Abstract
Thermal refuges are becoming increasingly influential for dictating the population status and spatial distribution of cold-water stenotherm salmonids in the mid- to southern extent of their range. The global climate is predicted to continue to warm, and therefore, the overall thermal suitability of freshwater habitats for stream salmonids is predicted to decline in concert. However, stream and river thermal heterogeneity will offer considerable resiliency for these populations. Thermal refuges are formed by many physical processes; common natural refuges include cold tributary plumes, groundwater springs, alcoves, and hyporheic upwellings. However, many anthropogenically formed refuges (such as stratified reservoirs or cold-water tailrace outflows) also exist in hydropower-regulated rivers. The significance of these refuges to stream salmonids depends on their size and temperature differential, but also other habitat characteristics such as their depth, flow velocity, Froude number, and many biotic factors within the refuges. Modern technologies such as drone-mounted thermal infrared cameras and other remote sensing techniques allow for the efficient identification of such refuges, and inexpensive options include the identification of refuges during ice cover using orthophotographs. Behavioural thermoregulation, i.e., salmonids aggregating in cold-water refuges, can be either facultative or obligate and the timing of these events is governed by life stage, species, and population-specific physiologically regulated cumulative thresholds that are inherently related to the recent thermal history, or hysteresis, of each individual. Salmonids appear to have an excellent spatial cognition for locating and relocating cold-water refuges, and their spatial distribution is largely affected by the availability of the cold-water refuges during the warm-water period in many thermally stressed rivers. Gregarious behaviour is the norm for salmonid fishes within the thermal refuges; however, the size/microhabitat hierarchy appears to dictate the within-refuge distribution at the micro-scale. There continues to be a great impetus for protecting—and in carefully determined cases creating—cold-water refuges in the future. A thorough understanding of what a “goldilocks” refuge is for various salmonids and their different life stages will be imperative as cold-water restoration is gaining popularity. Finally, disentangling the roles of the climate-induced and landscape activity-induced warming potential of fluvial freshwater will be important to ensure continued environmentally responsible landscape activities in future waterscapes.
Key Contribution:
This paper synthesizes over two decades of work in Atlantic Canada regarding cold-water refuge use by stream salmonids with comparisons to similar research on stream salmonids elsewhere. The article introduces the concept of facultative and obligate behavioural thermoregulation, and therefore, expands the previous concept by allowing for a better understanding of the reasons and timing of the cold-water refuge-seeking behaviour.
1. Introduction
Climate change has become the environmental phenomenon epitomizing the early 21st century and it has directed much of the research questions and hypotheses examined by contemporary environmental scientists. Climate change is, and likely more so in the future, causing a variety of changes in climate patterns, the frequency of extreme observations, and the severity of climate-correlated events [1]. Thousands of research articles, especially those since the 1990s, have been written about how predicted changes in the future climate will affect aquatic resources, including cold-water stenothermic fishes and particularly stream salmonids (e.g., Salmo, Oncorhynchus, Salvelinus spp.; e.g., the current special issue [2], and reviews such as [3] for Salmo spp. [4] for winter perspectives [5]). Generally speaking, warming air temperature is predicted to lead into concomitant increases in water temperature in both freshwater [6,7] and in oceanic systems [8]. It is the warming of water that is predicted to have the largest cascading effects on the ecology of stream salmonids in summer [3,9]. In winter, at least in seasonal climates, the water temperature may still generally remain at or close to 0 °C, but the duration of this relatively stable period may become shorter with potential consequences for winter-adapted salmonids [10,11]. Climate change is also predicted to cause changes in other weather parameters, such as changes in precipitation patterns with concomitant effects on stream discharge and the frequency and severity of ice break-up [1,12]. Many environmental parameters affecting freshwater resources act in concert in response to climate change, and some of these effects may be cumulative (e.g., less summer precipitation → lower discharge → higher cumulative effect on water temperature) [13], whereas some may be antagonistic (e.g., higher water temperature → lower ice thickness → less severe ice break-ups) [4].
One of the main concerns regarding climate warming is its potential effect on cold-water stenothermic fishes, such as the Salmonidae—a socio-economically important family of anadromous, potamodromous, and resident fishes in many jurisdictions [3]. With warming water, a declining overall habitat suitability for a number of salmonids has been documented [5,14,15,16,17]. However, it has been long recognized that despite the general trend of warming freshwater temperatures, rivers and streams possess an inherent thermal habitat heterogeneity wherein the generally uniform temperature of a given river reach may be “punctuated” with cold-water inputs of various types [18,19,20,21,22] and that these cold-water anomalies have the potential to act as refuges for salmonids during otherwise adverse temperature conditions in summer [13,23,24,25]. With a warming climate, the ecological significance of these refuges has increased for many salmonid populations in the southern-to-middle extent of their ranges, and related research has similarly increased in the last two decades for both the physical and biological aspects governing thermal refuges and their utility for salmonids [26,27].
In this article, our aim was not to conduct a quantitative meta-analysis or a systematic review of cold-water habitat use or the thermal capabilities of stream salmonids. Other recent reviews in related topics exist, for example, the work by [2,26,27,28]. Rather, we synthesized some of the insights the authors observed and concluded in the rivers of Atlantic Canada over the last two decades while conducting research into cold-water refuges and stream salmonid behavioural thermoregulation (see definition below). Further, we related this synthesis to the research of others for corollaries, parallels, or at times contrasting or supplementary perspectives. Our work mostly focused on Atlantic salmon Salmo salar L., 1758, and the examples that follow are primarily focused on this species, although the discussed concepts may be applicable more widely to other stream salmonids. We further narrowed down the focus by only considering thermal refuges from the perspective of the behaviour related to cold-water stenothermic salmonids in response to warm-water conditions, i.e., summer, while recognizing that the thermal heterogeneity, and often the very same thermal anomalies, may play a crucial, yet drastically different role in the winter biology of salmonids [4,29,30].
We start with a short description of the physical aspects of typical thermal refuges in Atlantic Canada and their detection and mapping using contemporary tools at different scales. We then review the concept of behavioural thermoregulation for common stream salmonids and synthesize the movement ecology and behavioural aspects related to using cold-water refuges by salmonids. We conclude by providing insights related to the management of cold-water refuges with respect to the conservation of salmonid populations and offer some future directions for research and the conservation/restoration of cold-water refuges.
2. Thermal Refuges—Physical Environment
As implied by the name, thermal refuges are defined as cold-water patches across the riverscape that provide thermal relief for cold-water fishes during extreme heat events [18,31]. Such refuges may exist naturally, or may be a product of anthropogenic alterations, for example in (hydropower) regulated rivers [22].
In natural rivers, the predominant thermal refuge type described in the literature (but not the most abundant category by number [32]) is the tributary-derived thermal refuge [13,24,33]. These refuges occur at the confluence of a cold-water tributary and the warmer main river (Figure 1a,b). The temperature differential between the cold-water tributaries and the main river can be caused by multiple factors [7], but commonly includes elevational differences, groundwater influx, or sufficient shading from the riparian vegetation. The exact size of the cold-water plume, and thus, the thermal refuge is dictated by the amount of cold-water inflow relative to the main river discharge, the temperature differential between the two mixing waterbodies, and a complex set of hydrodynamic physical properties [21,34]. Generally speaking, however, the tributary-derived cold-water plumes are often the most commonly used and sizeable thermal refuges for salmonids and may exceed hundreds of square metres (Figure 1b). These refuges, in fact, may have the potential to even qualify as refugia (as opposed to just refuges; as per the terminology defined in [26]), i.e., a thermal refuge so significant that it may act as a last ‘stronghold’ wherein a salmonid population in a cold tributary can persist generation after generation while the remainder of a river may become uninhabitable for the species.
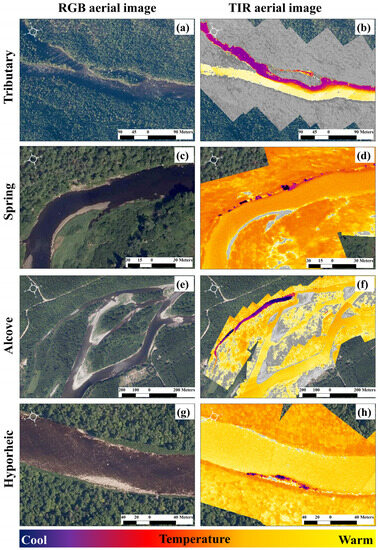
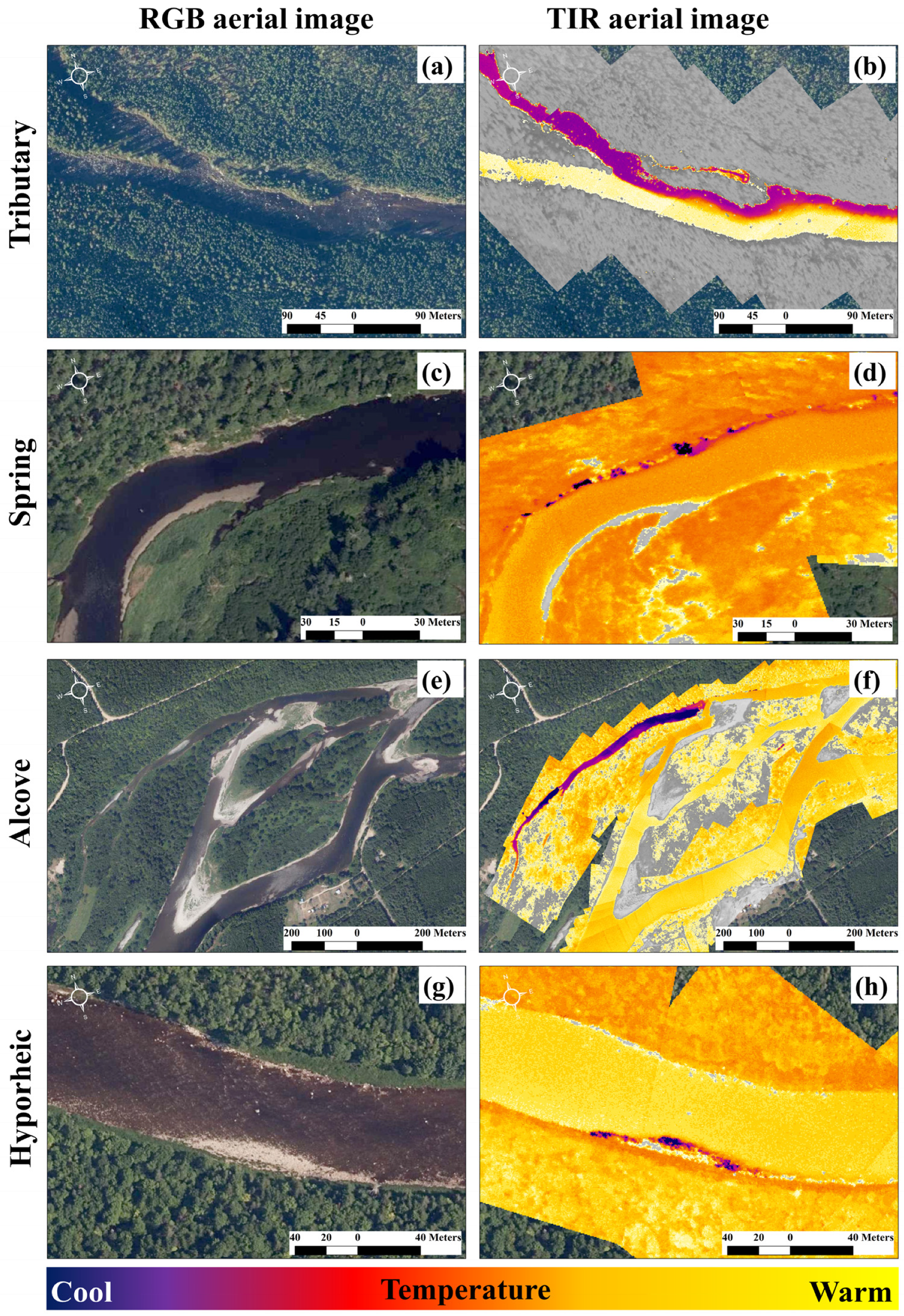
Figure 1.
Illustrations of four thermal refuge classes, where the red–green–blue (RGB) aerial image is paired with a corresponding thermal infrared (TIR) image. A tributary confluence thermal refuge is shown in (a,b), where the influence of the tributary is > 200 m in the main river. Panels (c,d) display a groundwater spring refuge, where the influence of the spring has a markedly small footprint. An alcove thermal refuge is presented in (e,f). These areas can be groundwater dominant and have low dissolved oxygen values and can also be disconnected from the main river during low flow. The last thermal refuge class is the hyporheic discharge refuge (g,h). These present as very fine-scale discharges and are likely to be the least energetically favorable thermal refuges for salmonids due to the hydrological processes that govern their presence [35]. The example images are from the Miramichi River, NB, Canada.
There are several other important thermal refuge types that have been observed to hold salmonids during thermally stressful conditions. These include (1) groundwater springs (Figure 1c,d [25]); (2) groundwater alcoves (Figure 1e,f [34,36]), and (3) hyporheic discharge points (Figure 1g,h [37]), as well as other types of cold-water patches and/or thermal refuges as described by [19,21,26,32].
Groundwater springs can be excellent thermal refuges for salmonids [25]; however, these refuge types represent fine-scale (small) cold-water patches. Although high densities of fishes can be observed in springs, they generally have a limited capacity to hold large quantities of salmonids due to their limited area and depth [18,19,37] (Figure 1d).
Other thermal refuges can also hold salmonids during thermally stressful conditions; however, these refuge types can be limited by other physical characteristics. For instance, alcove thermal refuges (Figure 1e,f) may have favorable low velocities and macrophytes that offer protection from predation. However, a low dissolved oxygen content can limit their usefulness [34,38]. Further, these refuges can become disconnected from the main river during low discharge (Figure 1f)—a critical criterion differentiating a cold-water patch from a thermal refuge. The least efficient thermal refuge type, and most under-reported in the literature, is a hyporheic discharge thermal refuge (Figure 1g,h). This is, perhaps, unsurprising as hyporheic discharge points are associated with changes in hydraulic gradients [39] and typically occur along gravel bars and at the downstream end of a riffle [35]. These areas are likely to be shallow (Figure 1h) if they present along a gravel bar or will be associated with downwelling currents from the riffle, which can induce higher velocities that would increase swimming costs [40].
In addition to thermal refuges in natural rivers, anthropogenically created thermal refuges may be found in (hydropower) regulated rivers. First, hydropower dams generate impoundments such as reservoirs or “headponds” that—depending on their size, depth, and flow regulation regime of the affiliated dam—may essentially act as lentic rather than lotic waterbodies and can thermally stratify due to their relatively slow flows and large depths (relative to the same body of water in an unimpounded condition). In rivers that frequently exceed the thermal tolerances of stream salmonids, the thermal stratification in impoundments can act as a thermal refuge, e.g., a 45 m deep, 37 km long reservoir in the Wolastoq|Saint John River in NB, Canada [41]. In regulated river systems, thermal refuges (or alternatively ‘cold-water pollution’, sensu [42]) may also be created downstream of impoundments via the release of cold hypolimnetic water from deep intakes into the tailrace or other adjacent areas [43]. In the Wolastoq|Saint John River system, for example, cold hypolimnetic water is mixed with groundwater that is further pumped into a biodiversity facility associated with the Mactaquac Dam. The outflow of this facility serves as an important thermal refuge for adult Atlantic salmon [44]. Finally, thermal refuges may be created in regulated systems where inter-system water transfers are used to route the flows between tributaries of different sizes in an effort to funnel flows to central hydropower generation units. Such inter-system transfers are common in Norway, for example [11,45]. It is important to note that while anthropogenically created thermal refuges may offer survival benefits to salmonids in regulated rivers with regard to protection from excessive water temperature, the repercussions of manipulated river temperatures may be far reaching, and a system-level assessment of the effects should be undertaken in cases where anthropogenic thermal manipulations occur [42].
