Parasitic Helminths and Freshwater Fish Introduction in Europe: A Systematic Review of Dynamic Interactions
Abstract
1. Introduction
- (1)
- Parasite loss during translocation of their host: this can occur through two mechanisms, either by “missing the boat” (Figure 1a.1), when the introduced hosts do not carry the parasite, or by “drowning on arrival”, when the host or the parasite fails to establish in the novel habitat (early extinction following host establishment, lack of suitable intermediate and/or final hosts in the recipient area) (Figure 1a.2) [18,19]. These mechanisms contribute to the often diminished parasite diversity observed in non-native organisms, e.g., [20,21]. This “release from the enemy” is often cited as a key factor in the success of non-native hosts in their new range [19,22,23]. The discretion of this specific case makes it rather difficult to monitor in the wild.
- (2)
- Co-introduction of parasites with their host without transmission to native fauna (Figure 1b): Co-introduced parasites can establish and spread in their new range by only infecting their original host and not switching to native hosts. This absence of host switch can be attributed to a lack of suitable hosts in the recipient area and the parasite’s host specificity [24].
- (3)
- Transmission of novel parasites to native host (Figure 1c): Non-native hosts carrying parasites can transmit their parasites to native hosts, a host-switching mechanism termed ‘parasite spillover’ [12,24,25]. Pathogen co-introductions may give rise to particularly severe consequences because of the lack of co-evolution between non-native parasites and a native host, which thus does not possess adequate immune response to the infection [26].
- (4)
- Parasite acquisition by non-native fish host: Depending on the host specificity of parasites already present in the recipient area (either native or previously introduced), a non-native host may acquire new parasites in their introduced range [12]. These new interactions can result in parasite spillback, when a non-native species is a competent host for a native parasite and the presence of this additional host results in an increased opportunity to impact native hosts (Figure 1d.1) [27]. Non-native species can also act as sink hosts by being less suitable for native parasites, and thus reduce transmission to native hosts through a dilution effect (Figure 1d.2) [23,28]. The case where a newly acquired parasite can bear noticeable pathogenicity on a non-native should also be noted, e.g., [29], a case which is termed “suppressive spillover” by Chalkowsky et al. [30].
2. Material and Methods
- Did the study include a European country? Yes/No
- Did the study include a fish host? Yes/No
- Did the study include an introduced parasite helminth or a parasite helminth of an introduced fish host? Yes/No
- Did the study include a freshwater environment? Yes/No
- Were the studied fish wild-caught and the infestations natural? Yes/No
- Main information concerning the study: title, date, authors.
- Location: country, watershed, number of sampling sites, habitat type (e.g., river, lake, reservoir), and island/mainland situation.
- Parasite-related information: taxonomy (phylum, class, subclass, order and species), status (native/non-native, and native distribution range for non-native), life cycle (direct/indirect and found on intermediate/final hosts), host specificity, microhabitat, zoonotic status, impact on fish host, socio-economic and/or ecological impact, both shown by the considered study and/or reported with the bibliography.
- Fish host-related information: taxonomy (family, species), status (native/non-native, and native distribution range for non-native), IUCN status, habitat type (e.g., demersal, benthopelagic) and diet, as retrieved from the Fishbase database (https://www.fishbase.se (accessed on 19 June 2023, [32]), and number of hosts examined.
- Methodology: method of detection and identification of parasites.
3. Bibliographical Analysis
4. Co-Introduction of Parasites with Their Fish Host without Transmission to Native Fish
4.1. The Case of North American Centrarchidae and Their Monogenea
4.2. Other Notable Co-Introductions of Fish Parasites in Europe
5. Co-Introduction of Parasites with Their Fish Host with Transmission to Native Fish
5.1. The Case of Anguillicola crassus
5.1.1. Pathogenicity of Anguillicola crassus
5.1.2. Advances in Detection Methods
5.1.3. Factors Involved in A. crassus Success and Importance of Paratenic Hosts
| Family | Species | Origin | Habitat | Diet | Locality |
|---|---|---|---|---|---|
| Centrarchidae | Lepomis gibbosus | Non-native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Detritus | Belgium [94], Czech Republic [21], Hungary [112,115,116,117] |
| Cichlidae | Oreochromis niloticus | Non-native | Benthopelagic | Zoobenthos, Zooplankton, Detritus, Plants | Belgium [94] |
| Amatitlania nigrofasciata | Non-native | Benthopelagic | Nekton, Zoobenthos, Detritus | Germany [118] | |
| Cyprinidae | Alburnus alburnus | Native | Benthopelagic | Zoobenthos, Zooplankton, Detritus, Plants | Belgium [94], Hungary [112,115,116,117] |
| Blicca bjoerkna | Native | Demersal | Zoobenthos, Zooplankton, Detritus, Plants | Hungary [112,116,117] | |
| Carassius carassius | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Detritus | Hungary [116,117] | |
| Carassius gibelio | Unclear | Benthopelagic | Zoobenthos, Detritus | Hungary [112,116,117] | |
| Cyprinus carpio | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Detritus, Plants | Hungary [112,115,116,117] | |
| Leuciscus aspius | Native | Benthopelagic | Zooplankton, Nekton | Hungary [112,116,117] | |
| Phoxinus phoxinus | Native | Demersal | Nekton, Zoobenthos, Zooplankton, Detritus, Plants | France [130] | |
| Pseudorasbora parva | Non-native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Plants | Hungary [112,115,116,117] | |
| Rhodeus amarus | Native | Benthopelagic | Zoobenthos, Plants | Hungary [112,116,117] | |
| Romanogobio albipinnatus | Native | Benthopelagic | Zoobenthos | Hungary [116,117] | |
| Scardinius erythrophthalmus | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Detritus, Plants | Belgium [94], Hungary [112,116,117] | |
| Tinca tinca | Native | Demersal | Nekton, Zoobenthos, Zooplankton, Detritus | Belgium [94], Hungary [112,116,117] | |
| Esocidae | Esox lucius | Native | Pelagic | Nekton, Zoobenthos, Zooplankton | Hungary [112,116] |
| Gasterosteidae | Gasterosteus aculeatus | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Plants | Belgium [94], France [130] |
| Pungitius pungitius | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton | France [130] | |
| Gobiidae | Babka gymnotrachelus | Non-native | Benthopelagic | Nekton, Zoobenthos | Poland [119] |
| Neogobius fluviatilis | Non-native | Benthopelagic | Nekton, Zoobenthos, Zooplankton | Germany [119,120], Hungary [112,116,117] | |
| Neogobius melanostomus | Non-native | Demersal | Zoobenthos | Austria [121,122,131], Croatia [122], Czech Republic [113], Germany [119,120,132], Slovakia [122] | |
| Ponticola kessleri | Non-native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Plants | Germany [119,120], Slovakia [131,133] | |
| Proterorhinus semilunaris | Non-native | Benthopelagic | No data available | Germany [119,120] | |
| Gobionidae | Gobio gobio | Native | Benthopelagic | Zoobenthos, Zooplankton, Plants | Belgium [94], Hungary [112] |
