Abstract
Selenium, an essential trace element, exerts beneficial effects on aquatic animals when present in suitable concentrations. This study investigates the effect of dietary nanometer selenium (Nano-Se) on the muscle selenium accumulation, nutrient composition, and antioxidant ability of Paramisgurnus dabryanus spp. Nano-Se was supplemented in the basal diets at levels of 0, 0.1, 0.2, 0.4, and 0.6 mg/kg. Three hundred fish, averaging 5.21 ± 0.06 g, were randomly divided into five groups and fed the experimental diet for 6 weeks. Fish with a dietary Nano-Se supplement of 0.2 mg/kg exhibited activities of SOD, GSH-Px, AKP, and CAT in the liver, which were significantly higher (p < 0.05) compared to the control diet, while MDA content was significantly lower (p < 0.05) in the 0.2 mg/kg group. The muscle selenium content significantly increased (p < 0.05) at ≥0.2 mg/kg Nano-Se levels. The highest levels of essential amino acids, EAA/TAA, and EAA/NEAA ratios were observed in fish fed 0.2 mg of Nano-Se. Thus, this study recommends incorporating 0.2 mg of Nano-Se per kg in the diet to enhance antioxidant defense, selenium content, and nutrient composition.
Keywords:
Paramisgurnus dabryanus spp.; nanometer selenium; antioxidant enzyme activity; amino acid composition Key Contribution:
The proper amount of Nano-Se can elevate the nutritional value and antioxidant capacity of Paramisgurnus dabryanus spp.
1. Introduction
Selenium constitutes a vital nutritional element that is essential for the consistent growth of animals, exerting favorable effects on their physiological processes. Insufficient selenium levels in animals have been linked to changes in pathological tissue [1,2] and decreased immune enzyme activity [3]. Appropriate selenium supplementation can stimulate animal growth and enhance antioxidant and immune functions [4]. Nonetheless, selenium’s therapeutic window is narrow, lying between nutritional requirements and toxic thresholds; excessive selenium can prove highly toxic to animals [5,6]. Research indicates that suboptimal selenium doses can diminish sperm quality, trigger oxidative stress, and induce DNA damage in sperm [7].
Numerous studies underscore the essential role of selenium as a trace nutrient for promoting the robust growth of aquatic organisms [8,9]. The positive effects of selenium extend to enhancing antioxidant capacity and immune competence in aquatic animals. Selenium’s presence is integral to the constitution of glutathione peroxidase (GPH-Px), a crucial antioxidant enzyme safeguarding the cell membrane structure and function against oxidative damage by catalyzing the decomposition of hydrogen peroxide [10,11]. Incorporating suitable selenium into diets substantially enhances aquatic animal’s antioxidant capacity [12,13]. Moreover, mounting evidence supports selenium-supplemented diets in enhancing the nutritional profile of aquatic organisms [14,15]. Hao et al. (2020) [16] demonstrated significant alterations in muscle selenium concentrations in fish-fed selenium-enriched diets, which is consistent with findings in Morone saxatilis [17] and Cyprinus carpio [18]. Nano-Se administration was found to elevate total and essential amino acid contents in Eriocheir sinensis [15]. These investigations collectively suggest that selenium-enriched aquatic meat holds potential as a functional food source [19].
Currently, aquaculture predominantly employs either inorganic or organic selenium; however, both forms exhibit limitations in terms of their bioavailability, solubility, and adhesion [20]. In light of nanotechnology advancements, the integration of selenium nanoparticles into fish diets has garnered significant attention for its positive impact on fish well-being. Nano-Se offers distinct advantages over inorganic selenium, including heightened biological activity, reduced toxicity, enhanced particle dispersion, an expansive surface area, and an elevated absorption rate [21]. Consequently, the development of Nano-Se-enriched aquatic feed, characterized by high efficiency and minimal toxicity, holds the potential to optimize the health and productivity of aquatic organisms.
Loach, recognized for its substantial nutritional and medicinal value, holds significance as a freshwater economic fish. Notably, China’s loach output reached 367,086 tons in 2021 (Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture and Rural Affairs, 2022) [22]. Taiwanese loach (Paramisgurnus dabryanus spp.) has not yet reached significant species differentiation and is an endemic population of Paramisgurnus dabryanus [23]. Paramisgurnus dabryanus, a widely cultured loach variant, stands out due to its favorable nutritional composition, robust growth, and market demand [24,25]. However, intensive loach farming exposes it to a range of diseases [26]. Selenium, an essential nutrient, not only enhances growth performance but also bolsters disease resistance, thereby augmenting the economic viability of loach cultivation. While Nano-Se serves as a premium selenium source, its application in loach feed remains underexplored. This study aims to determine the optimal dietary Nano-Se requirement for Paramisgurnus dabryanus spp., elucidating the effects of Nano-Se dietary supplementation on antioxidant enzyme activity, muscle selenium concentration, and muscle amino acid content.
