Abstract
The effects of three medicinal plants in the feeds of juvenile Nile tilapia (Oreochromis niloticus) were investigated on growth, survival, immunity, and digestive histology at various inclusion levels: (A) Guiera senegalensis at 0, 1, 2, 4, and 8%; (B) Pluchea odorata at 0, 0.5, 1, 2, and 4%; (C) Piliostigma reticulatum at 0, 1, and 2%; and (D) a mixture of the three at 0, 1, and 2%. After 28 days of feeding, fish were infected with Aeromonas hydrophila for 12 days. The results showed that, except for G. senegalensis, the inclusion of the medicinal plants, alone or combined, enhanced the immune response. The diet with 4% P. odorata significantly increased plasma lysozyme and bactericidal activities without affecting feed conversion efficiency or growth. Despite improved immunity, none of the treatments enhanced post-infection survival rates. However, fish fed the 1% mixture showed healthier livers and intestines, with reduced cell swelling and normal lipid deposits, followed by the 2% mixture and the control. These results highlight the potential benefits of incorporating P. odorata and the plant mixture into the diets of Nile tilapia juveniles for enhancing their immune response against A. hydrophila.
Key Contribution:
Supplementation with medicinal plant resources promotes fish welfare and immunity and contributes to the sustainability of aquaculture by limiting the use of antibiotics against pathogens and their associated impacts on the aquatic environment.
1. Introduction
Global aquaculture production has dramatically increased from 21.8 million metric tons of aquatic animals in the 1990s to 94.4 million metric tons in 2022 [1], mainly due to the improvement and intensification of production systems and the availability of high-quality aquafeeds. However, the intensification of aquaculture systems has led to an increase in diseases, which are currently one of the primary challenges faced by the industry. In fact, between 40 and 50% of losses in aquaculture production can be attributed to diseases [2,3]. This increase in diseases has led to the widespread use of antibiotics, which, in turn, has caused serious threats such as the spread of drug-resistant pathogens, suppression of aquatic animal immunity, and adverse environmental effects [4]. As a result, international organizations (UN, FAO, WHO, OIE, EFSA) have warned of the need to reduce the use of antibiotics and develop alternatives based on biosecurity and preventive methods. Medicinal plants are considered suitable alternatives in this regard due to their ability to promote fish growth and enhance immunity. They act as antibacterial and antiviral agents, strengthen the host immune system, and contain bioactive compounds such as terpenoids, tannins, alkaloids, and flavonoids, which possess antibacterial properties [5]. In Africa, for instance, herbs and medicinal plants are extensively used for healthcare, with over 80% of the rural population relying on them [6]. Moreover, they are not only cost effective and efficient but also have fewer side effects and pose no environmental or hazardous risks [7,8]. They are generally used individually or in combination with other plants or drugs to treat human and animal diseases [9]. Their use in aquaculture has garnered significant global attention, with more than 60 species being studied for their potential in enhancing fish health and disease management, and ongoing scientific research continues to actively investigate this area [10,11,12,13].
Nile tilapia (Oreochromis niloticus L., 1758) is one of the most widely farmed fish worldwide and plays a crucial role in improving nutrition and livelihoods, especially in developing countries [1], due to its rapid growth, easy reproduction, and resistance to handling. Despite its resistance, Nile tilapia remains susceptible to infectious diseases caused by viral or bacterial agents [14]. Aeromonas hydrophila, for instance, is one of the primary pathogenic bacteria in tropical aquaculture, affecting both marine and freshwater fish, including Nile tilapia. This opportunistic bacterium causes motile Aeromonas septicemia and includes symptoms such as cloacal hemorrhage, ascites, gastroenteric hemorrhage, and septicemic ulceration on the skin. Current antibiotic treatments have proven ineffective in managing this bacterial infection in aquaculture [5,15]. In this context, several medicinal plants or their extracts have been tested for their antibacterial activities. Examples include Murraya koenigii, Pandanus odoratissimus, Colocasia esculenta, and Euphorbia hirta, which inhibit the growth of A. hydrophila, and Prunus mume, Fructus toosendan, Artemisia argyi, Polygonum aviculare, Cephalanoplos segetum, Artemisia capillaries, Piper betle, Piper sarmentosum, and Piper nigrum, which inhibit the bacterial activity of A. hydrophila [5]. On the other hand, medicinal plants have also been shown to enhance immune response and disease resistance in Nile tilapia [16,17,18], as well as in other fish species [19,20,21].