| Ictaluridae | Ameiurus nebulosus | Non-native | Demersal | Nekton, Zoobenthos, Zooplankton, Plants | Belgium [94], Hungary [116] |
| Leuciscidae | Abramis brama | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Detritus, Plants | Hungary [112,115,116] |
| Chondrostoma nasus | Native | Benthopelagic | Detritus, Plants | Belgium [94] | |
| Leuciscus idus | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton | Belgium [94] | |
| Leuciscus leuciscus | Native | Benthopelagic | Zoobenthos, Zooplankton, Detritus, Plants | Belgium [94] | |
| Rutilus rutilus | Native | Benthopelagic | Zoobenthos, Zooplankton, Detritus, Plants | Belgium [94], Hungary [112,115,116,117] | |
| Squalius cephalus | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Plants | Belgium [94] | |
| Osmeridae | Osmerus eperlanus | Native | Pelagic-neritic | Nekton, Zoobenthos, Zooplankton | Netherlands [100] |
| Percidae | Gymnocephalus cernua | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton, Detritus, Plants | Belgium [94], Germany [114], Hungary [112,115,116,117], Poland [114], United Kingdom [134] |
| Perca fluviatilis | Native | Demersal | Nekton, Zoobenthos, Zooplankton | Belgium [94], Hungary [112,116] | |
| Sander lucioperca | Native | Pelagic | Nekton, Zoobenthos, Zooplankton | Belgium [94], Hungary [112,115,116] | |
| Siluridae | Silurus glanis | Native | Benthopelagic | Nekton, Zoobenthos, Zooplankton | Hungary [112,116,117] |
5.2. The Case of Gyrodactylus salaris in Norway
5.3. Other Notable Co-Introductions of Parasites to Native Fish in Europe
6. Non-Native Fish Host and Parasite Acquisition in the Recipient Area
6.1. Ponto-Caspian Gobiidae
6.1.1. Acquisition and Subsequent Spread of Native Parasite
6.1.2. Acquisition of Previously Introduced Parasites
6.2. Other Notable Acquisitions of Parasites by Non-Native Fish in Europe: Perccottus glenii
7. Fish Introduction and Zoonosis
7.1. Eustrongylides
7.2. Anisakids: Contracaecum and Anisakis
7.3. Clinostomum complanatum
7.4. Metagonimus yokogawai
7.5. Dibothriocephalus dendriticus (Syn. Diphyllobothrium dendriticum)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cambray, J.A. Impact on Indigenous Species Biodiversity Caused by the Globalisation of Alien Recreational Freshwater Fisheries. Hydrobiologia 2003, 500, 217–230. [Google Scholar] [CrossRef]
- Clavero, M.; Garcia-Berthou, E. Invasive Species Are a Leading Cause of Animal Extinctions. Trends Ecol. Evol. 2005, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.G.; Erős, T.; Falka, I.; Ferreira, M.T.; Fox, M.G.; Freyhof, J.; Gozlan, R.E.; Grabowska, J.; et al. To Be, or Not to Be, a Non-Native Freshwater Fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- Ellender, B.; Weyl, O. A Review of Current Knowledge, Risk and Ecological Impacts Associated with Non-Native Freshwater Fish Introductions in South Africa. Aquat. Invasions 2014, 9, 117–132. [Google Scholar] [CrossRef]
- Tadese, D.A.; Wubie, A. Impact of the Introduction and Domestication of Alien Fishes. Int. Res. J. Eng. Technol. 2021, 08, 8. [Google Scholar]
- Witkowski, A.; Grabowska, J. The Non-Indigenous Freshwater Fishes of Poland: Threats for Native Ichthyofauna and Consequence for Fishery: A Review. Acta Ichthyol. Piscat. 2012, 42, 77–87. [Google Scholar] [CrossRef]
- Roche, B.; Mattei, J. Les espèces animales introduites dans les eaux douces de Corse. Bull. Fr. Pêche Piscic. 1997, 344–345, 233–239. [Google Scholar] [CrossRef]
- Ribeiro, F.; Leunda, P.M. Non-Native Fish Impacts on Mediterranean Freshwater Ecosystems: Current Knowledge and Research Needs. Fish. Manag. Ecol. 2012, 19, 142–156. [Google Scholar] [CrossRef]
- Goedknegt, M.A.; Feis, M.E.; Wegner, K.M.; Luttikhuizen, P.C.; Buschbaum, C.; Camphuysen, K.; van der Meer, J.; Thieltges, D.W. Parasites and Marine Invasions: Ecological and Evolutionary Perspectives. J. Sea Res. 2016, 113, 11–27. [Google Scholar] [CrossRef]
- Taraschewski, H. Hosts and Parasites as Aliens. J. Helminthol. 2006, 80, 99–128. [Google Scholar] [CrossRef]
- Lambert, A. Introduction de Poissons Dans Les Milieux Aquatiques Continentaux:«Quid de Leurs Parasites?». Bull. Fr. Pêche Piscic. 1997, 344–345, 323–333. [Google Scholar] [CrossRef]
- Prenter, J.; MacNeil, C.; Dick, J.T.A.; Dunn, A.M. Roles of Parasites in Animal Invasions. Trends Ecol. Evol. 2004, 19, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H. Encyclopedia of Parasitology, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-43978-4. [Google Scholar]
- Dove, A.D.M.; Fletcher, A.S. The Distribution of the Introduced Tapeworm Bothriocephalus acheilognathi in Australian Freshwater Fishes. J. Helminthol. 2000, 74, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Balon, E.K. Origin and Domestication of the Wild Carp, Cyprinus carpio: From Roman Gourmets to the Swimming Flowers. Aquaculture 1995, 129, 3–48. [Google Scholar] [CrossRef]
- Lehtonen, H. Alien Freshwater Fishes of Europe. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 153–161. ISBN 978-94-015-9956-6. [Google Scholar]
- Holčík, J. Fish Introductions in Europe with Particular Reference to Its Central and Eastern Part. Can. J. Fish. Aquat. Sci. 1991, 48, 13–23. [Google Scholar] [CrossRef]
- MacLeod, C.J.; Paterson, A.M.; Tompkins, D.M.; Duncan, R.P. Parasites Lost—Do Invaders Miss the Boat or Drown on Arrival? Ecol. Lett. 2010, 13, 516–527. [Google Scholar] [CrossRef]
- Torchin, M.E.; Lafferty, K.D.; Dobson, A.P.; McKenzie, V.J.; Kuris, A.M. Introduced Species and Their Missing Parasites. Nature 2003, 421, 628–630. [Google Scholar] [CrossRef]
- Havlatova, L.; Ondrackova, M.; Prikrylova, I. Monogenean Parasites of Lepomis gibbosus Linnaeus Introduced into the River Durance, France. Helminthologia 2015, 52, 323–330. [Google Scholar] [CrossRef]
- Ondračková, M.; Bartáková, V.; Kvach, Y.; Bryjová, A.; Trichkova, T.; Ribeiro, F.; Carassou, L.; Martens, A.; Masson, G.; Zechmeister, T.; et al. Parasite Infection Reflects Host Genetic Diversity among Non-Native Populations of Pumpkinseed Sunfish in Europe. Hydrobiologia 2021, 848, 2169–2187. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic Plant Invasions and the Enemy Release Hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Dunn, A.M. Chapter 7 Parasites and Biological Invasions. In Advances in Parasitology; Elsevier: London, UK, 2009; Volume 68, pp. 161–184. ISBN 978-0-12-374787-7. [Google Scholar]
- Lymbery, A.J.; Morine, M.; Kanani, H.G.; Beatty, S.J.; Morgan, D.L. Co-Invaders: The Effects of Alien Parasites on Native Hosts. Int. J. Parasitol. Parasites Wildl. 2014, 3, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Gozlan, R.E. Introduction of Non-Native Freshwater Fish: Is It All Bad? Fish Fish. 2008, 9, 106–115. [Google Scholar] [CrossRef]
- Kelly, D.W.; Paterson, R.A.; Townsend, C.R.; Poulin, R.; Tompkins, D.M. Parasite Spillback: A Neglected Concept in Invasion Ecology? Ecology 2009, 90, 2047–2056. [Google Scholar] [CrossRef]
- Kopp, K.; Jokela, J. Resistant Invaders Can Convey Benefits to Native Species. Oikos 2007, 116, 295–301. [Google Scholar] [CrossRef]
- Mierzejewska, K.; Kvach, Y.; Wozniak, M.; Kosowska, A.; Dziekonska-Rynko, J. Parasites of an Asian Fish, the Chinese Sleeper Perccottus glenii, in the Wloclawek Reservoir on the Lower Vistula River, Poland: In Search of the Key Species in the Host Expansion Process. Comp. Parasitol. 2012, 79, 23–29. [Google Scholar] [CrossRef]