2. Materials and Methods
2.1. Diet Preparation
The basic diet used in this experiment is shown in Table 1. Nano-Se (provided by Guangzhou Bosio Biotechnology Co., Ltd., Guangzhou, China) was added to the basic diet at levels of 0 (control), 0.1, 0.2, 0.4, and 0.6 mg/kg, respectively. Ingredients were filtered through an 80-mesh sieve, mixed evenly, and made into granules with a diameter of 1.0 mm by a portable meat grinder; these were oven-dried at 55 °C for 24 h and stored at −20 °C for further use.

Table 1.
Ingredients and proximate composition of basal diets (%).
2.2. Experimental Design
Healthy fish were purchased from Loach Breeding Industrial Park in Lianyungang, China. The fish were acclimatized for 2 weeks prior to the experiment.
Before the feeding trial, 300 healthy fish of similar size (average body weight of 5.21 ± 0.06 g) were randomly distributed in fifteen tanks at a density of 20 individuals per tank. The 15 aquariums were divided into 5 diet groups with 3 triplicates for each group. Each group of fish was fed with the corresponding feed mentioned above, respectively.
The fish were hand-fed to satiation twice daily (9:00 and 17:00) during 6 weeks of the feeding trial. The feeding amount was appropriately adjusted according to the feeding behavior. It was appropriate to finish feeding within 1 h.
One-third of the fresh water was changed at 15:00 every day, and residual feed and feces were removed simultaneously. The water temperature was kept at 26 ± 1 °C, dissolved oxygen was 5.43 ± 0.76 mg/L, ammonia nitrogen was 0.28 ± 0.04 mg/L, pH = 7.48 ± 0.04, alkalinity was 2.43 ± 0.12 mmol/L, and natural lighting was used (12 h light/12 h dark).
2.3. Sample Collection
At the end of the feeding trial, the fish were starved for 24 h before sampling and then collected from the tanks. All fish were counted and weighed at the end of the trial to calculate the relative indices. Growth performance parameters were calculated according to the following formulae: survival rate (SR, %) = 100 × (final fish number/initial fish number), specific growth rate (SGR, %/d) = 100 × [(Ln (final body weight) − Ln (initial body weight))/days], weight gain rate (WGR, %) = 100 × (final body weight − initial body weight)/initial body weight, condition factor (CF, g/cm3) = 100 × [(final body weight)/(final body length)3], and feed conversion ratio (FCR) = dry feed fed/wet weight gain.
2.4. Determination of Whole Fish Body Composition
The moisture, crude protein, and crude lipid were analyzed according to the standard methods of AOAC (1995) [27]. Specifically, moisture was determined via drying at 105 °C to constant weight; crude protein was analyzed using the Kjeldahl method after digestion with sulfuric acid; crude lipid was determined via petroleum ether extraction using the Soxhlet method.
2.5. Determination of Selenium and Amino Acid Content
Selenium concentrations in diets and muscles were measured using inductively coupled plasma mass spectrometry (ICP-MS) (Thermo ICAP RQ; Thermo Fisher Scientific, Waltham, MA, USA).
The concentration of amino acid in the muscle was analyzed using a Waters e2695 HPLC system (Waters Technologies, Milford, MA, USA) fitted with a Waters AccQ-Tag Amino Acids C18 Column (3.9 mm × 150 mm, 4 μm) and a Waters 2475 fluorescence detector (Waters Technologies, Milford, MA, USA) at an excitation wavelength of 250 nm and emission wavelength of 395 nm at 37 °C. A sodium acetate buffer solution, acetonitrile, and water were used as mobile phases A, B, and C, respectively, for gradient elution, and the injection volume was 10 μL.
2.6. Hepatic Antioxidant Enzyme Activity Assay
The weighted liver tissues (100 mg) were homogenized in 10 volumes (w/v) of ice-cold physiological saline and then centrifuged at 4000 rpm for 10 min at 4 °C. The supernatant was carefully collected and frozen at −80 °C for enzyme activity analysis. The activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), alkaline phosphatase (AKP), and the content of malondialdehyde (MDA) were measured using the commercially available assay kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Enzyme activity was analyzed using a BioTek Synergy HT Multi-Detection Microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
2.7. Statistical Analysis
The experimental data were presented as the mean ± standard deviation (S. D.) for each group. Statistical analyses of experimental data were performed using SPSS 26.0. Significant differences among the groups were tested using one-way analyses of variance (ANOVAs), followed by least significant difference (LSD) multiple comparison tests. The significance level was set at p < 0.05.
