Abstract
The molly fish is a member of viviparous teleosts that are characterized by the fusion of the right and left ovaries during their early embryonic development. This fusion results in a singular and saccular ovary, where the germinal epithelium lines the internal lumen. The present study aimed to identify the immune cells in the ovarian stroma of Molly fish during the breeding season using histological and immunohistochemical analysis. Histological examination of the ovaries displayed oocytes at all different stages of development and degeneration. The ovocoel, a lymph-filled space, remains in the center of the ovary and branches posteriorly, creating the lumen of the gonoduct. The ovarian wall is composed of three layers: the mesothelium, tunica albuginea, and germinal epithelium. The developing ova were held together by the stroma, which consisted of vascular collagenous connective tissue clustered with immune cells. Immunohistochemical analysis revealed the presence of clusters of macrophages expressing APG5, IL-1β, TGF-β, S100, NF-κB, CD68, Iba-1, and Ach. Monocytes demonstrated positive immunoreactivity for both APG5 and IL-1β, whereas dendritic cells expressed only APG5. Furthermore, rodlet cells exhibited immunoreactivity for S100 protein, IL-1β, NF-κB, CD68, Nrf2, Ach, myostatin, SOX9, and Iba-1. In contrast, stem cells displayed immunoreactivity for Nrf2, myostatin, and SOX9. In conclusion, the ovarian stroma of Molly fish demonstrated a notable presence of immune cells, indicating their active involvement in immune reactions.
Key Contribution:
The expressions of various inflammatory and stem cell markers in the ovarian stroma of Molly fish highlight the high prevalence of immune cells in the ovary, indicating the contribution of the ovarian stroma in immune reactions.
1. Introduction
Immunity and reproduction are fundamental aspects of an individual’s fitness. Competitive allocation of these two functions can profoundly influence an organism’s life-history strategies. In recent years, there has been a growing interest among researchers in exploring this topic [1,2,3]. Despite having immune systems comparable to higher vertebrates, fish primarily rely on non-specific immune systems to survive in their environment from the early embryonic stage. While innate immunity is responsible for identifying pathogen-associated molecular patterns of pathogenic microorganisms, acquired immunity functions through antigen-specific receptors [4]. In teleosts, numerous immune cells were observed, including natural killer cells, macrophages, granular leukocytes, non-specific cytotoxic cells, thrombocytes, dendritic cells, lymphocytes, mast cells, monocytes, and eosinophilic granule cells [5]. The immune system has a crucial role in regulating gonad function [6]. Within the female reproductive system, immune cells including macrophages, dendritic cells, granulocytes, and T and B lymphocytes were found specifically in the ovaries [7,8]. These immune cells are actively involved in various reproductive processes in the ovaries, including follicle growth, ovulation, and corpus luteum production and regression [9]. The ovarian stroma is a supportive framework comprising common constituents, incorporating immune cells [10,11], blood vessels [12], nerves [13], and lymphatic vessels [14] as well as ovary-specific components.
Immune cells play a vital role in maintaining the physiological activities of the ovaries. In immature or dormant ovaries, there are low numbers of immune cells, such as mast cells, eosinophils, and macrophages, distributed sparsely throughout the stroma. However, as the ovaries become active, especially around ovulation and in the vicinity of the theca vasculature, the levels of these immune cells often increase. Ovarian immune cells are implicated in numerous processes, including phagocytosis, antigen presentation, tissue remodeling through proteolytic enzymes, and the release of soluble signals like cytokines, chemokines, and growth factors [15,16]. Among these immune cells, macrophages are the most densely distributed inside the ovary [6]. Research on the distribution of leukocytes in aquatic species has been limited, but it suggests that ovarian leukocytes may act as potential local regulators of ovarian function through the secretion of regulatory soluble substances. These substances include various cytokines, mainly produced by immune cells within the ovary [6].
The ovary is typically an unpaired hollow organ in viviparous teleosts, and its cavity is continuous with the gonoduct, a caudal extension that opens to the outside at the gonopore. Teleost females do not generate Müllerian ducts during development; therefore, when they reach sexual maturity, they lack oviducts [17,18]. The germinal epithelium, stroma, smooth muscle, and peritoneum (serosa) are the four histological layers that make up the ovarian wall of Molly fish. The ovarian stroma contains oocytes in various stages of development, including chromatin nucleolus (CN), primary growth (PG), and secondary growth (SG). The ovarian shape changes depending on the stage of pregnancy. These stages are classified as (i) previtellogenic, which signifies ovaries with oocytes within follicles during PG; (ii) vitellogenic, which indicates ovaries with oocytes in follicles during SG; and (iii) pregnant, indicates ovaries with intrafollicular gestation [19].
A Molly fish, scientifically known as Poecilia sphenops, is a type of freshwater teleost that exhibits viviparity. Mollies have been employed in experimental investigations quite frequently in recent years [20,21]. Populations of Poecilia sphenops are found throughout Central America, from Colombia to Mexico, including certain Caribbean islands, where they are primarily found in shallow areas, hardly ever being in coastal brackish waters. As omnivores, short-finned mollies generally consume algae, other plant materials, and also bloodworms [22]. Depending on the temperature, gestation duration might range from 3 to 4 weeks. Additionally, females can store sperm and give birth repeatedly during the year [22]. Little information is currently available concerning immune cells within the ovarian stroma. Consequently, this study employs histological and immunohistochemical analysis to provide novel insights into the presence of immune cells in the ovarian stroma of Molly fish during the breeding season.
2. Materials and Methods
2.1. Ethical Statement
The present study was conducted in compliance with both Egyptian legal regulations and the animal care guidelines established by the University of Sohag. The approval for all procedures in this study was granted by the Veterinary Medical Research Ethics Committee of the Faculty of Veterinary Medicine at Sohag University, Egypt, under ethical number Soh.un.vet/00045R (11/10/2023).
2.2. Sample Collection
A total of 14 adult female Molly fish (Poecilia sphenops) specimens were used for the study, during their breeding season (June–August). The fishes were procured from an ornamental fish shop and measured approximately 4.20 cm ± 4.0 cm in standard length and 10.60 g ± 1.70 g in body weight. The healthy fishes underwent a two-week acclimation period in the laboratory and were kept within aerated water tanks under a natural light/dark cycle. The fishes were fed twice a day with commercial pellets (37.2% crude protein, 4.3% lipid, and 1.9% fibers) [23], and their swimming and feeding behaviors were closely observed during the experiment. Before tissue sampling, the 14 fish samples were randomly chosen from the tanks and humanely euthanized using an overdose of MS-222.
2.3. Histological Analysis
The ovaries were promptly dissected from 7 fishes and were rapidly immersed in Bouin’s fixative solution for 22 h. Following fixation, the samples were prepared for light microscopy analysis using a Leica Dialux 20 microscope and a Canon digital camera (Candison Powershot A95). The samples were processed for dehydration with ethanol, cleared with methyl benzoate, and embedded in paraffin wax. Subsequently, thin sections of approximately 5 μm thickness were cut from both sagittal and transverse planes and were stained using Harris hematoxylin and Eosin as well as Crossmon’s trichrome staining techniques [24].
2.4. Immunohistochemical Analysis
Small ovarian samples intended for immunohistochemical analysis were dissected from 7 fishes and were promptly fixed in a 10% neutral buffered formalin solution for 24 h. The fixed samples were dehydrated using an increasing ethanol series, cleared with methyl benzoate, and embedded in paraffin wax, and transverse sections at thicknesses ranging from 3 to 5 μm were cut. For immunohistochemical analysis of these sections, the Pierce Peroxidase Detection Kit (36000, Thermo Fisher Scientific, Horsham, UK) was utilized following established protocols [25,26]. In brief, the sections were deparaffinized with xylene, rehydrated through graded ethanol, and rinsed with distilled water. To enhance epitope exposure, the sections were subjected to a 15 min heat treatment in a sodium citrate buffer (0.01 M, pH 6.0) using a microwave and were then allowed to cool at room temperature for 30 min. The sections were washed with wash buffer (Tris-buffered saline with 0.05% Tween-20 detergent) and were incubated for 30 min in a peroxidase suppressor to inhibit the activity of endogenous peroxidase. Following two 3 min washes with wash buffer, the tissues were blocked for 30 min at room temperature using the Universal Blocker™ blocking buffer in TBS.
The sections were then incubated overnight at 4 °C with primary antibodies against autophagy protein 5 (APG5, Santa Cruz Biotechnology “sc”, sc-133158, Heidelberg, Germany, diluted 1:100), transforming growth factor beta (TGF-β, sc-220, diluted 1:100), Interleukin 1 beta (IL-1β, sc-7884, diluted 1:100), nuclear factor kappa B (NF-κB, sc-8008, diluted 1:100), nuclear factor erythroid 2-related factor 2 (Nrf2, sc-722, diluted 1:100), SRY-Box transcription factor 9 (SOX9 Sigma-Aldrich, Madrid, Spain, AB5535, diluted 1:100), CD68 (sc-17832, diluted 1:100), Ionized calcium-binding adaptor molecule (Iba-1; sc-32725, diluted 1:200), Nicotinic Acetylcholine R alpha 7 (α7nAchR, also known as Ach, ABclonal, A7844, diluted 1:100), and S100 protein (Dako, Glostrup, Denmark, Z0311, diluted 1:100). In addition, ovarian specimens in which primary antibodies were omitted and replaced with the Universal Blocker™ blocking buffer in TBS were used as negative controls to prevent false-positive labeling of the primary antibodies used (Figures S1A–D and S2A–F). The slides were then washed twice for 3 min each with wash buffer and were subsequently incubated for 30 min at room temperature with secondary antibodies, which include goat anti-rabbit IgG (Invitrogen, Waltham, MA, USA, 65-6140, diluted 1:1000) and goat anti-mouse IgG (Invitrogen, Waltham, MA, USA, 31800, diluted 1:100). After that, slides underwent three consecutive washes, 3 min each, with wash buffer. The tissues were then subjected to a 30 min incubation with Avidin-HRP (Invitrogen, Waltham, MA, USA, 43-4423, diluted 1:500) in a universal blocker blocking buffer. Following another three washes with wash buffer, the tissues were exposed to a 1X metal-enhanced DAB substrate working solution (prepared by dissolving the stable peroxide buffer into the 10X DAB/Metal concentrate) for 5–15 min until the optimum staining intensity was obtained. Ultimately, the sections underwent two 3 min washes with wash buffer, received counterstaining with Harris modified Hematoxylin, and were mounted using mounting media. The sections were examined by a Leica Dialux 20 microscope and photographs were obtained with a Canon digital camera (Candison Powershot A95). Different cells in the ovarian stroma of Molly fish were identified based on their morphological characters, location, and immunohistochemical reactions as previously described [4,6,27,28].