- Chalkowski, K.; Lepczyk, C.A.; Zohdy, S. Parasite Ecology of Invasive Species: Conceptual Framework and New Hypotheses. Trends Parasitol. 2018, 34, 655–663. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase 2000: Concepts, Design and Data Sources; ICLARM: Los Baños, Philippines, 2000. [Google Scholar]
- Ondrackova, M.; Davidova, M.; Prikrylova, I.; Pecinkova, M. Monogenean Parasites of Introduced Pumpkinseed Lepomis gibbosus (Centrarchidae) in the Danube River Basin. J. Helminthol. 2011, 85, 435–441. [Google Scholar] [CrossRef]
- Hockley, F.A.; Williams, C.F.; Reading, A.J.; Taylor, N.G.H.; Cable, J. Parasite Fauna of Introduced Pumpkinseed Fish Lepomis gibbosus: First British Record of Onchocleidus Dispar (Monogenea). Dis. Aquat. Org. 2011, 97, 65–73. [Google Scholar] [CrossRef][Green Version]
- Sokolov, S.G.; Protasova, E.N.; Reshetnikov, A.N. Parasite Fauna of Rotan Perccottus glenii Dybowski, 1877 (Osteichthyes, Odontobutidae) in Some Waterbodies of European Russia. Biol. Bull 2013, 40, 862–871. [Google Scholar] [CrossRef]
- Reshetnikov, A.N.; Sokolov, S.G.; Protasova, E.N. The Host-Specific Parasite Nippotaenia mogurndae Confirms Introduction Vectors of the Fish Perccottus glenii in the Volga River Basin. J. Appl. Ichthyol. 2011, 27, 1226–1231. [Google Scholar] [CrossRef]
- Mihok, T.; Košuth, P.; Kočišová, A.; Pekárik, L.; Bártová, E.; Major, P. The Intestinal Parasite Pseudocapillaria tomentosa (Dujardin, 1843) of the Invasive Fish Species Topmouth Gudgeon, Pseudorasbora parva (Temminck and Schlegel), in Slovakia. J. Fish Dis. 2011, 34, 711–714. [Google Scholar] [CrossRef]
- Britton, J.R.; Pegg, J.; Williams, C.F. Pathological and Ecological Host Consequences of Infection by an Introduced Fish Parasite. PLoS ONE 2011, 6, e26365. [Google Scholar] [CrossRef] [PubMed]
- Bazsalovicsová, E.; Králová-Hromadová, I.; Štefka, J.; Scholz, T.; Hanzelová, V.; Vávrová, S.; Szemes, T.; Kirk, R. Population Study of Atractolytocestus huronensis (Cestoda: Caryophyllidea), an Invasive Parasite of Common Carp Introduced to Europe: Mitochondrial Cox1 Haplotypes and Intragenomic Ribosomal ITS2 Variants. Parasitol. Res. 2011, 109, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Antal, L.; Szekely, C.; Molnar, K. Parasitic Infections of Two Invasive Fish Species, the Caucasian Dwarf Goby and the Amur Sleeper, in Hungary. Acta Vet. Hung. 2015, 63, 472–484. [Google Scholar] [CrossRef]
- Mineeva, O.V. Infestation of Fish with the Alien Parasite Nicolla skrjabini (Iwanitzky, 1928) (Trematoda, Opecoelidae) in the Saratov Reservoir. Russ. J. Biol. Invasions 2016, 7, 268–274. [Google Scholar] [CrossRef]
- Rahel, F.J. Homogenization of Freshwater Faunas. Annu. Rev. Ecol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef]
- Moyle, P.B.; Mount, J.F. Homogenous Rivers, Homogenous Faunas. Proc. Natl. Acad. Sci. USA 2007, 104, 5711–5712. [Google Scholar] [CrossRef]
- Kvach, Y.; Drobiniak, O.; Kutsokon, Y.; Hoch, I. The Parasites of the Invasive Chinese Sleeper Perccottus glenii (Fam. Odontobutidae), with the First Report of Nippotaenia mogurndae in Ukraine. Knowl. Manag. Aquat. Ecosyst. 2013, 409, 05. [Google Scholar] [CrossRef]
- Kosuthova, L.; Kosco, J.; Letkova, V.; Kosuth, P.; Manko, P. New Records of Endoparasitic Helminths in Alien Invasive Fishes from the Carpathian Region. Biologia 2009, 64, 776–780. [Google Scholar] [CrossRef]
- Mierzejewska, K.; Martyniak, A.; Kakareko, T.; Hliwa, P. First Record of Nippotaenia mogurndae Yamaguti and Miyata, 1940 (Cestoda, Nippotaeniidae), a Parasite Introduced with Chinese Sleeper to Poland. Parasitol. Res. 2010, 106, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Ondrackova, M.; Matejusova, I.; Grabowska, J. Introduction of Gyrodactylus perccotti (Monogenea) into Europe on Its Invasive Fish Host, Amur Sleeper (Perccottus glenii, Dybowski 1877). Helminthologia 2012, 49, 21–26. [Google Scholar] [CrossRef]
- Stoyanov, B.; Mutafchiev, Y.; Pankov, P.; Georgiev, B.B. Helminth Parasites in the Alien Lepomis gibbosus (L.) (Centrarchidae) from the Lake Atanasovsko Wetlands, Bulgaria: Survey of Species and Structure of Helminth Communities. Acta Zool. Bulg. 2017, 69, 555–574. [Google Scholar]
- Ondrackova, M.; Kvach, Y.; Martens, A.; Jurajda, P. Limited Parasite Acquisition by Non-Native Lepomis gibbosus (Actinopterygii: Centrarchidae) at Two Ponds in the Upper Rhine Basin, Germany. J. Helminthol. 2019, 93, 453–460. [Google Scholar] [CrossRef]
- Sterud, E.; Jørgensen, A. Pumpkinseed Lepomis gibbosus (Linnaeus, 1758) (Centrarchidae) and Associated Parasites Introduced to Norway. Aquat. Invasions 2006, 1, 278–280. [Google Scholar] [CrossRef]
- Šimková, A.; Navrátilová, P.; Dávidová, M.; Ondračková, M.; Sinama, M.; Chappaz, R.; Gilles, A.; Costedoat, C. Does Invasive Chondrostoma nasus Shift the Parasite Community Structure of Endemic Parachondrostoma toxostoma in Sympatric Zones? Parasites Vectors 2012, 5, 200. [Google Scholar] [CrossRef] [PubMed]
- Galli, P.; Stefani, F.; Benzoni, F.; Crosa, G.; Zullini, A. New Records of Alien Monogeneans from Lepomis gibbosus and Silurus glanis in Italy. Parassitologia 2003, 45, 147–150. [Google Scholar]
- Galli, P.; Stefani, F.; Benzoni, F.; Zullini, A. Introduction of Alien Host-Parasite Complexes in a Natural Environment and the Symbiota Concept. Hydrobiologia 2005, 548, 293–299. [Google Scholar] [CrossRef]
- Galli, P.; Strona, G.; Benzoni, F.; Crosa, G.; Stefani, F. Monogenoids from Freshwater Fish in Italy, with Comments on Alien Species. Comp. Parasitol. 2007, 74, 264–272. [Google Scholar] [CrossRef]
- Pettersen, R.A.; Østbye, K.; Holmen, J.; Vøllestad, L.A.; Mo, T.A. Gyrodactylus Spp. Diversity in Native and Introduced Minnow (Phoxinus phoxinus) Populations: No Support for “the Enemy Release” Hypothesis. Parasites Vectors 2016, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.A.; Kristoffersen, R.; Knudsen, R.; Jakobsen, J.; Marcogliese, D.J.; Locke, S.A.; Primicerio, R.; Amundsen, P.-A. Parasite Communities of Two Three-Spined Stickleback Populations in Subarctic Norway-Effects of a Small Spatial-Scale Host Introduction. Parasitol. Res. 2015, 114, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Wielgoss, S.; Taraschewski, H.; Meyer, A.; Wirth, T. Population Structure of the Parasitic Nematode Anguillicola crassus, an Invader of Declining North Atlantic Eel Stocks. Mol. Ecol. 2008, 17, 3478–3495. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. Why Do Introduced Species Appear to Devastate Islands More Than Mainland Areas?! Pac. Sci. 1995, 49, 87–97. [Google Scholar]
- Vié, J.-C.; Hilton-Taylor, C.; Stuart, S.N.; IUCN—The World Conservation Union, IUCN Species Survival Commission (Eds.) Wildlife in a Changing World: An Analysis of the 2008 IUCN Red List of Threatened Species; IUCN: Gland, Switzerland; Lynx Edicions: Barcelona, Spain, 2009; ISBN 978-2-8317-1063-1. [Google Scholar]
- Copp, G.H.; Fox, M.G. Growth and Life History Traits of Introduced Pumpkinseed (Lepomis gibbosus) in Europe, and the Relevance to Its Potential Invasiveness. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Invading Nature—Springer Series in Invasion Ecology; Gherardi, F., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 289–306. ISBN 978-1-4020-6029-8. [Google Scholar]
- Kvach, Y.; Ondrackova, M.; Kutsokon, Y.; Dzyziuk, N. New Record of Monogenean Parasites on Non-Indigenous Fishes in the Ukrainian Danube Delta. BioInvasions Rec. 2018, 7, 65–72. [Google Scholar] [CrossRef]
