The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Analysis
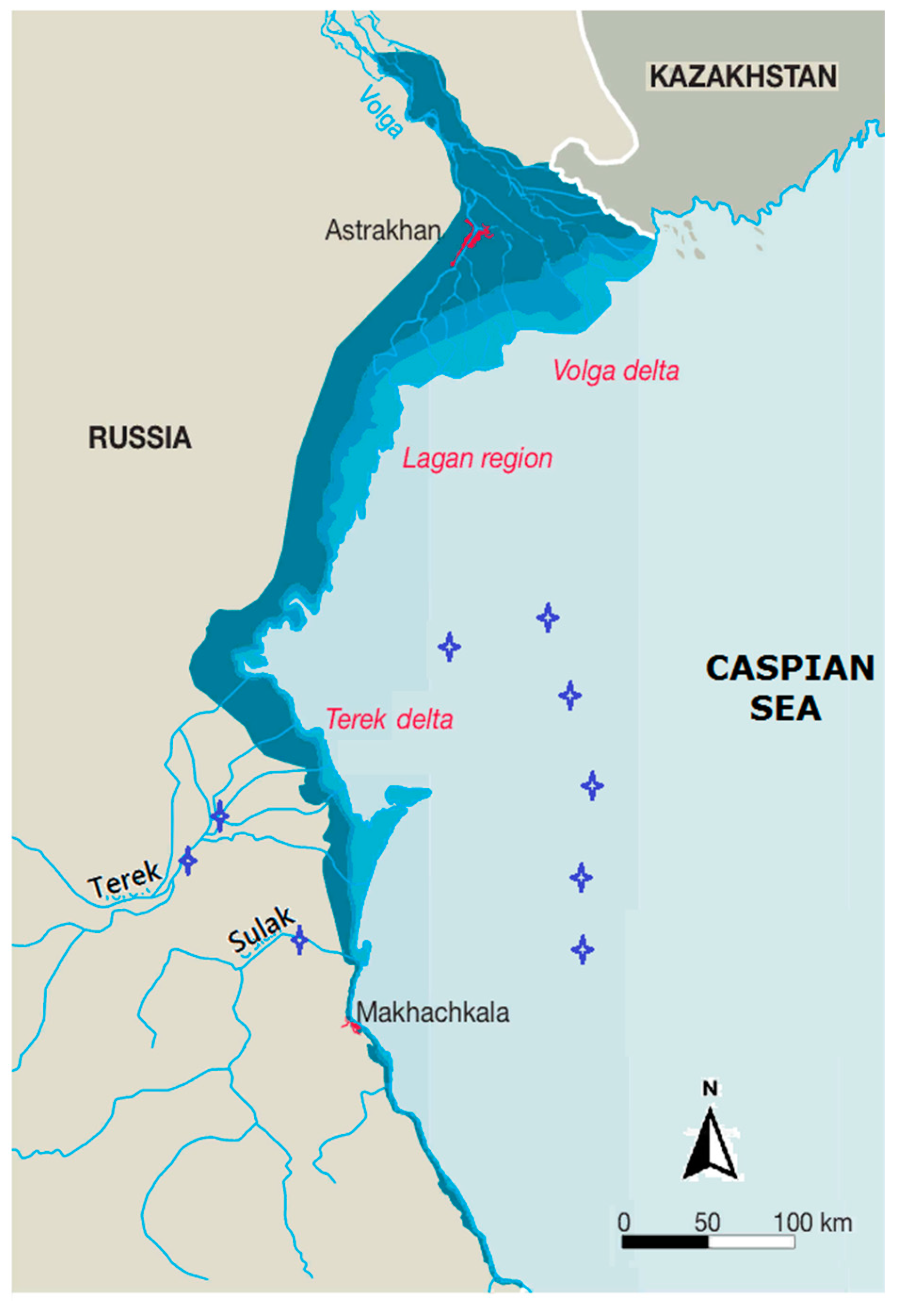
2.2. Study Area
2.3. Pike-Perch Sampling and Diet Analysis
2.4. Pike-Perch Age Determination Methodology
2.5. Statistical Analysis
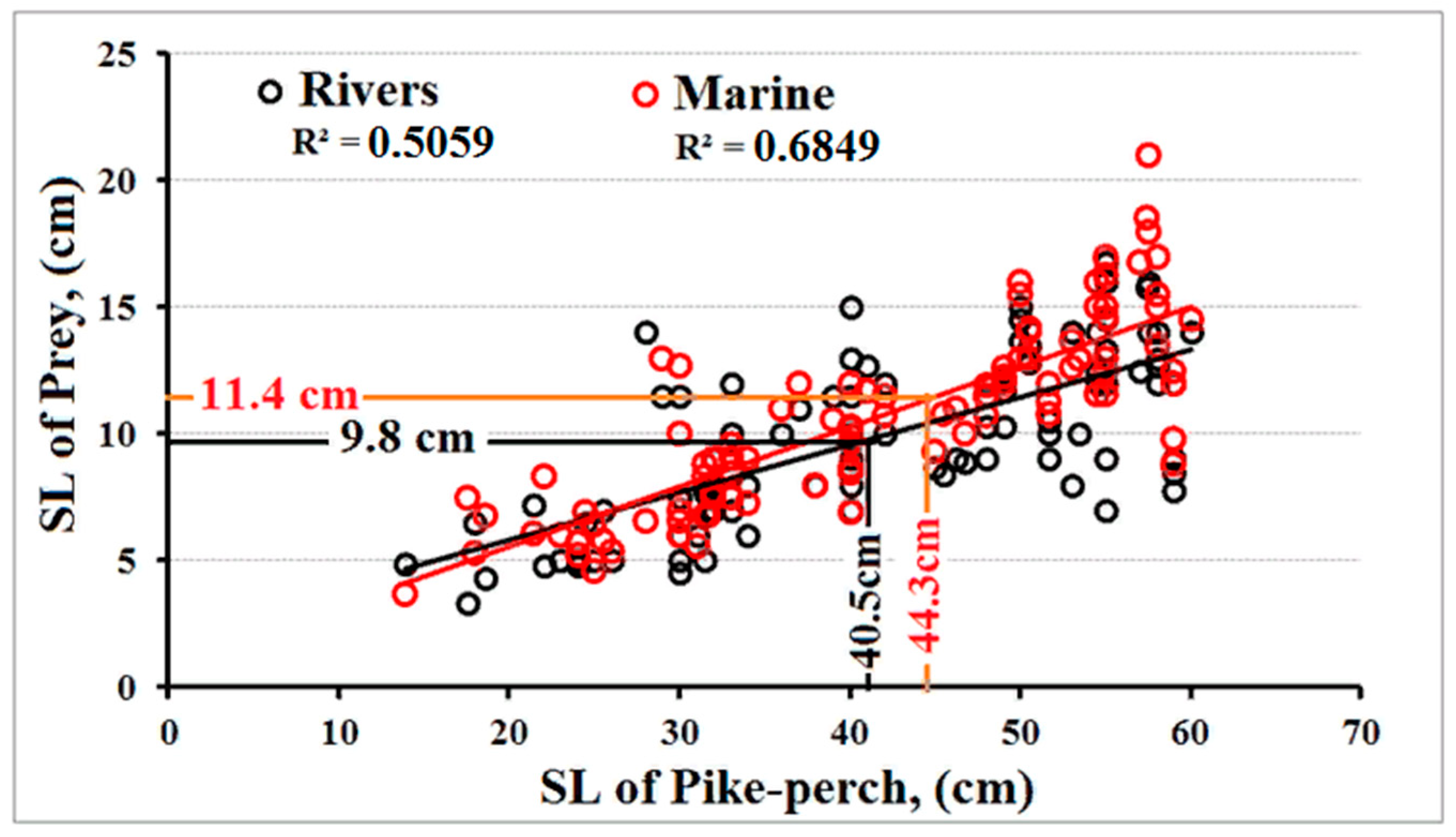
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bănăduc, D.; Simić, V.M.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simi, S.B. Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef] [PubMed]
- Wahltinez, S.J.; Kroll, K.J.; Behringer, D.C.; Arnold, J.E.; Whitaker, B.; Newton, A.L.; Edmiston, K.; Hewson, I.; Stacy, N.I. Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors. Fishes 2023, 8, 51. [Google Scholar] [CrossRef]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple freshwater stressors-Key drivers for the future of freshwater environments. Front. Environ. Sci. 2023, 11, 92. [Google Scholar] [CrossRef]
- Navarro-Ortega, A.; Acuña, V.; Bellin, A.; Burek, P.; Cassiani, G.; Choukr-Allah, R.; Dolédec, S.; Elosegi, A.; Ferrari, F.; Ginebreda, A.; et al. Managing the effects of multiple stressors on aquatic ecosystems under water scarcity. The GLOBAQUA project. Sci. Total Environ. 2015, 503–504, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Curtean-Bănăduc, A.; Olosutean, H.; Bănăduc, D. Influence of Environmental Variables on the Structure and Diversity of Ephemeropteran Communities: A Case Study of the Timiș River, Romania. Acta Zool. Bulg. 2016, 68, 215–224. [Google Scholar]
- Wheeler, C. The Ecosystem Role of Fishes in Lotic Environments. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2014; p. 3694. [Google Scholar]
- Villéger, S.; Brosse, S.; Mouchet, M.A.; Mouillot, D.; Vanni, M.J. Functional ecology of fish: Current approaches and future challenges. Aquat. Sci. 2017, 79, 783–801. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Zubcov, N.; Zubcov, E.; Schnenk, D. The dynamics of metals in fish from Nistru and Prut rivers (Moldova). Transylv. Rev. Syst. Ecol. Res. 2008, 6, 51–58. [Google Scholar]
- Curtean-Bănăduc, A.; Marić, S.; Gabor, G.; Didenko, A.; Rey Planellas, S.; Bănăduc, D. Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians—Conservation elements. Turk. J. Zool. 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Jeeva, V.; Kumar, S.; Verma, D.; Rumana, H.S. River fragmentation and connectivity problems in Gange River of upper Himalayas: The effect on the fish communities (India). Transylv. Rev. Syst. Ecol. Res. 2011, 12, 75–90. [Google Scholar]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The role of aquatic refuge habitats for fish, and threats in the context of climate change and human impact, during seasonal hydrological drought in the Saxon Villages area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Sosai, A.S. Illegal fishing in southern Mannar Island coastal area (Sri Lanka). Transylv. Rev. Syst. Ecol. Res. 2015, 17, 95–108. [Google Scholar]
- Zare-Shahraki, M.; Ebrahimi-Dorche, E.; Bruder, A.; Flotermersch, J.; Blocksom, K.; Bănăduc, D. Fish species composition, distribution and community structure in relation to environmental variation in a semi-arid mountainous river basin, Iran. Water 2022, 14, 2226. [Google Scholar] [CrossRef]
- Khoshnood, Z. Effects of environmental pollution on fish: A short review. Transylv. Rev. Syst. Ecol. Res. 2017, 19, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Bourillon, B.; Feunteun, E.; Acou, A.; Trancart, T.; Teichert, N.; Belpaire, C.; Dufour, S.; Bustamante, P.; Aarestrup, K.; Walker, A.; et al. Anthropogenic Contaminants Shape the Fitness of the Endangered European Eel: A Machine Learning Approach. Fishes 2022, 7, 274. [Google Scholar] [CrossRef]
- Baker, S.M.; Reyier, E.A.; Ahr, B.J.; Cook, G.S. Assessing the Effects of Physical Barriers and Hypoxia on Red Drum Movement Patterns to Develop More Effective Management Strategies. Fishes 2023, 8, 171. [Google Scholar] [CrossRef]
- Kar, D. Wetlands and their fish diversity in Assam (India). Transylv. Rev. Syst. Ecol. Res. 2019, 21, 47–94. [Google Scholar] [CrossRef] [Green Version]