3. Results
3.1. Histological Analysis
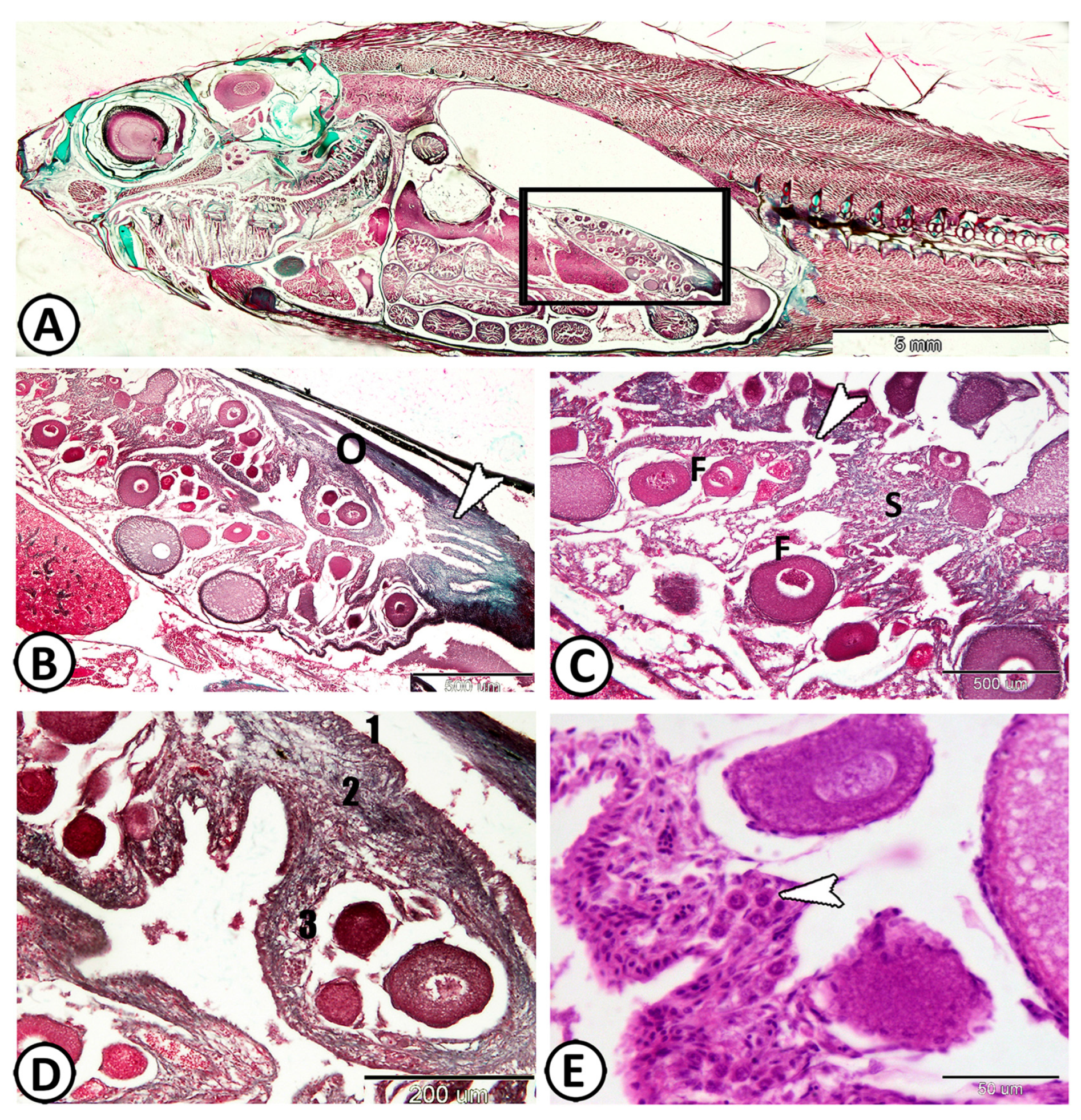
The Molly fish’s ovary is comprised of a single saccular structure located on the dorsal mesentery in the dorsolateral lining of the peritoneal cavity (Figure 1A). The histological examination of the ovaries displayed oocytes at various stages of development and degeneration, including atresia (Figure 1B). The ovocoel (ovarian cavity) is a lymph-filled space that remains in the center of the ovary as an irregular space and is lined with germinal epithelium (simple squamous epithelium), where oogonia originate (Figure 1C). The ovocoel extended posteriorly, forming the lumen of the gonoduct that is characterized by numerous mucosal folds (Figure 1B). The ovarian wall is composed of three layers; the outermost is a thin layer of the mesothelium, and the middle layer is the tunica albuginea, which is made up of collagenous, elastic fibers, in addition to smooth muscle cells with associated blood capillaries. The innermost layer is the germinal epithelium, which projects into ovocoel creating finger-like ovarian lamellae that contain many oocytes at different stages of development (Figure 1D). The developing ova were held together by the stroma, which also harbors vascular collagenous connective tissue and clusters of immune cells (Figure 1E).
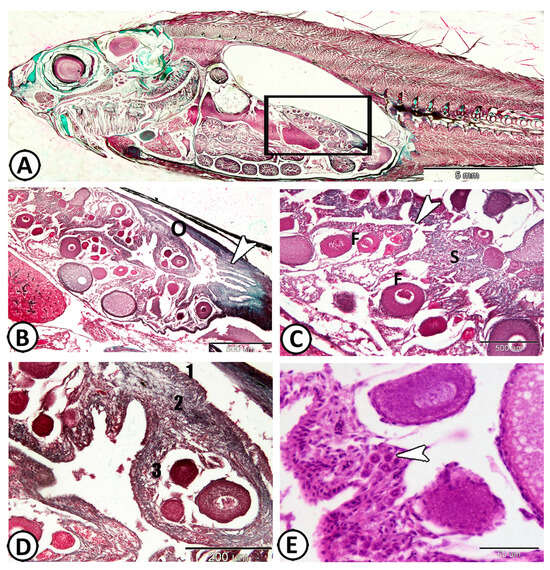
Figure 1.
Histological analysis of the Molly fish (Poecilia sphenops) ovary. (A) The ovary of Molly fish consists of a single saccular structure located on the dorsolateral lining of the peritoneal cavity (boxed area). (B) The caudal part of the ovary (O) branched posteriorly, forming the lumen of the gonoduct (arrowhead). (C) The ovocoel (arrowhead) remained in the center of the ovary, making an irregular space. Note the presence of different stages of ovarian follicles (F), held by the stroma (S). (D) The ovarian wall consisted of three layers: the mesothelium (1), the middle layer known as the tunica albuginea (2), and the innermost layer identified as the germinal epithelium (3). (E) The ovarian stroma housed clusters of immune cells (arrowhead).