3. Results
3.1. Growth Performance
The results of growth performance and feed utilization are shown in Table 2. The survival rate of the 0.4 mg/kg group was the highest and significantly higher than that of the control group and the 0.1 mg/kg group (p < 0.05), but there was no significant difference among the 0.2, 0.4, and 0.6 mg/kg groups (p > 0.05). The weight gain rate (WGR) and specific growth rate (SGR) first increased and then decreased with the increase in Nano-Se in the diet. The highest WGR and SGR values were observed for the group with the supplementation of 0.2 mg/kg, which was significantly higher than the other groups (p < 0.05). The feed conversion rate (FCR) of the 0.2 mg/kg group was the lowest at 1.51 and significantly lower than the control, 0.1 and 0.4 mg/kg groups (p < 0.05). According to the results of the condition factor (CF), the 0.2 mg/kg group reached the highest level, but there were no significant differences among the groups (p > 0.05).

Table 2.
Effect of dietary Nano-Se supplementation on the growth performance of Paramisgurnus dabryanus spp.
3.2. Antioxidant Ability
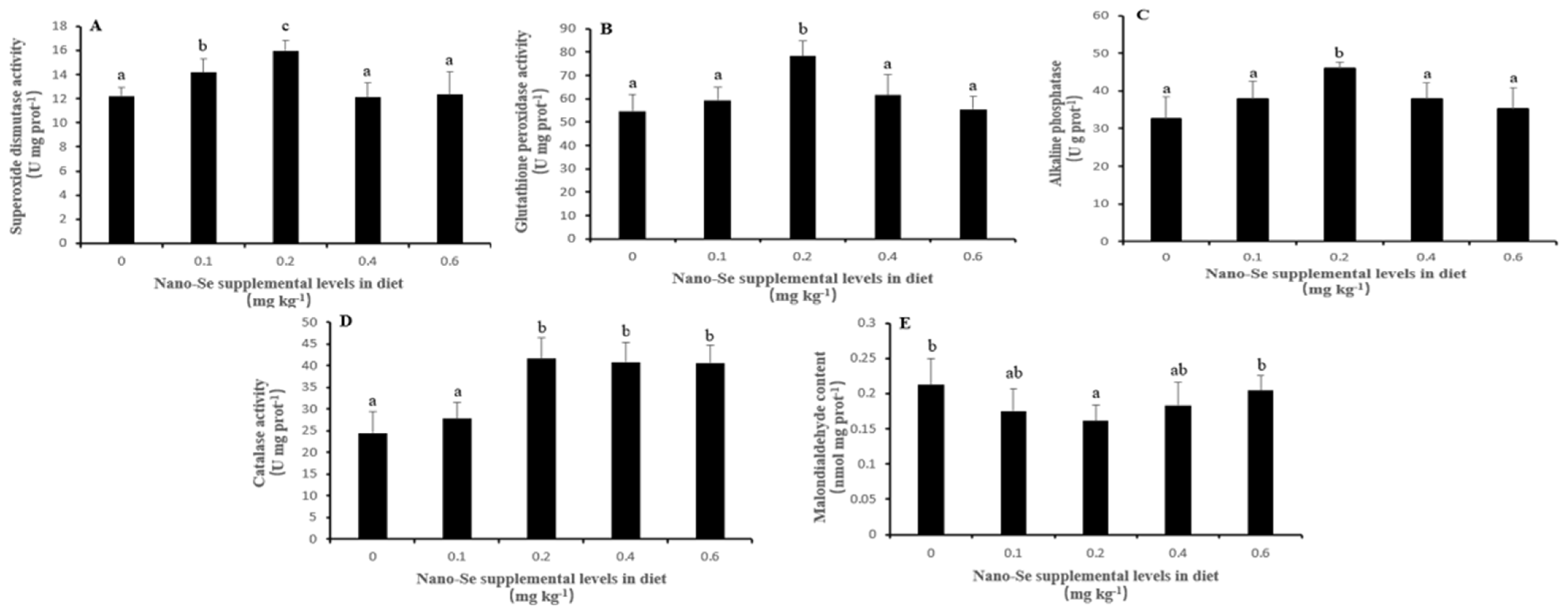
The antioxidant enzyme abilities in the liver of P. dabryanus spp. are shown in Figure 1. The SOD (Figure 1A), GSH-Px (Figure 1B), and AKP (Figure 1C) enzyme activities showed a trend of first increasing and then decreasing with the increase in the Nano-Se addition, with enzyme activities reaching the highest in the 0.2 mg/kg group, which was significantly higher than other groups (p < 0.05). The activity of the CAT (Figure 1D) enzyme increased gradually at first and then maintained a steady trend after the addition of Nano-Se reached 0.2 mg/kg. However, the MDA (Figure 1E) content in the liver of the 0.2 mg/kg group was the lowest and significantly lower than that of the control group and 0.6 mg/kg group (p < 0.05).
Figure 1.
Effects of Nano-Se on activities of antioxidant enzyme in the liver of Paramisgurnus dabryanus spp. (A) SOD. (B) GSH-Px. (C) AKP. (D) CAT. (E) MDA. Values with different lowercase letters are significantly different (p < 0.05).