- Cech, G.; Sandor, D.; Molnar, K.; Paulus, P.; Papp, M.; Preiszner, B.; Vital, Z.; Varga, A.; Szekely, C. New Record of Metacercariae of the North American Posthodiplostomum centrarchi (Digenea, Diplostomidae) in Pumpkinseed (Lepomis gibbosus) in Hungary. Acta Vet. Hung. 2020, 68, 20–29. [Google Scholar] [CrossRef]
- Rubtsova, N.Y. First Record of Onchocleidus dispar, an Alien Monogenean from Introduced Pumpkinseed Fish Lepomis gibbosus (Pisces, Centrarchidae) in Ukraine. Sci Parasitol. 2015, 16, 83–88. [Google Scholar]
- Kvach, Y.; Tkachenko, M.Y.; Bartáková, V.; Zięba, G.; Ondračková, M. The Role of the Non-Indigenous Pumpkinseed Lepomis gibbosus (Actinopterygii: Centrarchidae) in the Life Cycle of Bothriocephalus claviceps (Cestoda: Bothriocephalidae) in Europe. Parasitol. Res. 2021, 120, 3163–3171. [Google Scholar] [CrossRef]
- Brinker, A.; Hamers, R. First Description of Pumpkinseed Lepomis gibbosus (L.) as a Possible Second Intermediate Host for Triaenophorus nodulosus (Pallas, 1781) (Cestoda, Pseudophyllidea) in Germany. Bull. Eur. Assoc. Fish Pathol. 2000, 20, 83–86. [Google Scholar]
- Masson, G.; Vanacker, M.; Fox, M.G.; Beisel, J.-N. Impact of the Cestode Triaenophorus nodulosus on the Exotic Lepomis gibbosus and the Autochthonous Perca fluviatilis. Parasitology 2015, 142, 745–755. [Google Scholar] [CrossRef]
- Kvach, Y.; Seifertová, M.; Carassou, L.; Ondračková, M. First Record of the American Cestode Proteocephalus ambloplitis (Leidy, 1887) (Proteocephalidae) in Europe. J. Helminthol. 2020, 94, e144. [Google Scholar] [CrossRef] [PubMed]
- Kvach, Y.; Jurajda, P.; Bryjová, A.; Trichkova, T.; Ribeiro, F.; Přikrylová, I.; Ondračková, M. European Distribution for Metacercariae of the North American Digenean Posthodiplostomum cf. minimum centrarchi (Strigeiformes: Diplostomidae). Parasitol. Int. 2017, 66, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ondrackova, M.; Pravdova, M.; Seifertova, M.; Peikrylova, I.; Kvach, Y.; Ribeiro, F. Onchocleidus principalis (Monogenea: Ancyrocephalidae) Co-Introduced to Europe with Centrarchid Fish. Acta Parasitolog. 2020, 65, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Maitland, P.S.; Price, C.E. Urocleidus principalis (Mizelle, 1936), a North American Monogenetic Trematode New to the British Isles, Probably Introduced with the Largemouth Bass Micropterus salmoides (Lacépède, 1802). J. Fish Biol. 1969, 1, 17–18. [Google Scholar] [CrossRef]
- Cusack, R.; Cone, D.K. A Report of Bacterial Microcolonies on the Surface of Gyrodactylus (Monogenea). J. Fish Dis. 1985, 8, 125–127. [Google Scholar] [CrossRef]
- Reshetnikov, A.N. The Fish Perccottus glenii: History of Introduction to Western Regions of Eurasia. Hydrobiologia 2004, 522, 349–350. [Google Scholar] [CrossRef]
- Reshetnikov, A.N. The Current Range of Amur Sleeper Perccottus glenii Dybowski, 1877 (Odontobutidae, Pisces) in Eurasia. Russ. J. Biol. Invasions 2010, 1, 119–126. [Google Scholar] [CrossRef]
- Ondrackova, M.; Davidova, M.; Blazek, R.; Gelnar, M.; Jurajda, P. The Interaction between an Introduced Fish Host and Local Parasite Fauna: Neogobius kessleri in the Middle Danube River. Parasitol. Res. 2009, 105, 201–208. [Google Scholar] [CrossRef]
- Kvach, Y.; Kutsokon, Y.; Janac, M.; Dykyy, I.; Dzyziuk, N.; Dudliv, I.; Nazaruk, K. Parasites of the Invasive Chinese Sleeper Perccottus glenii (Actinopterygii: Odontobutidae) in the Region of the First Introduction of the Carpathian Population. Oceanol. Hydrobiol. Stud. 2022, 51, 1–9. [Google Scholar] [CrossRef]
- Kvach, Y.; Kutsokon, Y.; Stepien, C.A.; Markovych, M. Role of the Invasive Chinese Sleeper Perccottus glenii (Actinopterygii: Odontobutidae) in the Distribution of Fish Parasites in Europe: New Data and a Review. Biologia 2016, 71, 941–951. [Google Scholar] [CrossRef]
- Taraschewski, H.; Moravec, F.; Lamah, T.; Anders, K. Distribution and Morphology of Two Helminths Recently Introduced into European Eel Populations: Anguillicola crassus (Nematoda, Dracunculoidea) and Paratenuisentis ambiguus (Acanthocephala, Tenuisentidae). Dis. Aquat. Org. 1987, 3, 167–176. [Google Scholar] [CrossRef]
- Kirk, R.S. The Impact of Anguillicola crassus on European Eels. Fish. Manage. Ecol. 2003, 10, 385–394. [Google Scholar] [CrossRef]
- Lefebvre, F.; Fazio, G.; Mounaix, B.; Crivelli, A.J. Is the Continental Life of the European Eel Anguilla anguilla Affected by the Parasitic Invader Anguillicoloides crassus? Proc. R. Soc. B Biol. Sci. 2013, 280, 20122916. [Google Scholar] [CrossRef] [PubMed]
- Gargouri Ben Abdallah, L.; Maamouri, F. Spatio-Temporal Dynamics of the Nematode Anguillicola crassus in Northeast Tunisian Lagoons. C. R. Biol. 2006, 329, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Loukili, A.; Belghyti, D. The Dynamics of the Nematode Anguillicola crassus, Kuvahara 1974 in Eel Anguilla anguilla (L. 1758) in the Sebou Estuary (Morocco). Parasitol. Res. 2007, 100, 683–686. [Google Scholar] [CrossRef]
- Kennedy, C.R. The Pathogenic Helminth Parasites of Eels. J. Fish Dis. 2007, 30, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.; Crook, V.; Gollock, M. IUCN Red List of Threatened Species: Anguilla anguilla. IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2020. [Google Scholar]
- Drouineau, H.; Durif, C.; Castonguay, M.; Mateo, M.; Rochard, E.; Verreault, G.; Yokouchi, K.; Lambert, P. Freshwater Eels: A Symbol of the Effects of Global Change. Fish Fish. 2018, 19, 903–930. [Google Scholar] [CrossRef]
- Würtz, J.; Taraschewski, H. Histopathological Changes in the Swimbladder Wall of the European Eel Anguilla anguilla Due to Infections with Anguillicola crassus. Dis. Aquat. Org. 2000, 39, 121–134. [Google Scholar] [CrossRef]
- Currie, H.A.L.; Martin, N.F.; Garcia, G.E.; Davis, F.M.; Kemp, P.S. A Mechanical Approach to Understanding the Impact of the Nematode Anguillicoloides crassus on the European Eel Swimbladder. J. Exp. Biol. 2020, 223, jeb219808. [Google Scholar] [CrossRef]
- Békési, L.; Hornok, S.; Székely, C. Attempts to Analyse Anguillicola crassus Infection and the Humoral Host Response in Eels (Anguilla anguilla) of Lake Balaton, Hungary. Acta Vet. Hung. 1997, 45, 439–445. [Google Scholar]
- Molnár, K.; Székely, C.; Perényi, M. Dynamics of Anguillicola crassus (Nematoda: Dracunculoidea) Infection in Eels of Lake Balaton, Hungary. Folia Parasitol. 1994, 41, 193–202. [Google Scholar]
- Evans, D.W.; Matthews, M.A.; McClintock, C.A. The Spread of the Eel Swimbladder Nematode Anguillicola crassus through the Erne System, Ireland. J. Fish Biol. 2001, 59, 1416–1420. [Google Scholar] [CrossRef]
- Molnár, K.; Baska, F.; Csaba, G.; Glávits, R.; Székely, C. Pathological and Histopathogical Studies of the Swimbladder of Eels Anguilla anguilla Infected by Anguillicola crassus (Nematoda: Dracunculoidea). Dis. Aquat. Org. 1993, 15, 41–50. [Google Scholar] [CrossRef]
- Beregi, A.; Molnár, K.; Békési, L.; Székely, C. Radiodiagnostic Method for Studying Swimbladder Inflammation Caused by Anguillicola crassus (Nematoda: Dracunculoidea). Dis. Aquat. Org. 1998, 34, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K.; Székely, C.; Ferenc, B. Mass Mortality of Eel in Lake Balaton Due to Anguillicola crassus Infection. Bull. Eur. Assoc. Fish Pathol. 1991, 11, 211–2012. [Google Scholar]
- Schabuss, M.; Kennedy, C.R.; Konecny, R.; Grillitsch, B.; Reckendorfer, W.; Schiemer, F.; Herzig, A. Dynamics and Predicted Decline of Anguillicola crassus Infection in European Eels, Anguilla anguilla, in Neusiedler See, Austria. J. Helminthol. 2005, 79, 159–167. [Google Scholar] [CrossRef]
