PAHs, Physicochemical and Microbiological Analyses of Trout Processed by Traditional Smoking, in Different Types of Packaging
Abstract
:1. Introduction
2. Materials and Methods
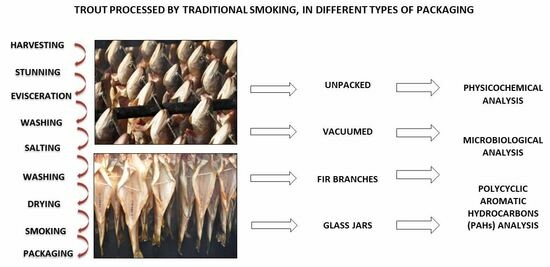
2.1. Fish and Experimental Protocol
2.2. Physicochemical Analysis
2.3. Microbiological Analysis
2.4. Polycyclic Aromatic Hydrocarbons (PAHs) Analysis
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Parameters
3.2. Microbiological Parameters
3.3. Polycyclic Aromatic Hydrocarbons (PAHs)
4. Discussion
4.1. Physico-Chemical Parameters
4.2. Microbiological Analysis
4.3. Polycyclic Aromatic Hydrocarbons (PAHs)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- D’Agaro, E.; Gibertoni, P.; Esposito, S. Recent trends and economic aspects in the rainbow trout (Oncorhynchus mykiss) sector. Appl. Sci. 2022, 12, 8773. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation. 2022. Available online: www.fao.org (accessed on 10 October 2022).
- Krešić, G.; Dujmić, E.; Lončarić, D.; Zrnčić, S.; Liović, N.; Pleadin, J. Fish Consumption: Influence of Knowledge, Product Information, and Satisfaction with Product Attributes. Nutrients 2022, 14, 2691. [Google Scholar] [CrossRef]
- Kitts, D.D.; Pratap-Singh, A.; Singh, A.; Chen, X.; Wang, S. A Risk–Benefit Analysis of First Nation’s Traditional Smoked Fish Processing. Foods 2023, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Smoking of fish and seafood: History, methods and effects on physical, nutritional and microbiological properties. Food Bioprocess Technol. 2012, 5, 831–853. [Google Scholar] [CrossRef]
- European Commission. EUMOFA (European Market Observatory for Fisheries and Aquaculture Products). In Country Analyses; 2020 Edition; Publications Office of the European Union: Luxembourg, 2021; 59p, ISBN 978-92-76-28896-1. [Google Scholar] [CrossRef]
- Bilgin, Ş.; Ünlüsayın, M.; İzci, L.; Günlü, A. The determination of the shelf life and some nutritional components of gilthead sea bream (Sparus aurata L., 1758) after cold and hot smoking. Turk. J. Vet. Anim. Sci. 2008, 32, 49–56. [Google Scholar]
- Messina, C.M.; Arena, R.; Ficano, G.; La Barbera, L.; Morghese, M.; Santulli, A. Combination of freezing, low sodium brine, and cold smoking on the quality and shelf-life of sea bass (Dicentrarchus labrax L.) fillets as a strategy to innovate the market of aquaculture products. Animals 2021, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency (USEPA). Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons; EPA/600/R-93/089; Office of Research and Development, U.S. Environmental Protection Agency: Washington, DC, USA, 1993; 20p.