- Bănăduc, D.; Maric, S.; Cianfaglione, K.; Afanasyev, S.; Somogyi, D.; Nyeste, K.; Antal, L.; Kosco, J.; Caleta, M.; Wanzenbock, J.; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species drive a Fish to the Edge of Extinction. Sustainability 2022, 14, 13493. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Ahammad, A.K.S.; Mahalder, B.; Alam, M.M.; Hasan, N.A.; Bashar, A.; Biswas, J.C.; Haque, M.M. Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study. Fishes 2022, 7, 270. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Mihuț, C.; Burcea, A.; McCall, G.S.; Matei, C.; Bănăduc, D. Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem; Chondrostoma nasus (Linnaeus, 1758) Case Study. Water 2023, 15, 1578. [Google Scholar] [CrossRef]
- Taiwo, I.O.; Olopade, O.A.; Bamidele, N.A. Heavy metal concentration in eight fish species from Epe Lagoon (Nigeria). Transylv. Rev. Syst. Ecol. Res. 2019, 21, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Reid, D.F.; Orlova, M.I. Geological and evolutionary underpinnings for the success of Ponto-Caspian species invasions in the Baltic Sea and North American Great Lakes. Can. J. Fish. Aquat. Sci. 2002, 59, 1144–1158. [Google Scholar] [CrossRef]
- Zonn, I.S.; Kosarev, A.N.; Glantz, M.H.; Kostianoy, A.G. The Caspian Sea Encyclopedia; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Vinetskaya, N.I. Salinity of the waters of the Northern Caspian. Bull. VNIRO 1959, 38, 26–52. [Google Scholar]
- Mankova, N.Y. Ecological and Biological Features of the Formation of Stocks of Common Pike Perch in the Volga-Caspian Region. Ph.D. Thesis, Astrakhan State Technical University, Astrakhan, Russia, 2003; 149p. [Google Scholar]
- Glazovsky, N.F. Salt balance of the Caspian Sea. Nature 1972, 10, 63–70. [Google Scholar]
- Sudakov, G.A.; Katunin, D.N.; Khodorevskaya, R.P. Environmental Monitoring Studies at the Severny License Area of Lukoil-Nizhnevolzhskneft LLC (1997–2006); KaspNIRKh Publishing House: Astrakhan, Russia, 2007; 432p. [Google Scholar]
- Guidance Document RD 52.15.880-2019. Guidelines for the Organization and Conduct of Observations, Assessment of the State and Pollution of the Marine Environment in the Areas of Exploration and Development of Offshore Oil and Gas Fields; Federal Service for Hydrometeorology and Environmental Monitoring (FSHEM), Caspian Marine Scientific Research Center (CMScRC): Astrakhan, Russia, 2019; 62p.
- Matishov, D.G.; Yaitskaya, N.A. Changes in the temperature and salinity of the waters of the Caspian Sea in the XX century. Oceanology 2018, 58, 864–874. [Google Scholar] [CrossRef]
- Aladin, N.; Plotnikov, I. Hydrobiology of the Caspian Sea, Lake Basin Management Initiative Thematic Paper; Springer: Dordrecht, The Netherlands, 2004; 29p. [Google Scholar]
- Kharchenko, T.A. Biodiversity of the Ponto-Caspian Relict Fauna in the Danube Basin (Review). Hydrobiol. J. 2005, 41, 57–79. [Google Scholar] [CrossRef]
- Abdurakhmanov, G.M.; Abdulmedzhidov, A.A.; Israpilov, I.M.; Guseinova, S.A. Ecological and zoogeographical assessment of biodiversity of the Caspian Sea. South Russ. Ecol. Dev. 2014, 7, 10–27. [Google Scholar] [CrossRef]
- Bănăduc, D.; Rey, S.; Trichkova, T.; Lenhardt, M.; Curtean-Bănăduc, A. The Lower Danube River—Danube Delta—North West Black Sea: A pivotal area of major interest for the past, present and future of its fish fauna—A short review. Sci. Total Environ. 2016, 545–546, 137–151. [Google Scholar] [CrossRef]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea coast area lakes, a broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Riede, K. Global Register of Migratory Species—From Global to Regional Scales; Final Report of the R&D-Projekt 808 05 081; Federal Agency for Nature Conservation: Bonn, Germany, 2004; 329p. [Google Scholar]
- Gerstmeier, R.; Romig, T. Die Süßwasserfische Europas: Für Naturfreunde und Angler; Franckh-Kosmos Verlag: Stuttgart, Germany, 1998; 368p. [Google Scholar]
- Oţel, V. Atlasul Peştilor Din Rezervaţia Biosferei Delta Dunării; Centrul de Informare Tehnologică Delta Dunării: Tulcea, Romania, 2007; 481p. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof: Berlin, Germany, 2007; 646p. [Google Scholar]