3.3. Body Composition
There were no significant differences in the muscle moisture and crude protein content of P. dabryanus spp. when fed with a different Nano-Se supplementation (Table 3). Unlike water and crude protein, the crude lipid content in muscle was affected by dietary Nano-Se inclusion. The highest content of crude lipid was observed in the 0.2 mg/kg group, which was significantly higher than the control, 0.1 and 0.6 mg/kg groups (p < 0.05).

Table 3.
Effect of dietary Nano-Se supplemental level on the whole-body composition of Paramisgurnus dabryanus spp.
3.4. Selenium Accumulation in Muscle
The selenium content in the muscle of P. dabryanus spp. gradually increased with the increase in Nano-Se addition (Table 4). The muscle selenium content in the 0.2, 0.4, and 0.6 mg/kg groups were significantly higher than that in the control and 0.1 mg/kg groups (p < 0.05), but there were no differences between the 0.4 and 0.6 mg/kg groups (p > 0.05).

Table 4.
Effect of dietary Nano-Se on selenium content in the muscle of Paramisgurnus dabryanus spp.
3.5. Amino Acid Analysis in Muscle
A total of 17 amino acids were detected in the muscle of P. dabryanus spp., including 7 essential amino acids, 10 non-essential amino acids, and 6 flavor amino acids (Table 5). The total amount of muscle amino acids in all groups fed with Nano-Se was significantly higher than that in the control group (p < 0.05), but there were no significant differences among the four Nano-Se addition groups (p > 0.05). The highest content of essential amino acids in the 0.2 mg/kg group was observed, and the essential amino acid contents of all groups with the addition of Nano-Se were significantly higher than that in the control group (p < 0.05). The contents of flavor amino acids in the four groups with added Nano-Se were significantly higher than that of the control group (p < 0.05), with the highest content observed in the 0.1 mg/kg group.

Table 5.
Effect of dietary Nano-Se on amino acid composition in the muscle of Paramisgurnus dabryanus spp.
Among the 17 amino acids, the highest content was glutamic acid, with the highest content of 3.14 g/100 g in the 0.4 mg/kg group. The glutamic acid contents in all groups with Nano-Se addition were significantly higher than the control group (p < 0.05). In addition, amino acids with a higher content included lysine, leucine, aspartic acid, and arginine. Cysteine was the lowest among the 17 amino acids, with a content of only about 0.16 g/100 g, and there were no significant differences among all the experimental groups (p > 0.05). The EAA/TAA in each group range were 39.91–40.39%, and the EAA/NEAA were 66.42–67.75%.
4. Discussion
Selenium stands as a crucial trace element for aquatic organisms, and prior research revealed that incorporating an optimal selenium amount into their diet enhances the growth performance of aquatic animals [16,18]. This is attributed to selenium’s role as a cofactor for enzymes engaged in digestive enzyme synthesis, leading to the heightened activity of these enzymes and an improved absorption rate, directly fostering growth [28]. Furthermore, selenium’s presence as a key constituent in the deiodinase enzyme indirectly stimulates the growth of hormone synthesis and secretion, thus expediting animal growth and protein synthesis [29,30]. Additionally, research has underscored variances in the impacts of diverse selenium sources on aquatic animal growth. For instance, in Eriocheir sinensis, supplementation with 0.2 mg/kg of Nano-Se significantly enhances their specific growth rate and survival, comparable to the effects of a 1.0 mg/kg selenium yeast group [15]. Similarly, a study by Ibrahim et al. demonstrated that Nano-Se outperforms bulk selenium in enhancing the growth performance of Nile tilapia (Oreochromis niloticus) when incorporated into a commercial feed [31]. In our study, supplementing the diet of P. dabryanus spp. with Nano-Se displays a positive influence on the final body weight, weight gain rate (WGR), specific growth rate (SGR), and reduced feed conversion rate (FCR). Notably, the 0.2 mg/kg dosage group exhibits the most pronounced effects on P. dabryanus spp.’s growth and feed utilization efficiency. These outcomes align with prior research, underscoring the efficacy of judicious Nano-Se supplementation in promoting the growth performance of P. dabryanus spp.