- Simko, P. Determination of polycyclic aromatic hydrocarbons in smoked meat products and smoke flavouring food additives. J. Chromatogr. B 2002, 770, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Siskos, I.; Zotos, A.; Taylor, K.D.A. The effect of drying, pressure and processing time on the quality of liquid-smoked trout (Salmo gairdnerii) fillets. J. Sci. Food Agric. 2005, 85, 2054–2060. [Google Scholar] [CrossRef]
- Moret, S.; Conte, L.; Dean, D. Assessment of polycyclic aromatic hydrocarbon content of smoked fish by means of a fast HPLC/HPLC method. J. Agric. Food Chem. 1999, 47, 1367–1371. [Google Scholar] [CrossRef]
- Tongo, I.; Ogbeide, O.; Ezemonye, L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 2017, 4, 55–61. [Google Scholar] [CrossRef]
- Tilgner, D.J.; Miler, K. The possibilities of eliminating carcinogens from curing smoke. Przemysł Spożywczy 1963, 17, 85–90. [Google Scholar]
- Stołyhwo, A.; Sikorski, Z.E. Polycyclic aromatic hydrocarbons in smoked fish—A critical review. Food Chem. 2005, 91, 303–311. [Google Scholar] [CrossRef]
- Mihalca, G.L.; Tita, O.; Tita, M.; Mihalca, A. Polycyclic aromatic hydrocarbons (PAHs) in smoked fish from three smoke-houses in Brasov County. J. Agroaliment. Process. Technol. 2011, 17, 392–397. [Google Scholar]
- Coroian, C.O.; Coroian, A.; Becze, A.; Longodor, A.; Mastan, O.; Radu-Rusu, R.-M. Polycyclic Aromatic Hydrocarbons (PAHs) Occurrence in Traditionally Smoked Chicken, Turkey and Duck Meat. Agriculture 2023, 13, 57. [Google Scholar] [CrossRef]
- European Commission (EC). Directive 2005/10/EC of 4 February 2005 laying down sampling methods and the methods of analysis for the official control of the levels of benzo(a)pyrene in foodstuffs. Off. J. Eur. Union 2005, L34, 15. [Google Scholar]
- The Romanian parliament. Legea nr. 43/2014 privind protecţia animalelor utilizate în scopuri ştiinţifice. Monitorul Oficial al României 2014, 326, 229. [Google Scholar]
- European Commission (EC). Directive 2010/63/EU of the European parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79. [Google Scholar]
- Kraemer, E.O.; Stamm, A.J. Mohr’s Method for the Determination of Silver and Halogens in other than Neutral Solutions. J. Am. Chem. Soc. 1924, 46, 2707–2709. [Google Scholar]
- SR ISO 937, 2007; PS-18, IL-18-07, Ed.3: Meat and Meat Products. Determination of Nitrogen Content (Reference Method). International Organization for Standardization (ISO): Geneva, Switzerland, 2007; 3p.
- SR ISO 1443, 2008; PS-20, IL-20-01, Ed.2: Meat and Meat Products. Determination of Total Lipids Content (Reference Method). International Organization for Standardization (ISO): Geneva, Switzerland, 2008; 2p.
- SR ISO 1442, 2010; PS-20, IL-20-06, Ed.2: Meat and Meat Products. Determination of Moisture Content (Reference Method). International Organization for Standardization (ISO): Geneva, Switzerland, 2010; 4p.
- SR ISO 2917, 2007; PS-19, IL-19-08, Ed.2: Meat and Meat Products. Determination of pH (Reference Method). International Organization for Standardization (ISO): Geneva, Switzerland, 2007; 6p.
- Ludorf, W.; Meyer, V. Fische und Fischerzeugnisse; Paul Parey Verlag: Berlin/Hamburg, Germany, 1973; 309p. [Google Scholar]
- European Commission (EC). Directive 95/149/EC: Commission Decision of 8 March 1995 fixing the total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Off. J. Eur. Union 1995, L97, 84–87. [Google Scholar]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemist (AOAC): Washington, DC, USA, 2000. [Google Scholar]
- EN ISO 11290-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes—Part 1: Detection. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- EN ISO 10273:2017; Microbiology of the Food Chain—Horizontal Method for the Detection of Pathogenic Yersinia enterocolitica. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- BS ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-chloro-3-indoyl β-D-glucuronide. International Organization for Standardization (ISO): Geneva, Switzerland, 2001.
- SR EN ISO 17993/2006; Water Quality. Determination of 15 Polycyclic Aromatic Hydrocarbons [PAH) in Water by HPLC with Fluorescence Detection after Liquid-Liquid Extraction. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 13 July 2022).