3.2. Immunohistochemistry
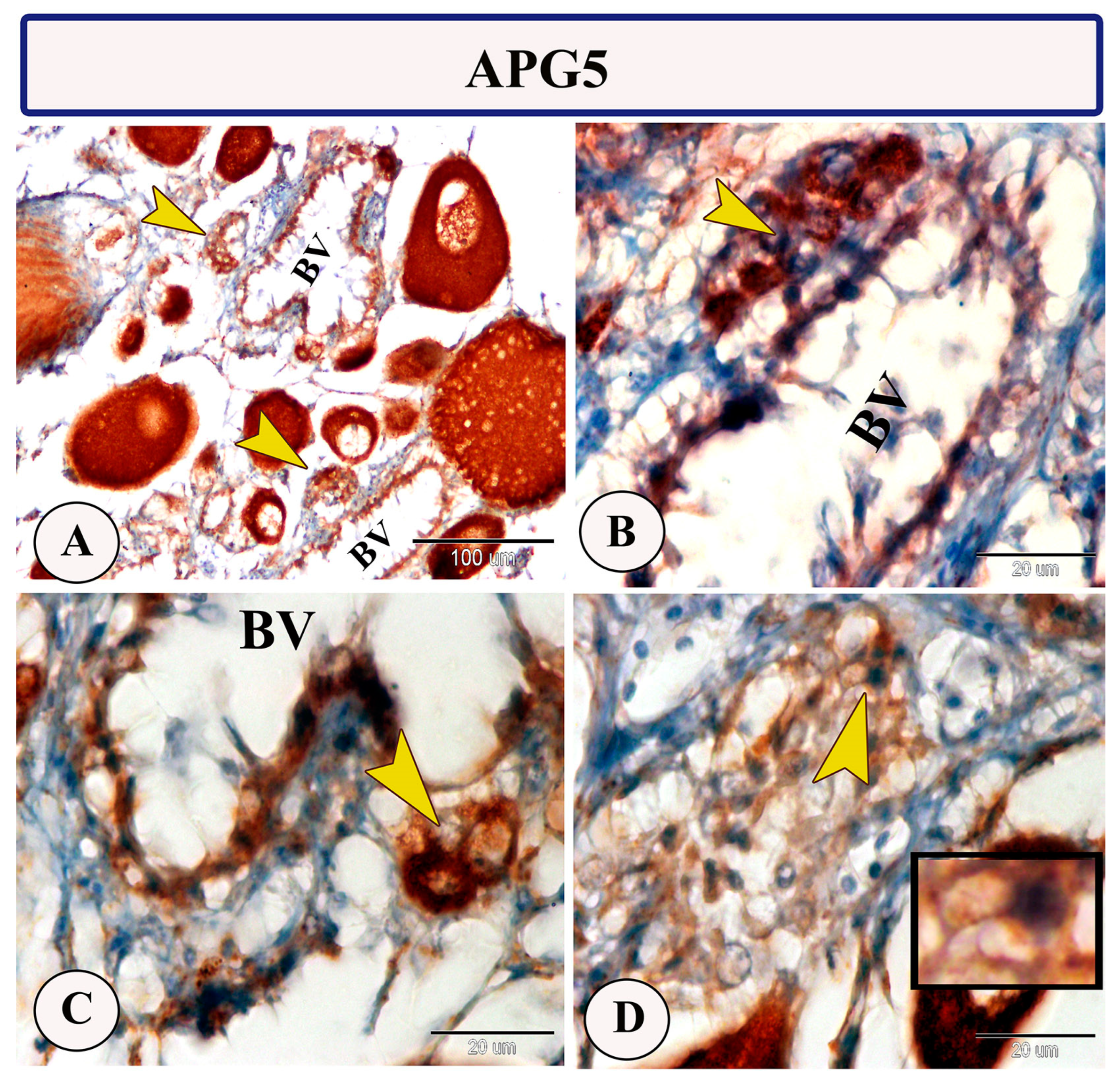
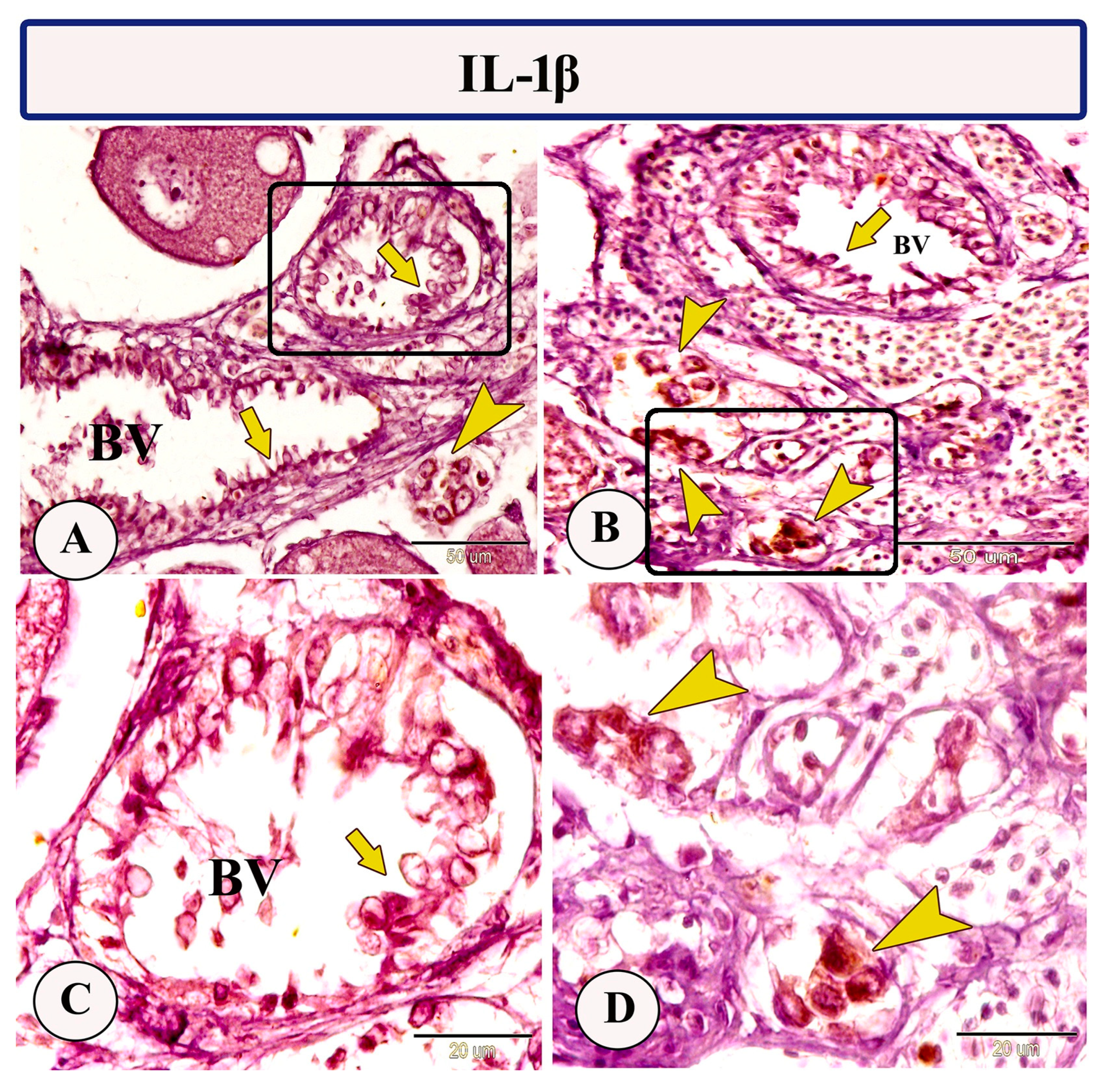
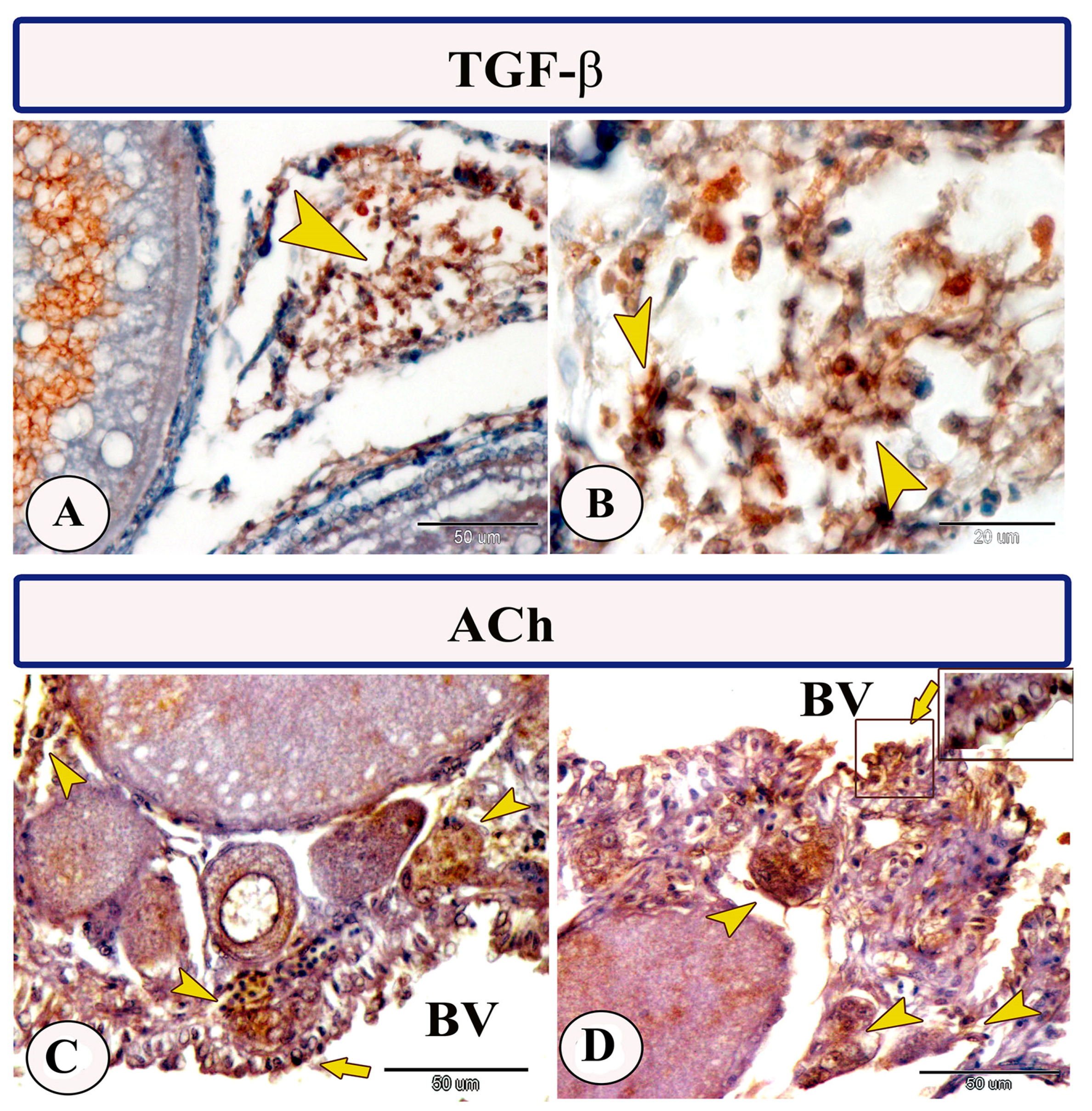
Immunohistochemical staining of the ovarian stroma against APG5 revealed highly positive macrophage cells that aggregated alongside the blood vessels as well as positive monocytes and dendritic cells. The macrophages that are usually found in clusters near the blood vessels and their cytoplasm showed strong immunoreactivity to APG5. The monocytes were characterized by their large size, whereas the dendritic cells were small cells with fine dendrite-like processes (Figure 2A–D). In addition, IL-1β immunostaining demonstrated a high percentage of positive-macrophage cells, monocytes, and rodlet cells (Figure 3A–D). The rodlet cells were observed in the wall of blood vessels and consisted of a capsule and intracytoplasmic inclusions. Only their capsules were characterized by positive immunoreactivity to IL-1β (Figure 3C). Immunolabeling of highly reactive macrophage cells with TGF-β was also detected (Figure 4A,B). The expression of Ach in macrophage and rodlet cells was also observed (Figure 4C,D).
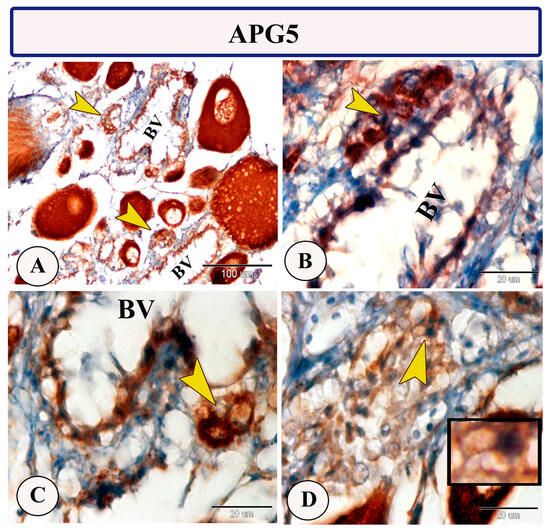
Figure 2.
Expression of APG5 in the ovarian stroma of Molly fish (Poecilia sphenops). (A,C) Groups of macrophage cells near blood vessels (BV) show high APG5 expression (arrowhead). (B) Monocytes displaying positive staining for APG5 (arrowheads). (D) Dendritic cells exhibiting positive staining for APG5, with cytoplasmic processes (arrowhead, boxed area).
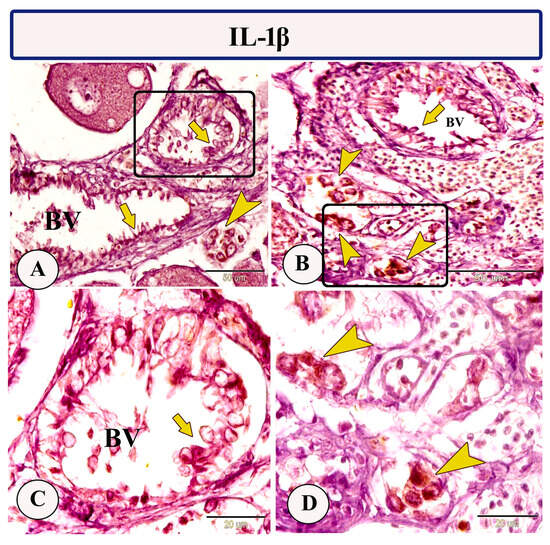
Figure 3.
Expression of IL-1β in the ovarian stroma of Molly fish (Poecilia sphenops). (A) Heavy IL-1β-positive clusters of macrophage cells (arrowhead) and rodlet cells (arrows). (B) Monocytes (arrowheads) and rodlet cells (arrow) exhibited an IL-1β-positive reaction. (C,D) Higher magnifications of the insets in A and B show IL-1β immunopositive-stained rodlet cells (arrow) and monocytes (arrowheads). Blood vessels (BV).