The objective of the present study was to evaluate the phytobiotic potential of three medicinal plants of West Africa, namely Guiera senegalensis, Pluchea odorata, and Piliostigma reticulatum, in Nile tilapia farming. Guiera senegalensis, belonging to the Combretaceae family, is one of the most popular medicinal plants in West Africa. It has been extensively used in traditional medicine to treat various ailments, particularly malaria and intestinal disorders. Within the Poular community, G. senegalensis is commonly used in veterinary medicine, often in combination with Heeria insignis and Crossopteryx febrifuga, to enhance weight, reproductive capacity, and milk secretion of animals [22]. Pluchea odorata, a member of the Asteraceae family, is locally known as “soigne tout”, which translates to “cure-all” in French. It is widely used in traditional medicine to treat headaches, allergies, fever, and muscle pain. Some studies have demonstrated the biological activity of Pluchea spp. extracts, including antimicrobial [23,24,25], antioxidant [26,27], and anti-leishmanial [28] properties, thereby validating its traditional uses. Piliostigma reticulatum (D.C.) Hochst, locally known as “Nguiguis” in Wolof, is an evergreen shrub belonging to the Caesalpiniaceae family (Leguminosae). It occurs naturally in the Sudano-Sahelian and Sudanese regions, ranging from Mauritania and Senegal in the west to Sudan in the east. The leaves of P. reticulatum are employed in pharmacopeia to treat various diseases [29,30]. Phytochemical analyses of P. reticulatum leaf extracts have revealed the presence of antimicrobial substances that act against bacteria such as Staphylococcus aureus and Escherichia coli, as well as fungi like Aspergillus niger and Candida albicans [31]. In the present study, different dietary inclusion levels of each individual medicinal plant and in combination were evaluated to assess their effects on growth performance, immunity, histopathology, and survival of Nile tilapia juveniles after a 28-day feeding trial followed by a 12-day infection challenge with A. hydrophila.
2. Materials and Methods
2.1. Medicinal Plant Collection and Experimental Diet Formulations
The medicinal plants used in this study (G. senegalensis, P. odorata, and P. reticulatum) were selected based on their frequent use in traditional medicine in Senegal. Fresh leaves of G. senegalensis were collected at Nguekokh (Thies) (14°30′11.8″ N 17°00′09.4″ W), Pluchea odorata at Mbour (Thies) (14°24′06.5″ N 16°57′30.7″ W), and P. reticulatum at the botanical garden of the Faculty of Medicine at Cheikh Anta Diop University (Dakar) (14°41′13.0″ N 17°28′01.6″ W). The leaves were washed and sun-dried for 72 h before being transformed into a powder using a mortar. The resulting powder was then sieved to obtain a homogeneous consistency and stored until further processing.
Four independent experiments were conducted to evaluate various inclusion levels of the three plants, both individually and in combination, in a commercial diet (extruded Carp-Coul 2, Le Gouessant, Lamballe-Armor, France; 32% protein, 9% lipid, 20.5% carbohydrate). In experiment A, G. senegalensis was added at 1, 2, 4, and 8% of dry matter (DM). In experiment B, P. odorata was included at 0.5, 1, 2, and 4% DM. In experiment C, P. reticulatum was included at 1 and 2% DM. Experiment D tested two mixtures of all three plants at 1 and 2% DM for each plant. Each experiment included a control diet without any medicinal plants. The inclusion levels of the plants were based on previous research in the field [19,32].
To prepare the experimental diets, the commercial diet and plant leaf powder were mixed with approximately 40% water and 25 mL of sunflower oil added per kg of feed. The mixture was processed into spaghetti filaments using a Santos meat grinder. The spaghetti filaments were manually spread on trays and placed in a dryer at 35 °C for approximately 18 h. After drying, the filaments were broken into small pellets and stored at 4 °C until use.