3. Detection and Mapping of Thermal Refuges
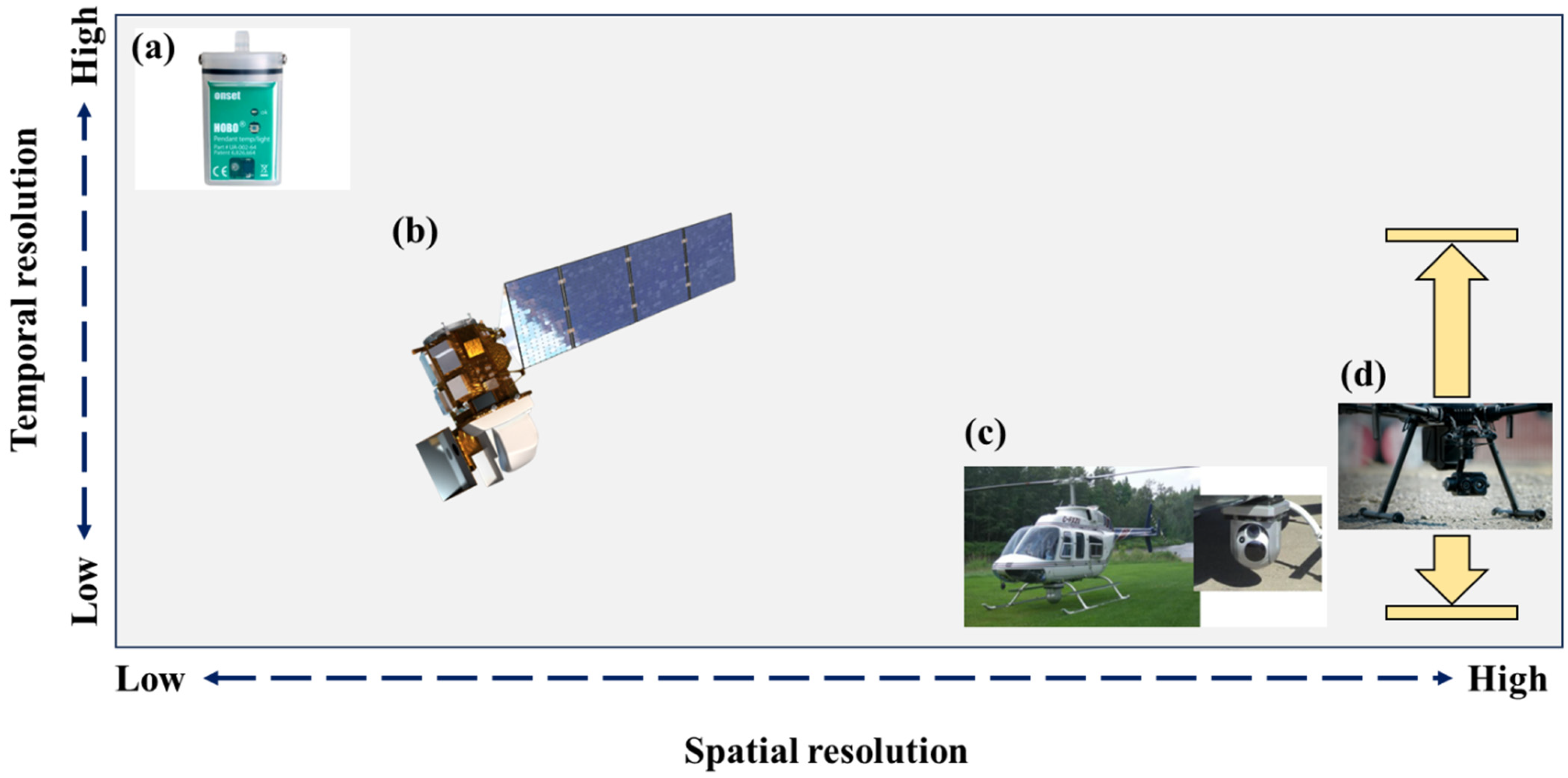
The methods for detecting and mapping thermal refuges vary at spatial and temporal scales (Figure 2). The most common method for detecting and further understanding the function and utility of thermal refuges has been via the deployment of thermographs, or temperature loggers [23,36,46] (Figure 2a). These data loggers are relatively inexpensive and provide researchers with insights into how a thermal refuge’s temperature regime varies through time using high temporal resolution. Temperature loggers have been deployed across broad spatial scales to better understand how different spatial locations across entire catchments may, or may not, provide thermal refuge for salmonids [27,47,48]. Typically, the data from a large network of temperature loggers is assembled and various modelling tools are then used to provide meaningful inference about the spatial patterns of the water temperature [49,50]. Spatial statistical network (SSN) models have proven useful in many cases because of the spatial autocorrelation of the water temperature data in dendritic networks of streams and rivers [50,51]. However, catchments with complex geologies may severely affect the simple predictable continuum of spatial autocorrelation patterns, and the current generation of SSN models (where spatial autocorrelation is assumed to be positive) may fail to perform well in such complex hydro-topo-geological settings [52]. In such hydro-geological complex situations, the water temperature logger data are better inferred using other modelling tools, such as machine learning Random Forest algorithms [52]. Temperature loggers are also naturally used at finer spatial scales—e.g., a tributary confluence—to capture the spatio-temporal variability of thermal refuges [53,54]. The trade-off with data loggers, however, is that they only represent a single point across a thermally heterogenous riverscape [5].
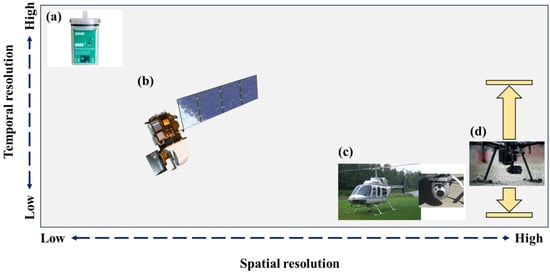
Figure 2.
A schematic depicting both the temporal and spatial scale that different temperature sensors can capture. Different spatio-temporal resolution combinations of water temperature are achieved by using temperature loggers (a), satellites (b) and manned- (c) and unmanned (d) airborne thermal infrared imagers. The use of these sensors is discussed in depth in the main text.
In recent decades, remote sensing has become a critical tool in the river ecologist’s/hydrologist’s toolbox. Satellites, such a Landsat 8 with two thermal bands, can provide spatially continuous data across broad swaths of rivers [55]. However, this comes at the cost of temporal resolution as the revisit rate for Landsat 8 is ~16 days [56] (Figure 2b). The major limitation of using thermal satellite images is their resolution. For instance, the Landsat 8 thermal bands have a 100 m ground sampling distance (GSD). While useful for large rivers, these data are not adequate for mapping the fine-scale heterogeneity that characterizes thermal refuges, especially in smaller streams that are often obscured by riparian vegetation. In a seminal paper, Torgersen et al. [31] illustrated how airborne thermal infrared imagers (or TIR) could be used to map entire riverscapes at a sub-metre resolution to identify thermal refuges (Figure 2c). Even though Torgersen et al. [31] were not the first to use TIR for mapping river temperatures [57], they formalized the process that has become common in mapping thermal refuges and other cold-water patches. Beginning in the 2010s, a sharp rise in the use of TIR for mapping potential thermal refuges commenced. Dugdale et al., [32,58] collected airborne TIR to classify potential thermal refuges across an entire catchment (the Restigouche River, NB, Canada). Similarly, airborne TIR has been used to elucidate the landscape controls on potential thermal refuges [52,59,60], whereas others have coupled TIR data with temperature loggers to develop broader understandings of thermalscape heterogeneity [22,61]. Further, others still have used airborne TIR to identify the location of thermal refuges to guide behavioural ecology studies [25,62]. Nonetheless, airborne TIR imagery remains expensive and provides data for only a snapshot in time.
Recently, relatively cheap TIR imagers (mostly uncooled microbolometers) have been paired with unmanned aerial vehicles, or drones, to map the reach-scale thermal heterogeneity of rivers [63,64] (Figure 2d). The efficacy of drone-based thermal mapping lies in its ability for users to collect high spatial resolution data (<0.2 m) at a high temporal resolution. For example, O’Sullivan et al. [65] coupled a TIR imager and a drone to gain repeated insights into how adult salmon hydraulic habitat selection varied as a function of temperature over a three-week period in the summer. Morgan and O’Sullivan [66] found that the holding positions of two salmonid species in a thermal plume differed by age class and species, and this was explained by the fine-scale thermal variability mapped via drone-based TIR. On a cautionary side, drone-based TIR imagers can suffer from large thermal drift [63]. However, the recent work by O’Sullivan and Kurylyk [67] found that the addition of tin foil to the outside of the TIR imager and flying the drone during clear sky conditions can largely alleviate the temperature bias related to thermal drift.
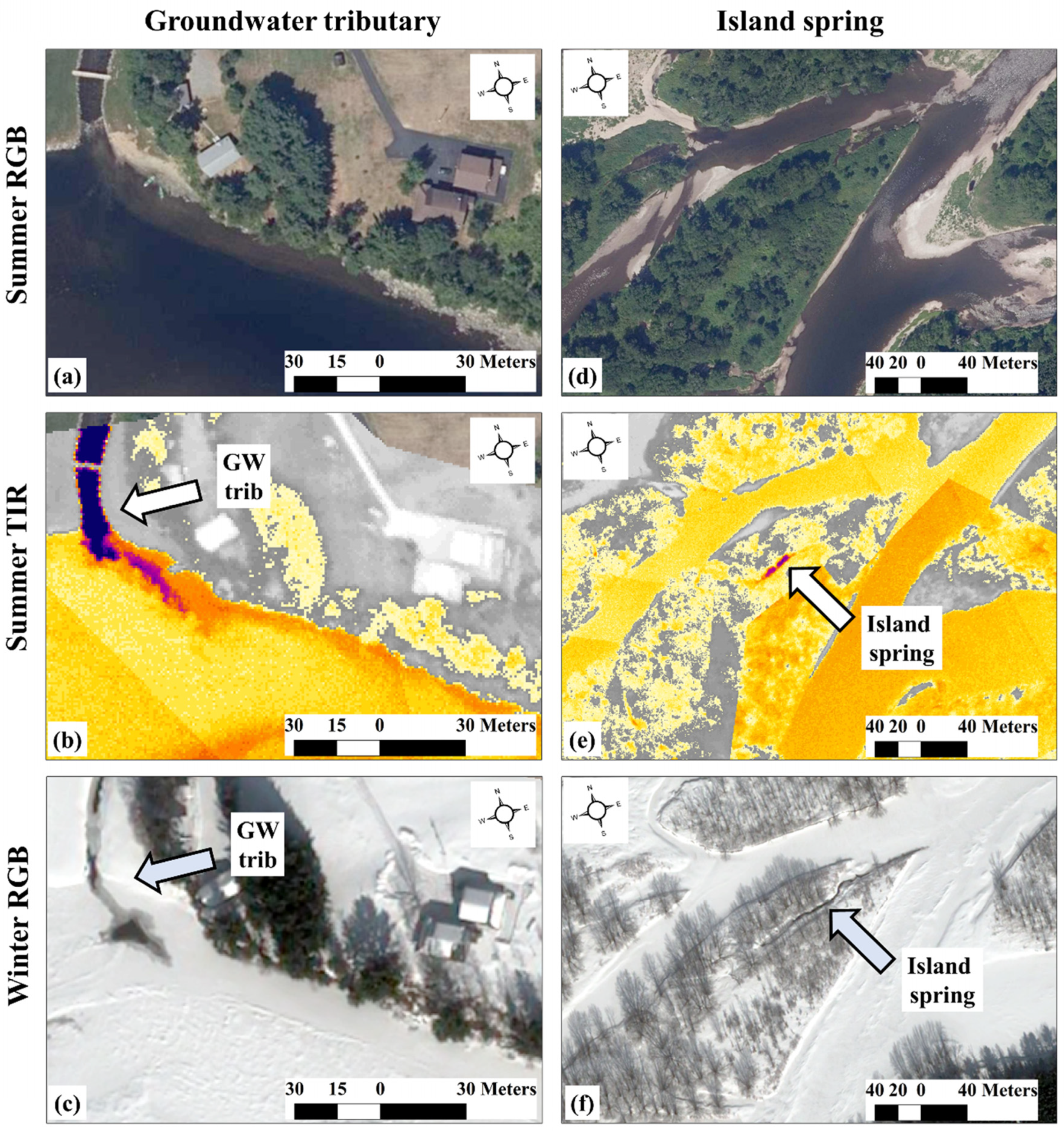
In northern climates where rivers freeze during winter, true colour (red, green, blue—RGB) satellite imagery can also be used to identify (summer) thermal refuges. Relatively deep groundwater will not freeze in the winter. Using this simple fact, O’Sullivan et al. [68] showed how sub-metre resolution (0.5 m) true colour satellite images collected when ice cover exists (ICE) can be used to identify potential (summer) thermal refuges with very high success (Figure 3). This method is illustrated by the identification of both tributary and spring-derived thermal refuges by comparing true colour summertime aerial images (Figure 3a,d), their corresponding airborne TIR images (Figure 3b,e), and winter satellite RGB images (Figure 3c,f). Since the ICE method utilizes freely available Google Earth imagery, it provides a much more inexpensive method for identifying potential thermal refuges in comparison to the expensive TIR method, making the identification of potential thermal refuges accessible to almost everyone, provided that iced-over conditions exist in winter.
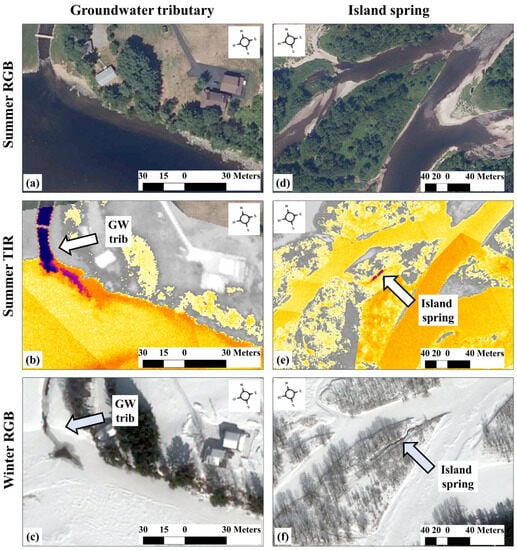
Figure 3.
In frozen environments, discrete groundwater (GW) discharges (dependent upon the depth the groundwater is sourced from [68]) can remain free-flowing during iced-over conditions. This is detailed for a groundwater tributary in panels (a–c) and for an island spring in panels (d–f), where the thermal images (b,e) reflect the summer conditions, and the high-resolution satellite images (Worldview 3) reflect the winter conditions (c,f).
4. Behavioural Thermoregulation; Elaborating the Concept
In the widest sense, behavioural thermoregulation takes place continuously in all free ranging poikilotherms, including fishes. Fish will generally align themselves in habitats that are conductive to their optimized metabolism, and ideally, fish will live in areas where their aerobic capacity is optimized (i.e., “optimal temperature for growth”; Topt). Another article in this special issue synthesized these optimal ranges for many stream salmonids [2]. All the movement of fish into such a suitable thermal habitat is, in a sense, behavioural thermoregulation: that is, fish use cognitive processes to select habitats within suitable or even optimal temperature ranges. However, in the thermal stress literature, including the main purpose of this current article (and to which the term henceforth refers), behavioural thermoregulation is a concept used to highlight the behaviour where stream salmonids aggregate (often in high numbers) in a patch of generally cooler water than the ambient water temperature during conditions when the ambient water temperature exceeds some physiologically disadvantageous criteria. The purpose of behavioural thermoregulation in response to heat stress appears to be two tiered and is reflected by the timing when different life stages start increasingly using cold-water refuges.