The biological function of selenium is mainly realized by enzymes containing selenium. It was found that selenium is the active center of many enzymes, such as glutathione peroxidase (GSH-Px). GSH-Px is an important peroxidase widely existing in the body, and its active center is selenocysteine, and selenium plays an important role in the catalytic reaction of GSH-Px [32]. Selenium can prevent the ROS-induced lipid peroxidation of the subcellular unit membrane structure from producing unsaturated fatty acids and causing damage to cells by improving antioxidant enzyme activity [33]. It becomes evident that the judicious addition of selenium contributes to sustaining GSH-Px enzyme activity, effectively curbing peroxide accumulation in the organism. Our study revealed a notable rise in SOD and GSH-Px activities within the 0.2 mg/kg group, indicating that appropriate dietary selenium effectively increases the oxidative resistance of fish [16]. This may be because selenium is part of the active center of GSH-Px, which can catalyze a reduction in peroxides [34]. Moreover, significantly heightened CAT activity was observed in fish administered with doses of 0.2, 0.4, and 0.6 mg/kg. The MDA content can reflect the degree of cell damage and lipid peroxidation. In the current study, the lowest level of MDA was observed in the 0.2 mg/kg group. Similar results were also observed in other fish, such as Megalobrama amblycephala [16] and Cyprinus carpio [18]. These findings indicate that Nano-Se enhances GSH-Px, SOD, and CAT values while diminishing MDA values. Notably, the dietary inclusion of 0.2 mg of Nano-Se/kg proves more efficacious in fortifying the antioxidant system against oxidative stress compared to other Nano-Se levels. Comparable results have been documented in crucian carp [35], common carp [18,36], and red sea bream [37].
The nutritional quality of fish is primarily contingent on the protein and fat content in the muscle [38]. In this study, there were no significant alterations in the moisture and crude protein content of the entire fish, which is consistent with findings in Carassius auratus [35], Seriola lalandi [39], Cyprinus carpio [18], Cynoglossus semilaevis [40], and Megalobrama amblycephala [16]. However, the crude lipid content in the 0.2 mg/kg group notably surpassed that in the control group, and with the increase in Nano-Se, the crude lipid content gradually decreased. These trends align with results from studies on Japanese seabass by Guo et al. (2018) [41] and Chinese mitten crabs by Shi et al. (2015) [15]. Therefore, selenium has an effect on the body lipid metabolism of P. dabryanus spp. to some extent, but its mechanism needs further study. Divergent patterns, however, have emerged in other aquatic species. For instance, selenium yeast supplementation in diets enhanced the crude protein content while reducing crude fat in GITF tilapia [42]. Similar outcomes were identified in Ctenopharyngodon idella [43]. Discrepancies between our findings and those mentioned above may arise from fish species and specifications, selenium addition methods and levels, and distinct breeding environments.
The selenium concentration in muscle increased proportionally with the dietary amount of Nano-Se. Remarkable shifts in the muscle selenium content occurred when the dietary Nano-Se supplementation level exceeded 0.2 mg/kg. However, the rise in the selenium content exhibited a gradual trend in fish fed with Nano-Se supplementation, reaching 0.4–0.6 mg/kg. This pattern suggests that the deposition rate of selenium in the P. dabryanus spp. muscle diminishes after a certain concentration threshold is reached. These findings align with those observed in juvenile carp [44], common carp [18], and juvenile blunt snout bream [16]. Consequently, consuming P. dabryanus spp. fed with a Nano-Se content of 0.2 mg/kg feed can effectively serve as a means for humans to supplement the trace element selenium and enhance immune function.
The amino acid composition in fish muscles is influenced by the physiological status, breeding environment, water area, salinity, and feed, with feed exerting the most substantial impact [45]. Amino acids are categorized as essential and non-essential, where the former cannot be synthesized by the body and must be derived from food [41]. The content and proportion of essential amino acids significantly contribute to proteins’ nutritional value [46]. In this study, each group exhibited EAA/TAA ratios ranging from 39.91% to 40.39% and EAA/NEAA ratios between 66.42% and 67.75%, aligning with the FAO/WHO’s ideal protein pattern of approximately 40% for EAA/TAA and over 60% for EAA/NEAA. Notably, the 0.2 mg/kg group displayed the highest EAA/TAA and EAA/NEAA ratios, at 40.58% and 68.31%, respectively, indicating the superior utilization of essential amino acids compared to other groups. This underscores the benefit of incorporating 0.2 mg/kg of Nano-Se into the diet for optimizing muscle-protein ratios [42]. The content of flavor amino acids stands as a crucial indicator of meat freshness and nutritional value [47]. Glutamic acid and aspartic acid contribute to a fresh taste, while glycine and alanine are notable sweet characteristic amino acids. Prior research demonstrates the significance of glutamic acid in fish meat flavor and its hepatoprotective role through glutamine synthesis, which aids in detoxification [48,49]. In this study, glutamic acid and aspartic acid emerged as the most abundant flavors of amino acids, and their levels in all Nano-Se-supplemented groups significantly surpassed those in the control group. This signifies that Nano-Se supplementation enhances glutamic acid and aspartic acid content in the muscles, thereby enhancing the freshness of P. dabryanus spp. muscles. Additionally, these outcomes emphasize that both the flavor amino acid and essential amino acid content in Nano-Se-fed P. dabryanus spp. muscles surpass those in the control group. This emphasizes that deliberate Nano-Se supplementation can elevate the nutritional value and flavor of P. dabryanus spp. muscles.