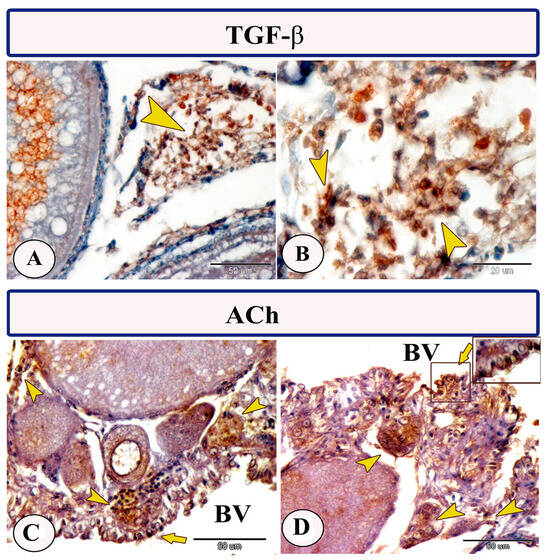
Figure 4.
Expression of TGF-β and ACh in the ovarian stroma of Molly fish (Poecilia sphenops). (A,B) Macrophage cells displaying positive immunostaining for TGF-β (arrowheads). (C,D) Macrophage cells (arrowheads) and rodlet cells (arrows) highly expressed ACh. Blood vessels (BV).
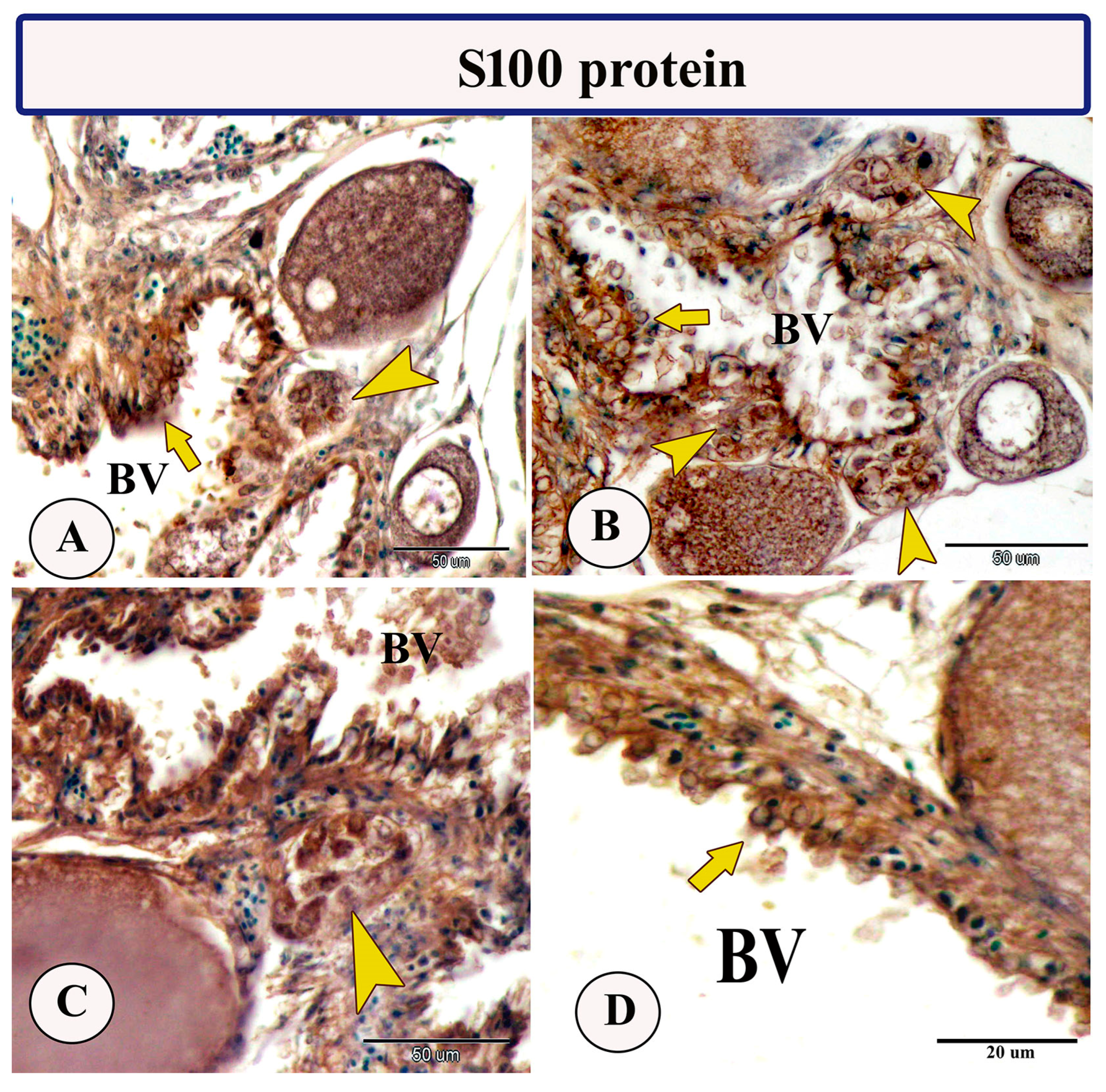
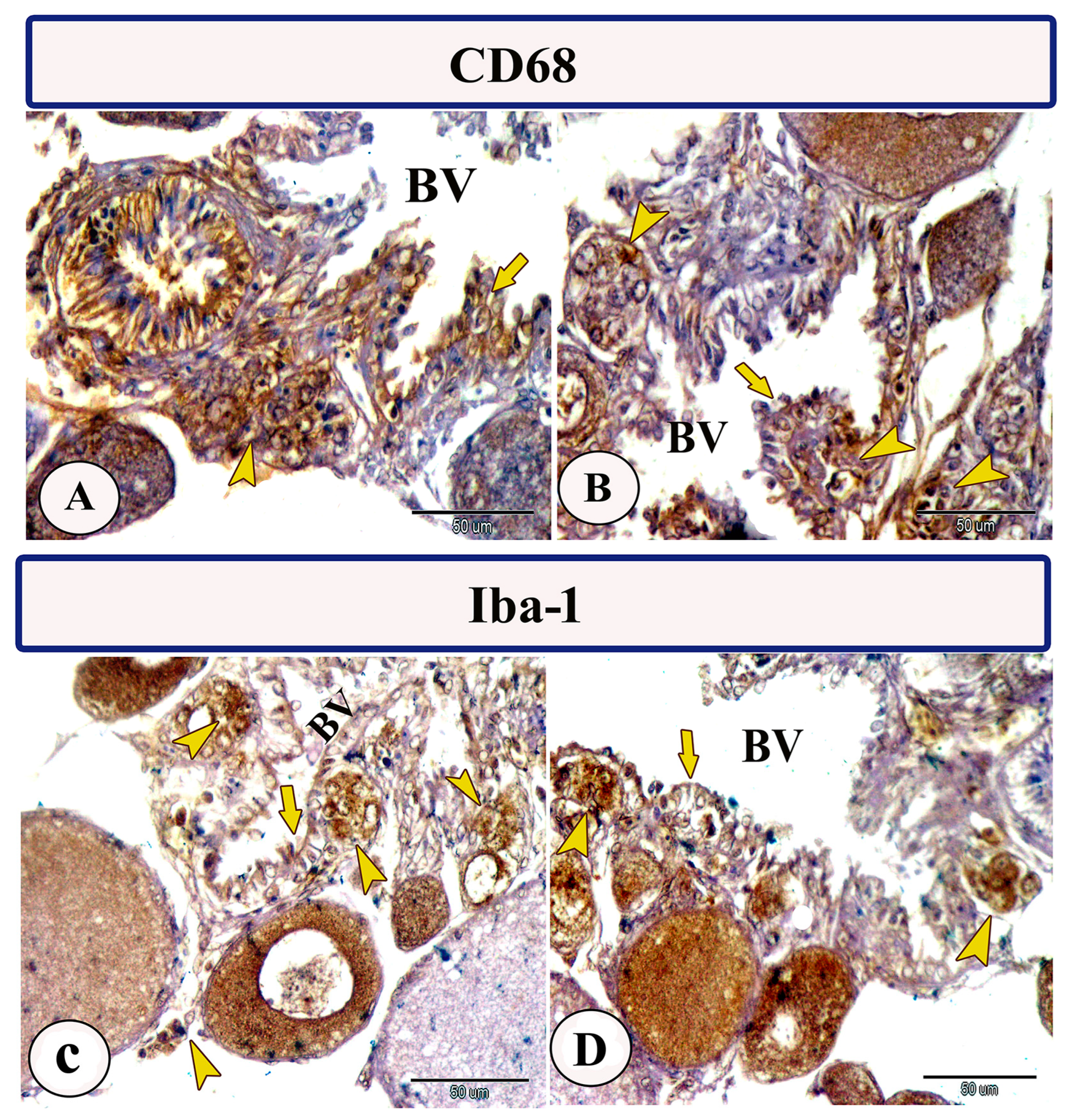
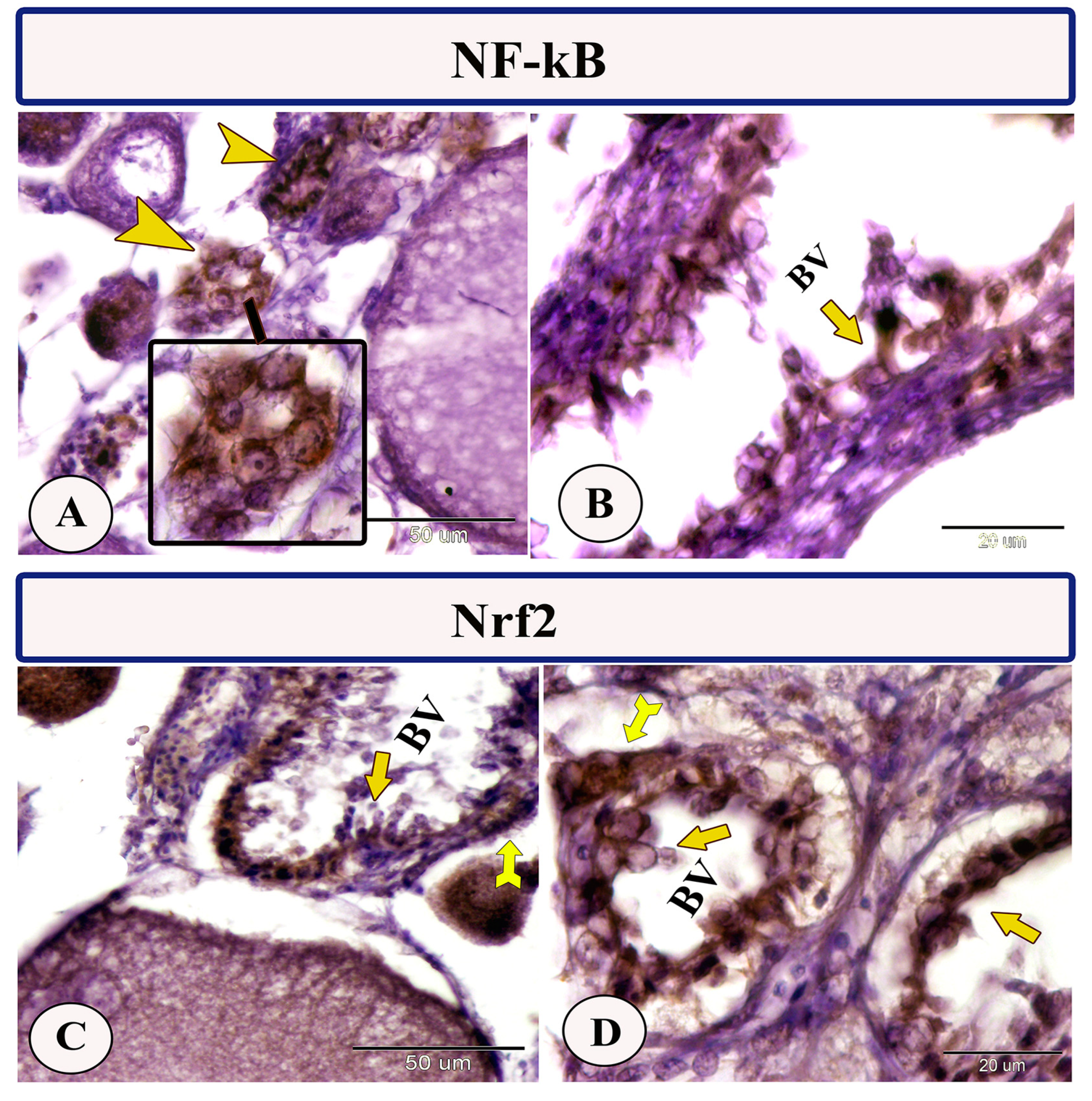
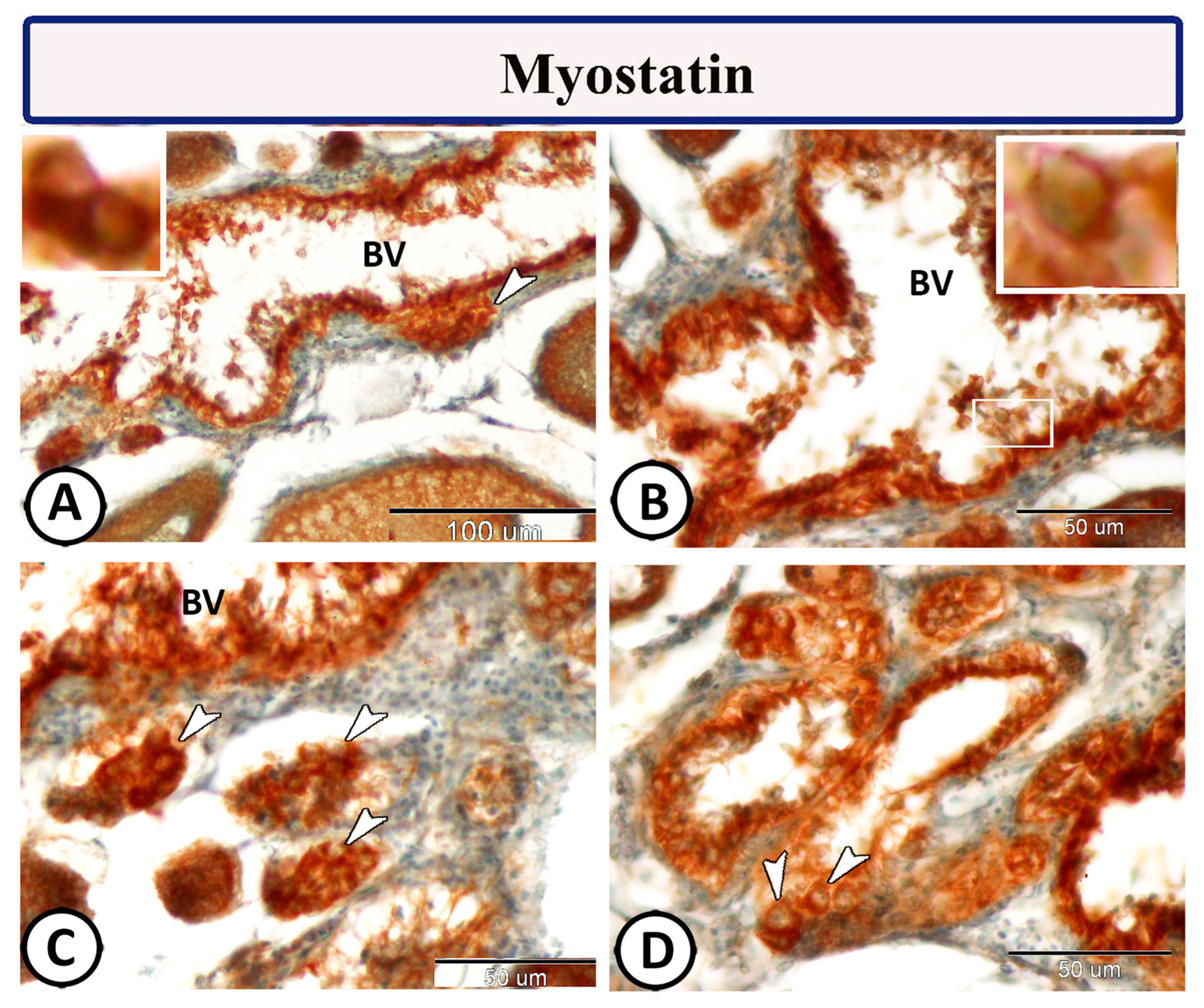
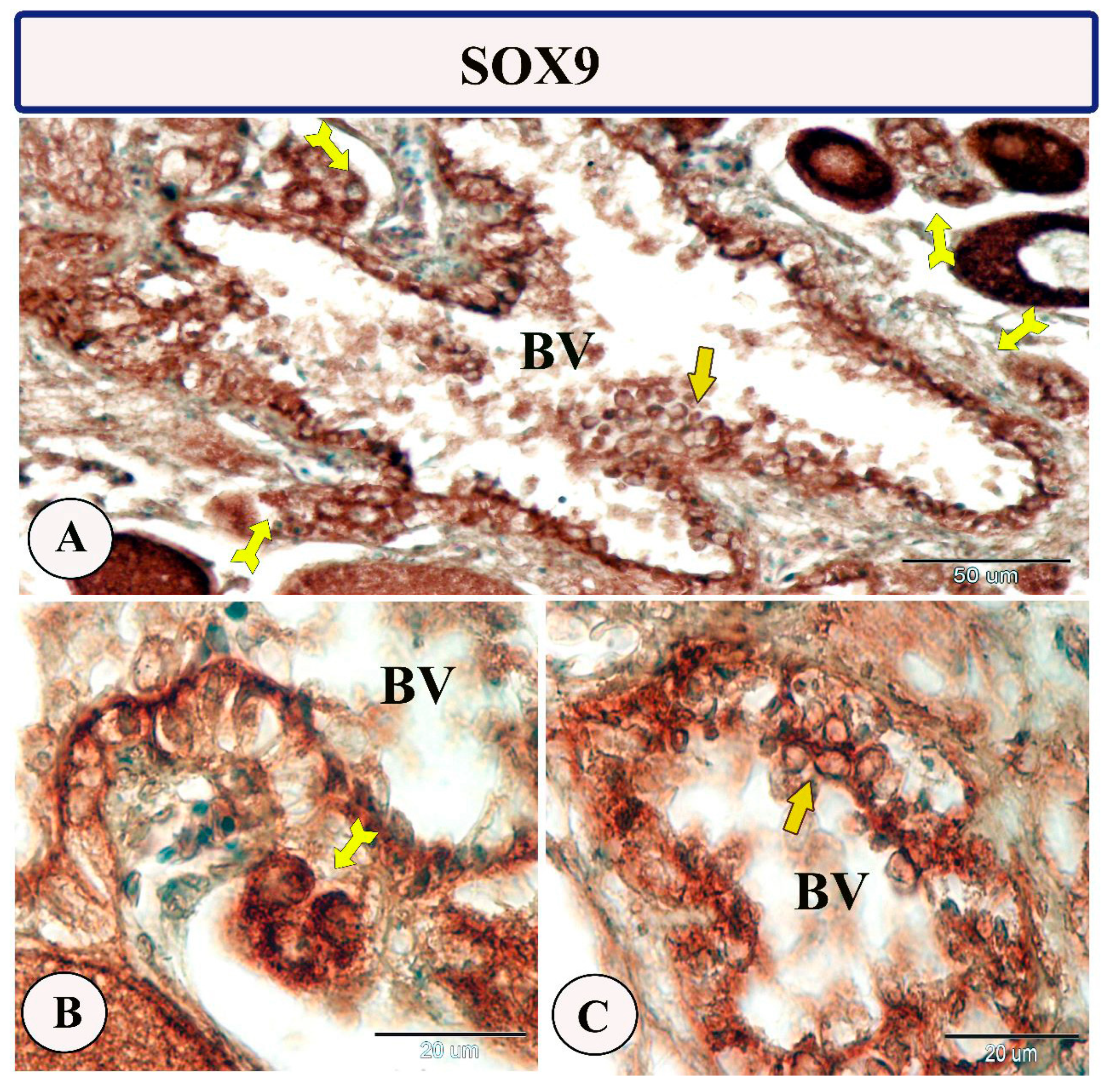
Immunohistochemical staining of the ovarian stroma against S100 proteins revealed highly positive groups of macrophage cells and rodlet cells (Figure 5A–D). Furthermore, CD68 and Iba-1 expressions revealed immunopositive reactions in both macrophage cells and rodlet cells (Figure 6A–D). In addition, both macrophages and rodlet cells expressed NF-κB (Figure 7A,B). Alternatively, Nrf2 immunostaining revealed a high percentage of positive stem and rodlet cells in the ovarian stroma (Figure 7C,D). Moreover, myostatin expression (Figure 8A–D) and SOX9 expression (Figure 9A–D) were detected within clusters of stem cells and rodlet cells. In Figure 9A, a large blood vessel showed many rodlet cells within its wall. The capsule of these cells expressed SOX9.
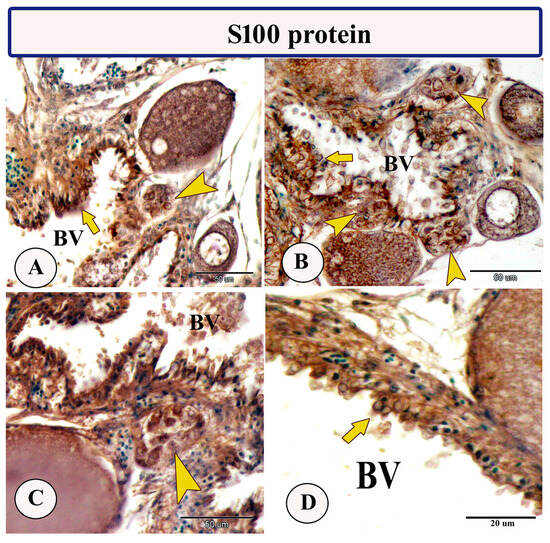
Figure 5.