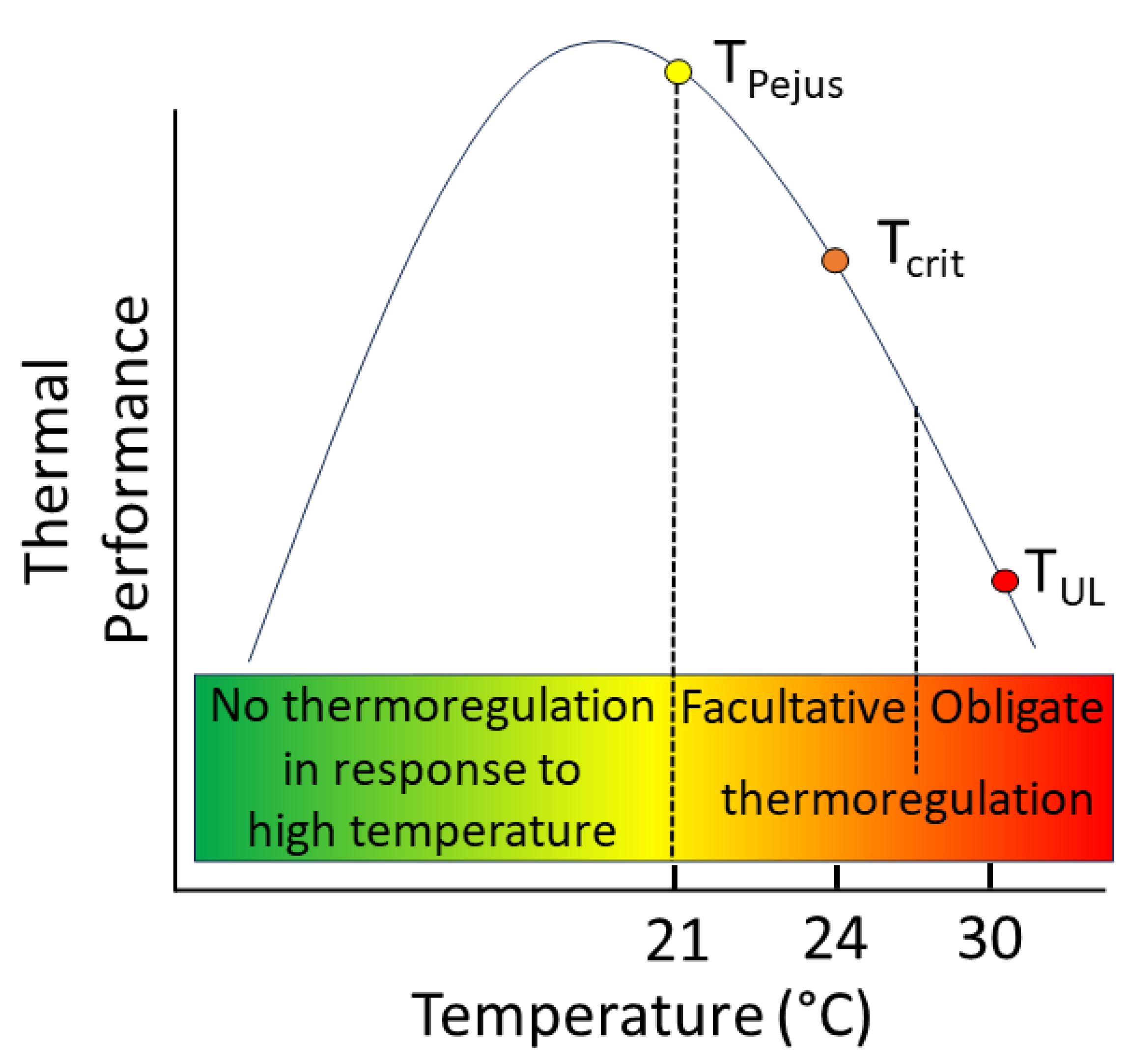
The two tiers of behavioural thermoregulation are herein defined as facultative and obligate behavioural thermoregulation. Conceptually, facultative thermoregulation is proposed to become increasingly common (yet, remains “optional”) under conditions when the water temperature exceeds the species/life stage/population-specific pejus water temperature (Tpejus). This demarcates a threshold when the water temperature exceeds the upper limit of an optimal temperature range and when the thermal conditions start to get increasingly worse for the individual fish (Figure 4). Facultative thermoregulation may continue to be observed slightly beyond the critical temperature (TCrit; Figure 4); a temperature threshold at which anaerobiosis starts to dominate the energy supply as the aerobic scope approaches zero. Within this range of water temperatures, the thermal conditions in the ambient river are increasingly beyond those that are physiologically suitable. Therefore, salmonids achieve a better thermal habitat by seeking out cold-water refuges in comparison to remaining in the ambient water temperature (but where they could still survive).
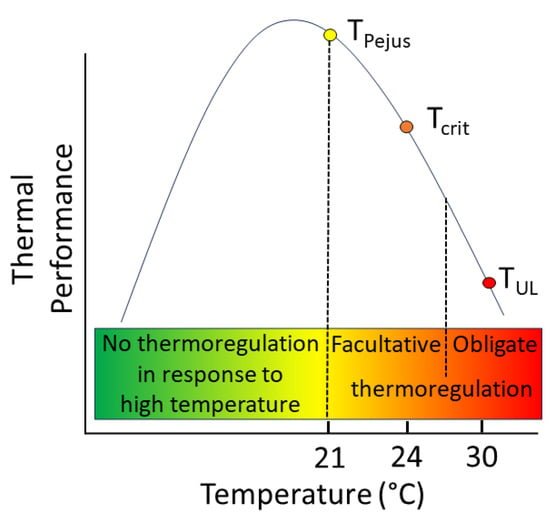
Figure 4.
Conceptual model of behavioural thermoregulation in response to high water temperature events differentiating non-aggregating behaviour and facultative vs. obligate behavioural thermoregulation. Facultative thermoregulation is proposed to (voluntarily) begin to occur under conditions when the water temperature exceeds the species/life stage/population-specific pejus water temperature (Tpejus) and may be observed slightly beyond the critical temperature (TCrit). Obligate behavioural thermoregulation takes over at some temperature > TCrit but in advance of the ultimate lethal temperature (TUL) and continues at temperatures exceeding the initial trigger temperature because a fish can no longer survive without behavioural thermoregulation. The example approximates the published literature values for juvenile Atlantic salmon to illustrate the conceptual difference between the two modes of behavioural thermoregulation [69,70].
Contrastingly, obligate behavioural thermoregulation occurs at some time (but not necessarily at a crisp “threshold”) when the ambient water temperature exceeds TCrit. As the water temperature continues to rise, and anaerobic metabolism produces accumulating concentrations of by-products (e.g., lactate) in fishes’ bodies, causing stream salmonids to enter a mode of obligate behavioural thermoregulation; a phenomenon that is necessary for the animal to avoid imminent death (Figure 4). As the metabolic by-products start to accumulate at an accelerating rate when the water temperatures exceed TCrit, the timing of the initiation of obligatory thermoregulation occurs at some temperature around TCrit but, supported by observations in field, well in advance of the ultimate lethal temperature (TUL), i.e., the temperature that fish cannot tolerate for more than 10 min [70]. Obligate behavioural thermoregulation continues under all water temperatures exceeding the initial trigger temperature (Figure 4).
It is important to recognize that not all the life stages of a salmonid species necessarily respond to high temperature events by both facultative and obligate thermoregulation. This is highly related to life-stage-specific behavioural patterns. For example, juvenile Atlantic salmon establish territories that are defended against conspecifics [71]. Due to their territoriality, juvenile Atlantic salmon may remain in their territories even when conditions exceed the TCrit values established in the laboratory [69], and generally aggregate (at least initially but see the concept of thermal hysteresis below; [72]) when temperatures exceed TCrit by 2–4 °C. This means that the juvenile life stage due to their inherent territorial nature seems to forgo facultative behavioural thermoregulation, and only exhibits obligate thermoregulation under conditions where remaining in territories becomes impossible due to the imminent probability of physiologically induced death (Figure 5). By contrast, adult Atlantic salmon return from the ocean and ascend their natal rivers typically in three distinct migratory phases [73]. Especially during the active upstream migratory phase, it appears common to encounter adult salmon in cold-water refuges soon after Tpejus is exceeded. However, and importantly, under these conditions adult Atlantic salmon can also be commonly encountered in ambient water temperatures, especially in reaches where cold-water refuges are not immediately close by. Such circumstances highlight facultative behavioural thermoregulation. Adult salmon do not have to remain in the cold-water refuge; however, if a refuge is available it may offer a thermal habitat that is physiologically more favorable than the ambient river water, provided that other physical habitat needs are met (e.g., depth, velocity, dissolved oxygen). For example, in the Northwest Miramichi River, NB, we observed adult Atlantic salmon beginning to use thermal refuges at 19 °C. However, other adult salmon continued to remain in ambient water temperatures up to 26 °C during the early part of their run (i.e., June [74]). This would represent a case of facultative behavioural thermoregulation, although it is also possible that the fish had to cope with the temperature stress due to the lack of a nearby cold-water refuge. However, in the Southwest Miramichi River, NB, we witnessed very strong obligatory behavioural thermoregulation in adult Atlantic salmon already at a water temperature slightly exceeding 19 °C (Figure 5 [65]), albeit this was observed in August by adults that had likely been in the river for a number of weeks already, and therefore, may have had pre-existing “thermal fatigue”, i.e., previously accumulated physiological by-products in their bodies [25,72]. We also observed a similar transition from facultative to obligate behavioural thermoregulation for brook trout Salvelinus fontinalis (Mitchill, 1814) in the tributaries of the Miramichi River (Figure 5) [25]. A relatively straightforward way to differentiate whether behavioural thermoregulation is facultative or obligate under a given situation can be inferred from the number of individual fishes in cold-water refuges vs. those remaining in ambient water temperatures. During facultative use, the individuals were often readily observed in both the thermal refuge and in ambient river habitats, whether their territories (e.g., juvenile salmonids [23,25]) or in non-refuge pool/deep run mesohabitats for adults (e.g., adult Atlantic salmon [74]). During obligate behavioural thermoregulation, effectively all the individuals of a given life stage aggregated in thermal refuges—typically en masse—or were in the process of doing so (Figure 5) and temperature-induced mortalities were also observed during these events (Figure 6). However, care has to be taken when using such field observations to determine the severity of behavioural thermoregulation because the relative abundance of fish in the area naturally dictates how many fish will be observed in a thermal refuge. That is, even one fish in a thermal refuge may represent obligate behavioural thermoregulation “aggregation” if it happens to be the lone fish present in the river reach. Such a consideration would have appeared ridiculous historically, but sadly with dwindling population densities in areas such as the Inner and Outer Bay of Fundy and even in many rivers in Atlantic Canada, small aggregations of very few Atlantic salmon should not be dismissed as non-important events.

Figure 5.
Examples of obligate behavioural thermoregulation in the Miramichi River, NB, Canada. (a,b) Adult Atlantic salmon in size-segregated aggregation in an inflow of 14.3 °C and when the adjacent main river temperature was 19.1 °C. (c) An aggregation of 1+ and 2+ Atlantic salmon parr in an inflow of 21.0 °C; main river 31.5 °C. (d) An aggregation of adult brook trout in an inflow of 20.4 °C; main river 26.0 °C.

Figure 6.
Example of adult Atlantic salmon mortality in direct response to a high temperature stress event in the Little Southwest Miramichi River, NB, Canada when the water temperature exceeded 30 °C, illustrating the grave consequences to salmonid populations if access to cold-water refuges is compromised.
When do stream salmonids have to aggregate in thermal refuges, i.e., what factors affect the timing of obligate behavioural thermoregulation? At the proximate level, the need for stream salmonids for obligate thermoregulation has an intricate physiological basis and the basic thermal biology of salmonids is described thoroughly elsewhere. Cardiac function/collapse or possibly hypoxia in the brain play a pivotal role as a limiting factor for upper temperature tolerance [75,76,77]. Since the need for behavioural thermoregulation is rooted in the physiological demands of the fish, there is variability in the timing when this phenomenon occurs. First, different stream salmonids have their species-specific thresholds for thermal habitat suitability (see this special issue [2]), and not surprisingly, the water temperatures at which various authors have observed behavioural thermoregulation either in situ or in experimental settings reflect these inter-species differences (Table 1). Some of the variability in the aggregation temperatures observed in the previous work stemmed from the fact that the data did not always represent a so-called “threshold” when behavioural thermoregulation was triggered, but simply described the temperatures at which the behaviour was observed to occur (Table 1). Additional variability may result from the authors who possibly describe both facultative and obligate behavioural thermoregulation. Similarly, it has been well established that the need for behavioural thermoregulation is linked to body size, translating into different timings for the phenomenon between the life stages within each species (Table 1). In effect, the larger, and therefore, typically older life stages respond earlier (i.e., in lower water temperatures) than smaller, younger life stages (Table 1). Breau et al. [69] discussed this size-dependent difference in timing for behavioural thermoregulation in the context of a larger surface-to-volume ratio in the younger life stages, and the consequent improved ability to uptake oxygen across the skin surface. A larger surface-to-volume ratio also creates a faster heat transfer, and therefore, a lower internal body temperature; one of the main predictions of Allen’s rule originally drawn for endotherms [78], but with a high relevance to ectotherms in thermally heterogenous environments, such as avoiding overheating in hot microhabitats [79]. In addition to species- and size-specific differences, there is also evidence that the timing when obligate behavioural thermoregulation is initiated may differ between populations within species, as would be expected due to the population-specific physiological differences in thermal tolerance [80,81,82]. For example, Corey et al. [13] examined the timing of obligate behavioural thermoregulation in prominent Atlantic salmon rivers in Eastern Canada that differed in their extent of warming and concluded that the aggregation response was not universal across rivers. They observed that the onset temperature for juvenile Atlantic salmon aggregations in thermal refuges occurred 1 °C sooner in the relatively cooler river (Little Southwest Miramichi; LSW) in comparison to the warmer Ouelle River, indicating a population-specific phenotypic plasticity in thermal abilities [13]. The preliminary data similarly suggested that in comparisons of a warm (LSW) to a relatively “cool” river (Restigouche, NB, Canada), the same pattern held wherein the thermoregulation was initiated earlier in the cooler river [83], which validated the assumptions made by Breau [82]. To predict that such differences exist between the populations of different thermal backgrounds, and especially across latitudinal clines, seems logical because these variations may be related to differences in the metabolic rates across a latitudinal gradient, as often described for many ectotherms (see e.g., [84] and references therein). Some laboratory experiments conducted on Atlantic salmon suggested that, with respect to their thermal capabilities, the differences between the populations did not appear to be evidence of local adaptation but rather were a result of high cardiac plasticity wherein the acclimation temperature (i.e., differences in the ambient water temperature between rivers) allowed for cardiac functions to compensate for the warmer temperatures [85]. However, laboratory experiments have also shown that there was a high phenotypic variation between individuals, families [86], and populations [80] with respect to the thermal tolerance where the variation had a significant genetic basis, which may allow for adaptation, at least under conditions of artificial selection (e.g., supplementation programs or aquaculture). There is some evidence that the sex of the fish also affects the ability to withstand or respond to high water temperatures. It has been suggested that females are relatively more vulnerable than males [87,88], and therefore, the timing or duration of behavioural thermoregulation may be affected by sex.