- Muus, B.J.; Dahlström, P. Süßwasserfische; BLV Verlagsgesellschaft: München, Germany, 1968; 224p. [Google Scholar]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Bănăduc, D. The benthic trophic corner stone compartment in POPs transfer from abiotic environment to higher trophic levels—Trichoptera and Ephemeroptera pre-alert indicator role. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Jiang, R.; Deng, Z.; Li, J.; Xiao, Y.; Xu, Y.; Wang, J.; Li, T.; Zhang, C. The “Journey” of Microplastics across the Marine Food Web in China’s Largest Fishing Ground. Water 2023, 15, 445. [Google Scholar] [CrossRef]
- Bănăduc, D.; Oprean, L.; Bogdan, A.; Curtean-Bănăduc, A. The analyse of the trophic resources utilisation by the congeneric species Barbus barbus (Linnaeus, 1758) and Barbus meridionalis Risso, 1827 in Târnava River Basin (Transylvania, Romania). Transylv. Rev. Syst. Ecol. Res. 2011, 12, 101–118. [Google Scholar]
- Qin, Q.; Zhang, F.; Liu, F.; Wang, C.; Liu, H. Food Web Structure and Trophic Interactions Revealed by Stable Isotope Analysis in the Midstream of the Chishui River, a Tributary of the Yangtze River, China. Water 2021, 13, 195. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Bănăduc, D. Trophic elements regarding the non-indigenous Pseudorasbora parva (Schlegel) 1842 fish species spreading success—Olt River Basin, a case study. J. Bioloy Zoo. 2008, 6, 185–196. [Google Scholar]
- Latorre, D.; Masó, G.; Hinckley, A.; Verdiell-Cubedo, D.; Castillo-García, G.; González-Rojas, A.G.; Black-Barbour, E.N.; Vila-Gispert, A.; García-Berthou, E.; Miranda, R.; et al. Interpopulation Variability in Dietary Traits of Invasive Bleak Alburnus alburnus (Actinopterygii, Cyprinidae) Across the Iberian Peninsula. Water 2020, 12, 2200. [Google Scholar] [CrossRef]
- Mazzoni, M.; Ferrario, C.; Bettinetti, R.; Piscia, R.; Cicala, D.; Volta, P.; Borgå, K.; Valsecchi, S.; Polesello, S. Trophic Magnification of Legacy (PCB, DDT and Hg) and Emerging Pollutants (PFAS) in the Fish Community of a Small Protected Southern Alpine Lake (Lake Mergozzo, Northern Italy). Water 2020, 12, 1591. [Google Scholar] [CrossRef]
- Qin, J.; Xie, S.; Cheng, F. Broad Diet Composition and Seasonal Feeding Variation Facilitate Successful Invasion of the Shimofuri Goby (Tridentiger bifasciatus) in a Water Transfer System. Water 2020, 12, 3411. [Google Scholar] [CrossRef]
- He, C.; Deng, H.; Ba, J.; Li, S.; Chen, Z.; Tao, Y.; Duan, X.; Liu, S.; Li, Y.; Chen, D. Food Chain Length Associated with Environmental Factors Affected by Large Dam along the Yangtze River. Water 2020, 12, 3157. [Google Scholar] [CrossRef]
- Schlumberger, O.; Proteau, J.-P. Reproduction of pike-perch (Stizosterdion lucioperca) in captivity. J. Appl. Ichthyol. 1996, 12, 149–152. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Frankiewicz, P.; Dabrowski, K.; Martyniak, A.; Zalewski, M. Cannibalism as a regulatory force of pike-perch, Stizostedion lucioperca (L.), population dynamics in the lowland Sulejów reservoir (Central Poland). Hydrobiologia 1999, 408/409, 47–55. [Google Scholar] [CrossRef]
- Lappalainen, J.; Olin, M.; Vinni, M. Pike-perch cannibalism: Effects of abundance, size and condition. Ann. Zool. Fenn. 2006, 43, 35–44. [Google Scholar]
- Lehtonen, H.; Hansson, S.; Winkler, H.M. Biology and exploitation of pike-perch, Stizostedion lucioperca (L.), in the Baltic Sea area. Ann. Zool. Fenn. 1996, 33, 525–535. [Google Scholar]
- Persson, A.; Brönmark, C. Foraging capacity and resource synchronization in an ontogenetic diet switcher, pike-perch (Stizostedion lucioperca). Ecology 2002, 83, 3014–3022. [Google Scholar] [CrossRef]
- Samoilov, K.Y. Population Structure and Phenetic Diversity of the Walleye Sander lucioperca (L.) of the Volga-Akhtuba System of the Lower Volga. Ph.D. Thesis, Moscow State University, Moscow, Russia, 2017; 155p. (In Russian). [Google Scholar]