- Belichovska, D.; Belichovska, K.; Pejkovski, Z. Smoke and Smoked Fish Production. Sci. J. Meat Technol. 2019, 60, 37–43. [Google Scholar] [CrossRef]
- Wiernasz, N.; Gigout, F.; Cardinal, M.; Cornet, J.; Rohloff, J.; Courcoux, P.; Vigneau, E.; Skírnisdottír, S.; Passerini, D.; Pilet, M.-F.; et al. Effect of the Manufacturing Process on the Microbiota, Organoleptic Properties and Volatilome of Three Salmon-Based Products. Foods 2021, 10, 2517. [Google Scholar] [CrossRef] [PubMed]
- Tümerkan, A.E.T. Investigations of the Polycyclic Aromatic Hydrocarbon and Elemental Profile of Smoked Fish. Molecules 2022, 27, 7015. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Kuzgun, N.K. Quality changes of nugget prepared from fresh and smoked rainbow trout during chilled storage. Br. Food J. 2018, 120, 2080–2087. [Google Scholar] [CrossRef]
- Sava, A.; Uiuiu, P.; Răducu, C.; Cocan, D.; Constantinescu, R.; Lațiu, C.; Coroian, A.; Ihuț, A.; Mireșan, V. Meat quality of traditionally smoked trout from Trecătoarea Ursului salmonid farm, Brașov County. Sci. Papers Ser. D Anim. Sci. 2020, 63, 427–432. [Google Scholar]
- Tosun, Ș.Y.; Özden, Ö. Survey of inhibition of Listeria monocytogenes in hot-smoked rainbow trout fillets for food safety. J. Food Process. Preserv. 2014, 38, 338–346. [Google Scholar] [CrossRef]
- Fıcıcılar, B.B.; Genccelep, H. A characterization study of hot smoked rainbow trout for each production stages. Int. J. Agric. Innov. Res. 2017, 6, 411–418. [Google Scholar]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Erkan, N.; Tosun, S.Y.; Özden, Ö.; Ulusoy, S. Effects of modified atmosphere and vacuum packaging on inhibition of Listeria monocytogenes and quality in hot-smoked rainbow trout fillets. Arch. Lebensmittelhyg. 2009, 60, 23–29. [Google Scholar]
- Çoban, Ö.E.; Patir, B.; Yilmaz, Ö. Protective effect of essential oils on the shelf life of smoked and vacuum packed rainbow trout (Oncorhynchus mykiss W.1792) fillets. J. Food Sci. Technol. 2014, 51, 2741–2747. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolińska, W.; Grela, E.R.; Bik-Małodzińska, M. Nutrient and mineral profile of chosen fresh and smoked fish. Nutrients 2019, 11, 1448. [Google Scholar] [CrossRef]
- Maga, J.A. Smoke in Food Processing; CRC Press: Boca Raton, FL, USA, 1988; 168p. [Google Scholar]
- Commission Regulation (EC). No 2074/2005 of 5 December 2005 laying down implementing measures for certain products under Regulation (EC) No 853/2004 of the European Parliament and of the Council and for the organization of official controls under Regulation (EC) No 854/2004 of the European Parliament and of the Council and Regulation (EC) No 882/2004 of the European Parliament and of the Council, derogating from Regulation (EC) No 852/2004 of the European Parliament and of the Council and amending Regulations (EC) No 853/2004 and (EC) No 854/2004. Off. J. Eur. Union 2005, L338, 27–59. [Google Scholar]
- Du, G.; Gai, Y.; Zhou, H.; Fu, S.; Zhang, D. Assessment of spoilage microbiota of rainbow trout (Oncorhynchus mykiss) during storage by 16S rDNA sequencing. J. Food Qual. 2022, 2022, 5367984. [Google Scholar] [CrossRef]