Table 1.
A compilation of water temperatures (°C) when behavioural thermoregulation (as defined in the text) was observed to occur for various stream salmonids, stratified by life stage.
Since the obligate behavioural thermoregulation is dependent on the species, life stage, and population, is there then “a fixed temperature threshold” that triggers an aggregation response into thermal refuges? Early work on the phenomenon provided some evidence to such fixed temperature thresholds [58] and, certainly, much of the current conservation management is based on simple water temperature thresholds targeting specific activities. The “fixed temperature threshold model” provides a relatively good approximation to the likely timing of the initial obligate behavioural thermoregulation events, for example, during the first thermal stress event of a summer or after a sufficiently long period of recovery, allowing for any previously accumulated physiological metabolites to dissipate. However, to explain the apparent variability in the water temperatures at which fish are aggregating into thermal refuges, O’Sullivan et al. [72] recently developed a model based on the concept of thermal hysteresis. As proposed in the previous studies, the accumulating physiological by-products due to anaerobic metabolism remain in the bodies of salmonids even after critically stressful water temperature conditions subside, and the presence of these by-products may compromise the ability to cope with subsequent heat events [25,110]. This has been termed “thermal fatigue”, “thermal baggage”, or “thermal hysteria” [25,72]. The existence of these accumulated anaerobic by-products is the proximate reason why the thermal threshold to aggregate into cold-water refuges seem to decline during subsequent thermal events and it can be sufficiently modelled by taking into account the time since the previous obligate behavioural thermoregulation event and the frequency of these events [72]. Therefore, the concept of thermal hysteresis captures the idea that the timing of obligate behavioural thermoregulation is inherently dependent on the history of previous obligate thermoregulation events resulting in a variable, rather than static, threshold temperature [72].
5. Behaviour Leading to, during, and after Behavioural Thermoregulation Event
5.1. Finding Refuges
Stream salmonids are known for their extreme spatial cognition as long-distance migrants [111]. However, their spatial capabilities as juveniles during their freshwater phase are also remarkable [112], with a sub-metre ability to return to prior territories after >5 month periods [11]. The searching ability of spatially sparse thermal refuges corroborate these earlier works that the spatial capabilities of at least juvenile Atlantic salmon appear excellent, and their movements to and from thermal refuges are not by chance. Breau [113] and Corey et al. [62] both examined individually tagged juvenile (age 1+ and 2+) Atlantic salmon leading to and during obligate behavioural thermoregulation events. Breau [113] designed a study with a maximum distance away from a known monitored cold-water refuge of 714 m; the tagged salmon parr were able to find the cold-water refuge from these distances. Continuing the previous work, Corey et al. [62] followed 636 passive integrated transponder-tagged Atlantic salmon parr and documented their movement of up to ~7.5 km to cold-water refuges. In both studies, the majority of the fishes’ movement to refuges was relatively localized and occurred within the reach of study; however, the movement distances regularly exceeded hundreds of metres [62,113]. How far the 0+ juveniles came to the thermal refuges remains unknown. Adult salmonids are capable of moving longer distances in response to thermally stressful events. For example, Carrow [74] observed a rapid migration of adult Atlantic salmon through relatively warmer downstream reaches in the Northwest Miramichi River into generally cooler upstream locations (~50 km) before the water temperature reached conditions necessitating obligate behavioural thermoregulation. Facultative thermoregulation was observed in the refuges that the migrating salmon encountered on their way to the cooler upstream reaches. As the water temperature continued to rise, migration further upstream became limited by concomitantly low water levels that prevented a search for cold-water refuges further upstream, and the adult salmon then started searching for cold-water refuges in the downstream direction [74]. However, if adult salmonids identified reaches wherein cold-water refuges are located during the later phases of their migration, they engaged in a similar behaviour as the juvenile life stages wherein they remained “conveniently” close to the refuges that they repeatedly accessed as the water temperature increased [9,65].
The mechanisms that salmonids use to find cold-water refuges during water temperature-related events are not fully understood. Upstream-migrating adults presumably continue swimming upstream until they encounter a gradient of cooler water temperature where behavioural thermoregulation is possible. For territory holding Atlantic salmon parr, both Breau [113] and Corey et al. [62] observed that individually tagged juveniles that located cold-water refuges came both from up and downstream directions, and across a river stemming >30 m in width. The likelihood of finding refuges either in the up or downstream direction was dependent on the reach of the juvenile Atlantic salmon, and similarly the movement distances in either the up or downstream direction appeared to be study reach-specific [62]. Leading up to obligate thermoregulation events, Atlantic salmon parr have been observed to assume positions off the stream substratum, actively swimming in water columns within their territories (but not feeding), and then grouping to small clusters of few parr, and traveling towards presumed cold-water refuges in small aggregations [113]. The possible explanations for traveling towards cold-water refuges in groups were hypothesized as potential safety in numbers, migration with kin, or the possibility of “following the leader”, wherein only some individuals knew location of the refuges, and others followed the conspecifics [113]. It has also been hypothesized that social cues, whether olfactory signals or visual cues of other aggregating fish, lead into an advertisement of the refuge locations [114]. Naturally, it is also possible that the refuge seeking fish may start aimlessly swimming in the direction where other conspecifics are moving, and they haphazardly encounter a cold-water refuge. The specific mechanisms during travel towards cold-water refuges warrant further research.
5.2. Behaviour in the Refuges and Post-Aggregation Movements
While the ultimate reason for the obligate use of thermal refuges is to survive past the thermal conditions that exceed the species/life stage/population-specific physiological criteria, it is clear that behaviour and spatial distribution of aggregating salmonids while within the thermal refuge is governed by a number of criteria. While it is recognized that the anadromous adult salmonids do not generally feed during their freshwater migration [115,116], the cessation of feeding and aggression has also been observed for many adult non-anadromous fish and the older juvenile life stages of anadromous salmonids when obligate behavioural thermoregulation takes place [23,113]. Such cessation results from the anaerobiosis and lack of energy available for specific dynamic action (i.e., metabolism related to digestion). All the available energy is used to maintain critical basal metabolism, and thus, to survive. Species-specific differences naturally occur and, for example, Brewitt et al. [117] showed that during facultative thermoregulation, the use of tributary confluence refuges allowed rainbow trout to move into the warmer main river to forage for prey and then retreat back to the cool thermal refuge.
During obligate thermoregulation events, it is notable that while energy preservation is at prime, the aggregating fish, and especially the juveniles, form their aggregations in the low(er) water column and continue to swim against the current as opposed to resting at the stream bottom or hiding within the substratum, as one would suspect from an energy conservation point of view (Figure 4c). The same behaviour has been observed in laboratory studies [69]. Breau et al. [69] hypothesized that the maintenance of swimming may have a role in recycling anaerobic waste products such as lactic acid in their muscles and could, therefore, have an active role in reducing physiological oxygen debt [118]. However, the spatial distribution of salmonids in the cold-water refuge is not independent of energy conservation or other biotic interactions. The research points towards many additional physical habitat characteristics that define what one might term the “optimal thermal refuges”. This is indicated by the fact that while the primary reason for salmonids to occupy cold-water refuges is indeed to encounter cooler temperatures than the ambient water temperature in the river, it is very common that the fish aggregations are not in the coolest part of the refuge [33,53,65,66,69,119]. In lieu of the coldest locations within the refuge, O’Sullivan et al. [65] observed adult Atlantic salmon consistently aggregating in specific hydraulic fields defined by a narrow range of a Froude number that was significantly smaller than the Froude number in the locations during non-thermally stressful events. In addition, they observed salmon aggregating in tight geometric formations—thermal-pelotons—modulating the hydrodynamics, especially for the individuals in the centre and back of the formations [65]. They proposed that this was likely linked to the interplay of the hydraulic and thermodynamic controls on the bioenergetic cost of holding differing positions in the thermal refuge [65]. Perhaps not surprisingly, the individuals in most energetically profitable positions (in the center of the peloton) were the large multi-sea winter salmon, while the smaller adults had to “sacrifice” themselves in higher energy-consuming positions in the front of the aggregation. This observation supports the notion that the spatial distribution within the cold-water refuges appears to be, at least in part, determined by intra-specific competition for the most profitable (energetic) positions. Similarly, under extreme water temperature conditions (>31.5 °C), hierarchical species and age-specific structuring within the cold-water refuge has been observed [66]. While still not necessarily occupying the coldest possible locations within the refuge, the species (brook trout and Atlantic salmon) and different age classes hierarchically structured themselves along a temperature gradient from relatively cool (21.8 °C) to warm (30.1 °C), corresponding to size and structured from largest (adults) to smallest (0+ juveniles), with 2+ and 1+ parr structured by age in between, further indicating that the more dominant (larger) individuals were able to exert control over spatial structuring within the refuge [66]. Aggregating salmonids may also remain in their typical tight clusters due to the physiological advantages of remaining in groups, i.e., the calming effect wherein aggregating may reduce the high oxygen demand associated with high temperature events [23]. The microhabitat parameters also affect the spatial distribution of aggregating salmonids, with access to relatively deeper locations in the refuge [23,119] or the availability of deeper microhabitats adjacent to the refuge [25] having been shown to be of particular importance. Why salmonids primarily aggregate in tributary-derived cold-water refuge plumes and do not generally appear to proceed upstream into the cold-water tributaries that generate these refuges may also be explained by the necessity to remain adjacent to deeper water or by other aforementioned energetic benefits that may be achieved by staying in the confluence of the cold-water tributaries and the main river.
When the obligate behavioural thermoregulation events dissipate, stream salmonids generally leave the refuge, albeit facultative behavioural thermoregulation may continue, and some individuals may find the refuge a physiologically better option than continuing into the ambient river. For territorial juveniles such as Atlantic salmon, the evidence suggests that the fish have the capability to “anticipate” the relative risk of future thermally stressing events, and if future thermal events can be foreseen, the fish may decide to establish territories in proximity to the areas where cold-water refuges are available. Breau [113] documented that while juvenile Atlantic salmon abandoned thermal refuges when the water temperature decreased below their physiological thresholds (e.g., during the nights between subsequent thermoregulation events), the fish did not return to their original tagging locations but remained on “standby” in some adjacent location where returning to the thermal refuge was facilitated. Corey et al. [62] observed that a very high proportion (96–100%) of juvenile Atlantic salmon remained in their original tagging reaches post-thermal events if they contained thermal refuges, but a lower proportion (62%) returned to a reach where no cold-water refuges were located. This finding led Corey et al. [120] to investigate the long-term effects of cold-water refuge availability on the relative abundance of juvenile Atlantic salmon along a 17-km segment of river and they found that while the relative abundance increased in the reaches with thermal refuges in summer, an equilibrium in the abundance was regained later in autumn when subsequent thermal events became unlikely. In the same study, Corey et al. [120] revealed that the tributary confluence thermal refuges with the highest temperature difference from the main river had the highest effect on the relative abundance of Atlantic salmon across five thermal refuges during the summer season. Similar observations regarding a preference towards a larger temperature differential between the mainstem and tributary has also been seen for juvenile rainbow (steelhead) trout [100].
5.3. Consequences of Behavioural Thermoregulation
The consequences of using thermal refuges are presumably clear. Salmonids that find and use cold-water refuges during thermally stressful events are able to survive through the bottleneck periods and use physiologically more benign environments in comparison to conspecifics that are not thermoregulating [69,87,121,122]. The consequence of a failure to find a thermal refuge during thermal events that necessitate obligate behavioural thermoregulation is also clear. Widespread mortality events of both adult and juvenile salmonids have been widely reported for decades [36], with numerous contemporary reports of mortalities resulting in a direct response to high water temperature events in combination with other human activities [62,121,123,124] (Figure 6). The consequences of refuge use on somatic growth are presumably small as behavioural thermoregulation occurs during conditions when there is no longer an aerobic scope for growth, feeding has ceased, and therefore, growth is arrested even during the time spent in the thermal refuges. We did not find published work examining the direct growth effect of refuge use by stream salmonids. Many others who have reported biological consequences have examined situations where the stream salmonid performance was compared between warm vs. relatively cooler water exposure, and therefore, the results can be inferred to apply to a situation wherein fish would have used cold-water refuges. Crossin et al. [87] noted a lower migration success in (chronically) thermally challenged female sockeye salmon, and similarly, Hinch et al. [88] reported a higher mortality in female Pacific salmonids relative to males, which they associated, in part, to high temperatures. This indicates that, at least for adults, females may benefit relatively more from using cold-water refuges and this may be explained by the fact that the stresses related to a higher investment of the gonadal development that occurs during migration [125] may be partially offset by cold-water refuge use. Gametes are also less viable in thermally challenged adult female salmonids [126], and similarly, negative effects of warm acclimation have been observed in male brown trout sperm, albeit the effects manifested only early in the spawning season [127]. The use of cold-water refuges have benefits related to energy conservation. For example, Berman and Quinn [128] estimated a 12 to 20% decrease in the basal metabolic demand for chinook salmon that exhibited facultative behavioural thermoregulation. Such energy conservation from the use of cold-water refuges is presumably beneficial as it allows for saved energy for spawning, and in the case of iteroparous salmonids, may allow for better survival post-spawning. However, the simulation work by Snyder et al. [129] in a heavily hydropower-regulated Columbia River system did not indicate significant contributions of cold-water refuge use to energy savings by migrating adults, and therefore, the energetic consequences of behavioural thermoregulation appear to be context specific.
While using thermal refuges is generally thought to provide benefits for salmonids, there also appear to be some negative consequences. The delay of migration for anadromous adult salmonids have been reported in numerous studies [41,129]. Babin et al. [41] followed acoustically tagged adult Atlantic salmon in a deep and large thermally stratified hydropower reservoir in the Wolastoq|Saint John River and observed that upon leaving the cold hyporheic water of the reservoir for a warmer riverine section, some Atlantic salmon turned back when the river temperatures exceeded 22 °C. They repeated such behaviour multiple times over the course of a month. Snyder et al. [129] similarly showed through a simulation exercise in the Columbia River system that behavioural thermoregulation contributed significantly to the total migration time (56% of total migration time) in summer-run steelhead. However, the effect was much more benign for fall-run Chinook (6% of total migration time). While these observations may be interpreted as a migration delay due to an “ecological trap” phenomenon [130], with a potential further consequence that these fish may not be able to reach their purported upstream spawning grounds, the delay may also ensure that the fish arrive in appropriate locations only after the temperature regime of the putative spawning grounds have declined into a suitable range [129]. Aside from being delayed in their migration, the salmonids aggregating in a cold-water refuge may also become exposed to high fishing pressure as many refuges are locally well-known fishing locations [119]. For example, Keefer et al. [98] documented a high rate for harvesting steelhead trout in the Columbia River in refuge tributaries. It is also plausible that large aggregations of salmonids in cold-water refuges attract predators, thus increasing the natural predation rate. However, while a plausible possibility, to our surprise we have in fact never personally documented either avian or mammalian predators at cold-water refuges even during the densest aggregation events despite having collectively observed a large number of these events. The only documented direct observations have been those of American crows (Corvus brachyrhynchos) that have been feeding on moribund or already dead juvenile Atlantic salmon floating downstream in the Miramichi River during water temperature-related events.
