 0.40 b | 0.89 ± 0.05 b | 30.63 ± 2.14 b | 100 a |
| T2 | 7.11 ± 0.24 a | 10.26 ± 0.31 a | 1.25 ± 0.10 a | 45.51 ± 4.43 a | 100 a |
| Parameter | Leaf Fresh Weight (g plant−1) | Fresh Root Weight (g plant−1) | Chlorophyll (SPAD) |
|---|---|---|---|
| T0 | 32.80 ± 0.50 b | 6.93 ± 0.42 a | 31.64 ± 0.78 b |
| T2 | 34.33 ± 0.65 a | 7.64 ± 0.36 a | 34.40 ± 0.43 a |
| Parameter | pH | Dissolved Oxygen (mg/L) | Nitrate (mg/L) | Nitrite (mg/L) |
|---|---|---|---|---|
| T0 | 7.05 ± 0.03 c | 7.01 ± 0.05 a | 3.40 ± 0.13 c | 0.08 ± 0.01 c |
| T1 | 7.52 ± 0.04 a | 5.56 ± 0.10 c | 6.83 ± 0.10 a | 0.24 ± 0.01 a |
| T2 | 7.19 ± 0.01 b | 6.71 ± 0.03 b | 5.19 ± 0.09 b | 0.12 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, H.; Wang, B.; Zhao, J.; Wang, Y.; Du, X.; Shi, Q. Influences of Aquaponics System on Growth Performance, Antioxidant Parameters, Stress Parameters and Gene Expression of Carassius auratus. Fishes 2023, 8, 360. https://doi.org/10.3390/fishes8070360
Mao H, Wang B, Zhao J, Wang Y, Du X, Shi Q. Influences of Aquaponics System on Growth Performance, Antioxidant Parameters, Stress Parameters and Gene Expression of Carassius auratus. Fishes. 2023; 8(7):360. https://doi.org/10.3390/fishes8070360
Chicago/Turabian StyleMao, Hanping, Bin Wang, Jian Zhao, Yafei Wang, Xiaoxue Du, and Qiang Shi. 2023. "Influences of Aquaponics System on Growth Performance, Antioxidant Parameters, Stress Parameters and Gene Expression of Carassius auratus" Fishes 8, no. 7: 360. https://doi.org/10.3390/fishes8070360
APA StyleMao, H., Wang, B., Zhao, J., Wang, Y., Du, X., & Shi, Q. (2023). Influences of Aquaponics System on Growth Performance, Antioxidant Parameters, Stress Parameters and Gene Expression of Carassius auratus. Fishes, 8(7), 360. https://doi.org/10.3390/fishes8070360