Expression of S100 protein in the ovarian stroma of Molly fish (Poecilia sphenops). (A–D) Clusters of macrophage cells (arrowheads) and rodlet cells (arrows) show an S100-positive reaction. Blood vessels (BV).
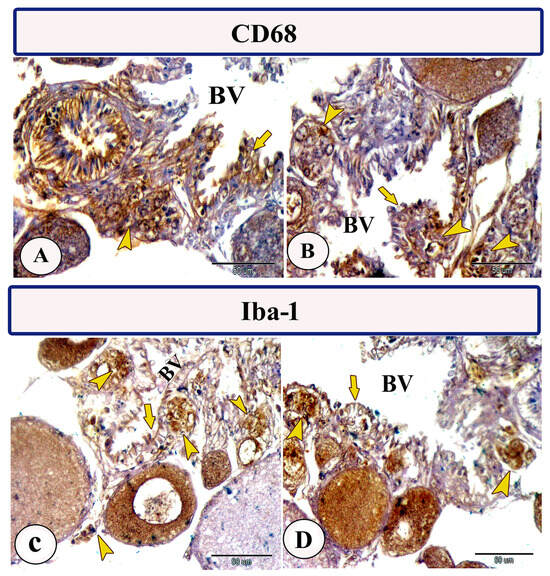
Figure 6.
Expression of CD68 and Iba-1 in the ovarian stroma of Molly fish (Poecilia sphenops). (A–D) Macrophage cells (arrowheads) and rodlet cells (arrows) exhibit positive immunoreactivity for CD68 and Iba-1. Blood vessels (BV).
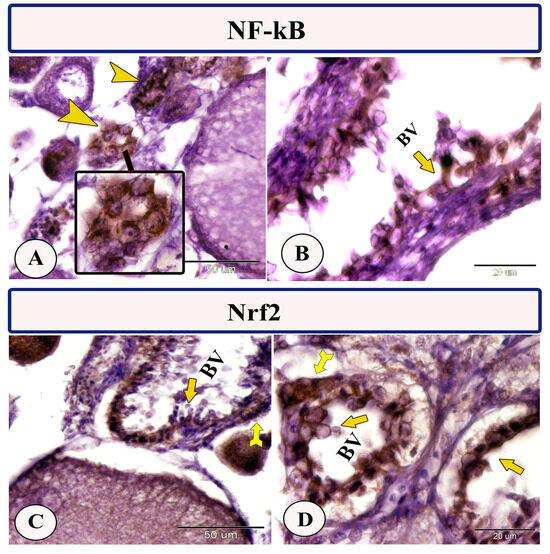
Figure 7.
Expression of NF-κB and Nrf2 in the ovarian stroma of Molly fish (Poecilia sphenops). (A,B) Macrophage cells (arrowheads) and rodlet cells (arrow) exhibit strong immunopositivity for NF-κB. (C,D) Stem cells (forked arrows) and rodlet cells (arrows) displayed strongly positive signals in response to Nrf2 immunostaining. Blood vessels (BV).
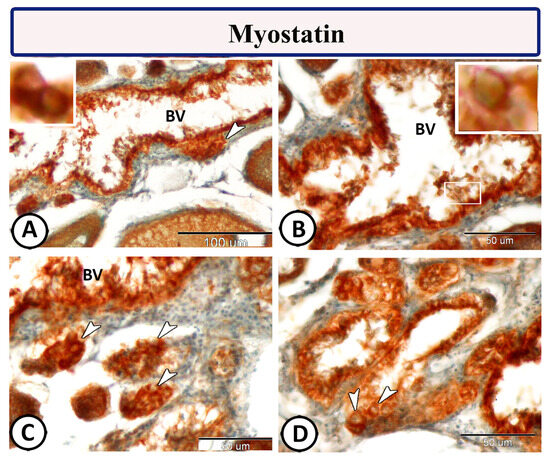
Figure 8.
Myostatin expression of the ovarian stroma in Molly fish (Poecilia sphenops). (A–D) Groups of stem cells (arrowheads) and rodlet cells (boxed areas) demonstrate positive myostatin reactions. Blood vessels (BV).
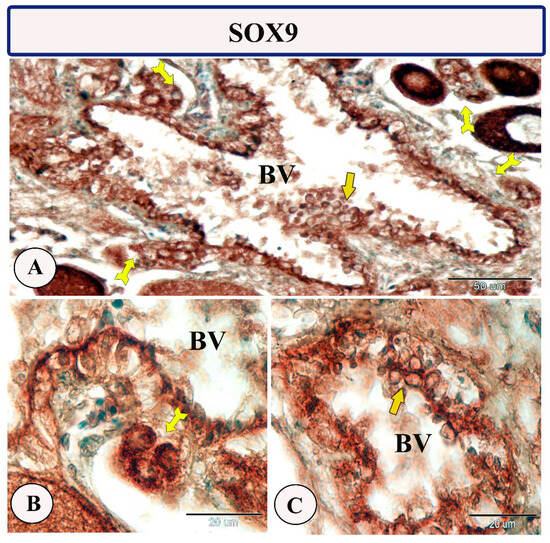
Figure 9.
Expression of SOX9 in the ovarian stroma of Molly fish (Poecilia sphenops). (A–C) Highly positive stem cells (forked arrows) and rodlet cells (arrows). Blood vessels (BV).
4. Discussion
As observed in the present study, the ovary of Molly fish is a singular saccular structure situated on the dorsal mesentery within the dorsolateral lining of the peritoneal cavity. Furthermore, the ovaries displayed oocytes at various stages of both development and degeneration. The observed pattern of folliculogenesis and follicular atresia, characterized by asynchronous oocyte development, has been identified in tilapia, cyprinids, and other teleosts known for multiple spawning [27]. While some fish species undergo simultaneous ovulation and spawning, others, like the Salmonidae, retain the ovulated eggs in their peritoneal cavity, resulting in a delayed spawning process [27].
In the ovarian stroma of Molly fish, monocytes and dendritic cells revealed strong APG5 expression. In fish, monocytes display phagocytosis and non-specific cytotoxic activities. They are considered transient blood cells due to their migration during the inflammatory phase to the connective tissue, where they are transformed into macrophages [29]. Furthermore, dendritic cells, characterized by their dendritic appearance, can function as antigen-presenting cells, possess phagocytic capabilities, and exhibit T-cell stimulatory characteristics [30]. Among the principal players in the autophagy process, APG5 plays an essential role in various processes including the formation of the autophagic vesicles, development and proliferation of the lymphocytes, the differentiation of the adipocytes, and also apoptosis [31]. Due to its involvement in different biological processes, APG5 expression has been widely observed in various Molly fish tissues [25,26,32,33].
Macrophages play fundamental roles in many immune reactions, involving lymphocyte activation, and phagocytosis; they also contribute to the production of growth factors, cytokines, and chemokines, in addition to their involvement in foreign antigen degradation, tissue remodeling, and presenting antigens to T cells [28,34,35]. The arrangement of macrophages within the ovarian stroma of Molly fish implies their involvement in various intra-ovarian processes. In the Molly fish ovary, expression of IL-1β was identified in macrophage cells, monocytes, and rodlet cells; hence, it is one of the primary cytokines identified in teleosts [36,37]. It is essential for regulating the inflammatory process [38] and works as a key initiator for local and systemic responses to various stimuli [39]. Moreover, increased production of IL-1β has been detected during various cellular processes including cell proliferation and differentiation [40].
Interestingly, the macrophage cells in the ovarian stroma of Molly fish demonstrated TGF-β expression. The TGF-β is an immune and non-hematopoietic cell-produced cytokine that plays an essential role in numerous biological processes encompassing cell proliferation, differentiation, immune response suppression, and oncogenesis [41,42]. Three different TGF-β proteins have been characterized in diverse fish species, with TGF-β1 considered the primary immune-active isoform. It exhibits the highest expression in lymphoid tissues, macrophages, and gills [41,43,44,45,46,47].
The macrophage and rodlet cells in the ovary of Molly fish exhibited strong alpha 7 nicotinic acetylcholine receptor (α7nAchR, Ach) expression. The α7nAchR is a prime receptor of the cholinergic anti-inflammatory pathway [48], which is broadly distributed around various non-neuronal cells such as macrophages, endothelial cells, and dendritic cells [49,50]. In our previous investigation, we identified the presence of α7nAchR in fish gut macrophages [51]. It is established that acetylcholine, which is released from efferent fibers of the vagus nerve inhibits the secretion of diverse pro-inflammatory cytokines by interacting with α7nAChR expressed by various immune cells and macrophages [52]. Stimulation of α7nAChR has been documented to reduce inflammatory responses in peripheral tissues and restore impaired function of immune cells [53,54,55]. Moreover, a previous study reported the inhibition of IL-1β gene and protein expression using α7nAChR agonist therapy, where the latter can inhibit the development of endometriosis [56]. In addition, failure of the activated cholinergic system to attenuate the inflammatory responses has been detected in α7nAchR-deficient mice [57].
In addition, rodlet cells and macrophages in the ovary of Molly fish exhibited positive immunoreactivity to the S100 protein and CD68 and Iba-1. The S-100 expression was reported in various tissues of Molly fish, including the ependymal cells [32] and pancreas [58]. The S100 protein is involved in defense systems across numerous species, exhibiting both extracellular and intracellular functionalities, and plays a fundamental role in pro-inflammatory stimulation, cytoskeleton rearrangement, and free radical scavengers [59]. Moreover, S100 proteins are associated with various regulatory mechanisms of cellular functions, including cell proliferation and differentiation, apoptosis, invasion, enzyme activation, and energy metabolism [59,60,61].
The heavily glycosylated transmembrane glycoprotein (type I), CD68, is primarily linked to the endosomal/lysosomal compartment [62]. It is a surface marker that is myeloid-specific and is widely expressed in cells belonging to the mononuclear phagocyte lineage, such as microglia, osteoclasts, macrophages, and myeloid dendritic cells [63,64]. Macrophages expressing CD68+ are recognized as essential elements in the foreign body reaction [65]. The preferential localization of CD68 during late endosomes suggests its involvement in peptide transport/antigen processing [66]. In contrast, Iba1, an actin-cross-linking protein, is recognized as a newly discovered EF-hand protein found to be exclusively expressed in cells belonging to monocytic lineages, such as microglia. It is also expressed in macrophages, where it is essential for the induction of membrane ruffling by the macrophage colony-stimulating factor [67,68,69,70]. It has been also suggested that Iba1 is involved in calcium signaling pathways and is considered a principal molecule in the process of membrane ruffling and phagocytosis of macrophages/microglia [68].
The macrophage and rodlet cells in the ovary of Molly fish also exhibited strong NF-κB expression. Furthermore, a high level of Nrf2 immunoreaction was observed in the rodlet cells. The transcription factor NF-κB drives the production of pro-inflammatory cytokines within the innate immune response and is essential for numerous signaling pathways [71], where it is involved in the regulation of cell proliferation, adhesion, and cell apoptosis [72]. Moreover, Nrf2 is a key protein in various functions in fish, including osmoregulation, antioxidation, immunopotentiation, cell proliferation, and differentiation [73,74].