6. Management and Conservation Actions in Response to a Warming Climate, Thermally Challenging Conditions and Cold-Water Refuges–Future of Stream Salmonids
Since thermally stressful events are common and may become increasingly so in future, the management of the cold-water patches and refuges is becoming increasing important. Mejia et al. [28] recently outlined advice for bridging the gap between science and management in this field. Many rivers that sustain fisheries for stream salmonids have implemented management strategies to protect salmonid populations during these warm events. The purpose of the management actions with respect to warm-water events is commonly geared towards the protection of the adult life stages from exposure to recreational fishery [124,131], although actions also exist to protect specific cold-water refuges overall [26]. The evidence that the post-release survival of adult salmonids decreases as a function of increasing water temperature after species/population-specific thresholds are surpassed is convincing [131,132,133,134]. Depending on what management measures are implemented to protect the adults, the juvenile life stages may also benefit from these measures. For example, temporary closures of known cold-water refuges for recreational fishing will also protect the juveniles that aggregate there from additional “foot traffic” in these locations during thermally stressful times. Such protections may be necessary. During obligate behavioural thermoregulation, the aggregating juveniles are often physiologically stressed to a point that any additional stressor (such as tactile exhibitions by anglers who may “pick up” or poke juvenile fishes out of curiosity) may be detrimental. The protection of aggregating juveniles in key cold-water refuges may, therefore, be necessary in their own right. We have observed, for example, events where recreational vehicles (such as four-wheelers) are driven across dense juvenile aggregations in shallow refuges by unsuspecting recreational fishermen. However, extreme care must be practiced when protecting cold-water refuges so that the protective actions would not have unintended consequences. Indicating where cold-water refuges are by their closure (or by publicly sharing thermal imagery) may “backfire” by attracting audiences with non-laudable intentions. With thermal imagery becoming more readily available and shareable through open-source publications, thought must be directed as to whether or not the locations of the cold-water refuges should be made publicly known. Through autonomous underwater video camera imagery, we have indirectly observed poaching events of anadromous brook trout when the fish have been aggregating in cold-water refuges. In our example, a large number of anadromous brook trout were repeatedly observed in subsequent frames of underwater camera imagery (for >13 h) when the water temperature conditions exceeded the conditions for obligate behavioural thermoregulation (28 °C) only to abruptly, completely, and permanently disappear from the refuge even though the water temperature conditions remained unchanged. Concurrently, the “surface foam” covering the cold-water refuge also disappeared between the photograph frames. It is conceivable that the fish were abruptly removed by a poacher’s gill net maneuvered through the cold-water refuge. While such poaching events or individuals engaged in such illegal activity may be sparse, it is nevertheless important to carefully consider whether the spatial location of critical cold-water refuges should be publicly advertised as they may attract unwanted attention to these important locations that may otherwise stay hidden to the public eye, and especially to the individuals who have affinity for illegal harvests. Alternatively, local river guardians, conservation officers, or automated (infrared) security cameras may be required to ensure the protection of cold-water refuges from the “two-legged predator”.
VanLeeuwen et al. [131] recently reviewed when and how water temperature-related fishery closures are best implemented. The trade-offs in these management strategies are clear. Since salmonid fishing may represent one of the most socio-economically significant activities in rural communities, the closure of recreational angling that is too lenient may have a severe financial consequence on the local economy. However, on the reverse, the non-closure may have a drastic consequence on the salmonid population due to exposure to said fishing during an exceedingly stressful time. Various protocols are used to implement water temperature-related protections, and common strategies include closures of selected cold-water pools, closures of whole or larger sections of river, or restricting the timing of fishing activities within the day after pre-determined temperature thresholds are exceeded [131]. One of the strategies that appear to offer a promising balance between continued fishing vs. temperature-related closure is the implementation of a “morning only” fishery that would safeguard fishing opportunities for anglers who may have traveled a long distance for their fishing opportunity, while protecting the salmonid population during the most stressful time of the day [131]. Hourly water temperature consistently reaches its minimum at a predictable morning hour, e.g., 9:00 AM in the Miramichi River [135]. The best protection/management strategy depends, however, on the status of the salmonid stock, fishing pressure, and the overall temperature regime of the river that may remain too high throughout the diel period for any fishery in certain locations [131]. To help manage water temperature-related fishery closures in the future, it will be useful to better understand the population-specific plasticity with regard to their requirements to behaviourally thermoregulate [13]. The current temperature thresholds when fisheries closures are contemplated have often been derived from research that took place in relatively warmer rivers where populations may have slightly higher tolerance for temperature-induced behavioural thermoregulation. However, in northern latitudes or relatively cooler rivers, the physiological processes in response to water temperature may start earlier in such cold-evolved populations. The hysteresis curves may take a drastically different shape or the inflection points where stress begins to accumulate may occur much earlier in these rivers, necessitating earlier management intervention.
The opportunities for cold-water refuge management/manipulation is also presented by hydropower-regulated rivers through hypolimnetic water releases or other cold-water outflows released into the tailrace. On one hand, such artificial cold-water releases can create an opportunity for salmonid fisheries in rivers that would otherwise not be thermally suitable [43]. On the other hand, such cold-water plumes may become locations where upstream-migrating salmonids spend excessive time; therefore, significantly delaying or even stopping further upstream migration. These cases have been aptly named thermal pollution [42] or thermal traps [41]. The management of the cold-water regime in hydropower-regulated rivers can be particularly complex as the quantity of water flow is often tied to the energy demands and market conditions, and the protection/enhancement of salmonid populations via these mechanisms adds another layer of complexity. It also has to be recognized that toying with the natural temperature regime of a river using cold hypolimnetic releases is likely to have cascading repercussions throughout other aquatic biota and the ecosystem, and the overall impacts of changes in the thermal regime should be fully evaluated before cold-water releases are used to benefit salmonid fisheries. The trade-offs in tailwater thermal habitat management are often complex [43].
Management decisions aside, another increasingly common conservation measure aimed at protecting salmonid populations from stressful warmwater events is cold-water refuge restoration [21]. This practice is becoming particularly popular in Atlantic Canada among non-government environmental organizations, First Nation conservation groups, and fishing clubs, and can fall under the categories of improving/enhancing existing cold-water refuges or potentially creating new ones. While promising, and possibly a generally useful and desired activity, there are a few important considerations that need to be taken into account when cold-water refuge restoration in contemplated.
First, if the addition of new cold-water refuges (e.g., via groundwater pumping [21]) is contemplated, a very careful and multi-faceted consideration of the consequences will be necessary. For creating new cold-water refuges, the first important question regards the location of the proposed refuge. Presumably, new cold-water refuges could be contemplated in reaches where a river network analysis (e.g., thermal imagery) indicates a near-complete lack of current refuges. Creating a new refuge could, under these circumstances, have an important protective effect if situated in the reaches where the current nearest refuge is beyond the movement ability of e.g., juvenile life stages when obligate behavioural thermoregulation is triggered. In this context, the approach utilized by Snyder et al. [129] appears particularly useful for assessing different cold-water refuge addition scenarios. Such simulation tools allow for an assessment of the contribution of thermal refuges on the migration timing, distance, energy conservation of migrating/moving salmonids, and their eventual success, and therefore, allows for a determination of the utility of the added (or removed) thermal refuges on fish survival and health [129].
The second important consideration when contemplating the creation of new cold-water refuges is why a refuge would be created in a particular location. If the location is determined based on a large-scale strategic assessment and is determined to be a location in a reach where no other refuges exist, as above, then the location may be justified as a conservation measure. However, perhaps the creation of a new cold-water refuge is to retain more fish adjacent to a fishing lodge for the purpose of improved recreational angling opportunities? In such cases, the intent would be counterintuitive from the conservation perspective, even when the intent would be to practice catch-and-release fishing since mortality rates do increase with increasing water temperature [133,134]. If creating new cold-water refuges increasingly becomes a practice for the purpose of retaining fish close to a recreational fishing lodge, there is a real risk of such practice further exploding into an arms race between fishing establishments with the “most fish going to the person with the largest pump” and should generally be discouraged since groundwater sources are a finite resource. In fact, the third important consideration with regard to creating new cold-water refuges is whether such practice is advisable at all, as the use of groundwater to create a new refuge will explicitly mean that the groundwater will be “away” from naturally percolating into the river in another location. The action of pumping groundwater also takes water away from the terrestrial ecosystem. At periods when water levels are low and temperatures are high, water-induced forest stress is also likely [136]. Further, the impacts may resonate to domestic water supplies in regions where groundwater wells provide potable water. This is an aspect with no easy solution; even all the authors of the current article do not fully agree on the advice of groundwater pumping. Kurylyk et al. [21] presented different scenarios for creating new cold-water refuges through groundwater pumping and the simulated effects on river depletion. It is important to emphasize that the intent of this work was to suggest the creation of cold-water refuges only on an extreme temporary basis, i.e., activated automatically but temporarily when water temperature exceeds conditions that are extremely likely to cause obligate behavioural thermoregulation events and in areas where no other refuges are present. Implemented in this way, a new refuge would be temporarily created during the hours of day when the alternative scenario for stream salmonids is likely to be physiologically induced death but would minimize the overall effect on the amount of groundwater and river depletion.
With regard to projects that aim to enhance or restore cold-water refuges, multiple interesting avenues exist for the future. As our understanding increases regarding what characteristics conform a “goldilocks refuge”, some cold-water refuges can possibly be made significantly more suitable through even small changes [21]. However, conservation groups should also keep in mind that the old adage of “fix it only if it is broken” may prove useful in this context. One potentially promising avenue for restoration/enhancement work may regard cold-water alcove refuges, which may become disconnected from the main rivers during low discharge conditions but may hold large volumes of cold water (Figure 1f). The cold-water alcoves may also be oxygen-depleted due to high groundwater concentrations and a very slow flow, but artificial oxygenation through an air pump may alleviate these situations or modifications in the alcove could induce drops that entrain air and thereby provide natural oxygenation. These possibilities are ripe for exploration in the future.
If the emphasis of cold-water restoration is on maximizing the net benefits for aggregating salmonids, then one must implicitly understand what the fish are looking for in a thermal refuge. While the secondary physical habitat characteristics may temporarily become less important in certain cases, e.g., during obligate behavioural thermoregulation events (“life-or-death situation”) when access to some cold water is better than no access at all, we documented through numerous examples that a cold-water patch does not necessarily mean cold-water refuge, let alone an ideal refuge. As outlined earlier, Wilbur et al. [25] showed that access to relatively deeper water adjacent to the cold water was a major determinant separating a cold-water patch from a refuge for large-bodied brook trout. O’Sullivan et al. [65] demonstrated a specificity towards unique hydraulic conditions, and not necessarily the coolest water for adult Atlantic salmon, further indicating that knowledge of the thermal refuge habitat and hydraulic requirements is necessary for most successful cold-water refuge restoration. We propose that it may be necessary to mandate before–after monitoring protocols for cold-water refuge restoration/enhancement, which could include documenting both the tangible benefits in terms of the gained physical cold-water habitat but possibly also show that the enhancement activity at least does not reduce the density or absolute number of salmonids using the refuges post-enhancement. While these projects almost always proceed with the best future for the salmonid population in mind, the outcome is not necessarily guaranteed if the restorative work is conducted by untrained local groups without expertise in hydraulics, habitats, or salmonid ecology. Particularly, if the restorative work is conducted without the knowledge of what the criteria for the salmonid population/life stage in question even are, the restorative work may prove to be simply “feel good” projects for participating organizations with limited tangible benefits. If public funds are dedicated to these restoration projects, the groups should involve individuals with appropriate expertise (hydrologists, hydromorphologists, ecologists) to minimize/avoid negative impacts, and there should be accountability for any tangible benefits.
At a larger (river network) scale, temperature network models—either spatial statistical network or random forest models, depending on the hydrogeological setup combined with other physical and biological variables and correlates—allow for species distribution modelling, and therefore, predictions of the species occurrence or abundance in future climates [27]. While such models ignore the heterogeneity of the thermal habitats at the micro-scale (i.e., the presence or absence and frequency of thermal refuges), they allow resource managers to predict “mean responses” and allow for the identification of the likelihood of aquatic species disappearing from parts of a river network as a function of a warming climate [27]. Such exercises have been shown to be useful in conservation as they will allow for the identification of sections of rivers (e.g., tributaries, sub-catchments) that are primary targets for added conservation or mitigation measures, or areas that will be likely candidates for species resiliency if rivers get warmer over time. On the other hand, the information derived from such models can also potentially be used in the future to identify areas of rivers that can be “sacrificed” if certain sections are predicted to become uninhabitable for a species due to climate warming-induced water temperature increases alone. Then, such areas may be prioritized for anthropogenic development while allowing the more resilient areas for the species presence be left as conservation reaches. Such prioritization vs. sacrificing may sound apocalyptic but may similarly provide a balance between the development and conservation in catchments where increasing anthropogenic pressures are inevitable, where the overall thermal habitat has become extremely limited, and therefore, where “everything cannot be saved”.
The warming climate and the consequently warming freshwater is predicted to make life more difficult for stream salmonids in the southern extreme or lower elevational part of their range. Much attention, or even blame, has been directed toward landscape activities (e.g., agriculture, forestry), which may often be the only prominent anthropogenic activities in otherwise pristine catchments with salmonid populations, for their potential role in the warming of freshwater, and therefore, their potential role of being the reason or at least further exaggerating the negative trend on thermal habitat suitability [137,138]. It has been long recognized that such landscape activities, when poorly conducted and/or especially when occurring without appropriate mitigations such as appropriate riparian zones, can indeed have a significant and long-term warming effect on freshwater streams [139,140]. On the contrary, when landscape activities are undertaken on a moderate scale with mitigations (e.g., harvesting of 7% of a stream basin in the Catamaran Brook catchment, NB, Canada), the effects of freshwater warming have not been observed [141,142]. At least in Atlantic Canada, it is currently difficult to find unregulated landscape activities in areas where salmonids occur. Therefore, it may be more prudent to shift the focus of pressure from landscape users to landscape regulators (often government agencies stipulating laws, regulations, and permits) and ask whether there should be a concern regarding the effect of the landscape activity on warming of freshwater. It is the regulators who can establish the “bowling lane” of what (and how) the landscape users can do in agro-forested landscapes, and then ensure through a process of adaptive management that the regulations are effective in protecting cold-water habitats by also taking into account the cumulative effects in the interconnected waterways. Ideally, both landscape users and regulators would work together in the best interest of the fish.
It will also be crucially important to thoroughly quantify the role of freshwater warming that is attributable to climate patterns vs. landscape activities, especially in catchments that have regionally significant salmonid populations. There continues to be a prevailing misconception in many jurisdictions that landscape activities have a similar effect on the water temperature across the catchment’s landscape [138], and therefore, that the management of landscape activities can follow similar regulations and mitigations irrespective of the spatial location of such activities within or between catchments. Examples of such erroneous views are, e.g., the fixed percentage of an allowable harvest of the catchment surface area or fixed width buffer zones across whole jurisdictions (e.g., Canadian Atlantic provinces). The “one-size-fits-all” approach is false; large river catchments are most likely to have different hydrological response areas and hydrological units that respond differently to landscape activities [52,136,143,144,145,146]. If the potential effect of landscape activities is of concern to fisheries management due to the warming effects on freshwater, then it is imperative that there is a thorough understanding of the science and the pathways of the potential warming effect that is stratified by appropriate hydrological units. It may turn out that the landscape activity has nothing to do with the purported or observed warming. Conversely, it may become apparent that landscape activities are driving broad shifts in hydrological processes. It is our belief that if these pathways are understood and the potential effects can be quantified and effectively communicated to both landscape managers, landscape operators, and the general public, a positive outcome can be achieved, and environmentally conscientious practices can be pursued while minimizing and/or limiting the impacts on freshwater resources. As for the effects of climate change on the warming of freshwater, only time will tell whether the current warming patterns can be curbed by implementing intergovernmental policies and whether stream salmonids have sufficient time and physiological/genetic scope to adapt to changing thermalscapes.