5. Conclusions
In summary, this study demonstrated that the supplementation of Nano-Se in the diet at a ratio of 0.2 mg/kg could enhance the antioxidant ability, increase the muscle selenium content, and improve essential amino acid, EAA/TAA, and EAA/NEAA ratios. Therefore, the data of the present study recommend adding 0.2 mg of Nano-Se per kg of the basic diet to improve antioxidant defense, selenium content, and nutrient composition.
Author Contributions
Conceptualization, J.C., H.F., H.W., F.L. and M.Z.; Data curation, J.C., S.X. and H.W.; Formal analysis, J.C. and S.X.; Funding acquisition, H.W., F.L., J.C. and M.Z.; Investigation, S.X., H.X. and H.Z.; Project administration, J.C., H.F. and H.W.; Visualization, J.C., H.X. and H.Z.; Writing—original draft, J.C., S.X. and H.X.; writing—Reviewing and editing, H.F., F.L. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Leading Talent Project of the Training Plan for Academic and Technical Leaders of Major Disciplines in Jiangxi Province (20225BCJ2201), the Integrated Breeding of Loach and its Industrial Promotion and Application (2023yyzygg-05), the Special Fund Project for the Transformation of Scientific and Technological Achievements of Lianyungang Municipality (CA202202), and the Introduction of the talent research start-up fund of Jiangsu Ocean University (KQ21010).
Institutional Review Board Statement
This study was approved by the Animal Care and Use Committee of Jiangsu Ocean University (protocol no. 2020-37; approval date: 1 September 1 2019). All sampling procedures were performed according to the ethical guidelines for Experimental Animals of Jiangsu Ocean University.
Data Availability Statement
All data generated during this study are included in this article.
Conflicts of Interest
Haihua Wang was employed by the Jiujiang Xingxiang Aquaculture Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bell, J.G.; Cower, C.B.; Adron, J.W.; Pirie, B.J.S. Some effects of selenium deficiency on enzyme activities and indices of tissue peroxidation in Atlantic salmon (Salmon salar). Aquaculture 1987, 65, 43–54. [Google Scholar] [CrossRef]
- Bell, J.G.; Pirie, B.J.S.; Adron, J.W.; Cowey, C.B. Some effects of selenium deficiency on glutathione peroxidase (EC 1.11.1.9) activity and tissue pathology in rainbow trout (Salmo gairdneri). Br. J. Nutr. 1986, 55, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Peng, C.Z.; Jin, M.C.; Huang, J.L. Effects of selenium deficiency on the physiological and biochemical indices of blood in common carp Cyprinus carpio. J. Dalian Fish. Univ. 2009, 24, 283–287. [Google Scholar] [CrossRef]
- Leanne, K.C.; Collin, E.S.; Anna, K.H.; Molly, K.; Dave, S. Selenium: Mercury molar rations in freshwater fish in the Columbia river basin: Potential applications for specific fish consumption advisories. Biol. Trace Elem. Res. 2017, 178, 136–146. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aima, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar] [CrossRef]
- Narayanan, G.; Baskaralingam, V.; Ravichandran, R.; Sekar, V.; Caterina, F. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2018, 162, 147–159. [Google Scholar] [CrossRef]
- Seyedi, J.; Kalbassi, M.R.; Esmaeilbeigi, M.; Tayemh, M.B.; Amiri, M.J. Toxicity and deleterious impacts of selenium nanoparticles at supranutritional and imbalance levels on male goldfish (Carassius auratus) sperm. J. Trace Elem. Med. Biol. 2021, 66, 126758. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, N.P. Effect of dietary selenium on immuno-biochemical plasticity and resistance against Aeromonas veronii biovar sobria in fish reared under multiple stressors. Fish Shellfish Immunol. 2019, 84, 38–47. [Google Scholar] [CrossRef]
- Zheng, L.; Feng, L.; Jiang, W.D.; Wu, P.; Tang, L.; Kuang, S.Y.; Zeng, Y.Y.; Zhou, X.Q.; Liu, Y. Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 77, 53–70. [Google Scholar] [CrossRef]
- Rider, S.A.; Davies, S.J.; Jha, A.N.; Fisher, A.A.; Knight, J.; Sweetman, J.W. Supra-nutritional dietary intake of selenite and selenium yeast in normal and stressed rainbow trout (Oncorhynchus mykiss): Implications on selenium status and health responses. Aquaculture 2009, 295, 282–291. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swamson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Scicence 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Ma, C.; Dai, Y.Y.; Wang, X.Y.; Bai, D.Q.; Shang, X.D.; Jia, L. Effect of dietary nano-Se on oxidation resistance of juvenile tongue sole (Cynogiossus semilaevis). Feed Res. 2019, 42, 25–30. [Google Scholar] [CrossRef]