The proximate composition of the experimental diets, including crude protein, lipids, ash, and dry matter, was analyzed following the procedures of the Association of Official Analytical Chemists (AOAC). The samples were dried to constant weight at 105 °C for 24 h to determine moisture and dry matter contents. Crude protein (total nitrogen x 6.25) was determined using the micro-Kjeldahl method (Kjeltec System 1002 distillation unit, Tecator, Hoeganaes, Sweden). Lipids were extracted using the Soxhlet method, and ash content was determined by incinerating the samples in a muffle furnace at 550 °C for 6 h. The results were expressed as a percentage of dry matter (% DM) (Table 1). The composition of the three plants, as reported in published sources, is provided in Supplementary Table S1.

Table 1.
Proximate composition of the different experimental diets (in % DM). DM, dry matter.
2.2. Fish Rearing
The four experiments were conducted at the approved aquatic experimental platform PLATAX of the Institute of Evolutionary Science of Montpellier (ISEM) c/o IRSTEA, located in Montpellier, France (14°38′46.4″ N 3°52′28.2”E). The experiments were conducted in compliance with the Guidelines of the European Union Council (2010/63/EU) on the protection of animals used for scientific purposes and were approved by the French Ministry of Higher Education and Research (project authorization APAFIS#28283-2020112412125734 v2). Juveniles of Nile tilapia were distributed in 250 L aquariums connected to a recirculation aquaculture system with mechanical and biological filters, following a randomized design. Each diet was tested in triplicate for 28 days. The temperature of the water tanks was maintained at 28 ± 1 °C using a 2 kW heater and a temperature controller. Physicochemical parameters including temperature, oxygen, and pH were monitored and recorded twice daily for each aquarium. The specific rearing conditions for each experiment are summarized in Table 2. Fish were manually fed at a rate of 3% of their biomass twice daily, at 09:00 a.m. and 04:00 p.m., throughout the duration of the experiment. The aquarium bottoms were cleaned daily in the morning prior to feeding and the water volume was readjusted as necessary.

Table 2.
Overview of rearing conditions of the four experiments performed in a recirculation aquaculture system (n = 3). DO, dissolved oxygen (mg/l); Ni, initial number of fish per treatment; T, water temperature (°C); WWi, initial wet weight.
2.3. Infection Challenge
After the 28-day feeding period, a 12-day bacterial infection challenge was conducted with an A. hydrophila strain (Reference: 8581) provided by the “Laboratoire Vétérinaire de l’Hérault” (Montpellier, France), which was originally isolated from a diseased carp (Cyprinus carpio). The selection of this bacterial species is based on its widespread occurrence in existing research on experimental infections in aquaculture, which aids in facilitating comparisons with other studies, and its prevalence in tropical regions such as Senegal. The strain was cultured at 30 °C for 24 h in TSB (22092 500, Sigma, Saint Quentin Fallavier, France) to achieve a final concentration of 7.108 CFU/mL. At the end of the 28-day feeding period, 22 (trial A) and 25 individuals per aquarium (trials B, C, and D) were anaesthetized with eugenol (0.8 µL/L) and then subjected to an intramuscular injection on the left side of their bodies. Each fish received 0.1 mL (trials A and B) or 0.2 mL (trials C and D) of A. hydrophila at a concentration of 5.107 CFU/mL (trial A), 4.109 CFU/mL (trial B) or 5.108 CFU/mL (trials C and D). Different doses of the bacterium were tested in a preliminary experiment to determine the lethal dose (LD50) for the bacterial challenge. During the challenge period, each group of fish continued to be fed their respective diets from the feeding trial. As negative controls, at least one individual per aquarium received 0.1 or 0.2 mL of sterile TSB (n = 20). All the negative control fish were pooled together in a single aquarium and fed the control diet. Mortality was monitored twice daily.