- Verreth, J.; Kleyn, K. The effect of biomanipulation of the zooplankton on the growth, feeding and survival of pike-perch (Stizostedion lucioperca) in nursing ponds. J. Appl. Ichthyol. 1987, 3, 13–23. [Google Scholar] [CrossRef]
- Steffens, W.; Geldhauser, F.; Gerstner, P.; Hilge, V. German experiences in the propagation and rearing of fingerling of pike-perch (Stizostedion lucioperca). Ann. Zool. Fenn. 1996, 33, 627–634. [Google Scholar]
- Antalfi, A. Propagation and Rearing of Pike Perch in Pond Culture; EIFAC Technical Paper; FAO: Rome, Italy, 1979; Volume 35, pp. 120–125. [Google Scholar]
- Woynarowich, E. Rearing of pike perch larvae up to predatory fish age. Z. Fisch. 1960, 9, 73–83. (In German) [Google Scholar]
- Dörner, H.; Hülsmann, S.; Hölker, F.; Skov, C.; Wagner, A. Size-dependent predator-prey relationships between pike-perch and their prey fish. Ecol. Freshwat. Fish. 2007, 16, 307–314. [Google Scholar] [CrossRef]
- Van Densen, W.L.T. Piscivory and the development of bimodality in the size distribution of 0+ pike-perch (Stizostedion lucioperca L.). J. Appl. Ichthyol. 1985, 1, 119–131. [Google Scholar] [CrossRef]
- Persson, A.; Brönmark, C. Pike-perch Sander lucioperca trapped between niches: Foraging performance and prey selection in a piscivore on a planktivore diet. J. Fish. Biol. 2008, 73, 793–808. [Google Scholar] [CrossRef]
- Bogutskaya, N.G.; Kiyashko, V.; Naseka, A.M.; Orlova, M.I. Identification Keys for Fish and Invertebrates. In Fish and Shellfish; Scientific Press LLC: Moscow, Russia, 2013. (In Russian) [Google Scholar]
- Zhu, X.; Wastle, R.; Leonard, D.; Howland, K.; Carmichael, T.J.; Tallman, R.F. Comparison of Scales, Pectoral Fin Rays, and Otoliths for Estimating Age, Growth, and Mortality of Lake Whitefish, Coregonus clupeaformis, in Great Slave Lake; Canadian Science Advisory Secretariat: Ottawa, ON, Canada, 2017. [Google Scholar]
- Fortunatova, K.P.; Popova, O.A. Feeding and Food Relationships in Predatory Fishes in the River Volga Delta; Nauka: Moscow, Russia, 1973. [Google Scholar]
- Dorofeeva, E.A. Chromosome complexes of Sevan trout Salmo ischchan in connection with the karyosystematics of salmon. Zool. J. 1967, 46, 248–253. [Google Scholar]
- Abdusamadov, A.S.; Huseynova, S.A.; Dudurkhanova, L.A. Analysis of the state of reserves and fishing of biological resources of the western part of the Middle Caspian and prospects for using their resource potential. South Russ. Ecol. Dev. 2016, 2, 70–83. [Google Scholar] [CrossRef]
- Alibekova, Z.G.; Rabazanov, R.N. Pre-Caucasian trout (Salmo trutta ciscaucasicus)—As a necessary component of the Caspian ichthyofauna. Rybnoe hozyajstvo. Fisheries 2022, 2022, 64–68. [Google Scholar] [CrossRef]
- Abdusamadov, A.S. Prospects for the development of coastal fishing in the Western Caspian region of Russia. Fisheries (Rybnoe khozyaistvo) 2004, 6, 8–10. (In Russian) [Google Scholar]
- Abdusamadov, A.S. Fish stocks state and prospects for development of coastal fishing in Tersko Caspian region. Rybnoe khozyaistvo. Fisheries 2007, 3, 61–63. (In Russian) [Google Scholar]
- Shakirova, F.M.; Anokhina, O.K.; Smirnov, A.A.; Valieva, G.D. Modern commercial and biological characteristics of the Pike Perch of the Kuibyshev reservoir. Fishing Problems 2022, 23, 91–101. [Google Scholar] [CrossRef]
- Craig, J.F. Percid Fishes: Systematics, Ecology, and Exploitation; Blackwell Science: Oxford, UK, 2000. [Google Scholar]
- Bozek, M.A.; Baccante, D.A.; Lester, N.P. Walleye and sauger life history. In Biology, Management, and Culture of Walleye and Sauger; Barton, B.A., Ed.; American Fisheries Society: Bethesda, MD, USA, 2011; pp. 233–301. [Google Scholar]
- Bolotova, N.L.; Zuyanova, O.V.; Zuyanov, E.A.; Shitova, S.V. Acclimatization of the pikeperch Stizostedion lucioperca and its incorporation into the system of food relations in Lake Vozhe. Vopr. Ikhtiol. 1995, 35, 374–387. [Google Scholar]
- Larsen, L.K.; Berg, S. Invasive Alien Species Fact Sheet—Stizostedion lucioperca. Online Database of the North European and Baltic Network on Invasive Alien Species—NOBANIS, 2006. Available online: http://www.nobanis.org (accessed on 11 February 2023).