7. Conclusions
As the climate is predicted to continue to warm due to global climate change, it is very likely that high water temperature-related behavioural thermoregulation events, whether facultative or obligate, will continue to become a more common behaviour for stream salmonids. While this behaviour is already common in the southern part of the range for most stream salmonids, it is likely that the more northernly situated or higher elevation populations will also stat experiencing events that demand aggregation into cold-water refuges and the physiologically determined timing of these events may occur earlier than expected in these populations that have evolved in relatively colder water. It is imperative for fisheries management to carefully consider the locations and availability of current thermal refuges, determine management responses that are necessary in each location to protect salmonid populations, and work closely with land users to ensure the least possible impacts of high water temperature events on already stressed populations.
Author Contributions
Conceptualization, T.L.; investigation, all authors; data curation, T.L., A.M.O., E.M.C., E.N.C. and C.B.; writing—original draft preparation, T.L., A.M.O. and E.N.C.; writing—review and editing, T.L., A.M.O. and C.B.; visualization, T.L., A.M.O. and E.N.C.; funding acquisition, T.L., A.M.O., R.A.C. (R. Allen Curry) and R.A.C. (Richard A. Cunjak). All authors have read and agreed to the published version of the manuscript.
Funding
Over the years of research into cold-water refuge use by salmonids, our research programs have received funding from numerous organizations, including, but not limited to, the Natural Sciences and Engineering Research Council of Canada, the Foundation for Conservation of Atlantic Salmon, Fisheries and Oceans Canada, the New Brunswick Innovation Foundation, the Province of New Brunswick, J.D. Irving Ltd., the Miramichi Salmon Association (including Jack T.H. Fenety Scholarships for five of the authors over the years), and Mitacs.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors of this article represent “three academic generations” of scientists who have, all combined, worked on thermal refuges for a better part of last two decades in various combinations of tutelage. As such, numerous field technicians, both junior and senior, have greatly facilitated our understanding of thermal refuge use by stream salmonids and we are thankful for their hard work. We also thank Dan Isaak from the US Forest Service for helping to ensure the appropriate coverage of Pacific salmonid temperature thresholds, and we thank the three anonymous reviewers for their improvements to the earlier draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Synthesis Report of the IPCC Sixth Assessment Report (AR6); IPCC: Geneva, Switzerland, 2023; 85p. [Google Scholar]
- Jonsson, B. Thermal Effects on Ecological Traits of Salmonids. Fishes 2023, 8, 337. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. A Review of the Likely Effects of Climate Change on Anadromous Atlantic Salmon Salmo salar and Brown Trout Salmo trutta, with Particular Reference to Water Temperature and Flow. J. Fish Biol. 2009, 75, 2381–2447. [Google Scholar] [CrossRef]
- Linnansaari, T.; Cunjak, R.A. Fish: Freshwater Ecosystems. In Temperature Adaptation in a Changing Climate: Nature at Risk; Storey, K.B., Tanino, K.K., Eds.; CABI: Wallingford, UK, 2012; pp. 80–97. ISBN 978-1-84593-822-2. [Google Scholar]
- Isaak, D.J.; Wollrab, S.; Horan, D.; Chandler, G. Climate Change Effects on Stream and River Temperatures across the Northwest U.S. from 1980–2009 and Implications for Salmonid Fishes. Clim. Chang. 2012, 113, 499–524. [Google Scholar] [CrossRef]
- Heino, J.; Virkkala, R.; Toivonen, H. Climate Change and Freshwater Biodiversity: Detected Patterns, Future Trends and Adaptations in Northern Regions. Biol. Rev. 2009, 84, 39–54. [Google Scholar] [CrossRef]
- Caissie, D. The Thermal Regime of Rivers: A Review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Dahms, C.; Killen, S.S. Temperature Change Effects on Marine Fish Range Shifts: A Meta-analysis of Ecological and Methodological Predictors. Glob. Chang. Biol. 2023, 29, 4459–4479. [Google Scholar] [CrossRef]
- Moore, A.; Bendall, B.; Barry, J.; Waring, C.; Crooks, N.; Crooks, L. River Temperature and Adult Anadromous Atlantic Salmon, Salmo salar, and Brown Trout, Salmo trutta. Fish. Manag. Ecol. 2012, 19, 518–526. [Google Scholar] [CrossRef]
- Finstad, A.G.; Ugedal, O.; Forseth, T.; Næsje, T.F. Energy-Related Juvenile Winter Mortality in a Northern Population of Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2004, 61, 2358–2368. [Google Scholar] [CrossRef]
- Linnansaari, T.; Alfredsen, K.; Stickler, M.; Arnekleiv, J.V.; Harby, A.; Cunjak, R.A. Does Ice Matter? Site Fidelity and Movements by Atlantic Salmon (Salmo salar L.) Parr during Winter in a Substrate Enhanced River Reach. River Res. Appl. 2009, 25, 773–787. [Google Scholar] [CrossRef]
- Prowse, T.D.; Bonsal, B.R.; Duguay, C.R.; Lacroix, M.P. River-Ice Break-up/Freeze-up: A Review of Climatic Drivers, Historical Trends and Future Predictions. Ann. Glaciol. 2007, 46, 443–451. [Google Scholar] [CrossRef]
- Corey, E.; Linnansaari, T.; Dugdale, S.J.; Bergeron, N.; Gendron, J.; Lapointe, M.; Cunjak, R.A. Comparing the Behavioural Thermoregulation Response to Heat Stress by Atlantic Salmon Parr (Salmo salar) in Two Rivers. Ecol. Freshw. Fish 2020, 29, 50–62. [Google Scholar] [CrossRef]
- Wenger, S.J.; Isaak, D.J.; Luce, C.H.; Neville, H.M.; Fausch, K.D.; Dunham, J.B.; Dauwalter, D.C.; Young, M.K.; Elsner, M.M.; Rieman, B.E.; et al. Flow Regime, Temperature, and Biotic Interactions Drive Differential Declines of Trout Species under Climate Change. Proc. Natl. Acad. Sci. USA 2011, 108, 14175–14180. [Google Scholar] [CrossRef]
- Isaak, D.J.; Rieman, B.E. Stream Isotherm Shifts from Climate Change and Implications for Distributions of Ectothermic Organisms. Glob. Chang. Biol. 2013, 19, 742–751. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; Corey, E.; Cunjak, R.A.; Linnansaari, T.; Curry, R.A. Salmonid Thermal Habitat Contraction in a Hydrogeologically Complex Setting. Ecosphere 2021, 12, e03797. [Google Scholar] [CrossRef]
- Svenning, M.; Falkegård, M.; Dempson, J.B.; Power, M.; Bårdsen, B.; Guðbergsson, G.; Fauchald, P. Temporal Changes in the Relative Abundance of Anadromous Arctic Charr, Brown Trout, and Atlantic Salmon in Northern Europe: Do They Reflect Changing Climates? Freshw. Biol. 2022, 67, 64–77. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Liss, W.J.; Frissell, C.A. Thermal Heterogeneity, Stream Channel Morphology, and Salmonid Abundance in Northeastern Oregon Streams. Can. J. Fish. Aquat. Sci. 2003, 60, 1266–1280. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Liss, W.J.; Frissell, C.A. Cold Water Patches in Warm Streams: Physicochemical Characteristics and the Influence of Shading. J. Am. Water Resour. Assoc. 2003, 39, 355–368. [Google Scholar] [CrossRef]
- Torgersen, C.E.; Ebersole, J.L.; Keenan, D.M. Primer for Identifying Cold-Water Refuges to Protect and Restore Thermal Diversity in Riverine Landscapes; U.S. Environmental Protection Agency: Washington, DC, USA, 2012; p. 91.
- Kurylyk, B.L.; MacQuarrie, K.T.B.; Linnansaari, T.; Cunjak, R.A.; Curry, R.A. Preserving, Augmenting, and Creating Cold-water Thermal Refugia in Rivers: Concepts Derived from Research on the Miramichi River, New Brunswick (Canada). Ecohydrology 2015, 8, 1095–1108. [Google Scholar] [CrossRef]
- Mejia, F.H.; Torgersen, C.E.; Berntsen, E.K.; Maroney, J.R.; Connor, J.M.; Fullerton, A.H.; Ebersole, J.L.; Lorang, M.S. Longitudinal, Lateral, Vertical, and Temporal Thermal Heterogeneity in a Large Impounded River: Implications for Cold-Water Refuges. Remote Sens. 2020, 12, 1386. [Google Scholar] [CrossRef]
- Breau, C.; Cunjak, R.A.; Bremset, G. Age-Specific Aggregation of Wild Juvenile Atlantic Salmon Salmo salar at Cool Water Sources during High Temperature Events. J. Fish Biol. 2007, 71, 1179–1191. [Google Scholar] [CrossRef]
- Dugdale, S.J.; Franssen, J.; Corey, E.; Bergeron, N.; Lapointe, M.; Cunjak, R.A. Main Stem Movement of Atlantic Salmon Parr in Response to High Temperatures. Ecol. Freshw. Fish 2016, 25, 429–445. [Google Scholar] [CrossRef]
- Wilbur, N.M.; O’Sullivan, A.M.; MacQuarrie, K.T.B.; Linnansaari, T.; Curry, R.A. Characterizing Physical Habitat Preferences and Thermal Refuge Occupancy of Brook Trout (Salvelinus fontinalis) and Atlantic Salmon (Salmo salar) at High River Temperatures. River Res. Appl. 2020, 36, 769–783. [Google Scholar] [CrossRef]
- Sullivan, C.J.; Vokoun, J.C.; Helton, A.M.; Briggs, M.A.; Kurylyk, B.L. An Ecohydrological Typology for Thermal Refuges in Streams and Rivers. Ecohydrology 2021, 14, e2295. [Google Scholar] [CrossRef]
- Isaak, D.J.; Young, M.K. Cold-Water Habitats, Climate Refugia, and Their Utility for Conserving Salmonid Fishes. Can. J. Fish. Aquat. Sci. 2023, 80, 1187–1206. [Google Scholar] [CrossRef]
- Mejia, F.H.; Ouellet, V.; Briggs, M.A.; Carlson, S.M.; Casas-Mulet, R.; Chapman, M.; Collins, M.J.; Dugdale, S.J.; Ebersole, J.L.; Frechette, D.M.; et al. Closing the Gap between Science and Management of Cold-water Refuges in Rivers and Streams. Glob. Chang. Biol. 2023, 29, 5482–5508. [Google Scholar] [CrossRef]
- Cunjak, R.A. Winter Habitat of Selected Stream Fishes and Potential Impacts from Land-Use Activity. Can. J. Fish. Aquat. Sci. 1996, 53 (Suppl. S1), 267–282. [Google Scholar] [CrossRef]
- Cunjak, R.A.; Prowse, T.D.; Parrish, D.L. Atlantic Salmon (Salmo salar) in Winter: “The Season of Parr Discontent”. Can. J. Fish. Aquat. Sci. 1998, 55 (Suppl. S1), 161–180. [Google Scholar] [CrossRef]
- Torgersen, C.E.; Price, D.M.; Li, H.W.; Mcintosh, B.A. Multiscale Thermal Refugia and Stream Habitat Associations of Chinook Salmon in Northeastern Oregon. Ecol. Appl. 1999, 9, 301–319. [Google Scholar] [CrossRef]
- Dugdale, S.J.; Bergeron, N.; St-Hilaire, A. Temporal Variability of Thermal Refuges and Water Temperature Patterns in an Atlantic Salmon River. Remote Sens. Environ. 2013, 136, 358–373. [Google Scholar] [CrossRef]
- Sutton, R.J.; Deas, M.L.; Tanaka, S.K.; Soto, T.; Corum, R.A. Salmonid Observations at a Klamath River Thermal Refuge under Various Hydrological and Meteorological Conditions. River Res. Appl. 2007, 23, 775–785. [Google Scholar] [CrossRef]
- Gendron, J. Physical Controls on Summer Thermal Refuges for Salmonids in Two Gravel-Cobble Salmon Rivers with Contrasting Thermal Regimes: The Ouelle and Ste-Marguerite Rivers. Master’s Thesis, McGill University, Montreal, QC, Canada, 2013. [Google Scholar]
- Wondzell, S.M.; Gooseff, M. 9.13 Geomorphic Controls on Hyporheic Exchange across Scales: Watersheds to Particles. In Treatise on Geomorphology; Shroder, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 9, pp. 203–218. ISBN 978-0-08-088522-3. [Google Scholar]
- Huntsman, A.G. Death of Salmon and Trout with High Temperature. J. Fish. Res. Board Can. 1942, 5, 485–501. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Liss, W.J.; Frissell, C.A. Relationship between Stream Temperature, Thermal Refugia and Rainbow Trout Oncorhynchus mykiss Abundance in Arid-Land Streams in the Northwestern United States. Ecol. Freshw. Fish 2001, 10, 1–10. [Google Scholar] [CrossRef]
- Nielsen, J.L.; Lisle, T.E.; Ozaki, V. Thermally Stratified Pools and Their Use by Steelhead in Northern California Streams. Trans. Am. Fish. Soc. 1994, 123, 613–626. [Google Scholar] [CrossRef]
- Wondzell, S.M.; Ward, A.S. The Channel-Source Hypothesis: Empirical Evidence for in-Channel Sourcing of Dissolved Organic Carbon to Explain Hysteresis in a Headwater Mountain Stream. Hydrol. Process. 2022, 36, e14570. [Google Scholar] [CrossRef]
- Enders, E.C.; Boisclair, D.; Roy, A.G. The Effect of Turbulence on the Cost of Swimming for Juvenile Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2003, 60, 1149–1160. [Google Scholar] [CrossRef]
- Babin, A.B.; Peake, S.; Linnansaari, T.; Curry, R.A.; Ndong, M.; Haralampides, K.; Jones, R. Atlantic Salmon Upstream Migration Delay in a Large Hydropower Reservoir. N. Am. J. Fish. Manag. 2021, 41, 158–175. [Google Scholar] [CrossRef]
- Olden, J.D.; Naiman, R.J. Incorporating Thermal Regimes into Environmental Flows Assessments: Modifying Dam Operations to Restore Freshwater Ecosystem Integrity: Incorporating Thermal Regimes in Environmental Flows Assessments. Freshw. Biol. 2010, 55, 86–107. [Google Scholar] [CrossRef]
- Krause, C.W.; Newcomb, T.J.; Orth, D.J. Thermal Habitat Assessment of Alternative Flow Scenarios in a Tailwater Fishery. River Res. Appl. 2005, 21, 581–593. [Google Scholar] [CrossRef]
- Hill, C.R.; Harrison, P.; University of New Brunswick, Fredericton, NB, Canada. Personal Communication, 2023.