- Xu, Y.L.; Gao, Q.F.; Dong, S.L.; Mei, Y.P.; Li, X.Q. Effects of supplementary selenium and vitamin E on the growth performance, antioxidant enzyme activity, and gene expression of sea cucumber Apostichopus japonicus. Biol. Trace Elem Res. 2021, 199, 4820–4831. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Zaineldin, A.I.; Van, D.H.; Ahmed, H.A.; Elsabagh, M.; Abdeldaim, M.M. An evaluation of dietary selenium nanoparticles for red sea bream (Pagrus major) aquaculture: Growth, tissue bioaccumulation, and antioxidative responses. Environ. Sci. Pollut. Res. 2019, 26, 30876–30884. [Google Scholar] [CrossRef]
- Shi, M.M.; Qin, F.J.; Yuan, L.X.; Song, X.H.; Pu, Y.X.; Liu, Z.J.; Fu, J.G. Effects of nano-Se on growth performance, selenium content and nutrient composition of Chinese mitten crabs (Eriocheir sinensis). Feed Ind. 2015, 36, 21–25. [Google Scholar] [CrossRef]
- Hao, J.Y.; Lin, Y.; Pan, W.J.; Liu, B.; Miao, L.H.; Zhou, Q.L.; Liang, H.L.; Ge, X.P. Dietary selenium enhances the growth and anti-oxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2020, 101, 115–125. [Google Scholar] [CrossRef]
- Cotter, P.A.; Craig, S.R.; Mclean, E. Hyper accumulation of selenium in hybrid striped bass: A functional food for aquaculture? Aquacult. Nutr. 2008, 14, 215–222. [Google Scholar] [CrossRef]
- Ashouri, S.; Keyvanshokoon, S.; Salati, A.P.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29. [Google Scholar] [CrossRef]
- Durigon, E.G.; Kunz, D.F.; Peixoto, N.C.; Uczay, J.; Lazzari, R. Diet selenium improves the antioxidant defense system of juveniles Nile tilapia (Oreochromis niloticus L.). Braz. J. Microbiol. 2019, 79, 527–532. [Google Scholar] [CrossRef]
- Mechlaoui, M.; Dominguez, D.; Robaina, L.; Geraert, P.A.; Kaushik, S.; Saleh, R.; Briens, M.; Montero, D.; Lzquierdo, M. Effects of different dietary selenium sources on growth performance, liver and muscle composition, antioxidant status, stress response and expression of related genes in gilthead seabream (Sparus aurata). Aquaculture 2019, 507, 251–259. [Google Scholar] [CrossRef]
- Gopi, M.; Beulah, P.V.; Ramasamy, D.K.; Muthuvel, S.; Govindasamy, P. Role of nanoparticles in animal and poultry nutrition: Modes of action and applications in formulating feed additives and food processing. Int. J. Pharmacol. 2017, 13, 724–731. [Google Scholar] [CrossRef]
- Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture and Rural Affairs. 2022 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2022.
- Huang, Z.Z.; Ma, B.; Guo, X.L.; Wang, H.H.; Ma, A.J.; Sun, Z.B.; Wang, Q.M. Comparative transcriptome analysis of the molecular mechanism underling the golden red colour in mutant Taiwanese loach. Aquaculture 2021, 543, 736979. [Google Scholar] [CrossRef]
- Dong, Z.G.; Zhang, M.; Wei, S.F.; Ge, H.X.; Li, L.T.; Ni, Q.G.; Lin, Q.F.; Li, Y. Effect of farming patterns on the nutrient composition and farming environment of loach, Paramisgurnus dabryanus. Aquaculture 2018, 497, 214–219. [Google Scholar] [CrossRef]
- Zhang, B.Z. Dietary chitosan oligosaccharides modulate the growth, intestine digestive enzymes, body composition and nonspecific immunity of loach Paramisgurnus dabryanus. Fish Shellfish Immunol. 2019, 88, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Li, X.; Wang, M.J.; Wang, C.M.; Peng, Y.Q.; Wang, H.H.; Zhu, M. Molecular cloning and expression analysis of myd88 from oriental weatherfish (Misgurnus anguillicaudatus) in response to bacterial challenge. J. Fish. Biol. 2020, 96, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International, 16th ed.; Cunnif, P.A., Ed.; AOAC Int.: Arlington, VA, USA, 1995; Volume 1, pp. 31–65. [Google Scholar]
- Shenkin, A. The key role of micronutrients. Clin. Nutr. 2006, 25, 1–13. [Google Scholar] [CrossRef]
- Arthur, J.R.; Nicol, F.; Beckett, G.J. Hepatic iodothyronine 5′-deiodinase: The role of selenium. Biochem. J. 1990, 272, 537–540. [Google Scholar] [CrossRef]