2.4. Fish Sampling
At the end of the 28-day feeding period, 18 fish per dietary treatment were randomly selected for immunological analyses and anaesthetized using eugenol (0.8 µL/L). Approximately 500 μL of blood was collected from the tail vein of each fish using a 1 mL heparinized syringe. Six blood samples per dietary treatment were immediately used for respiratory burst activity analysis, while the remaining 12 samples were rapidly centrifuged at 3000× g at 4 °C for 10 min to separate the plasma. The plasma from these samples was collected in Eppendorf tubes and stored at −20 °C until further analyses of plasma lysozyme and bactericidal activities. For experiments C and D, 12 fish per dietary treatment were sampled for the histological analysis of the digestive system: six fish at the end of the 28-day feeding period and six fish at the end of the 12-day infection challenge. The livers and intestines were removed and fixed overnight in a 4% buffered formalin solution (pH 7.4).
2.5. Growth, Survival, and Feed Efficiency
All fish were individually weighed every two weeks to determine the average weight gain (AWG, in g), specific growth rate (SGR, in %/day), and feed conversion rate (FCR). Survival rate (SR, in %) was calculated at the end of the 28-day feeding trial and daily during the infection challenge. The equations used to calculate each parameter were as follows:
where Wf and Wi are the average final and initial weights, respectively, and T is the time in days.
2.6. Immunological Analyses
2.6.1. Respiratory Burst Activity
The production of oxygen radicals by blood phagocytes during respiratory burst activity was measured using the colorimetric test with nitroblue tetrazolium (NBT). Fresh heparinized blood from six fish per dietary treatment was used for the NBT assay, to which a 0.2% NBT solution (N5514, Sigma, Saint-Quentin-Fallavier, France) was added. The mixture was then incubated for 30 min at 25 °C. Following incubation, 50 μL of this mixture was combined with 1 mL of N, N-dimethyl (D455, Merck, Fontenay-Sous-Bois, France) in a glass tube and centrifuged at 3000× g for 5 min. The optical density of the supernatant was measured at 540 nm using a spectrophotometer (DR 3900, Hach, Colorado, USA). The values were expressed as mg NBT to formazan/mL.
2.6.2. Bactericidal Activity of Plasma
The bactericidal activity of plasma from six fish per dietary treatment was assessed using the A. hydrophila strain ATCC 35654 according to [33]. Equal volumes of plasma (50 µL) and the bacterial suspension (104 CFU/mL) were mixed and incubated at 30 °C for 1 h. A positive control was prepared by substituting the plasma with sterile Phosphate-buffered saline (PBS). After incubation, the mixture was diluted with Tryptic Soy Broth (TSB) (22092, Sigma, Saint-Quentin-Fallavier, France) at a ratio of 1:10. Then, 100 µL of this mixture was plated onto Tryptic Soy Agar (22091, Sigma, Saint-Quentin-Fallavier, France) and incubated at 30 °C for 24 h. The bactericidal activity was determined using the following formula:
where C+ is positive control count.
2.6.3. Plasma Lysozyme Activity
Plasma lysozyme activity from six fish per dietary treatment was determined by turbidometric assay according to [34], with a minor modification. Instead of NaH2PO4, PBS at pH 6.2 was used. Approximately 25 µL of plasma was added to 175 µL of Micrococcus lysodeikticus (M3770, Sigma, Saint-Quentin-Fallavier, France) suspended in PBS pH 6.2. Two absorbance measurements were performed at 450 nm using an Elisa reader (ELx808, Biotek, Vermont, USA), one immediately after mixing and the other after 4.5 min. One unit of lysozyme activity was defined as the amount of plasma lysozyme causing a decrease in absorbance of 0.001/min at 450 nm.
2.7. Histological Analyses
The fixed livers and intestines were dehydrated with a graded series of ethanol and embedded in paraffin using an automatic tissue processor (STP120, Myr, Francheville, France). Paraffin blocks were prepared and cut into 3 μm sections using an automatic microtome (Leica, RM 2235RT, Nussloch, Germany). The paraffin sections were kept at 40 °C overnight. Following this, the samples were deparaffinized using a graded series of xylene substitute and stained with hematoxylin and eosin for general morphological observations. The histological preparations were examined under a microscope equipped with a camera (Leica, DM6 B, Nussloch, Germany). The number of hepatocytes and lipid deposits in the liver, as well as the number of enterocytes and goblet cells in the intestine, were counted in 6 randomly chosen fields per specimen (50 × 50 μm and 100 μm per field, respectively). The length of the intestinal folds was measured in 6 randomly chosen fields per specimen (100 μm per field). Measurements on the histological slides were performed with the ImageJ software version 1.53g [35], and the data were expressed as the mean ± S.D. Additionally, histopathological observations were conducted on specimens from experiment D.