Notably, myostatin and SOX9 expressions were detected in groups of rodlet cells and stem cells and in the ovarian stroma of Molly fish. Myostatin is a member of the TGF-β superfamily and was first characterized as a negative regulator of growth in skeletal muscles [75]. Its expressions have been widely observed in several fish tissues, including pseudobranch, liver, and brain tissues [32,33,76]. In addition, myostatin immunoreactivity has been recently detected in both the granulosa and theca cells of zebrafish ovary [77]. Previous studies have reported the importance of myostatin in reproduction, where it has been involved in granulosa-cell proliferation and terminal differentiation, oocyte maturation, follicular development, and ovarian steroidogenesis [78,79]. Also, it has been reported that myostatin boosts the expression of the connective tissue growth factor [80].
The transcription factor SOX9 is critical for the development of different tissues and body organs [81]. Furthermore, it is implicated in sex differentiation and determination, early embryonic differentiation, and developmental and seasonal variations in the gonads [82,83]. In teleosts, two different forms of the SOX9 gene have been demonstrated: SOX9a1 and SOX9a2 [81,84,85]. Previous studies described SOX9 expression in the ovarian tissues of some fish species such as the medaka, zebrafish, rice field eel, and walking catfish [83,84,85,86]. Recently, we observed a robust expression of SOX9 in the ovarian stem cells and previtellogenic follicles of zebrafish [77]; these observations indicate the potential role of SOX9 in follicular development and stem cell maintenance, thus inducing embryonic development [83,87].
5. Conclusions
This study sheds light on the intriguing relationship between the immune system and gonad function, specifically highlighting the unique characteristics of immune cells within the ovarian stroma of Molly fish. The research reveals that various cells within the ovarian stroma, such as monocytes, macrophages, and rodlet cells, exhibit positive immunoreactivity to several key immunohistochemical markers, including APG5, IL-1β, TGF-β, Ach, S100 protein, CD68, Iba-1, NF-κB, Nrf2, myostatin, and SOX9. Furthermore, the expressions of myostatin and SOX9 have been identified in clusters of stem cells within the ovarian stroma, suggesting a potential link between these markers and the regulation of stem cell activity. These findings collectively suggest an active role of ovarian tissues in immune responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9010010/s1, Figure S1. (A–D) Negative controls of the ovarian tissues of the Molly fish, where (A) NF-κB, (B) SOX9, (C) myostatin, and (D) Nrf2 primary antibodies were omitted, and the tissue specimens were incubated with blocking buffer. Figure S2. (A–F) Negative controls of the ovarian tissues of the Molly fish where (A) APG5, (B) TGF, (C) S100, (D) CD68, (E) IBA, and (F) IL-1β primary antibodies were omitted and the tissue specimens were incubated with blocking buffer.
Author Contributions
Conceptualization, methodology, investigation, formal analysis, data curation, R.K.A.S., D.M.M., M.A.H., A.S.A., G.Z., M.A., A.A. and N.A.; writing—original draft preparation, R.K.A.S., D.M.M., M.A.H. and N.A.; writing—review and editing, A.S.A., G.Z., M.A. and A.A.; resources, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research was carried out in compliance with both Egyptian legal regulations and the animal care guidelines set by the University of Sohag. Approval for all procedures in this study was granted by the Veterinary Medical Research Ethics Committee of the Faculty of Veterinary Medicine at Sohag University, Egypt, under ethical number Soh.un.vet/00045R (11/10/2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hogarth, P.J. Reproduction and Immunity in the Female; Springer: Dordrecht, The Netherlands, 1982. [Google Scholar]
- Schmid-Hempel, P. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lawniczak, M.K.; Barnes, A.I.; Linklater, J.R.; Boone, J.M.; Wigby, S.; Chapman, T. Mating and immunity in invertebrates. Trends Ecol. Evol. 2007, 22, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Banu, H.; Prakash, A.; Tripathi, G. Immune system of fish: An evolutionary perspective. In Antimicrobial Immune Response; IntechOpen: London, UK, 2021; Volume 1. [Google Scholar]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Ellis, A.E. The leucocytes of fish: A review. J. Fish Biol. 1977, 11, 453–491. [Google Scholar] [CrossRef]
- Givan, A.L.; White, H.D.; Stern, J.E.; Colby, E.; Guyre, P.M.; Wira, C.R.; Gosselin, E.J. Flow cytometric analysis of leukocytes in the human female reproductive tract: Comparison of fallopian tube, uterus, cervix, and vagina. Am. J. Reprod. Immunol. 1997, 38, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.-J.; Kang, J.-H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef]
- Yang, X.; Gilman-Sachs, A.; Kwak-Kim, J. Ovarian and endometrial immunity during the ovarian cycle. J. Reprod. Immunol. 2019, 133, 7–14. [Google Scholar] [CrossRef]
- Wu, R.; Van der Hoek, K.H.; Ryan, N.K.; Norman, R.J.; Robker, R.L. Macrophage contributions to ovarian function. Hum. Reprod. Update 2004, 10, 119–133. [Google Scholar] [CrossRef]
- Mazzoni, T.S.; Lo Nostro, F.L.; Antoneli, F.N.; Quagio-Grassiotto, I. Action of the metalloproteinases in gonadal remodeling during sex reversal in the sequential hermaphroditism of the teleostei fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae). Cells 2018, 7, 34. [Google Scholar] [CrossRef]
- Reeves, G. Specific Stroma in the Cortex and Medulla of the Ovary: Cell Types and Vascular Supply in Relation to Follicular Apparatus and Ovulation. Obstet. Gynecol. 1971, 37, 832–844. [Google Scholar] [CrossRef]
- Neilson, D.; Jones, G.S.; Woodruff, J.D.; Goldberg, B. The innervation of the ovary. Obstet. Gynecol. Surv. 1970, 25, 889–904. [Google Scholar] [CrossRef]
- Brown, H.M.; Robker, R.L.; Russell, D.L. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology 2010, 151, 5446–5455. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.; Brannstrom, M. White cells and the ovary–incidental invaders or essential effectors? J. Endocrinol. 1994, 140, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, L.; Brayboy, L. Macrophages: An indispensable piece of ovarian health. Biol. Reprod. 2021, 104, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Wake, M. Oviduct structure and function in non-mammalian vertebrates. Fortschritte Zool. 1985, 30, 427–435. [Google Scholar]
- Wourms, J.P.; Grove, B.D.; Lombardi, J. 1 The Maternal-Embryonic Relationship in Viviparous Fishes. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 11, pp. 1–134. [Google Scholar]
- Torres-Martínez, A.; Hernández-Franyutti, A.; Uribe, M.C.; Contreras-Sánchez, W.M. Ovarian structure and oogenesis of the extremophile viviparous teleost Poecilia mexicana (P. oeciliidae) from an active sulfur spring cave in Southern Mexico. J. Morphol. 2017, 278, 1667–1681. [Google Scholar] [CrossRef]
- Osório, J.; Rétaux, S. The lamprey in evolutionary studies. Dev. Genes Evol. 2008, 218, 221–235. [Google Scholar] [CrossRef]
- Tembo, R.N. The sublethal effects of low-pH exposure on the chemoreception of Poecilia sphenops. Arch. Environ. Contam. Toxicol. 2009, 57, 157–163. [Google Scholar] [CrossRef]
- Wischnath, L. Atlas of Livebearers of the World; Tfh Pubns Inc.: Neptune, NJ, USA, 1993. [Google Scholar]
- Sarker, P.K.; Kapuscinski, A.R.; Bae, A.Y.; Donaldson, E.; Sitek, A.J.; Fitzgerald, D.S.; Edelson, O.F. Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus). PLoS ONE 2018, 13, e0201315. [Google Scholar] [CrossRef]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone Publisher: Edinburgh, UK, 2002; Volume 172, pp. 593–620. [Google Scholar]
- Mokhtar, D.M.; Hussein, M.M.; Sayed, R.K. Novel Identification and Microscopy of the Intestinal Bulb of Molly Fish (Poecilia sphenops) with a Focus on Its Role in Immunity. Microsc. Microanal. 2022, 28, 1827–1839. [Google Scholar] [CrossRef]
- Sayed, R.K.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and functional aspects of the spleen in molly fish Poecilia sphenops (Valenciennes, 1846): Synergistic interactions of stem cells, neurons, and immune cells. Biology 2022, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Secombes, C.; Wang, T. The innate and adaptive immune system of fish. In Infectious Disease in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–68. [Google Scholar]
- Tavares-Dias, M.; Schalch, S.H.C.; Martins, M.L.; Silva, É.D.; Moraes, F.R.; Perecin, D. Hematologia de teleósteos brasileiros com infecção parasitária. I. Variáveis do Leporinus macrocephalus (Garavelo e Britski, 1988) (Anostomidae) e Piaractus mesopotamicus (Holmberg, 1887) (Characidae). Acta Sci. 1999, 21, 337–342. [Google Scholar]
- Bassity, E.; Clark, T.G. Functional identification of dendritic cells in the teleost model, rainbow trout (Oncorhynchus mykiss). PLoS ONE 2012, 7, e33196. [Google Scholar] [CrossRef] [PubMed]
- Pierdominici, M.; Vomero, M.; Barbati, C.; Colasanti, T.; Maselli, A.; Vacirca, D.; Giovannetti, A.; Malorni, W.; Ortona, E. Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. FASEB J. 2012, 26, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Sayed, R.K.; Zaccone, G.; Albano, M.; Hussein, M.T. Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies. Cells 2022, 11, 2659. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Sayed, R.K.; Mokhtar, D.M. Structural and immunohistochemical analysis of the cellular compositions of the liver of molly fish (Poecilia sphenops), focusing on its immune role. Zool. Lett. 2023, 9, 1–13. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Hodgkinson, J.W.; Grayfer, L.; Belosevic, M. Biology of bony fish macrophages. Biology 2015, 4, 881–906. [Google Scholar] [CrossRef]
- Fujiki, K.; Shin, D.-H.; Nakao, M.; Yano, T. Molecular cloning and expression analysis of carp (Cyprinus carpio) interleukin-1β, high affinity immunoglobulin E Fc receptor γ subunit and serum amyloid A. Fish Shellfish Immunol. 2000, 10, 229–242. [Google Scholar] [CrossRef]