- Thomas, K.; Ollevier, F. Paratenic Hosts of the Swimbladder Nematode Anguillicola crassus. Dis. Aquat. Org. 1992, 13, 165–174. [Google Scholar] [CrossRef]
- Cruz, E.; Silva, P.; Grazina Freitas, M.S.; Carvalho-Varela, M. First Report of Anguillicola crassus in the European Eel in Portugal. Bull. Eur. Ass. Fish Pathol. 1992, 12, 154–156. [Google Scholar]
- Neto, A.F.; Costa, J.L.; Costa, M.J.; Domingos, I. Epidemiology and Pathology of Anguillicoloides crassus in European Eel Anguilla anguilla from the Tagus Estuary (Portugal). Dis. Aquat. Org. 2010, 88, 225–233. [Google Scholar] [CrossRef]
- Barry, J.; Mcleish, J.; Dodd, J.A.; Turnbull, J.F.; Boylan, P.; Adams, C.E. Introduced Parasite Anguillicola crassus Infection Significantly Impedes Swim Bladder Function in the European Eel Anguilla anguilla (L.). J. Fish Dis. 2014, 37, 921–924. [Google Scholar] [CrossRef]
- Würtz, J.; Knopf, K.; Taraschewski, H. Distribution and Prevalence of Anguillicola crassus (Nematoda) in Eels Anguilla anguilla of the Rivers Rhine and Naab, Germany. Dis. Aquat. Org. 1998, 32, 137–143. [Google Scholar] [CrossRef]
- Molnár, K. Formation of Parasitic Nodules in the Swimbladder and Intestinal Walls of the Eel Anguilla anguilla Due to Infections with Larval Stages of Anguillicola crassus. Dis. Aquat. Org. 1995, 20, 163–170. [Google Scholar] [CrossRef]
- Haenen, O.L.M.; van Banning, P.; Dekker, W. Infection of Eel Anguilla anguilla (L.) and Smelt Osmerus eperlanus (L.) with Anguillicola crassus (Nematoda, Dracunculoidea) in the Netherlands from 1986 to 1992. Aquaculture 1994, 126, 219–229. [Google Scholar] [CrossRef]
- Molnár, K. On Eels (Anguilla anguilla) Infected by Anguillicola crassus (Nematoda: Dracunculoidea). Acta Vet. Hung. 1993, 41, 349–360. [Google Scholar] [PubMed]
- Gollock, M.J.; Kennedy, C.R.; Brown, J.A. European Eels, Anguilla anguilla (L.), Infected with Anguillicola crassus Exhibit a More Pronounced Stress Response to Severe Hypoxia than Uninfected Eels. J. Fish Dis. 2005, 28, 429–436. [Google Scholar] [CrossRef]
- Schneebauer, G.; Hanel, R.; Pelster, B. Anguillicola crassus Impairs the Silvering-Related Enhancements of the ROS Defense Capacity in Swimbladder Tissue of the European Eel (Anguilla anguilla). J. Comp. Physiol. B 2016, 186, 867–877. [Google Scholar] [CrossRef][Green Version]
- Palstra, A.P.; Heppener, D.F.M.; van Ginneken, V.J.T.; Székely, C.; van den Thillart, G.E.E.J.M. Swimming Performance of Silver Eels Is Severely Impaired by the Swim-Bladder Parasite Anguillicola crassus. J. Exp. Mar. Biol. Ecol. 2007, 352, 244–256. [Google Scholar] [CrossRef]
- Newbold, L.R.; Hockley, F.A.; Williams, C.F.; Cable, J.; Reading, A.J.; Auchterlonie, N.; Kemp, P.S. Relationship between European Eel Anguilla anguilla Infection with Non-Native Parasites and Swimming Behaviour on Encountering Accelerating Flow. J. Fish Biol. 2015, 86, 1519–1533. [Google Scholar] [CrossRef]
- Gollock, M.J.; Kennedy, C.R.; Quabius, E.S.; Brown, J.A. The Effect of Parasitism of European Eels with the Nematode, Anguillicola crassus on the Impact of Netting and Aerial Exposure. Aquaculture 2004, 233, 45–54. [Google Scholar] [CrossRef]
- Jousseaume, T.; Roussel, J.-M.; Beaulaton, L.; Bardonnet, A.; Faliex, E.; Amilhat, E.; Acou, A.; Feunteun, E.; Launey, S. Molecular Detection of the Swim Bladder Parasite Anguillicola crassus (Nematoda) in Fecal Samples of the Endangered European Eel Anguilla anguilla. Parasitol. Res. 2021, 120, 1897–1902. [Google Scholar] [CrossRef]
- Frisch, K.; Davie, A.; Schwarz, T.; Turnbull, J.F. Comparative Imaging of European Eels (Anguilla anguilla) for the Evaluation of Swimbladder Nematode (Anguillicoloides crassus) Infestation. J. Fish Dis. 2016, 39, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.R.; Fitch, D.J. Colonization, Larval Survival and Epidemiology of the Nematode Anguillicola crassus, Parasitic in the Eel, Anguilla anguilla, in Britain. J. Fish Biol. 1990, 36, 117–131. [Google Scholar] [CrossRef]
- Moser, M.L.; Patrick, W.S.; Crutchfield Jr, J.U. Infection of American Eels, Anguilla rostrata, by an Introduced Nematode Parasite, Anguillicola crassus, in North Carolina. Copeia 2001, 2001, 848–853. [Google Scholar] [CrossRef]
- Marohn, L.; Prigge, E.; Hanel, R. Introduced American Eels Anguilla rostrata in European Waters: Life-History Traits in a Non-Native Environment. J. Fish Biol. 2014, 84, 1740–1747. [Google Scholar] [CrossRef]
- Székely, C. Paratenic Hosts for the Parasitic Nematode Anguillicola crassus in Lake Balaton, Hungary. Dis. Aquat. Org. 1994, 18, 11–20. [Google Scholar] [CrossRef]
- Kvach, Y.; Ondrackova, M.; Janac, M.; Jurajda, P. The Parasite Community of Round Goby Neogobius melanostomus (Pallas, 1814) (Actinopterygii: Gobiidae) Newly Introduced into the Upper Elbe. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 19. [Google Scholar] [CrossRef]
- Pietrock, M.; Meinelt, T. Dynamics of Anguillicola crassus Larval Infections in a Paratenic Host, the Ruffe (Gymnocephalus cernuus) from the Oder River on the Border of Germany and Poland. J. Helminthol. 2002, 76, 235–240. [Google Scholar] [CrossRef]
- Pazooki, J.; Székely, C. Survey of the Paratenic Hosts of Anguillicola crassus. Acta Vet. Hung. 1994, 42, 87–97. [Google Scholar]
- Székely, C. Dynamics of Anguillicola crassus (Nematoda: Dracunculoidea) Larval Infection in Paratenic Host Fishes of Lake Balaton, Hungary. Acta Vet. Hung. 1995, 43, 401–422. [Google Scholar]
- Székely, C.S.; Pazooki, J.; Molnár, K. Host Reaction in Paratenic Fish Hosts against 3rd Stage Larvae of Anguillicola crassus. Dis. Aquat. Org. 1996, 26, 173–180. [Google Scholar] [CrossRef]
- Emde, S.; Kochmann, J.; Kuhn, T.; Dörge, D.D.; Plath, M.; Miesen, F.W.; Klimpel, S. Cooling Water of Power Plant Creates “Hot Spots” for Tropical Fishes and Parasites. Parasitol. Res. 2016, 115, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ondrackova, M.; Janac, M.; Borcherding, J.; Grabowska, J.; Bartakova, V.; Jurajda, P. Non-Native Gobies Share Predominantly Immature Parasites with Local Fish Hosts. J. Vertebr. Biol. 2021, 70, 21050. [Google Scholar] [CrossRef]
- Ondrackova, M.; Valova, Z.; Hudcova, I.; Michalkova, V.; Simkova, A.; Borcherding, J.; Jurajda, P. Temporal Effects on Host-Parasite Associations in Four Naturalized Goby Species Living in Sympatry. Hydrobiologia 2015, 746, 233–243. [Google Scholar] [CrossRef]
- Ondrackova, M.; Simkova, A.; Civanova, K.; Vyskocilova, M.; Jurajda, P. Parasite Diversity and Microsatellite Variability in Native and Introduced Populations of Four Neogobius Species (Gobiidae). Parasitology 2012, 139, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Francová, K.; Ondračková, M.; Polačik, M.; Jurajda, P. Parasite Fauna of Native and Non-Native Populations of Neogobius melanostomus (Pallas, 1814) (Gobiidae) in the Longitudinal Profile of the Danube River. J. Appl. Ichthyol. 2011, 27, 879–886. [Google Scholar] [CrossRef]
- Hoglund, J.; Thomas, K. The Black Goby Gobius niger as a Potential Paratenic Host for the Parasitic Nematode Anguillicola crassus in a Thermal Effluent of the Baltic. Dis. Aquat. Org. 1992, 13, 175–180. [Google Scholar] [CrossRef]
- De Charleroy, D.; Grisez, L.; Thomas, K.; Belpaire, C.; Ollevier, F. The Life Cycle of Anguillicola crassus. Dis. Aquat. Org. 1990, 8, 77–84. [Google Scholar] [CrossRef]