- Bienkiewicz, G.; Tokarczyk, G.; Czerniejewska-Surma, B.; Suryn, J. Changes in EPA and DHA content and lipids quality parameters of rainbow trout (Oncorhynchus mykiss, Walbaum) and carp (Cyprinus carpio, L.) at individual stages of hot smoking. Heliyon 2019, 5, e02964. [Google Scholar] [CrossRef] [PubMed]
- El-Lahamy, A.A.; Khalil, K.I.; El-Sherif, S.A.; Mahmud, A.A. Influence of smoking methods and refrigeration storage on physicochemical quality parameters of catfish (Clarias gariepinus) fillets. Oceanogr. Fish. 2019, 8, 155–159. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef]
- Maillet, A.; Denojean, P.; Bouju-Albert, A.; Scaon, E.; Leuillet, S.; Dousset, X.; Jaffrès, E.; Combrisson, J.; Prévost, H. Characterization of Bacterial Communities of Cold-Smoked Salmon during Storage. Foods 2021, 10, 362. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–29. [Google Scholar]
- Mendonca, A.; Thomas-Popo, E.; Gordon, A. Microbiological considerations in food safety and quality systems implementation. In Food Safety and Quality Systems in Developing Countries; Academic Press: Cambridge, MA, USA, 2020; pp. 185–260. [Google Scholar] [CrossRef]
- Zaki, H.M.B.A.; Emara, M.M.T.; Abdallah, M.R.S. Effect of smoke duration on compositional analysis, deterioration criteria, microbial profile and sensory attributes of marine and freshwater fish: A comparative study. Adv. Anim. Vet. Sci. 2021, 9, 1259–1266. [Google Scholar]
- Jakhar, J.K.; Kumar, A.; Vardia, H.K. Hygienic and nutritional quality of Traditional dried and smoked fishes at Kawardha fish Market, Chhattisgarh, India. Bioscan 2015, 10, 1099–1102. [Google Scholar]
- Sulieman, A.M.E.; Mustafa, W.A.; Osman, O.A.; Shommo, S.A. Assessment of the Quality of Smoked Fish Obtained from White Nile River. Bull. Environ. Pharmacol. Life Sci. 2018, 7, 20–25. [Google Scholar]
- Abdul-Baten, M.D.; Won, N.E.; Mohibbullah, M.D.; Yoon, S.J.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Effect of hot smoking Treatment in improving Sensory and Physicochemical Properties of processed Japanese Spanish Mackerel (Scomberomorus niphonius). Food Sci. Nutr. 2020, 8, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; Mohibbullah, M.; Won, N.E.; Baten, M.A.; Sohn, J.H.; Kim, J.-S.; Choi, J.-S. Improved Hot Smoke Processing of Chub Mackerel (Scomber japonicus) Promotes Sensorial, Physicochemical and Microbiological Characteristics. Appl. Sci. 2021, 11, 2629. [Google Scholar] [CrossRef]
- Chen, B.H. Analysis, Formation and Inhibition of Polycyclic Aromatic Hydrocarbons in Foods. An Overview. J. Food Drug Anal. 1997, 5, 25–42. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K.; Monteau, F.; Fowler, S.W. Review of polycyclic aromatic hydrocarbons (PAHs) in fish and fisheries products; a Sri Lankan perspective. Environ. Sci. Pollut. Res. 2020, 27, 20663–20674. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation No. 1881/2006/EC of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs with amendments. Off. J. Eur. Union. 2006, L364, 5–24. [Google Scholar]
- Commission Regulation (EU). 2020/1255 of 7 September 2020 amending Regulation (EC) No 1881/2006 as regards maximum levels of polycyclic aromatic hydrocarbons (PAHs) in traditionally smoked meat and smoked meat products and traditionally smoked fish and smoked fishery products and establishing a maximum level of PAHs in powders of food of plant origin used for the preparation of beverages. Off. J. Eur. Union. 2020, L293/1, 1–4. [Google Scholar]