- Bird, S.; Wang, T.; Zou, J.; Cunningham, C.; Secombes, C.J. The first cytokine sequence within cartilaginous fish: IL-1β in the small spotted catshark (Scyliorhinus canicula). J. Immunol. 2002, 168, 3329–3340. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T.; Bird, S. The interleukins of fish. Dev. Comp. Immunol. 2011, 35, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- O’Léime, C.S.; Cryan, J.F.; Nolan, Y.M. Nuclear deterrents: Intrinsic regulators of IL-1β-induced effects on hippocampal neurogenesis. Brain Behav. Immun. 2017, 66, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Laing, K.; Cunningham, C.; Secombes, C. Genes for three different isoforms of transforming growth factor-β are present in plaice (Pleuronectes platessa) DNA. Fish Shellfish Immunol. 2000, 10, 261–271. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Pickard, K.M.; Rampton, D.; Kruidenier, L.; Rovedatti, L.; Leakey, N.A.; Corazza, G.R.; Monteleone, G.; MacDonald, T.T. Blockade of transforming growth factor β upregulates T-box transcription factor T-bet, and increases T helper cell type 1 cytokine and matrix metalloproteinase-3 production in the human gut mucosa. Gut 2008, 57, 605–612. [Google Scholar] [CrossRef]
- Harms, C.; Kennedy-Stoskopf, S.; Horne, W.; Fuller, F.; Tompkins, W. Cloning and sequencing hybrid striped bass (Morone saxatilis × M. chrysops) transforming growth factor-β (TGF-β), and development of a reverse transcription quantitative competitive polymerase chain reaction (RT-qcPCR) assay to measure TGF-β mRNA of teleost fish. Fish Shellfish Immunol. 2000, 10, 61–85. [Google Scholar]
- Zhan, Y.; Jimmy, K. Molecular isolation and characterisation of carp transforming growth factor β1 from activated leucocytes. Fish Shellfish Immunol. 2000, 10, 309–318. [Google Scholar] [CrossRef]
- Funkenstein, B.; Olekh, E.; Jakowlew, S.B. Identification of a novel transforming growth factor-β (TGF-β6) gene in fish: Regulation in skeletal muscle by nutritional state. BMC Mol. Biol. 2010, 11, 37. [Google Scholar] [CrossRef]
- Maehr, T.; Costa, M.M.; Vecino, J.L.G.; Wadsworth, S.; Martin, S.A.; Wang, T.; Secombes, C.J. Transforming growth factor-β1b: A second TGF-β1 paralogue in the rainbow trout (Oncorhynchus mykiss) that has a lower constitutive expression but is more responsive to immune stimulation. Fish Shellfish Immunol. 2013, 34, 420–432. [Google Scholar] [CrossRef]
- Wei, H.; Yin, L.; Feng, S.; Wang, X.; Yang, K.; Zhang, A.; Zhou, H. Dual-parallel inhibition of IL-10 and TGF-β1 controls LPS-induced inflammatory response via NF-κB signaling in grass carp monocytes/macrophages. Fish Shellfish Immunol. 2015, 44, 445–452. [Google Scholar] [CrossRef]
- Chao, R.; Tong, Y.-L.; Li, J.-C.; Lu, Z.-Q.; Yao, Y.-M. The protective effect of alpha 7 nicotinic acetylcholine receptor activation on critical illness and its mechanism. Int. J. Biol. Sci. 2017, 13, 46. [Google Scholar]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 13, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mathieu, S.L.; Harris, R.; Ji, J.; Anderson, D.J.; Malysz, J.; Bunnelle, W.H.; Waring, J.F.; Marsh, K.C.; Murtaza, A. Role of α7 nicotinic acetylcholine receptors in regulating tumor necrosis factor-α (TNF-α) as revealed by subtype selective agonists. J. Neuroimmunol. 2011, 239, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Alesci, A.; Mokhtar, D.M.; Aragona, M.; Guerrera, M.C.; Capillo, G.; Albano, M.; de Oliveira Fernandes, J.; Kiron, V.; Sayed, R.K. Localization of acetylcholine, alpha 7-nAChR and the antimicrobial peptide piscidin 1 in the macrophages of fish gut: Evidence for a cholinergic system, diverse macrophage populations and polarization of immune responses. Fishes 2023, 8, 43. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef]
- Huston, J.M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yuan, R.; Rosas-Ballina, M.; Ashok, M.; Goldstein, R.S.; Chavan, S.; Pavlov, V.A. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007, 35, 2762–2768. [Google Scholar]
- Pavlov, V.A.; Ochani, M.; Yang, L.-H.; Gallowitsch-Puerta, M.; Ochani, K.; Lin, X.; Levi, J.; Parrish, W.R.; Rosas-Ballina, M.; Czura, C.J. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007, 35, 1139–1144. [Google Scholar] [CrossRef]
- Yamada-Nomoto, K.; Yoshino, O.; Akiyama, I.; Ushijima, A.; Ono, Y.; Shima, T.; Nakashima, A.; Hayashi, S.; Kadowaki, M.; Osuga, Y. Alpha-7 nicotinic acetylcholine receptor (nAChR) agonist inhibits the development of endometriosis by regulating inflammation. Am. J. Reprod. Immunol. 2016, 76, 491–498. [Google Scholar] [CrossRef]
- van Maanen, M.A.; Stoof, S.P.; LaRosa, G.J.; Vervoordeldonk, M.J.; Tak, P.P. Role of the cholinergic nervous system in rheumatoid arthritis: Aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann. Rheum. Dis. 2010, 69, 1717–1723. [Google Scholar] [CrossRef]
- Hussein, M.M.; Sayed, R.K.; Mokhtar, D.M. Structural and immunohistochemical characterization of pancreas of Molly fish (Poecilia sphenops), with a special reference to its immune role. Microsc. Res. Technol. 2023, 58, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ali, S.A. Multifunctional role of S100 protein family in the immune system: An update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Q.; Guo, F.; Chen, M.; Tao, X.; Dong, D. S100 proteins in pancreatic cancer: Current knowledge and future perspectives. Front. Oncol. 2021, 11, 711180. [Google Scholar] [CrossRef] [PubMed]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef]
- Betjes, M.G.; Haks, M.C.; Tuk, C.W.; Beelen, R.H. Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology 1991, 183, 79–87. [Google Scholar] [CrossRef]
- Greaves, D.R.; Gordon, S. Macrophage-specific gene expression: Current paradigms and future challenges. Int. J. Hematol. 2002, 76, 6–15. [Google Scholar] [CrossRef]
- Klinge, U.; Dievernich, A.; Tolba, R.; Klosterhalfen, B.; Davies, L. CD68+ macrophages as crucial components of the foreign body reaction demonstrate an unconventional pattern of functional markers quantified by analysis with double fluorescence staining. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3134–3146. [Google Scholar] [CrossRef]
- Barois, N.; De Saint-Vis, B.; Lebecque, S.; Geuze, H.J.; Kleijmeer, M.J. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic 2002, 3, 894–905. [Google Scholar] [CrossRef]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel geneiba1in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J. Cell Sci. 2000, 113, 3073–3084. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Kumon, Y.; Watanabe, H.; Ohnishi, T.; Shudou, M.; Chuai, M.; Imai, Y.; Takahashi, H.; Tanaka, J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2008, 28, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.G.; Tergaonkar, V.; Ng, J.K.; Dubova, I.; Izpisua-Belmonte, J.C.; Verma, I.M. Characterization of NF-κΒ/IκΒ proteins in zebra fish and their involvement in notochord development. Mol. Cell. Biol. 2004, 24, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Viatour, P.; Merville, M.-P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, Z. Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol. Biotechnol. Equip. 2019, 33, 1453–1463. [Google Scholar] [CrossRef][Green Version]
- Zhu, J.; Wang, H.; Sun, Q.; Ji, X.; Zhu, L.; Cong, Z.; Zhou, Y.; Liu, H.; Zhou, M. Nrf2 is required to maintain the self-renewal of glioma stem cells. BMC Cancer 2013, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, B.D.; Weber, G.M.; Sullivan, C.V.; Levine, M.A. Isolation and characterization of myostatin complementary deoxyribonucleic acid clones from two commercially important fish: Oreochromis mossambicus and Morone chrysops. Endocrinology 2001, 142, 1412–1418. [Google Scholar] [CrossRef][Green Version]
- Mokhtar, D.M.; Sayed, R.K.; Zaccone, G.; Alesci, A.; Hussein, M.M. The potential role of the pseudobranch of molly fish (Poecilia sphenops) in immunity and cell regeneration. Sci. Rep. 2023, 13, 8665. [Google Scholar] [CrossRef]
- Mohamedien, D.; Mokhtar, D.M.; Abdellah, N.; Awad, M.; Albano, M.; Sayed, R.K. Ovary of Zebrafish during Spawning Season: Ultrastructure and Immunohistochemical Profiles of SOX9 and Myostatin. Animals 2023, 13, 3362. [Google Scholar] [CrossRef]
- Kobayashi, W. Communications of Oocyte-Granulosa Cells in the Chum Salmon Ovary Detected by Transmission Electron Microscopy: (Oocyte/granulosa cell/intercellular junction/salmonid ovary/teleost). Dev. Growth Differ. 1985, 27, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Cheewasopit, W.; Laird, M.; Glister, C.; Knight, P.G. Myostatin is expressed in bovine ovarian follicles and modulates granulosal and thecal steroidogenesis. Reproduction 2018, 156, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Harlow, C.R.; Hillier, S.G. Connective tissue growth factor in the ovarian paracrine system. Mol. Cell. Endocrinol. 2002, 187, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Klüver, N.; Kondo, M.; Herpin, A.; Mitani, H.; Schartl, M. Divergent expression patterns of SOX9 duplicates in teleosts indicate a lineage specific subfunctionalization. Dev. Genes Evol. 2005, 215, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Raghuveer, K.; Senthilkumaran, B. Isolation of SOX9 duplicates in catfish: Localization, differential expression pattern during gonadal development and recrudescence, and hCG-induced up-regulation of SOX9 in testicular slices. Reproduction 2010, 140, 477. [Google Scholar] [CrossRef]
- Bhat, I.A.; Rather, M.A.; Saha, R.; Pathakota, G.-B.; Pavan-Kumar, A.; Sharma, R. Expression analysis of SOX9 genes during annual reproductive cycles in gonads and after nanodelivery of LHRH in Clarias batrachus. Res. Vet. Sci. 2016, 106, 100–106. [Google Scholar] [CrossRef]
- Chiang, E.F.-L.; Pai, C.-I.; Wyatt, M.; Yan, Y.-L.; Postlethwait, J.; Chung, B.-C. Two SOX9 genes on duplicated zebrafish chromosomes: Expression of similar transcription activators in distinct sites. Dev. Biol. 2001, 231, 149–163. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, L.; Guo, Y.; Yu, H.; Cheng, H.; Huang, X.; Tiersch, T.R.; Berta, P. Similar gene structure of two SOX9a genes and their expression patterns during gonadal differentiation in a teleost fish, rice field eel (Monopterus albus). Mol. Reprod. Dev. 2003, 66, 211–217. [Google Scholar] [CrossRef]
- Yokoi, H.; Kobayashi, T.; Tanaka, M.; Nagahama, Y.; Wakamatsu, Y.; Takeda, H.; Araki, K.; Morohashi, K.I.; Ozato, K. SOX9 in a teleost fish, medaka (Oryzias latipes): Evidence for diversified function of SOX9 in gonad differentiation. Mol. Reprod. Dev. 2002, 63, 5–16. [Google Scholar] [CrossRef]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V. Toxic effects of zinc chloride on the bone development in Danio rerio (Hamilton, 1822). Front. Physiol. 2016, 7, 153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).