- Gagne, J.J. The Pikeperch and Its Culture; Ecole National Veterinaries de Toulouse: Toulouse, France, 1977; Volume 97, 161p. [Google Scholar]
- Freyhof, J.; Kottelat, M. Sander lucioperca. In IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2. 2008. Available online: http://www.iucnredlist.org/apps/redlist/details/20860/0 (accessed on 20 November 2022).
- Shakirova, F.M.; Severov, Y.A.; Udachin, S.A.; Valieva, G.D. Diet of pikeperch (Sander lucioperca (L, 1758)) of the central part of the Kuibyshev reservoir in different seasons of the year. Proc. Samara Sci. Cent. Russ. Acad. Sci. 2017, 2, 346–354. [Google Scholar]
- Linfield, R.S.J.; Rickards, R.B. The zander in perspective. Fish Manag. 1979, 10, 1–16. [Google Scholar] [CrossRef]
- Welcomme, R.L. International Introductions of Inland Aquatic Species; FAO fisheries technical paper; FAO: Rome, Italy, 1988; Volume 294, p. 318. [Google Scholar]
- Crivelli, A.J. Are fish introductions a threat to endemic fresh-water fishes in the Northern Mediterranean Region. Biol. Cons. 1995, 72, 311–319. [Google Scholar] [CrossRef]
- Saipulaev, I.M.; Eldarov, E.M. Water Resources of Dagestan: State and Problems; Dagestan Geographical Society: Makhachkala, Russia, 1996; 180p. [Google Scholar]
- Faridovna, K.N. The Current Ecological and Toxicological State of the Terek River and the Dagestan Coast of the Middle Caspian Sea; Abstract of the Dissertation for the PhD Thesis Degree of Candidate of Biological Sciences; University of Makhachkala: Makhachkala, Russia, 2004. [Google Scholar]
- Lavrova, O.Y.; Nazirova, K.R.; Alferyeva, Y.O.; Zhadanova, P.D.; Strochkov, A.Y. Comparison of plume parameters of the Sulak and Terek rivers based on satellite data and in situ measurements. Mod. Probl. Remote Sens. Earth Space 2022, 19, 264–283. [Google Scholar] [CrossRef]
- Zimnov, S.A.; Kerimov, A.A.; Shteinman, B.S. Delta-Formation Processes in Rivers on the Western Caspian Coast and the Problems of Rational Development of the Natural Resources in Mouth Areas, Leningrad; Gidrometeoizdat: Moscow, Russia, 1986. [Google Scholar]
- Baidin, S.S.; Skriptunov, N.A.; Shteinman, B.S.; Gan, G.N. Hydrology of Terek and Sulak Mouth Areas; Gidrometeoizdat: Moscow, Russia, 1971. [Google Scholar]
- Mikhailova, M.V. Present-Day Channel Deformations in the Mouth Areas of the Terek and Sulak. Tr. Gos. Okeanogr. Inst 1991, 198, 38–46. [Google Scholar]
- Korotaev, V.N.; Rychagov, G.I. Investigation of Relationships Between Geostructural Conditions and Morphogenetic Types of River Mouth Systems. In Proceeding of the International Scientific Conference Deltas: Genesis, Dynamics, Modeling and Sustainable Development, Istomino, Republic of Buryatia, Russian Federation, 21–25 July 2014; Publishing house «Red Box»: Ulan-Ude, Republic of Buryatia, 2014; 120p. [Google Scholar]
- Popova, O.A.; Sytina, L.A. Food and feeding relations of Eurasian perch (Perca Fluvatilis) and pike-perch (Stizostedion lucioperca) in various waters of the USSR. J. Fish. Res. Board Can. 1976, 34, 1559–1570. [Google Scholar] [CrossRef]