- Bogdanović, T.; Pleadin, J.; Petričević, S.; Listeš, E.; Sokolić, D.; Marković, K.; Ozogul, F.; Šimat, V. The occurrence of polycyclic aromatic hydrocarbons in fish and meat products of Croatia and dietary exposure. J. Food Compos. Anal. 2019, 75, 49–60. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Christensen, J.H.; Højgård, A.; Granby, K.; Timm-Heinrich, M. Influence of smoking parameters on the concentration of polycyclic aromatic hydrocarbons (PAHs) in Danish smoked fish. Food Addit. Contam. Part A 2010, 27, 1294–1305. [Google Scholar] [CrossRef]
- Ova, G.; Onaran, S. Polycyclic aromatic hydrocarbons contamination in salmon-trout and eel smoked by two different methods. Adv. Food Sci. 1998, 20, 168–172. [Google Scholar]








| Variable | Type of Comparison | Effect | DF * | χ2 Value/ F Value/W Value * | p Value * |
|---|---|---|---|---|---|
| Protein | ANCOVA | Time | 3 | 14.27 | <0.001 |
| Species | 1 | 7.92 | 0.012 | ||
| Time + Species | 3 | 2.45 | 0.100 | ||
| ANOVA | Packaging type | 3 | 0.79 | 0.511 | |
| Nitrogen | ANCOVA | Time | 3 | 9.37 | <0.001 |
| Species | 1 | 5.20 | 0.035 | ||
| Time + Species | 3 | 1.38 | 0.281 | ||
| ANOVA | Packaging type | 3 | 0.62 | 0.61 | |
| Total Lipids | Kruskal-Wallis | Time | 3 | 13.77 | 0.003 |
| Packaging type | 3 | 21.30 | 0.380 | ||
| Wilcoxon | Species | 1 | 57.00 | 0.166 | |
| Moisture | Kruskal-Wallis | Time | 3 | 5.28 | 0.153 |
| Packaging type | 3 | 6.147 | 0.105 | ||
| Wilcoxon | Species | 1 | 140.50 | 0.004 | |
| pH | Kruskal-Wallis | Time | 3 | 7.35 | 0.062 |
| Packaging type | 3 | 1.45 | 0.694 | ||
| Wilcoxon | Species | 1 | 129.00 | 0.023 | |
| TVB-N * | ANCOVA | Time | 3 | 148.29 | <0.001 |
| Species | 1 | 0.07 | 0.793 | ||
| Time + Species | 3 | 0.14 | 0.937 | ||
| ANOVA | Packaging type | 3 | 0.09 | 0.962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sava, A.; Uiuiu, P.; Lațiu, C.; Cocan, D.; Muntean, G.-C.; Papuc, T.; Ihuț, A.; Răducu, C.; Becze, A.; Craioveanu, C.; et al. PAHs, Physicochemical and Microbiological Analyses of Trout Processed by Traditional Smoking, in Different Types of Packaging. Fishes 2023, 8, 424. https://doi.org/10.3390/fishes8080424
Sava A, Uiuiu P, Lațiu C, Cocan D, Muntean G-C, Papuc T, Ihuț A, Răducu C, Becze A, Craioveanu C, et al. PAHs, Physicochemical and Microbiological Analyses of Trout Processed by Traditional Smoking, in Different Types of Packaging. Fishes. 2023; 8(8):424. https://doi.org/10.3390/fishes8080424
Chicago/Turabian StyleSava, Alexandru, Paul Uiuiu, Călin Lațiu, Daniel Cocan, George-Cătălin Muntean, Tudor Papuc, Andrada Ihuț, Camelia Răducu, Anca Becze, Cristina Craioveanu, and et al. 2023. "PAHs, Physicochemical and Microbiological Analyses of Trout Processed by Traditional Smoking, in Different Types of Packaging" Fishes 8, no. 8: 424. https://doi.org/10.3390/fishes8080424
APA StyleSava, A., Uiuiu, P., Lațiu, C., Cocan, D., Muntean, G.-C., Papuc, T., Ihuț, A., Răducu, C., Becze, A., Craioveanu, C., Munteanu, C., Constantinescu, R., & Mireșan, V. (2023). PAHs, Physicochemical and Microbiological Analyses of Trout Processed by Traditional Smoking, in Different Types of Packaging. Fishes, 8(8), 424. https://doi.org/10.3390/fishes8080424