- Hohenadler, M.A.A.; Honka, K.I.; Emde, S.; Klimpel, S.; Sures, B. First Evidence for a Possible Invasional Meltdown among Invasive Fish Parasites. Sci. Rep. 2018, 8, 15085. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive Interactions of Nonindigenous Species: Invasional Meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Green, P.T.; O’Dowd, D.J.; Abbott, K.L.; Jeffery, M.; Retallick, K.; Mac Nally, R. Invasional Meltdown: Invader–Invader Mutualism Facilitates a Secondary Invasion. Ecology 2011, 92, 1758–1768. [Google Scholar] [CrossRef]
- Hohenadler, M.A.A.; Nachev, M.; Thielen, F.; Taraschewski, H.; Grabner, D.; Sures, B. Pomphorhynchus laevis: An Invasive Species in the River Rhine? Biol. Invasions 2018, 20, 207–217. [Google Scholar] [CrossRef]
- Emde, S.; Rueckert, S.; Kochmann, J.; Knopf, K.; Sures, B.; Klimpel, S. Nematode Eel Parasite Found inside Acanthocephalan Cysts—A “Trojan Horse” Strategy? Parasites Vectors 2014, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Bonneau, S.; Biagianti, S.; Petter, A.J. Description of the Larval Stages of Anguillicola crassus (Nematoda, Dracunculoidea) Using Light and Scanning Electron Microscopy. Aquat. Living Resour. 1992, 5, 307–318. [Google Scholar] [CrossRef]
- Ondrackova, M.; Francova, K.; Davidova, M.; Polacik, M.; Jurajda, P. Condition Status and Parasite Infection of Neogobius kessleri and N. melanostomus (Gobiidae) in Their Native and Non-Native Area of Distribution of the Danube River. Ecol. Res. 2010, 25, 857–866. [Google Scholar] [CrossRef]
- Kvach, Y.; Ondrackova, M.; Janac, M.; Krasnovyd, V.; Seifertova, M.; Jurajda, P. Parasites of Round Goby, Neogobius melanostomus, Currently Invading the Elbe River. Oceanol. Hydrobiol. Stud. 2019, 48, 56–65. [Google Scholar] [CrossRef]
- Ondračková, M.; Dávidová, M.; Pečínková, M.; Blažek, R.; Gelnar, M.; Valová, Z.; Černý, J.; Jurajda, P. Metazoan Parasites of Neogobius Fishes in the Slovak Section of the River Danube. J. Appl. Ichthyol. 2005, 21, 345–349. [Google Scholar] [CrossRef]
- Pegg, J.; Andreou, D.; Williams, C.F.; Britton, J.R. Head Morphology and Piscivory of European Eels, Anguilla anguilla, Predict Their Probability of Infection by the Invasive Parasitic Nematode Anguillicoloides crassus. Freshw. Biol. 2015, 60, 1977–1987. [Google Scholar] [CrossRef]
- Johnsen, B.O.; Jensen, A.J. Infestations of Atlantic Salmon, Salmo salar, by Gyrodactylus salaris in Norwegian Rivers. J. Fish Biol. 1986, 29, 233–241. [Google Scholar] [CrossRef]
- Johnsen, B.O.; Jensen, A.J. Introduction and Establishment of Gyrodactylus salaris Malmberg, 1957, on Atlantic Salmon, Salmo salar L., Fry and Parr in the River Vefsna, Northern Norway. J. Fish Dis. 1988, 11, 35–45. [Google Scholar] [CrossRef]
- Sandodden, R.; Brazier, M.; Sandvik, M.; Moen, A.; Wist, A.; Adolfsen, P. Eradication of Gyrodactylus salaris Infested Atlantic Salmon (Salmo salar) in the Rauma River, Norway, Using Rotenone. Manag. Biol. Invasions 2018, 9, 67–77. [Google Scholar] [CrossRef]
- Cunningham, C.O.; Mo, T.A. Random Amplified Polymorphic DNA (RAPD) Analysis of Three Norwegian Gyrodactylus salaris Populations (Monogenea; Gyrodactylidae). J. Parasitol. 1997, 83, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, B.O. The Effect of an Attack by the Parasite Gyrodactylus salaris on the Population of Salmon Parr in the River Lakselva, Misvaer in Northern Norway. J. Arct. Biol. 1978, 11, 7–9. [Google Scholar]
- Pettersen, R.A.; Hytterød, S.; Vøllestad, L.A.; Mo, T.A. Osmoregulatory Disturbances in Atlantic Salmon, Salmo salar L., Caused by the Monogenean Gyrodactylus salaris. J. Fish Dis. 2013, 36, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.; Bachmann, L.; Bakke, T.A. Mitochondrial DNA Variation of Gyrodactylus Spp. (Monogenea, Gyrodactylidae) Populations Infecting Atlantic Salmon, Grayling, and Rainbow Trout in Norway and Sweden. Int. J. Parasitol. 2003, 33, 1471–1478. [Google Scholar] [CrossRef]
- Robertsen, G.; Hansen, H.; Bachmann, L.; Bakke, T.A. Arctic Charr (Salvelinus alpinus) Is a Suitable Host for Gyrodactylus salaris (Monogenea, Gyrodactylidae) in Norway. Parasitology 2007, 134, 257–267. [Google Scholar] [CrossRef]
- Adolfsen, P.; Bardal, H.; Aune, S. Fighting an Invasive Fish Parasite in Subarctic Norwegian Rivers The End of a Long Story? Manag. Biol. Invasions 2021, 12, 49–65. [Google Scholar] [CrossRef]
- Ieshko, E.P.; Shul’man, B.S.; Lebedeva, D.I.; Barskaya, Y.Y.; Niemela, E. Bullhead (Cottus gobio L.) Invasion in the Utsjoki River (Northern Finland): Parasitological Aspects. Russ. J. Biol. Invasions 2013, 4, 17–23. [Google Scholar] [CrossRef]
- Golovin, P.P. Monogeneans of Eel during Its Culture Using Heated Water. In Investigations of Monogenoidea in the USSR; USSR Acad. Sci.: Leningrad, Russia, 1977; pp. 144–150. [Google Scholar]
- Gérard, C.; Trancart, T.; Amilhat, E.; Faliex, E.; Virag, L.; Feunteun, E.; Acou, A. Influence of Introduced vs. Native Parasites on the Body Condition of Migrant Silver Eels. Parasite 2013, 20, 38. [Google Scholar] [CrossRef][Green Version]
- Kennedy, C.; Di Cave, D. Gyrodactylus anguillae (Monogenea): The Story of an Appearance and a Disappearance. Folia Parasitol. 2013, 45, 77–78. [Google Scholar]
- Morozinska-Gogol, J. Alien Species of Fish Parasites in the Coastal Lakes and Lagoons of the Southern Baltic. Oceanologia 2009, 51, 105–115. [Google Scholar] [CrossRef]
- Sures, B.; Knopf, K.; Würtz, J.; Hirt, J. Richness and Diversity of Parasite Communities in European Eels Anguilla anguilla of the River Rhine, Germany, with Special Reference to Helminth Parasites. Parasitology 1999, 119, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Mouillot, D. Host Introductions and the Geography of Parasite Taxonomic Diversity. J. Biogeogr. 2003, 30, 837–845. [Google Scholar] [CrossRef]
- Schatz, A.M.; Park, A.W. Host and Parasite Traits Predict Cross-Species Parasite Acquisition by Introduced Mammals. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210341. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, J. Reproductive Biology of Racer Goby Neogobius gymnotrachelus in the Włocławski Reservoir (Vistula River, Poland). J. Appl. Ichthyol. 2005, 21, 296–299. [Google Scholar] [CrossRef]
- Stráňai, I.; Andreji, J. The First Report of Round Goby, Neogobius melanostomus (Pisces, Gobiidae) in the Waters of Slovakia. Folia Zool. 2004, 53, 335–338. [Google Scholar]
- Ahnelt, H.; Bănărescu, P.; Spolwind, R.; Harka, A.; Waidbacher, H. Occurence and Distribution of Three Gobiid Species (Pisces: Gobiidae) in the Middle and Upper Danube Region—Example of Different Dispersal Patterns? Biológia 1998, 53, 661–674. [Google Scholar]
- Jurajda, P.; Černý, J.; Polačik, M.; Valová, Z.; Janáč, M.; Blažek, R.; Ondračková, M. The Recent Distribution and Abundance of Non-Native Neogobius Fishes in the Slovak Section of the River Danube. J. Appl. Ichthyol. 2005, 21, 319–323. [Google Scholar] [CrossRef]
- Kvach, Y.; Boldyrev, V.; Lohner, R.; Stepien, C.A. The Parasite Community of Gobiid Fishes (Actinopterygii: Gobiidae) from the Lower Volga River Region. Biologia 2015, 70, 948–957. [Google Scholar] [CrossRef]
- Mühlegger, J.M.; Jirsa, F.; Konecny, R.; Frank, C. Parasites of Apollonia melanostoma (Pallas 1814) and Neogobius kessleri (Guenther 1861) (Osteichthyes, Gobiidae) from the Danube River in Austria. J. Helminthol. 2010, 84, 87–92. [Google Scholar] [CrossRef]
- Moravec, F. Checklist of the Metazoan Parasites of Fishes of the Czech Republic and the Slovak Republic (1873–2000); Academia: Praha, Czech, 2001; ISBN 978-80-200-0907-4. [Google Scholar]