- Alfredsen, K.; Harby, A.; Linnansaari, T.; Ugedal, O. Development of an Inflow-Controlled Environmental Flow Regime for a Norwegian River: Inflow-Controlled Environmental Flow Regime. River Res. Appl. 2012, 28, 731–739. [Google Scholar] [CrossRef]
- Cunjak, R.A.; Caissie, D.; El-Jabi, N.; Hardie, P.; Conlon, J.H.; Pollock, T.L.; Giberson, D.J.; Komadina-Douthwright, S.M. The Catamaran Brook (New Brunswick) Habitat Research Project: Biological, Physical and Chemical Conditions (1990–1992). Can. Tech. Rep. Fish. Aquat. Sci. 1993, 1914, 81. [Google Scholar]
- Isaak, D.J.; Young, M.K.; Nagel, D.E.; Horan, D.L.; Groce, M.C. The Cold-Water Climate Shield: Delineating Refugia for Preserving Salmonid Fishes through the 21st Century. Glob. Chang. Biol. 2015, 21, 2540–2553. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; Wegscheider, B.; Helminen, J.; Cormier, J.G.; Linnansaari, T.; Wilson, D.A.; Curry, R.A. Catchment-Scale, High-Resolution, Hydraulic Models and Habitat Maps–a Salmonid’s Perspective. J. Ecohydraulics 2021, 6, 53–68. [Google Scholar] [CrossRef]
- Boyer, C.; St-Hilaire, A.; Bergeron, N.; Daigle, A.; Curry, R.A.; Caissie, D.; Gillis, C.-A. RivTemp: A Water Temperature Network for Atlantic Salmon Rivers in Eastern Canada. Water News 2016, 35, 10–15. [Google Scholar] [CrossRef]
- Isaak, D.J.; Wenger, S.J.; Peterson, E.E.; Ver Hoef, J.M.; Nagel, D.E.; Luce, C.H.; Hostetler, S.W.; Dunham, J.B.; Roper, B.B.; Wollrab, S.P.; et al. The NorWeST Summer Stream Temperature Model and Scenarios for the Western U.S.: A Crowd-Sourced Database and New Geospatial Tools Foster a User Community and Predict Broad Climate Warming of Rivers and Streams. Water Resour. Res. 2017, 53, 9181–9205. [Google Scholar] [CrossRef]
- Isaak, D.J.; Peterson, E.E.; Ver Hoef, J.M.; Wenger, S.J.; Falke, J.A.; Torgersen, C.E.; Sowder, C.; Steel, E.A.; Fortin, M.-J.; Jordan, C.E.; et al. Applications of Spatial Statistical Network Models to Stream Data: Spatial Statistical Network Models for Stream Data. Wiley Interdiscip. Rev. Water 2014, 1, 277–294. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; Devito, K.J.; Ogilvie, J.; Linnansaari, T.; Pronk, T.; Allard, S.; Curry, R.A. Effects of Topographic Resolution and Geologic Setting on Spatial Statistical River Temperature Models. Water Resour. Res. 2020, 56, e2020WR028122. [Google Scholar] [CrossRef]
- Wang, T.; Kelson, S.J.; Greer, G.; Thompson, S.E.; Carlson, S.M. Tributary Confluences Are Dynamic Thermal Refuges for a Juvenile Salmonid in a Warming River Network. River Res. Appl. 2020, 36, 1076–1086. [Google Scholar] [CrossRef]
- Ritter, T.D.; Zale, A.V.; Grisak, G.; Lance, M.J. Groundwater Upwelling Regulates Thermal Hydrodynamics and Salmonid Movements during High-temperature Events at a Montane Tributary Confluence. Trans. Am. Fish. Soc. 2020, 149, 600–619. [Google Scholar] [CrossRef]
- Handcock, R.N.; Torgersen, C.E.; Cherkauer, K.A.; Gillespie, A.R.; Tockner, K.; Faux, R.N.; Tan, J. Thermal Infrared Remote Sensing of Water Temperature in Riverine Landscapes. In Fluvial Remote Sensing for Science and Management; Carbonneau, P.E., Piegay, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 85–113. ISBN 978-0-470-71427-0. [Google Scholar]
- Roy, D.P.; Wulder, M.A.; Loveland, T.R.; Woodcock, C.E.; Allen, R.G.; Anderson, M.C.; Helder, D.; Irons, J.R.; Johnson, D.M.; Kennedy, R.; et al. Landsat-8: Science and Product Vision for Terrestrial Global Change Research. Remote Sens. Environ. 2014, 145, 154–172. [Google Scholar] [CrossRef]
- Atwell, B.H.; MacDonald, R.B.; Bartolucci, L.A. Thermal Mapping of Streams from Airborne Radiometric Scanning. J. Am. Water Resour. Assoc. 1971, 7, 228–243. [Google Scholar] [CrossRef]
- Dugdale, S.J.; Bergeron, N.E.; St-Hilaire, A. Spatial Distribution of Thermal Refuges Analysed in Relation to Riverscape Hydromorphology Using Airborne Thermal Infrared Imagery. Remote Sens. Environ. 2015, 160, 43–55. [Google Scholar] [CrossRef]
- Monk, W.A.; Wilbur, N.M.; Curry, R.A.; Gagnon, R.; Faux, R.N. Linking Landscape Variables to Cold Water Refugia in Rivers. J. Environ. Manag. 2013, 118, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, A.H.; Torgersen, C.E.; Lawler, J.J.; Faux, R.N.; Steel, E.A.; Beechie, T.J.; Ebersole, J.L.; Leibowitz, S.G. Rethinking the Longitudinal Stream Temperature Paradigm: Region-Wide Comparison of Thermal Infrared Imagery Reveals Unexpected Complexity of River Temperatures. Hydrol. Process. 2015, 29, 4719–4737. [Google Scholar] [CrossRef]
- Fuller, M.R.; Ebersole, J.L.; Detenbeck, N.E.; Labiosa, R.; Leinenbach, P.; Torgersen, C.E. Integrating Thermal Infrared Stream Temperature Imagery and Spatial Stream Network Models to Understand Natural Spatial Thermal Variability in Streams. J. Therm. Biol. 2021, 100, 103028. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.M.; Linnansaari, T.; O’Sullivan, A.M.; Curry, R.A.; Cunjak, R.A. Quantifying Movement Patterns of Wild Juvenile Atlantic Salmon (Salmo salar) in Relation to High Water Temperature and Proximity to Thermal Refuges. Ph.D. Thesis, University of New Brunswick, Fredericton, Canada, 2023. [Google Scholar]
- Dugdale, S.J.; Kelleher, C.A.; Malcolm, I.A.; Caldwell, S.; Hannah, D.M. Assessing the Potential of Drone-based Thermal Infrared Imagery for Quantifying River Temperature Heterogeneity. Hydrol. Process. 2019, 33, 1152–1163. [Google Scholar] [CrossRef]
- Casas-Mulet, R.; Pander, J.; Ryu, D.; Stewardson, M.J.; Geist, J. Unmanned Aerial Vehicle (UAV)-Based Thermal Infra-Red (TIR) and Optical Imagery Reveals Multi-Spatial Scale Controls of Cold-Water Areas over a Groundwater-Dominated Riverscape. Front. Environ. Sci. 2020, 8, 64. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; Linnansaari, T.; Leavitt, J.; Samways, K.M.; Kurylyk, B.L.; Curry, R.A. The Salmon-peloton: Hydraulic Habitat Shifts of Adult Atlantic Salmon (Salmo salar) Due to Behavioural Thermoregulation. River Res. Appl. 2022, 38, 107–118. [Google Scholar] [CrossRef]
- Morgan, A.M.; O’Sullivan, A.M. Cooler, Bigger; Warmer, Smaller: Fine-Scale Thermal Heterogeneity Maps Age Class and Species Distribution in Behaviourally Thermoregulating Salmonids. River Res. Appl. 2023, 39, 163–176. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; Kurylyk, B.L. Limiting External Absorptivity of UAV-Based Uncooled Thermal Infrared Sensors Increases Water Temperature Measurement Accuracy. Remote Sens. 2022, 14, 6356. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; Linnansaari, T.; Curry, R.A. Ice Cover Exists: A Quick Method to Delineate Groundwater Inputs in Running Waters for Cold and Temperate Regions. Hydrol. Process. 2019, 33, 3297–3309. [Google Scholar] [CrossRef]
- Breau, C.; Cunjak, R.A.; Peake, S.J. Behaviour during Elevated Water Temperatures: Can Physiology Explain Movement of Juvenile Atlantic Salmon to Cool Water?: Temperature and Physiology of Juvenile Salmon. J. Anim. Ecol. 2011, 80, 844–853. [Google Scholar] [CrossRef]
- Elliott, J.M.; Elliott, J.A. Temperature Requirements of Atlantic Salmon Salmo salar, Brown Trout Salmo trutta and Arctic Charr Salvelinus alpinus: Predicting the Effects of Climate Change. J. Fish Biol. 2010, 77, 1793–1817. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.W.A.; Kramer, D.L. Territory Size as a Predictor of the Upper Limit to Population Density of Juvenile Salmonids in Streams. Can. J. Fish. Aquat. Sci. 1990, 47, 1724–1737. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; Corey, E.M.; Collet, E.N.; Helminen, J.; Curry, R.A.; MacIntyre, C.; Linnansaari, T. Timing and Frequency of High Temperature Events Bend the Onset of Behavioural Thermoregulation in Atlantic Salmon (Salmo salar). Conserv. Physiol. 2023, 11, coac079. [Google Scholar] [CrossRef]
- Økland, F.; Erkinaro, J.; Moen, K.; Niemela, E.; Fiske, P.; McKinley, R.S.; Thorstad, E.B. Return Migration of Atlantic Salmon in the River Tana: Phases of Migratory Behaviour. J. Fish Biol. 2001, 59, 862–874. [Google Scholar] [CrossRef]
- Carrow, R.M. Freshwater Migration and Behaviour of Wild Adult Atlantic Salmon (Salmo salar) in the Miramichi River, New Brunswick, Canada. Master’s Thesis, University of New Brunswick, Department of Biology, Fredericton, NB, Canada, 2021. [Google Scholar]
- Farrell, A.P.; Eliason, E.J.; Sandblom, E.; Clark, T.D. Fish Cardiorespiratory Physiology in an Era of Climate Change. Can. J. Zool. 2009, 87, 835–851. [Google Scholar] [CrossRef]
- Eliason, E.J.; Clark, T.D.; Hinch, S.G.; Farrell, A.P. Cardiorespiratory Collapse at High Temperature in Swimming Adult Sockeye Salmon. Conserv. Physiol. 2013, 1, cot008. [Google Scholar] [CrossRef]
- Ern, R.; Andreassen, A.H.; Jutfelt, F. Physiological Mechanisms of Acute Upper Thermal Tolerance in Fish. Physiology 2023, 38, 141–158. [Google Scholar] [CrossRef]
- Allen, J.A. The Influence of Physical Conditions in the Genesis of Species. Radic. Rev. 1877, 1, 108–140. [Google Scholar]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Eliason, E.J.; Clark, T.D.; Hague, M.J.; Hanson, L.M.; Gallagher, Z.S.; Jeffries, K.M.; Gale, M.K.; Patterson, D.A.; Hinch, S.G.; Farrell, A.P. Differences in Thermal Tolerance among Sockeye Salmon Populations. Science 2011, 332, 109–112. [Google Scholar] [CrossRef]
- Anlauf-Dunn, K.; Kraskura, K.; Eliason, E.J. Intraspecific Variability in Thermal Tolerance: A Case Study with Coastal Cutthroat Trout. Conserv. Physiol. 2022, 10, coac029. [Google Scholar] [CrossRef]
- Breau, C. Knowledge of Fish Physiology Used to Set Water Temperature Thresholds for In-Season Closures of Atlantic Salmon (Salmo salar) Recreational Fisheries; Canadian Science Advisory Secretariat Research Document; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2013; p. 24.
- Collet, E.; O’Sullivan, A.M.; University of New Brunswick, Fredericton, NB, Canada. Personal Communication, 2023.