2.8. Statistical Analysis
The Shapiro–Wilk and Levene tests were initially conducted to check for normality and homogeneity of variances, respectively. For AWG, FCR, SGR, SR, and histological analyses, differences among regimes were analyzed using one-way ANOVA, followed by Tukey’s test to determine significant differences between groups. The Kruskal–Wallis non-parametric test was used to examine immunological differences among the experimental regimes as the data did not meet the requirements for normality and homoscedasticity. Mortality rates between treatments were compared using the Chi-square analysis, while survival curves were analyzed using the Kaplan–Meier survival analysis. The Mann–Whitney U test was used to compare the interaction of hepatocyte, lipid deposition, mucosal cell, and enterocyte numbers before and after infection. Statistical significance was set at p values < 0.05.
3. Results
3.1. Feeding Efficiency, Growth, and Survival
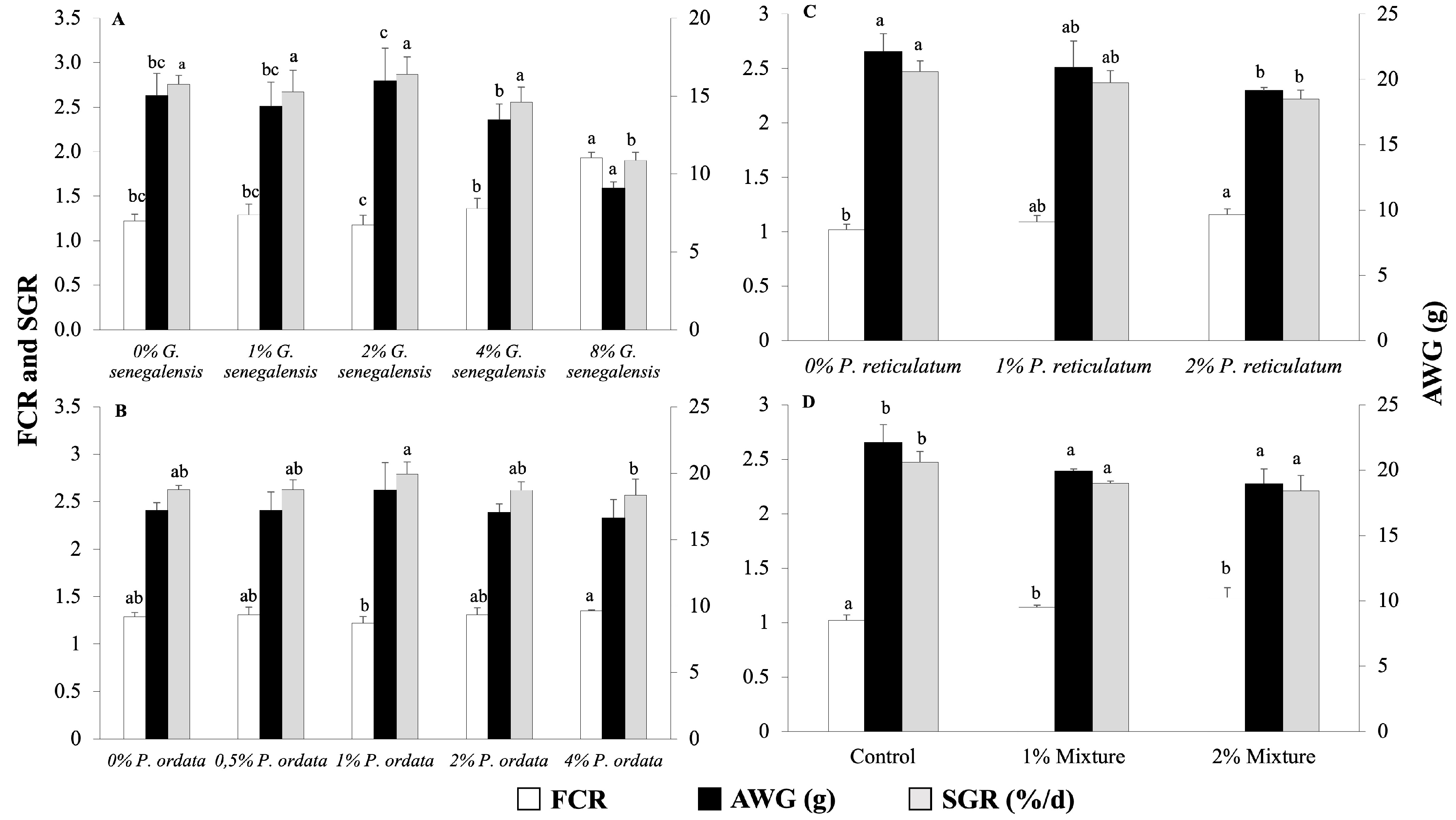
The feeding efficiency and growth of Nile tilapia juveniles in the four experiments are shown in Figure 1. The inclusion of G. senegalensis up to 4% in the diet had no significant effect on the FCR, growth, or survival of juvenile Nile tilapia at the end of the 28-day feeding trial. However, at the 8% dietary inclusion level, there was a significant increase in FCR and a decrease in fish growth and SGR compared to the control group (Figure 1A). Among the tested inclusion levels of P. odorata, AWG was not affected. The only significant differences were observed in FCR and SGR between the 1% and 4% inclusion levels, with the 4% inclusion level showing a higher FCR and lower SGR (Figure 1B). The 2% P. reticulatum diet significantly increased FCR and decreased SGR and AWG compared to the control diet (Figure 1C), while the mixture of plants significantly increased FCR and decreased SGR and AWG in a dose-dependent manner (Figure 1D). Survival rate was above 97% in all experiments and was not significantly affected by any of the tested diets (p > 0.05).