- Çiçek, S.; Özoğul, F. Effects of selenium nanoparticles on growth performance, hematological, serum biochemical parameters, and antioxidant status in fish. Anim. Feed. Sci. Technol. 2021, 281, 115099. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; EI-gendy, G.M.; Ahmed, A.I.; Elharoun, E.R.; Hassaan, M.S. Nanoselenium versus bulk selenium as a dietary supplement: Effects on growth, feed efficiency, intestinal histology, haemato-biochemical and oxidative stress biomarkers in Nile tilapia (Oreochromis niloticus Linaeus, 1758) fingerlings. Aquac. Res. 2021, 52, 5642–5655. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Maddox, J.F.; Mastro, A.M.; Scholz, R.W.; Hildenbrandt, G.; Reddy, C. Selenium deficiency alters the lipoxygenase pathway and mitogenic response in bovine lymphocytes. J. Nutr. 1992, 122, 2121–2127. [Google Scholar] [CrossRef]
- Orun, I.; Talas, Z.S.; Ozdemir, I.; Alkan, A.; Erdogan, K. Antioxidative role of selenium on some tissues of (Cd2+, Cr3+)-induced rainbow trout. Ecotoxicol Environ.Saf. 2008, 71, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.L.; Li, J.X.; Luo, Q.Y.; Wang, X.; Xiao, M.M.; Zhou, D.; Lu, Q.; Chen, X. Effect of supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front. Vet. Sci. 2021, 8, 813672. [Google Scholar] [CrossRef]
- Zhou, X.X.; Wang, Y.B.; Gu, Q.; Li, W.F. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 2009, 291, 78–81. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac. Nutr. 2017, 23, 611–617. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Zaineldin, A.I.; Van, D.H.; Moustafa, E.M.; Abdel-Daim, M.M.; Angeles, E.M.; Hassaan, M.S. Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol. Biochem. 2019, 45, 219–230. [Google Scholar] [CrossRef]
- Zhou, L.J.; Shen, D.X.; Zhan, H.X. Studies on the nutritional components of fish muscles and human health. Anim. Husbandry Feed Sci. 2013, 34, 69–71. [Google Scholar] [CrossRef]
- Le, K.T.; Fotedar, R. Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriola lalandi). Aquaculture 2014, 420, 57–62. [Google Scholar] [CrossRef]
- Chen, C.X.; Shang, X.D.; Ma, C.; Dai, Y.Y.; Jia, L.; Bai, D.Q. Effect of dietary nano-Se on growth, body composition and selenium content of juvenile tongue sole (Cynogiossus semilaevis). J. Agric. Sci. 2018, 46, 180–183. [Google Scholar] [CrossRef]
- Guo, Z.F.; Li, J.; Yang, X.Q. Effects of dietary selenium content on growth performance and antioxidant capacity of juvenile Japanese seabass (Lateolabrax japonicus). China Feed 2018, 6, 88–92. [Google Scholar] [CrossRef]
- Li, L.Q.; Luo, Y.J.; Xiao, J.; Huang, Y.F.; Yin, Q.L.; Wang, Z.F.; Tan, W.F. Effects of artificial addition of yeast selenium on protein, fat and amino acids in muscle of GITF tilapia. J. South. Agric. 2020, 51, 2856–2864. [Google Scholar] [CrossRef]
- Su, C.F.; Luo, L.; Wen, H.; Chen, X.C.; Sheng, X.S.; Chen, Z. Effects of dietary selenium on growth performance, quality and digestive enzyme activities of grass carp. J. Shanghai Fish. Univ. 2007, 16, 124–129. [Google Scholar]
- Elia, A.C.; Prearo, M.; Pacini, N.; Dörr, A.J.M.; Abete, M.C. Effect of selenium diets on growth, accumulation and antioxidant response in juvenile carp. Ecotoxicol. Environ. Saf. 2011, 74, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Sun, Z.H.; Zhao, J.; Zhou, J.X.; Liu, L.Y. A comparative study on amino acid composition and contents in the muscle of several fishes. Amino Acids Biot. Resour. 1996, 18, 37–42. [Google Scholar] [CrossRef]
- Bing, X.W.; Cai, B.Y.; Wang, L.P. Evaluation of nutritive quality and nutritional components in Spinibarbus sinensis muscle. J. Fish. Sci. China 2005, 12, 211–215. [Google Scholar]
- Ding, C.H.; Wang, Y.; Wang, W.L.; Cui, Z.F.; He, Y.Y.; Wu, W.H. Study on the contents of acidic and basic amino acids and flavor amino acids in fish meal. Feed Ind. 2018, 39, 49–52. [Google Scholar] [CrossRef]
- Hurson, M.; Regan, M.C.; Kirk, S.J.; Wasserkrug, H.L.; Barbul, A. Metabolic effects of arginine in a healthy elderly population. JPEN J. Parenter. Enteral. Nutr. 1995, 19, 227–230. [Google Scholar] [CrossRef]
- Park, J.N.; Watanabe, T.; Endoh, K.I.; Watanabe, K.S.; Abe, H. Taste-active components in a Vietnamese fish sauce. Fish Sci. 2002, 68, 913–920. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).