- Pörtner, H.O.; Bennett, A.F.; Bozinovic, F.; Clarke, A.; Lardies, M.A.; Lucassen, M.; Pelster, B.; Schiemer, F.; Stillman, J.H. Trade-Offs in Thermal Adaptation: The Need for a Molecular to Ecological Integration. Physiol. Biochem. Zool. 2006, 79, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Anttila, K.; Couturier, C.S.; Øverli, Ø.; Johnsen, A.; Marthinsen, G.; Nilsson, G.E.; Farrell, A.P. Atlantic Salmon Show Capability for Cardiac Acclimation to Warm Temperatures. Nat. Commun. 2014, 5, 4252. [Google Scholar] [CrossRef] [PubMed]
- Anttila, K.; Dhillon, R.S.; Boulding, E.G.; Farrell, A.P.; Glebe, B.D.; Elliott, J.A.K.; Wolters, W.R.; Schulte, P.M. Variation in Temperature Tolerance among Families of Atlantic Salmon (Salmo salar) Is Associated with Hypoxia Tolerance, Ventricle Size and Myoglobin Level. J. Exp. Biol. 2013, 216, 1183–1190. [Google Scholar] [CrossRef]
- Crossin, G.T.; Hinch, S.G.; Cooke, S.J.; Welch, D.W.; Patterson, D.A.; Jones, S.R.M.; Lotto, A.G.; Leggatt, R.A.; Mathes, M.T.; Shrimpton, J.M.; et al. Exposure to High Temperature Influences the Behaviour, Physiology, and Survival of Sockeye Salmon during Spawning Migration. Can. J. Zool. 2008, 86, 127–140. [Google Scholar] [CrossRef]
- Hinch, S.G.; Bett, N.N.; Eliason, E.J.; Farrell, A.P.; Cooke, S.J.; Patterson, D.A. Exceptionally High Mortality of Adult Female Salmon: A Large-Scale Pattern and a Conservation Concern. Can. J. Fish. Aquat. Sci. 2021, 78, 639–654. [Google Scholar] [CrossRef]
- Gibson, R.J. Some Factors Influencing the Distributions of Brook Trout and Young Atlantic Salmon. J. Fish. Res. Board Can. 1966, 23, 1977–1980. [Google Scholar] [CrossRef]
- Frechette, D.M.; Dugdale, S.J.; Dodson, J.J.; Bergeron, N.E. Understanding Summertime Thermal Refuge Use by Adult Atlantic Salmon Using Remote Sensing, River Temperature Monitoring, and Acoustic Telemetry. Can. J. Fish. Aquat. Sci. 2018, 75, 1999–2010. [Google Scholar] [CrossRef]
- Wehrly, K.E.; Wang, L.; Mitro, M. Field-Based Estimates of Thermal Tolerance Limits for Trout: Incorporating Exposure Time and Temperature Fluctuation. Trans. Am. Fish. Soc. 2007, 136, 365–374. [Google Scholar] [CrossRef]
- Hitt, N.P.; Snook, E.L.; Massie, D.L. Brook Trout Use of Thermal Refugia and Foraging Habitat Influenced by Brown Trout. Can. J. Fish. Aquat. Sci. 2017, 74, 406–418. [Google Scholar] [CrossRef]
- Kaya, C.M.; Kaeding, L.R.; Burkhalter, D.E. Use of a Cold-Water Refuge by Rainbow and Brown Trout in a Geothermally Heated Stream. Progress. Fish-Cult. 1977, 39, 37–39. [Google Scholar] [CrossRef]
- Dobos, M.E.; Corsi, M.P.; Schill, D.J.; DuPont, J.M.; Quist, M.C. Influences of Summer Water Temperatures on the Movement, Distribution, and Resource Use of Fluvial Westslope Cutthroat Trout in the South Fork Clearwater River Basin. N. Am. J. Fish. Manag. 2016, 36, 549–567. [Google Scholar] [CrossRef]
- Barrett, H.S.; Armstrong, J.B. Move, Migrate, or Tolerate: Quantifying Three Tactics for Coldwater Fish Coping with Warm Summers in a Large River. Ecosphere 2022, 13, e4095. [Google Scholar] [CrossRef]
- Hillyard, R.W.; Keeley, E.R. Temperature-Related Changes in Habitat Quality and Use by Bonneville Cutthroat Trout in Regulated and Unregulated River Segments. Trans. Am. Fish. Soc. 2012, 141, 1649–1663. [Google Scholar] [CrossRef]
- McCarrick, D.K.; Dillon, J.C.; High, B.; Quist, M.C. Spatial and Temporal Distribution and Habitat Selection of Native Yellowstone Cutthroat Trout and Nonnative Utah Chub. N. Am. J. Fish. Manag. 2022, 42, 939–951. [Google Scholar] [CrossRef]
- Keefer, M.L.; Peery, C.A.; High, B. Behavioral Thermoregulation and Associated Mortality Trade-Offs in Migrating Adult Steelhead (Oncorhynchus mykiss): Variability among Sympatric Populations. Can. J. Fish. Aquat. Sci. 2009, 66, 1734–1747. [Google Scholar] [CrossRef]
- Keefer, M.L.; Clabough, T.S.; Jepson, M.A.; Johnson, E.L.; Peery, C.A.; Caudill, C.C. Thermal Exposure of Adult Chinook Salmon and Steelhead: Diverse Behavioral Strategies in a Large and Warming River System. PLoS ONE 2018, 13, e0204274. [Google Scholar] [CrossRef]
- Brewitt, K.S.; Danner, E.M. Spatio-Temporal Temperature Variation Influences Juvenile Steelhead (Oncorhynchus mykiss) Use of Thermal Refuges. Ecosphere 2014, 5, art92. [Google Scholar] [CrossRef]
- Keefer, M.L.; Clabough, T.S.; Jepson, M.A.; Naughton, G.P.; Blubaugh, T.J.; Joosten, D.C.; Caudill, C.C. Thermal Exposure of Adult Chinook Salmon in the Willamette River Basin. J. Therm. Biol. 2015, 48, 11–20. [Google Scholar] [CrossRef]
- Goniea, T.M.; Keefer, M.L.; Bjornn, T.C.; Peery, C.A.; Bennett, D.H.; Stuehrenberg, L.C. Behavioral Thermoregulation and Slowed Migration by Adult Fall Chinook Salmon in Response to High Columbia River Water Temperatures. Trans. Am. Fish. Soc. 2006, 135, 408–419. [Google Scholar] [CrossRef]
- Armstrong, J.B.; Ward, E.J.; Schindler, D.E.; Lisi, P.J. Adaptive Capacity at the Northern Front: Sockeye Salmon Behaviourally Thermoregulate during Novel Exposure to Warm Temperatures. Conserv. Physiol. 2016, 4, cow039. [Google Scholar] [CrossRef]
- Biro, P.A. Staying Cool: Behavioral Thermoregulation during Summer by Young-of-Year Brook Trout in a Lake. Trans. Am. Fish. Soc. 1998, 127, 212–222. [Google Scholar] [CrossRef]
- Langeland, A.; L’Abee-Lund, J.H. An Experimental Test of the Genetic Component of the Ontogenetic Habitat Shift in Arctic Charr (Salvelinus alpinus). Ecol. Freshw. Fish 1998, 7, 200–207. [Google Scholar] [CrossRef]
- Sellers, T.J.; Parker, B.R.; Schindler, D.W.; Tonn, W.M. Pelagic Distribution of Lake Trout (Salvelinus namaycush) in Small Canadian Shield Lakes with Respect to Temperature, Dissolved Oxygen, and Light. Can. J. Fish. Aquat. Sci. 1998, 55, 170–179. [Google Scholar] [CrossRef]
- Howell, P.J.; Dunham, J.B.; Sankovich, P.M. Relationships between Water Temperatures and Upstream Migration, Cold Water Refuge Use, and Spawning of Adult Bull Trout from the Lostine River, Oregon, USA. Ecol. Freshw. Fish 2010, 19, 96–106. [Google Scholar] [CrossRef]
- Gutowsky, L.F.G.; Harrison, P.M.; Martins, E.G.; Leake, A.; Patterson, D.A.; Zhu, D.Z.; Power, M.; Cooke, S.J. Daily Temperature Experience and Selection by Adfluvial Bull Trout (Salvelinus confluentus). Environ. Biol. Fishes 2017, 100, 1167–1180. [Google Scholar] [CrossRef]
- Swanberg, T.R. Movements of and Habitat Use by Fluvial Bull Trout in the Blackfoot River, Montana. Trans. Am. Fish. Soc. 1997, 126, 735–746. [Google Scholar] [CrossRef]
- Gallant, M.J.; LeBlanc, S.; MacCormack, T.J.; Currie, S. Physiological Responses to a Short-Term, Environmentally Realistic, Acute Heat Stress in Atlantic Salmon, Salmo salar. Facets 2017, 2, 330–341. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Whoriskey, F.G.; Rikardsen, A.H.; Aarestrup, K. Aquatic Nomads: The Life and Migrations of the Atlantic Salmon. In Atlantic Salmon Ecology; Aas, Ø., Einum, S., Klemetsen, A., Skurdal, J., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 1–32. [Google Scholar]
- Braithwaite, V.A.; Armstrong, J.D.; McAdam, H.M.; Huntingford, F.A. Can Juvenile Atlantic Salmon Use Multiple Cue Systems in Spatial Learning? Anim. Behav. 1996, 51, 1409–1415. [Google Scholar] [CrossRef]
- Breau, C. The Ecophysiology, Behaviour and Movement Patterns of Juvenile Atlantic Salmon (Salmo salar) during Elevated Water Temperatures and the Importance of Cool Water Sites. Ph.D. Thesis, University of New Brunswick, Department of Biology, Fredericton, NB, Canada, 2011. [Google Scholar]
- Elvidge, C.K.; Cooke, E.L.L.; Cunjak, R.A.; Cooke, S.J. Social Cues May Advertise Habitat Quality to Refuge-Seeking Conspecifics. Can. J. Zool. 2017, 95, 1–5. [Google Scholar] [CrossRef]
- Johansen, M.; Erkinaro, J.; Amundsen, P.-A. The When What and Where of Freshwater Feeding. In Atlantic Salmon Ecology; Aas, Ø., Einum, S., Klemetsen, A., Skurdal, J., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 89–114. [Google Scholar]
- Johansen, M. Evidence of Freshwater Feeding by Adult Salmon in the Tana River, Northern Norway. J. Fish Biol. 2001, 59, 1405–1407. [Google Scholar] [CrossRef]
- Brewitt, K.S.; Danner, E.M.; Moore, J.W. Hot Eats and Cool Creeks: Juvenile Pacific Salmonids Use Mainstem Prey While in Thermal Refuges. Can. J. Fish. Aquat. Sci. 2017, 74, 1588–1602. [Google Scholar] [CrossRef]
- Milligan, C.L.; Hooke, G.B.; Johnson, C. Sustained Swimming at Low Velocity Following a Bout of Exhaustive Exercise Enhances Metabolic Recovery in Rainbow Trout. J. Exp. Biol. 2000, 203, 921–926. [Google Scholar] [CrossRef]
- Baird, O.E.; Krueger, C.C. Behavioral Thermoregulation of Brook and Rainbow Trout: Comparison of Summer Habitat Use in an Adirondack River, New York. Trans. Am. Fish. Soc. 2003, 132, 1194–1206. [Google Scholar] [CrossRef]
- Corey, E.; Linnansaari, T.; Cunjak, R.A. High Temperature Events Shape the Broadscale Distribution of Juvenile Atlantic Salmon (Salmo salar). Freshw. Biol. 2023, 68, 534–545. [Google Scholar] [CrossRef]
- Farrell, A.P.; Hinch, S.G.; Cooke, S.J.; Patterson, D.A.; Crossin, G.T.; Lapointe, M.; Mathes, M.T. Pacific Salmon in Hot Water: Applying Aerobic Scope Models and Biotelemetry to Predict the Success of Spawning Migrations. Physiol. Biochem. Zool. 2008, 81, 697–709. [Google Scholar] [CrossRef]
- Corey, E.; Linnansaari, T.; Cunjak, R.A.; Currie, S. Physiological Effects of Environmentally Relevant, Multi-Day Thermal Stress on Wild Juvenile Atlantic Salmon (Salmo salar). Conserv. Physiol. 2017, 5, cox014. [Google Scholar] [CrossRef]
- Bowerman, T.; Roumasset, A.; Keefer, M.L.; Sharpe, C.S.; Caudill, C.C. Prespawn Mortality of Female Chinook Salmon Increases with Water Temperature and Percent Hatchery Origin. Trans. Am. Fish. Soc. 2018, 147, 31–42. [Google Scholar] [CrossRef]
- Fisheries and Oceans Canada. Temperature Threshold to Define Management Strategies for Atlantic Salmon (Salmo salar) Fisheries under Environmentally Stressful Conditions. Can. Sci. Adv. Sec. Sci. Adv. Rep. 2012, 19, 17. [Google Scholar]
- Crossin, G.T.; Hinch, S.G.; Farrell, A.P.; Higgs, D.A.; Lotto, A.G.; Oakes, J.D.; Healey, M.C. Energetics and Morphology of Sockeye Salmon: Effects of Upriver Migratory Distance and Elevation. J. Fish Biol. 2004, 65, 788–810. [Google Scholar] [CrossRef]
- Fenkes, M.; Shiels, H.A.; Fitzpatrick, J.L.; Nudds, R.L. The Potential Impacts of Migratory Difficulty, Including Warmer Waters and Altered Flow Conditions, on the Reproductive Success of Salmonid Fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 193, 11–21. [Google Scholar] [CrossRef]
- Fenkes, M.; Fitzpatrick, J.L.; Ozolina, K.; Shiels, H.A.; Nudds, R.L. Sperm in Hot Water: Direct and Indirect Thermal Challenges Interact to Impact on Brown Trout Sperm Quality. J. Exp. Biol. 2017, 220, 2513–2520. [Google Scholar] [CrossRef]
- Berman, C.H.; Quinn, T.P. Behavioural Thermoregulation and Homing by Spring Chinook Salmon, Oncorhynchus tshawytscha (Walbaum), in the Yakima River. J. Fish Biol. 1991, 39, 301–312. [Google Scholar] [CrossRef]
- Snyder, M.N.; Schumaker, N.H.; Dunham, J.B.; Keefer, M.L.; Leinenbach, P.; Brookes, A.; Palmer, J.; Wu, J.; Keenan, D.; Ebersole, J.L. Assessing Contributions of Cold-Water Refuges to Reproductive Migration Corridor Conditions for Adult Salmon and Steelhead Trout in the Columbia River, USA. J. Ecohydraulics 2022, 7, 111–123. [Google Scholar] [CrossRef]
- Robertson, B.A.; Hutto, R.L. A Framework for Understanding Ecological Traps and an Evaluation of Existing Evidence. Ecology 2006, 87, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, T.E.; Lehnert, S.J.; Breau, C.; Fitzsimmons, M.; Kelly, N.I.; Dempson, J.B.; Neville, V.M.; Young, M.; Keefe, D.; Bird, T.J.; et al. Considerations for Water Temperature-Related Fishery Closures in Recreational Atlantic Salmon (Salmo salar) Catch and Release Fisheries: A Case Study from Eastern Canada. Rev. Fish. Sci. Aquac. 2023, 31, 598–619. [Google Scholar] [CrossRef]
- Gale, M.K.; Hinch, S.G.; Donaldson, M.R. The Role of Temperature in the Capture and Release of Fish: Temperature Effects on Capture-Release. Fish Fish. 2013, 14, 1–33. [Google Scholar] [CrossRef]
- Lennox, R.J.; Cooke, S.J.; Davis, C.R.; Gargan, P.; Hawkins, L.A.; Havn, T.B.; Johansen, M.R.; Kennedy, R.J.; Richard, A.; Svenning, M.-A.; et al. Pan-Holarctic Assessment of Post-Release Mortality of Angled Atlantic Salmon Salmo salar. Biol. Conserv. 2017, 209, 150–158. [Google Scholar] [CrossRef]
- Keefe, D.; Young, M.; Van Leeuwen, T.E.; Adams, B. Long-term Survival of Atlantic Salmon Following Catch and Release: Considerations for Anglers, Scientists and Resource Managers. Fish. Manag. Ecol. 2022, 29, 286–297. [Google Scholar] [CrossRef]
- Caissie, D.; Breau, C.; Hayward, J.; Cameron, P. Water Temperature Characteristics within the Miramichi and Restigouche Rivers; Canadian Science Advisory Secretariat Research Document; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2013; p. vii + 31.
- O’Sullivan, A.M.; Devito, K.J.; D’Orangeville, L.; Curry, R.A. The Waterscape Continuum Concept: Rethinking Boundaries in Ecosystems. WIREs Water 2022, 9, e1598. [Google Scholar] [CrossRef]
- Lynch, J.A.; Rishel, G.B.; Corbett, E.S. Thermal Alteration of Streams Draining Clearcut Watersheds: Quantification and Biological Implications. Hydrobiologia 1984, 111, 161–169. [Google Scholar] [CrossRef]
- Moore, R.D.; Spittlehouse, D.L.; Story, A. Riparian Microclimate and Stream Temperature Response to Forest Harvesting: A Review. J. Am. Water Resour. Assoc. 2005, 41, 813–834. [Google Scholar] [CrossRef]
- Johnson, S.L.; Jones, J.A. Stream Temperature Responses to Forest Harvest and Debris Flows in Western Cascades, Oregon. Can. J. Fish. Aquat. Sci. 2000, 57, 30–39. [Google Scholar] [CrossRef]
- Hall, J.D.; Cederholm, C.J.; Murphy, M.L.; Koski, K.V. Fish-Forestry Interactions in Oregon, Washington and Alaska, USA. In Fishes and Forestry; Worldwide Watershed Interactions and Management; Northcote, T.G., Hartman, G.F., Eds.; Blackwell Publishing: Oxford, UK, 2004; pp. 363–388. [Google Scholar]
- Cunjak, R.A.; Curry, R.A.; Scruton, D.A.; Clarke, K.D. Fish-Forestry Interactions in Freshwaters of Atlantic Canada. In Fishes and Forestry; Worldwide Watershed Interactions and Management; Northcote, T.G., Hartman, G.F., Eds.; Blackwell Publishing: Oxford, UK, 2004; pp. 439–462. [Google Scholar]
- Alexander, M.D.; MacQuarrie, K.T.B.; Caissie, D.; Butler, K.E. The Thermal Regime of Shallow Groundwater and a Small Atlantic Salmon Stream Bordering a Clearcut with a Forested Streamside Buffer. In Proceedings of the Annual Conference of the Canadian Society for Civil Engineering, Moncton, NB, Canada, 4–7 June 2003; p. 10. [Google Scholar]
- Winter, T.C.; Harvey, J.W.; Franke, O.L.; Alley, W.M. Ground Water and Surface Water a Single Resource; Diane Publishing: Collingdale, PA, USA, 1998; p. 88. [Google Scholar]
- Devito, K.J.; Creed, I.F.; Fraser, C.J.D. Controls on Runoff from a Partially Harvested Aspen-Forested Headwater Catchment, Boreal Plain, Canada. Hydrol. Process. 2005, 19, 3–25. [Google Scholar] [CrossRef]
- Devito, K.J.; Hokanson, K.J.; Moore, P.A.; Kettridge, N.; Anderson, A.E.; Chasmer, L.; Hopkinson, C.; Lukenbach, M.C.; Mendoza, C.A.; Morissette, J.; et al. Landscape Controls on Long-Term Runoff in Subhumid Heterogeneous Boreal Plains Catchments. Hydrol. Process. 2017, 31, 2737–2751. [Google Scholar] [CrossRef]
- Briggs, M.A.; Lane, J.W.; Snyder, C.D.; White, E.A.; Johnson, Z.C.; Nelms, D.L.; Hitt, N.P. Shallow Bedrock Limits Groundwater Seepage-Based Headwater Climate Refugia. Limnologica 2018, 68, 142–156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).