- Popova, O.A. The role of Predatory Fish in Ecosystems. Variability of Fish in Freshwater Ecosystems. Collection of Papers, Institute of Evolution, Morphology and Ecology of Animals Named after A.N. Severtsov; Nauka Publishing House: Moscow, Russia, 1979; pp. 13–47. [Google Scholar]
- Konovalov, A.F. The Role of Walleye (Stizostedion lucioperca (L.)) in the Ecosystems of Large Lakes of the Vologda Region: Dissertation of the Candidate of Master Thesis in Biological Sciences; University of Petrozavodsk: Petrozavodsk, Russia, 2004; 127p. [Google Scholar]
- Kovalenko, E.O. Morphobiological Characteristics of Pikeperch (Sander lucioperca, L.) and Its Role in the Ecosystem of the Krasnodar Reservoir: Dissertation Thesis of Candidate of Biological Sciences; KubGU: Krasnodar, Russia, 2015; 133p. (In Russian) [Google Scholar]
- Van Densen, W.L.T. Predator enhancement in freshwater fish communities. In Rehabilitation of Freshwater Fisheries; Cowx, I.G., Ed.; Blackwell Scientific Publications Ltd.: Oxford, UK, 1994; p. 102. [Google Scholar]
| Composition of Diet Elements | Terek and Sulak Rivers | Western Caspian Region | Significant |
|---|---|---|---|
| Caspian roach Rutilus caspicus | 20.5 + 1.25 A | 18.1 + 0.98 A | NS |
| Asp Leuciscus aspius | 13 + 0.78 B | 2.2 + 0.15 D | ** |
| European Perch Perca fluviatilis | 9.5 + 1.14 C | 2.4 + 0.56 D | ** |
| Terek nase Chondr.oxyrhynchum | 8.3 + 0.94 C | 11.2 + 1.25 B | * |
| Ruffe Gymnocephalus cernua | 7.5 + 0.87 C | 10.4 1.18 B | * |
| Pike-perch Sander lucioperca | 1.5 + 0.30 E | 1.3 + 0.25 D | NS |
| Freshwater bream Abramis brama | 7.2 + 0.8 C | 3.4 + 0.68 CD | * |
| Pike Esox lucius | 5.8 + 0.7 CD | 2.1 +0.40 D | * |
| Common carp Cyprinus carpio | 3.8 + 0.76 E | 3.1 + 0.65 D | NS |
| Sabrefish Pelecus cultratus | 1.7 + 0.34 E | 0.3 + 0.05 E | * |
| Bleak Alburnus alburnus | 3.5 + 0.75 E | - | |
| Caspian kutum Rutilus frisii | 3.3 +0.80 E | 2.2 + 0.44 D | NS |
| Mysida | 2.4 +0.6 E | 1.3 + 0.36 D | NS |
| Round goby Neogobius melanostomus | 1.3 0.39 E | 6.5 +0.81 C | ** |
| Caspian tyulka Clupeonella caspia | - | 3.2 +0.96 D | |
| Digested fish remains + | 3.4 0.70 E | 4.4% + 0.90 | ** |
| Age | 1+ | 2+ | 3+ | 4+ | 5+ | 6+ | 7+ | 8+ | 9+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| Species/elements in the alimentary tract | Zooplankton | 27.2 + 3.019 a | 9.13 + 1.1 B | – | – | – | – | – | ||
| Mysida | 18.31 + 2.5 A | 7.10 + 0.78 A | 5.6 + 0.65 B | 3.5 + 0.38 C | 1.7 + 0.51 C | 0.75 + 0.04 D | 1.1 + 0.34 D | - | - | |
| Chironomidae | 11.42 + 1.53 A | 10.42 1.4 A | 2.6 + 0.52 B | 0.57 + 0.04 C | 0.3 + 0.02 C | 0.65 + 0.03 C | 1.5 + 0.65 C | 0.53 + 0.06 C | - | |
| Fragments of crustaceans | 15.5 + 2.20 A | 8.7 0.92 B | 4.3 0.9 + 0.9 C | 4.1 + 1.5 C | 2.2 + 0.68 D | 2 + 0.4 D | 1.4 0.56 D | 0.6 0.04 C | - | |
| Fishes: the main ones are listed below | 27.57 + 2.84 C | 58.15 + 5.83 B | 87.7 + 5.94 A | 86.33 8.2 A | 90.8 10.4 D | 96.4 8.8 A | 95.4 + 98 A | 98.87 9.23 A | 100 +0.0 A | |
| Caspian roach Rutilus caspicus (Yakovlev, 1870) | 10.71+ 1.72 B | 14.86 + 3.0 B | 20.82 + 1.44 A | 18.62 2.8 A | 20.13 2.46 A | 18.14 + 2.36 A | 20.17 2.38 A | 18.5 2.40 A | 18.6 + 2.39 A | |