- Kvach, Y.; Kornyychuk, Y.; Mierzejewska, K.; Rubtsova, N.; Yurakhno, V.; Grabowska, J.; Ovcharenko, M. Parasitization of Invasive Gobiids in the Eastern Part of the Central Trans-European Corridor of Invasion of Ponto-Caspian Hydrobionts. Parasitol. Res. 2014, 113, 1605–1624. [Google Scholar] [CrossRef]
- Mierzejewska, K.; Kvach, Y.; Stańczak, K.; Grabowska, J.; Woźniak, M.; Dziekońska-Rynko, J.; Ovcharenko, M. Parasites of Non-Native Gobies in the Włocławek Reservoir on the Lower Vistula River, First Comprehensive Study in Poland. Knowl. Managt. Aquat. Ecosyst. 2014, 414, 1. [Google Scholar] [CrossRef]
- Kvach, Y.; Mierzejewska, K. Non-Indigenous Benthic Fishes as New Hosts for Bucephalus polymorphus Baer, 1827 (Digenea: Bucephalidae) in the Vistula River Basin, Poland. Knowl. Manag. Aquat. Ecosyst. 2011, 400, 2. [Google Scholar] [CrossRef]
- Ondračková, M.; Hudcová, I.; Dávidová, M.; Adámek, Z.; Kašnỳ, M.; Jurajda, P. Non-Native Gobies Facilitate the Transmission of Bucephalus polymorphus (Trematoda). Parasites Vectors 2015, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Hohenadler, M.A.A.; Nachev, M.; Freese, M.; Pohlmann, J.D.; Hanel, R.; Sures, B. How Ponto-Caspian Invaders Affect Local Parasite Communities of Native Fish. Parasitol. Res. 2019, 118, 2543–2555. [Google Scholar] [CrossRef]
- David, G.M.; Staentzel, C.; Schlumberger, O.; Perrot-Minnot, M.-J.; Beisel, J.-N.; Hardion, L. A Minimalist Macroparasite Diversity in the Round Goby of the Upper Rhine Reduced to an Exotic Acanthocephalan Lineage. Parasitology 2018, 145, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K. Some Remarks on Parasitic Infections of the Invasive Neogobius spp. (Pisces) in the Hungarian Reaches of the Danube River, with a Description of Goussia szekelyi sp. n. (Apicomplexa: Eimeriidae). J. Appl. Ichthyol. 2006, 22, 395–400. [Google Scholar] [CrossRef]
- Kvach, Y.; Janáč, M.; Nehring, S.; Ondračková, M.; Jurajda, P. Parasite Communities and Infection Levels of the Invasive Chinese Sleeper Perccottus glenii (Actinopterygii: Odontobutidae) from the Naab River Basin, Germany. J. Helminthol. 2017, 91, 703–710. [Google Scholar] [CrossRef]
- Reshetnikov, A.N.; Sokolov, S.G.; Chikhlyaev, I.V.; Fayzulin, A.I.; Kirillov, A.A.; Kuzovenko, A.E.; Protasova, E.N.; Skomorokhov, M.O. Direct and Indirect Interactions between an Invasive Alien Fish (Perccottus glenii) and Two Native Semi-Aquatic Snakes. Copeia 2013, 2013, 103–110. [Google Scholar] [CrossRef]
- Sokolov, S.G.; Protasova, E.N.; Reshetnikov, A.N. First Data on Parasites of the Rotan, Perccottus glenii Dybowski, 1877 (Perciformes: Odontobutidae), from Germany, with a Detection of the Previously Unknown Merocercoid of the Gryporhynchid Cestode Mashonalepis macrosphincter (Fuhrmann, 1909). Acta Zool. Bulg. 2015, 67, 557–560. [Google Scholar]
- Kvach, Y.; Kutsokon, I.; Roman, A.; Čeirāns, A.; Pupins, M.; Kirjušina, M. Parasite Acquisition by the Invasive Chinese Sleeper (Perccottus glenii Dybowski, 1877) (Gobiiformes: Odontobutidae) in Latvia and Ukraine. J. Appl. Ichthyol. 2020, 36, 785–794. [Google Scholar] [CrossRef]
- Moskvina, T.V.; Ermolenko, A.V. Helminth Infections in Domestic Dogs from Russia. Vet. World 2016, 9, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.G.; Protasova, E.N.; Reshetnikov, A.N.; Voropaeva, E.L. Interactions of the Introduced Rotan Perccottus glenii Dybowski, 1877 (Osteichthyes, Odontobutidae) with Aboriginal Fish Species: The Parasitological Aspect. Biol. Bull 2012, 39, 829–833. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Darwin Murrell, K.; Lymbery, A.J. Fish-Borne Parasitic Zoonoses: Status and Issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.C.; Pavanelli, G.C.; Takemoto, R.M.; Nawa, Y. Fish-Borne Nematodiases in South America: Neglected Emerging Diseases. J. Helminthol. 2018, 92, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Elsheikha, H.M. Biology, Epidemiology, Clinical Features, Diagnosis, and Treatment of Selected Fish-Borne Parasitic Zoonoses. Yale J Biol Med 2021, 94, 297–309. [Google Scholar]
- Bardhan, A. Fish-Borne Parasites Proficient in Zoonotic Diseases: A Mini Review. Insights Vet. Sci. 2022, 6, 005–012. [Google Scholar] [CrossRef]
- Guagliardo, S.; Viozzi, G.; Brugni, N. Pathology Associated with Larval Eustrongylides sp. (Nematoda: Dioctophymatoidea) Infection in Galaxias maculatus (Actinopterygii: Galaxiidae) from Patagonia, Argentina. Int. J. Parasitol. Parasites Wildl. 2019, 10, 113–116. [Google Scholar] [CrossRef]
- Menconi, V.; Riina, M.V.; Pastorino, P.; Mugetti, D.; Canola, S.; Pizzul, E.; Bona, M.C.; Dondo, A.; Acutis, P.L.; Prearo, M. First Occurrence of Eustrongylides spp. (Nematoda: Dioctophymatidae) in a Subalpine Lake in Northwest Italy: New Data on Distribution and Host Range. Int. J. Environ. Res. Public Health 2020, 17, 4171. [Google Scholar] [CrossRef]
- Xiong, F.; Wang, G.T.; Wu, S.G.; Nie, P. Development of Eustrongylides ignotus (Nematoda: Dioctophmida) in Domestic Ducks (Anas platyrhynchos domestica (L.)). J. Parasitol. 2009, 95, 1035–1039. [Google Scholar] [CrossRef]
- Williams, M.; Hernandez-Jover, M.; Shamsi, S. Parasites of Zoonotic Interest in Selected Edible Freshwater Fish Imported to Australia. Food Waterborne Parasitol. 2021, 26, e00138. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Hurwitz, H.; Sun, A.M.; Coletta, D. Intestinal Perforation Caused by Larval Eustrongylides (Nematoda: Dioctophymatoidae) in New Jersey. Am. J. Trop. Med. Hyg. 1989, 40, 648–650. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Ruiz-Tiben, E. Cutaneous Emergence of Eustrongylides in Two Persons from South Sudan. Am. J. Trop. Med. Hyg. 2014, 90, 315–317. [Google Scholar] [CrossRef]
- Wittner, M.; Turner, J.W.; Jacquette, G.; Ash, L.R.; Salgo, M.P.; Tanowitz, H.B. Eustrongylidiasis—A Parasitic Infection Acquired by Eating Sushi. N. Engl. J. Med. 1989, 320, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Moravec, F. Parasitic Nematodes of Freshwater Fishes of Europe, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; ISBN 978-0-7923-2172-9. [Google Scholar]
- Nikolic, V.; Zimonovic, P.; Znidarsic, T.K. First Record in Europe of a Nematode Parasite in Amur Sleeper Perccottus glenii Dybowski. Bull. Eur. Ass. Fish Pathol. 2007, 27, 36. [Google Scholar]
- Moravec, F. Misidentification of Nematodes from the Chinese Sleeper Perccottus glenii in Europe. Bull. Eur. Assoc. Fish Pathol. 2008, 28, 86–87. [Google Scholar]
- Dorucu, M.; Crompton, D.W.; Huntingford, F.A.; Walters, D.E. The Ecology of Endoparasitic Helminth Infections of Brown Trout (Salmo trutta) and Rainbow Trout (Oncorhynchus mykiss) in Scotland. Folia Parasitol. 1995, 42, 29–35. [Google Scholar]
- Kennedy, C.R.; Lie, S.F. The Distribution and Pathogenicity of Larvae of Eustrongylides (Nematoda) in Brown Trout Salmo trutta L. in Fernworthy Reservoir, Devon. J. Fish Biol. 1976, 8, 293–302. [Google Scholar] [CrossRef]
- Castiglione, D.; Di Maggio, M.; Guardone, L.; Ricci, E.; Tinacci, L.; Guglielmone, G.; Coltraro, M.; Susini, F.; Armani, A. Eustrongylides excisus in Fish Species Caught in the Massaciuccoli Lake (Northwest Tuscany, Italy): Implications for Freshwater Fish Quality and Public Health. Food Control 2023, 153, 109894. [Google Scholar] [CrossRef]