| European perch Perca fluviatilis (Linnaeus, 1758) | - | 6.44 + 0.5 B | 11.1 + 1.33 A | 6.15 + 0.75 B | 4.81 + 0.96 C | 7.88 + 1.43 B | 6.32 + 0.36 B | 5.3 + 1.06 B | 7.5 1.5 B | |
| Round goby Neogobius melanostomus (Pallas 1814) | 3.94 +0.97 B | 2.42 + 0.55 C | 6.40 + 0.74 A | 4.5 + 0.54 A | 3.48 + 1.43 B | 5.63 + 0.95 A | 5.85 + 0.95 A | 3.25 + 0.28 B | 4.8 + 1.5 B | |
| Caspian tyulka Clupeonella caspia (Svetividov, 1941) | 2.12 + 0.34 B | 4.23 + 0.5 B | 6.65 0.76 A | 3.5 0.85 B | 3.8 + 0.83 B | 5.8 0.68 A | 6.3 + 0.72 A | 6.1 + 1.3 A | 7.7 + 1.14 A | |
| Ruffe Gymnocephalus cernua (Linnaeus, 1758) | 0.41 + 0.04 B | 7.5 + 0.87 A | 7.7 + 0.84 A | 6.2 + 0.92 | 5.44 + 0.94 A | 6.53 + 0.98 A | 7.21 + 0.91 A | 7.75 + 1.25 A | 7.25 + 1.05 A | |
| Pike-perch Sander lucioperca (Linnaeus, 1758) | - | 2.7 + 0.36 C | 6.6 + 0.72 B | 5.21 + 0.71 B | 9.6 + 1.48 A | 3.72 + 0.9 C | 2.46 + 0.5 C | 4.15 + 0.55 B | 3.5 + 0.75 C | |
| Caspian kutum Rutilus frisii (Nordmann 1840) | - | - | - | 7.5 + 0.82 A | 5.25 + 1.75 B | 5.0 0.75 B | 4.5 + 0.55 B | 4.65 + 1.13 B | 4.5 + 0.416 B | |
| Sabrefish Pelecus cultratus (Linnaeus, 1758) | - | - | - | 3.12 + 0.42 a | 5.17 + 0.87 a | 4.48 + 0.93 a | 5.45 + 0.5 a | 4.75 + 1.2 a | 3.64 + 0.4 a | |
| Pike Esox lucius (Linnaeus, 1758) | - | - | - | 4.56 + 0.60 b | 6.52 + 6.52 + 0.92 a | 7.64 + 0.78 a | 7.35 + 1.45 a | 8.63 + 1.71 a | 7.46 0.96 a | |
| Terek nase Chondr. oxyrhynchum (Kessler, 1877) | - | - | - | 3.98 + 0.33 a | 3.83 + 0.36 a | 4.81 + 0.48 a | 3.90 + 0.52 a | 3.93 + 0.63 a | 2.36 + 0.129 a | |
| Bleak Alburnus alburnus (Linnaeus, 1758) | 9 + 0.89 a | 4.54 + 1.02 b | 8.82 + 1.44 a + 1.1 b | 6.2 + 5.7 + 0.97 b | 5.7 + 0.97 b | 5.35 + 0.85 b | 4.73 + 0.36 b | 6.26 + 0.38 b | 7.2 + 0.94 b | |
| Freshwater bream Abramis brama (Linnaeus, 1758) | - | - | - | - | 11.35 + 1.3 a | 7.85 + 1.8 b | 9.55 + 1.6 a | 10.35 + 1.4 a | 9.6 + 1.9 | |
| Asp Leuciscus aspius (Linnaeus, 1758) | - | - | 5.68 + 1.73 c | 4.25 + 0.65 c | 4.65 + 0.69 c | 8.53 + 1.38 b | 8.82 + 1.41 b | 12.33 + 0.99 a | 11.8 + 1.6 a | |
| Common carp Cyprinus carpio (Linnaeus, 1758) | - | - | - | 2.84 + 0.43 a | 1.07 + 0.47 a | 3.39 + 0.42 a | 1.71 0.81 a | 2.25 + 0.68 a | 2.79 + 0.81 a | |
| Digested fish remains + | 1.4 + 0.4 d | 15.4 + 1.4 a | 13.8 + 1.4 a | 9.7 + 1.1 b | 4.7 + 0.9 c | 1.6 + 0.13d | 1.1 + 0.2 d | 0.67 + 0.2 d | 1.3 + 0.3 d | |
| Months | % of Full Stomachs from the Total Number of Examined | Frequency of Occurrence of Objects (%) by Weight. (Numerator—Rivers, Denominator—Sea) | Lengths of Pike-Perch with Full Stomachs (cm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rivers | Marine | Roach | Asp | Perch | Nase | Ruffe | Pike-Perch | Freshwater Bream | Pike | Carp | Rivers | Marine | |
| March | 71.4 + 3.12 | - | 12.5–16.5 | – | |||||||||
| April | 77.3 + 3.17 A a | 69.2 + 3.34 A a | 9.4 + 3.6 Bb | 13.5 + 3.5 Bb | 4.7 + 2.8 Cc | 4.7 + 2.16 | 6.9 + 3.1 C | 1.5 + 1.05 D | 1.5 + 0.2 D | 1.4 + 0.1 D | 1.5 + 0.1 D | 13.5–61.5 | 15.5–21.7 |
| May | 84.3 + 4.21 A | 63.3 + 3.16 B | 15.4 + 3.65 C a | 15.3 + 3.5 D b | 1.79 + 1.3 D | 4.7 + 2.2 E | 6 + 2.8 E | 2.25 + 0.8 F | 3.9 + 0.8 | 2.5 + 0.3 F | 1.3 + 0.2 F | 21.5–53.5 | 21.5–31.5 |
| June | 62.1 + 2.62 A | 72.7 + 3.36 A | 14 + 3.6 B a | 8.93 + 3.8 C b | 5.4 + 3.2 D | 3.9 + 2.2 D | 4.9 + 2.8 D | 2.4 + 0.5 E | 5.6 + 1.2 D | 2.8 + 0.7 E | 1.7 + 0.01 | 41–53 | 38–49.8 |
| July | 56.2 + 2.82 B | 83.3 + 4.16 A | 11.6 + 3.5C b | 6 + 3.2 C b | 1.87 + 0.15 D | 2.4 + 1.6 D | 4.9 + 1.5 C | 1.8 + 0.7 D | 4 + 0.6 C | 3.3 + 0.4 D | 1.5 + 0.2 E | 23–53 | 23–47.5 |
| August | 45 + 2.8 B | 88.9 + 4.42 A | 6.0 + 1.7 D | 4.16 + 3.6 D b | 2.15 + 1.03 D | 7.4 + 3.2 C | 3 + 1 D | 2.7 + 0.7 D | 3.3 + 1.2 D | 1.35 + 0.2 D | 2 + 0.35 D | 29–42 | 31.5–53 |
| September | 57 + 2.83 B | 73 + 3.81 A | 11.4 + 4.6 C | 5.85 + 2.6 B | 3.7 + 0.8 D | 4.9 + 1.9 D | 6 + 1.4 D | 1.5 + 0.6 E | 5 + 0.7 D | 3.8 + 0.3 D | 3.6 + 0.7 D | 43.5–53 | 28.5–51.8 |
| October | 65.2 + 3.26 A | 63.6 + 3.18 A | 9.6 + 3.5 B | 10.5 + 3.6 B | 5.1 + 2.4 B | 5 + 2.2 B | 6.9 + 1.7 B | 1.3 + 0.2 c | 4.7 + 0.7 B | 3 + 0.3 C | 0.9 + 0.01 | 28.5–51.5 | 29.5–58.5 |
| November | 59.1 | 63.6 | 6.8/9.8 | 10.6/9.5 | 3.8/6.5 | 5.4/4.8 | 5.5/4.1 | 1.2/2.5 | 3.8/3.1 | 3.5/1.7 | 1.3/0.0 | 43.5–49.6 | 42.5–54.6 |
| 66.8 + 6.3 A | 72.7 + 6.5 | 12.8–61 | 15.5–58.5 | ||||||||||
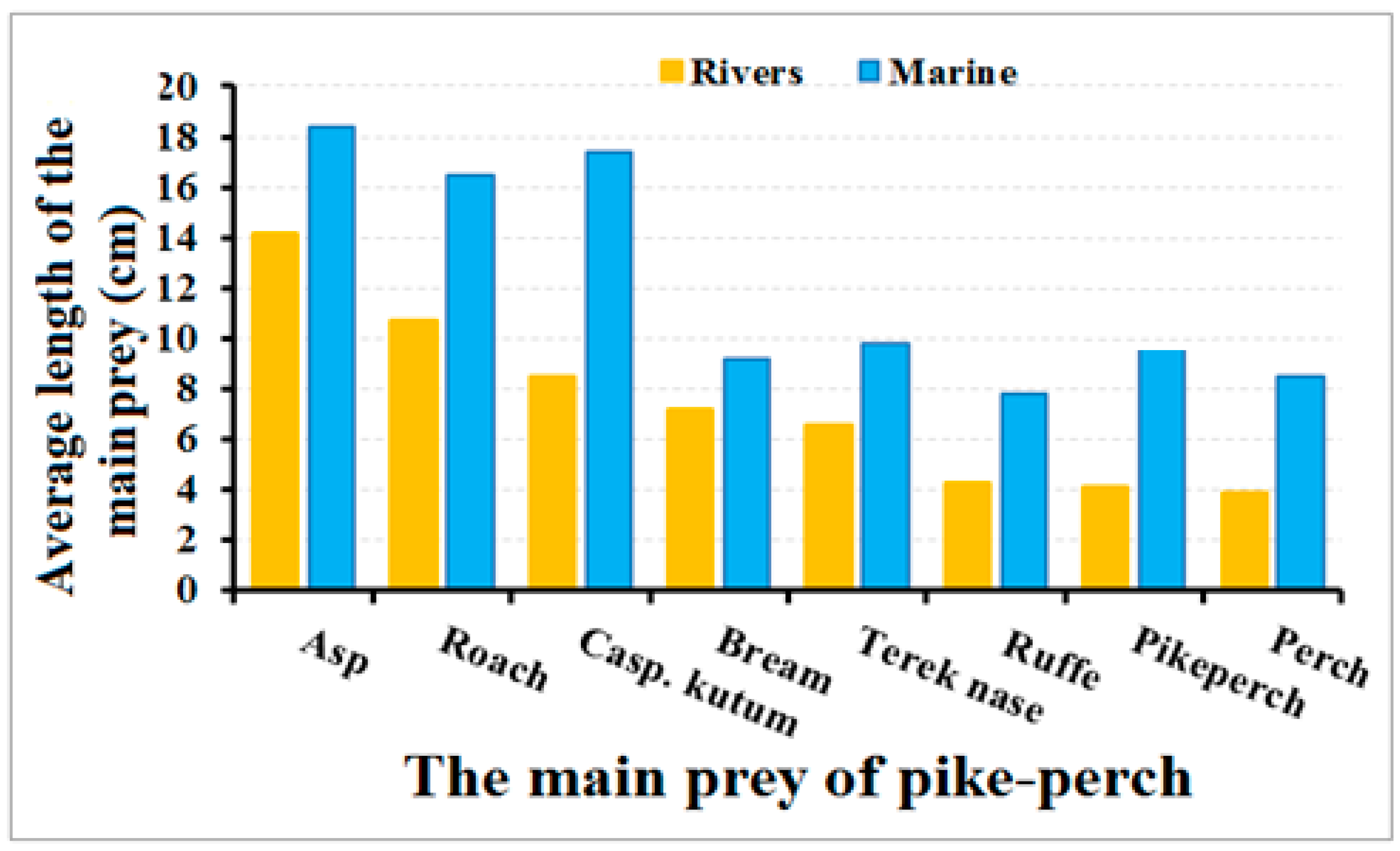
| Main Feeding Prey Species | The Ranges of the Length of Prey, cm | |
|---|---|---|
| Rivers | Sea | |
| Caspian roach | 10.7 ± 1.34/3–16.5 | 11.3 ± 1.24/6.5–17.3 |
| Asp | 14.8 ± 1.2/1.7–21.5 | 18.4± 0.36/8.5–23.2 |
| Perch | 3.92 ± 0.21/1.8–7.5 | 8.5 ± 0.96/5–12 |
| Nase | 6.55 ± 0.42/3.5–11.7 | 9.8 ± 0.81/6–18.5 |
| Ruffe | 4.3 ± 0.43/2.2–6.1 | 7.8 ± 1.16/5–9 |
| Goby | 6.2 ± 1.1/7–10 | 7.5 ± 0.78/6–14.5 |
| Pike-perch | 4.1 ± 0.62/1–4.5 | 9.6 ± 1.06/8–21 |
| Bream | 7.2 ± 0.25/3.5–12.5 | 9.2 ± 0.94/5–12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alieva, A.K.; Nasibulina, B.M.; Bakhshalizadeh, S.; Kurochkina, T.F.; Popov, N.N.; Barbol, B.I.; Bănăduc, D.; Jussupbekova, N.M.; Kuanysheva, G.A.; Ali, A.M. The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study. Fishes 2023, 8, 395. https://doi.org/10.3390/fishes8080395
Alieva AK, Nasibulina BM, Bakhshalizadeh S, Kurochkina TF, Popov NN, Barbol BI, Bănăduc D, Jussupbekova NM, Kuanysheva GA, Ali AM. The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study. Fishes. 2023; 8(8):395. https://doi.org/10.3390/fishes8080395
Chicago/Turabian StyleAlieva, Aminat K., Botagoz M. Nasibulina, Shima Bakhshalizadeh, Tatyana F. Kurochkina, Nikolai N. Popov, Bekzhan I. Barbol, Doru Bănăduc, Nurgul M. Jussupbekova, Gulnur A. Kuanysheva, and Attaala M. Ali. 2023. "The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study" Fishes 8, no. 8: 395. https://doi.org/10.3390/fishes8080395
APA StyleAlieva, A. K., Nasibulina, B. M., Bakhshalizadeh, S., Kurochkina, T. F., Popov, N. N., Barbol, B. I., Bănăduc, D., Jussupbekova, N. M., Kuanysheva, G. A., & Ali, A. M. (2023). The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study. Fishes, 8(8), 395. https://doi.org/10.3390/fishes8080395