- Mazzone, A.; Caffara, M.; Gustinelli, A.; Agnetti, F.; Sgariglia, E.; Lo Vaglio, G.; Quaglio, F.; Fioravanti, M.L. Morphological and Molecular Characterization of Larval and Adult Stages of Eustrongylides excisus (Nematoda: Dioctophymatoidea) with Histopathological Observations. J. Parasitol. 2019, 105, 882–889. [Google Scholar] [CrossRef]
- Shamsi, S.; Turner, A.; Wassens, S. Description and Genetic Characterization of a New Contracaecum Larval Type (Nematoda: Anisakidae) from Australia. J. Helminthol. 2018, 92, 216–222. [Google Scholar] [CrossRef]
- Dorny, P.; Praet, N.; Deckers, N.; Gabriel, S. Emerging Food-Borne Parasites. Vet. Parasitol. 2009, 163, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K.; Mehrdana, F. Effects of Anisakid Nematodes Anisakis simplex (s.l.), Pseudoterranova decipiens (s.l.) and Contracaecum osculatum (s.l.) on Fish and Consumer Health. Food Waterborne Parasitol. 2016, 4, 13–22. [Google Scholar] [CrossRef]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From Obscure Infectious Worm to Inducer of Immune Hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Køie, M.; Fagerholm, H.-P. The Life Cycle of Contracaecum osculatum (Rudolphi, 1802) Sensu Stricto (Nematoda, Ascaridoidea, Anisakidae) in View of Experimental Infections. Parasitol. Res. 1995, 81, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Salati, F.; Meloni, M.; Cau, M.; Angelucci, G. Presence of Contracaecum spp. in Teleosts Cultured and Fished in Sardinia. Vet. Parasitol. 2013, 196, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Wootten, R. The Metazoan Parasite-fauna of Fish from Hanningfield Reservoir, Essex in Relation to Features of the Habitat and Host Populations. J. Zool. 1973, 171, 323–331. [Google Scholar] [CrossRef]
- Pilecka-Rapacz, M.; Sobecka, E. Parasitic Nematodes of Pumpkinseed Sunfish [Lepomis gibbosus L., 1758] from Warm-Water Canal of a Power Plant in Szczecin, Poland. Wiad. Parazytol. 2008, 54, 213–216. [Google Scholar]
- Park, C.-W.; Kim, J.-S.; Joo, H.-S.; Kim, J. A Human Case of Clinostomum complanatum Infection in Korea. Korean J. Parasitol. 2009, 47, 401–404. [Google Scholar] [CrossRef]
- Hara, H.; Miyauchi, Y.; Tahara, S.; Yamashita, H. Human Laryngitis Caused by Clinostomum complanatum. Nagoya J. Med. Sci. 2014, 76, 181. [Google Scholar]
- Menconi, V.; Manfrin, C.; Pastorino, P.; Mugetti, D.; Cortinovis, L.; Pizzul, E.; Pallavicini, A.; Prearo, M. First Report of Clinostomum complanatum (Trematoda: Digenea) in European Perch (Perca fluviatilis) from an Italian Subalpine Lake: A Risk for Public Health? Int. J. Environ. Res. Public Health 2020, 17, 1389. [Google Scholar] [CrossRef]
- Caffara, M.; Locke, S.A.; Gustinelli, A.; Marcogliese, D.J.; Fioravanti, M.L. Morphological and Molecular Differentiation of Clinostomum complanatum and Clinostomum marginatum (Digenea: Clinostomidae) Metacercariae and Adults. J. Parasitol. 2011, 97, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Mott, K.E. Epidemiology and Morbidity of Food-Borne Intestinal Trematode Infections; World Health Organization: Geneva, Switzerland, 1994; p. 26. [Google Scholar]
- Chai, J.-Y. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea. V. Intestinal Pathology in Experimentally Infected Albino Rats. Seoul J. Med. 1979, 20, 104–117. [Google Scholar]
- Cakić, P.; Paunović, M.; Stojanović, B.; Ðikanović, V.; Kulišić, Z. Metagonimus yokogawai: A New Parasitic Trematoda Species in Ichtyoparasitofauna of the Serbia. Acta Vet. 2007, 57, 537–543. [Google Scholar] [CrossRef]
- Scholz, T.; Kuchta, R. Fish-Borne, Zoonotic Cestodes (Diphyllobothrium and Relatives) in Cold Climates: A Never-Ending Story of Neglected and (Re)-Emergent Parasites. Food Waterborne Parasitol. 2016, 4, 23–38. [Google Scholar] [CrossRef]


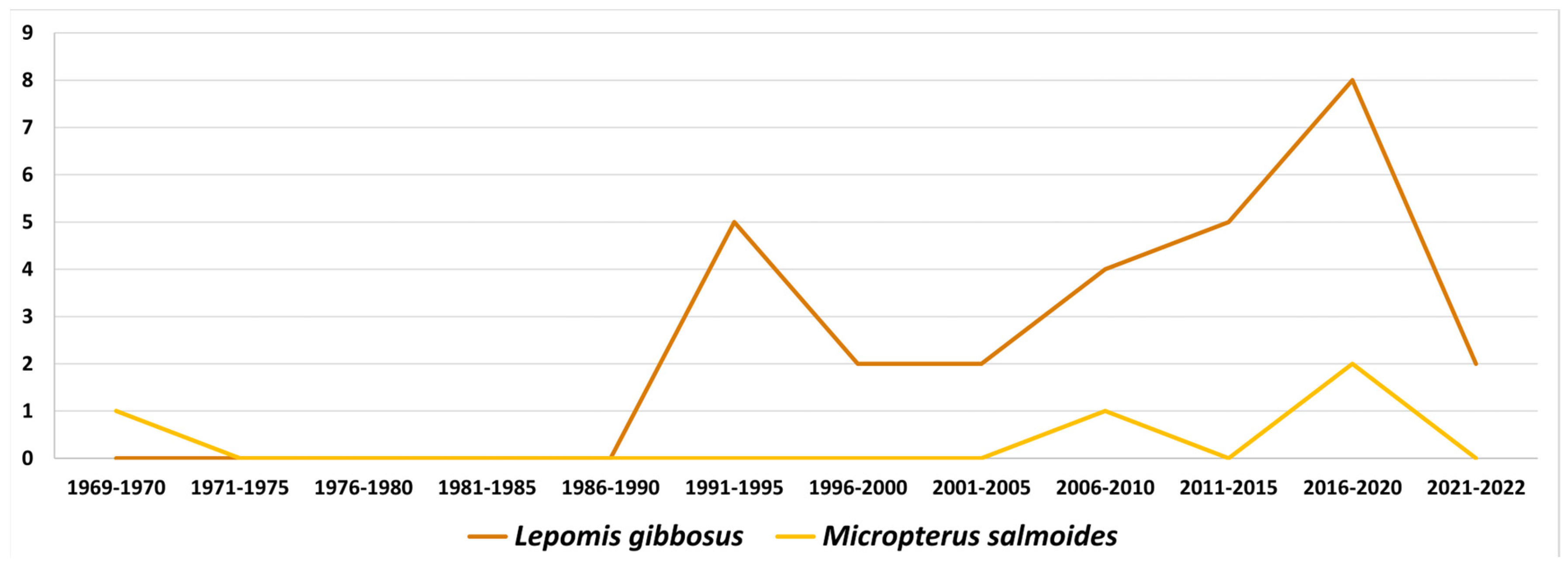

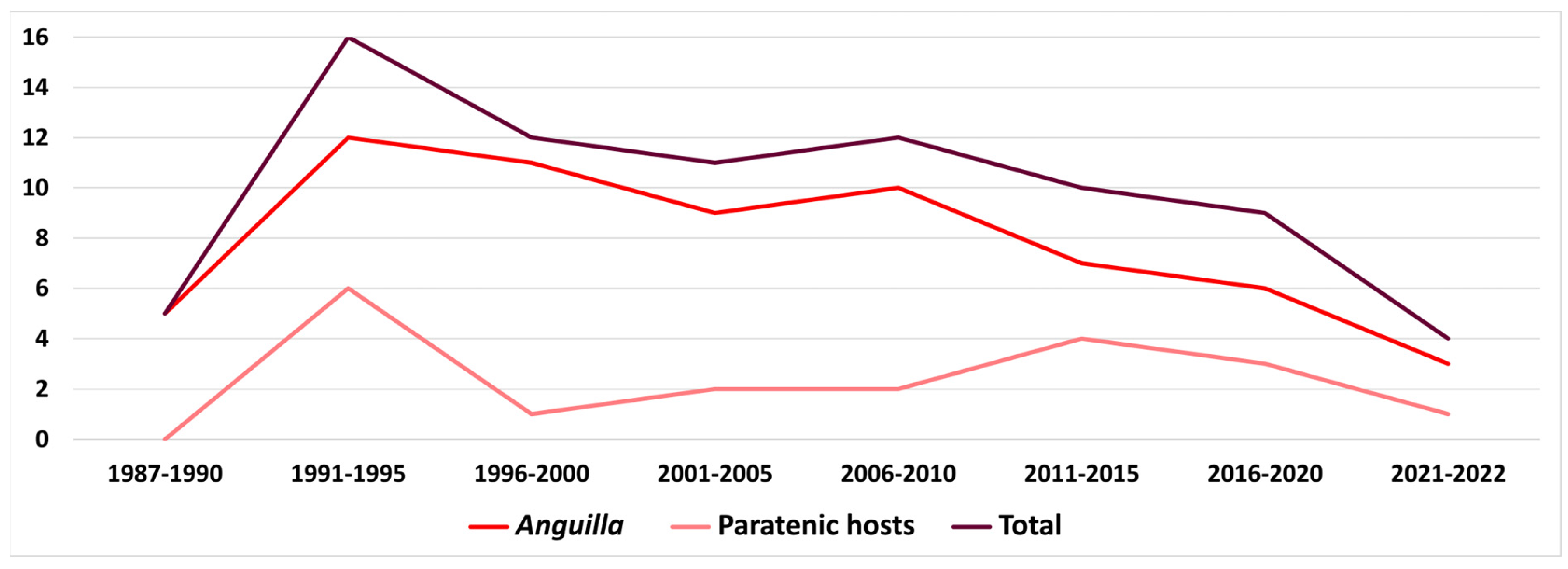
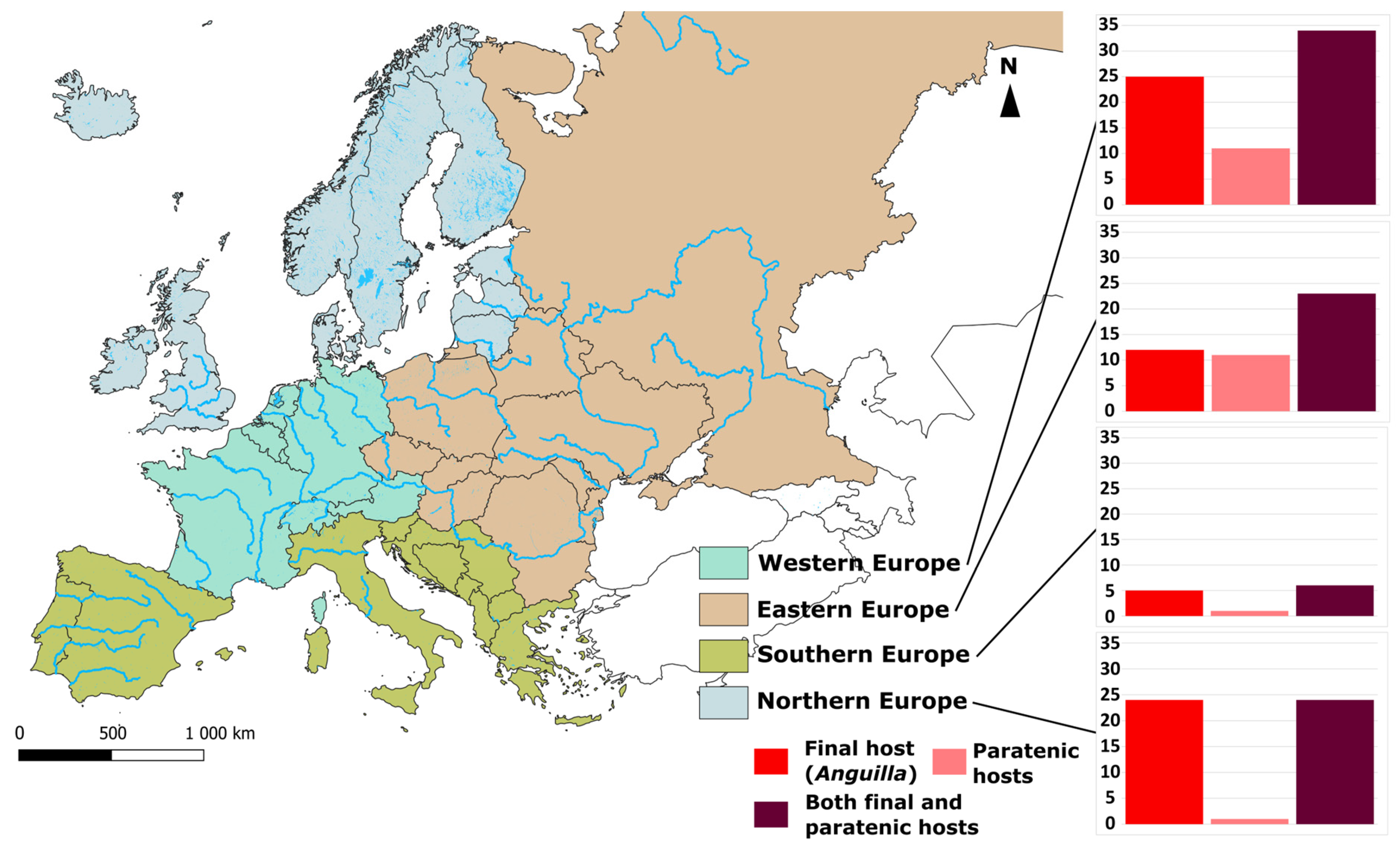
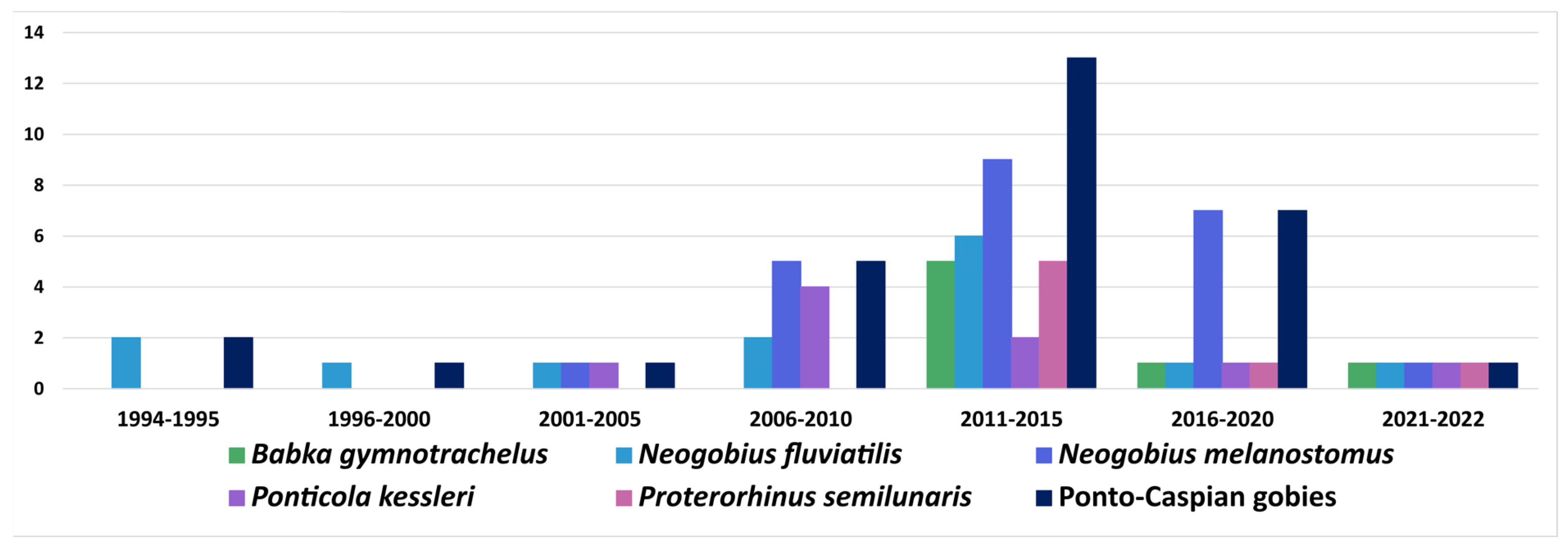

| Parasite | Host Species | Locality | |
|---|---|---|---|
| Family | Species | ||
| Ancyrocephalidae | Actinocleidus oculatus | Lepomis gibbosus | France [20,21], Germany [21,49], Italy [52,53,54] |
| Actinocleidus recurvatus | Lepomis gibbosus | Austria [21], Croatia [33], France [20,21], Germany [21,49], Italy [52,53,54], Slovakia [33] | |
| Actinocleidus sp. | Lepomis gibbosus | Austria [21], France [21], Germany [21,49] | |
| Cleidodiscus robustus | Lepomis gibbosus | France [20,21] | |
| Onchocleidus acer | Lepomis gibbosus | France [20] | |
| Onchocleidus dispar | Lepomis gibbosus | Austria [21], Bulgaria [21,33,48], Croatia [33], Czech Republic [21,33], France [20,21], Germany [21,49], Italy [52,53,54], Portugal [69], Slovakia [33], Ukraine [63], United Kingdom [34] | |
| Micropterus salmoides | Portugal [69] | ||
| Onchocleidus principalis | Lepomis gibbosus | Portugal [69] | |
| Micropterus salmoides | Italy [54], Portugal [69], United Kingdom [70] | ||
| Onchocleidus similis | Lepomis gibbosus | Austria [21], Bulgaria [21,33,48], Croatia [33], Czech Republic [21,33], France [20,21], Norway [50], Germany [21,49], Italy [52,53,54], Slovakia [33], Ukraine [61] | |
| Onchocleidus sp. | Lepomis gibbosus | Germany [21,49], Norway [50] | |
| Unidentified Ancyrocephalidae | Lepomis gibbosus | Austria [21], France [21] | |
| Gyrodactylidae | Gyrodactylus avalonia | Lepomis gibbosus | Ukraine [61] |
| Gyrodactylus macrochiri | Lepomis gibbosus | France [20,21] | |
| Parasite Species | Host Species | Locality |
|---|---|---|
| Eustrongylides excisus | Babka gymnotrachelus | Poland [159,160] |
| Neogobius fluviatilis | Poland [159], Ukraine [159] | |
| Neogobius melanostomus | Austria [122,131] | |
| Ponticola kessleri | Slovakia [74] | |
| Perccottus glenii | Poland [29] | |
| Eustrongylides mergorum | Perccottus glenii | Russia [35] |
| Eustrongylides tubifex | Babka gymnotrachelus | Poland [160] |
| Perccottus glenii | Poland [29], Ukraine [169] | |
| Eustrongylides sp. | Babka gymnotrachelus | Poland [119] |
| Neogobius fluviatilis | Poland [160] | |
| Neogobius melanostomus | Czech Republic [119] | |
| Proterorhinus semilunaris | Poland [160] | |
| Perccottus glenii | Serbia [184,185] | |
| Oncorhynchus mykiss | United Kingdom [186] | |
| Salmo trutta | United Kingdom [187] |
| Parasite Species | Host Species | Locality |
|---|---|---|
| Contracaecum ovale | Lepomis gibbosus | Germany [21,49] |
| Contracaecum rudolphii | Neogobius melanostomus | Czech Republic [113] |
| Contracaecum sp. | Lepomis gibbosus | Bulgaria [48], United Kingdom [34], Poland [197] |
| Neogobius fluviatilis | Slovakia [133] | |
| Chondrostoma nasus | France [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, A.; Foata, J.; Quilichini, Y. Parasitic Helminths and Freshwater Fish Introduction in Europe: A Systematic Review of Dynamic Interactions. Fishes 2023, 8, 450. https://doi.org/10.3390/fishes8090450
Esposito A, Foata J, Quilichini Y. Parasitic Helminths and Freshwater Fish Introduction in Europe: A Systematic Review of Dynamic Interactions. Fishes. 2023; 8(9):450. https://doi.org/10.3390/fishes8090450
Chicago/Turabian StyleEsposito, Anaïs, Joséphine Foata, and Yann Quilichini. 2023. "Parasitic Helminths and Freshwater Fish Introduction in Europe: A Systematic Review of Dynamic Interactions" Fishes 8, no. 9: 450. https://doi.org/10.3390/fishes8090450
APA StyleEsposito, A., Foata, J., & Quilichini, Y. (2023). Parasitic Helminths and Freshwater Fish Introduction in Europe: A Systematic Review of Dynamic Interactions. Fishes, 8(9), 450. https://doi.org/10.3390/fishes8090450