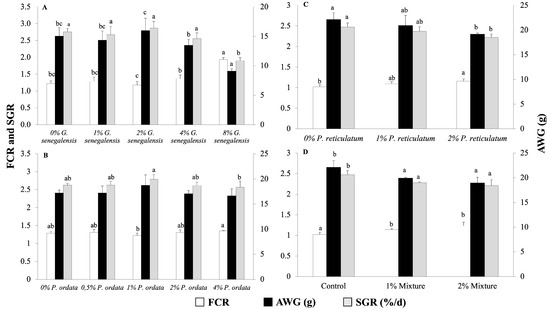
Figure 1.
Feed conversion ratio (FCR), average weight gain (AWG), and specific growth rate (SGR) of Nile tilapia juveniles fed different experimental diets for 28 days. (A) Groups fed 0, 1, 2, 4, and 8% G. senegalensis dietary inclusions; (B) groups fed 0, 0.5, 1, 2, and 4% P. odorata dietary inclusions; (C) groups fed 0, 1, and 2% P. reticulatum dietary inclusions; (D) groups fed 0, 1, and 2% mixture dietary inclusions. Data are expressed as mean ± SD (n = 3). Different letters indicate statistically significant differences among dietary groups (One way ANOVA followed by Tukey’s test, p < 0.05).
Dose–response curves have been calculated for the three parameters depicted in Figure 1A,B across the five tested levels of supplementation. However, these analyses revealed no significant correlation for P. odorata in terms of fish growth performance. Specifically, the dose–response relationships for Figure 1A demonstrated relatively high coefficients of determination with R2 values of 0.856 for FCR, 0.8415 for SGR, and 0.8309 for AWG, suggesting a possible trend, although not directly correlating to the dose. Conversely, Figure 1B exhibited much lower R2 values of 0.3358 for FCR, 0.1995 for SGR, and 0.1949 for AWG, indicating a weaker or no response to varying doses of the plant supplementation. These results suggest that while the response to supplementation levels was assessed, P. odorata did not significantly influence growth parameters in a dose-dependent manner.
3.2. Immunological Analyses
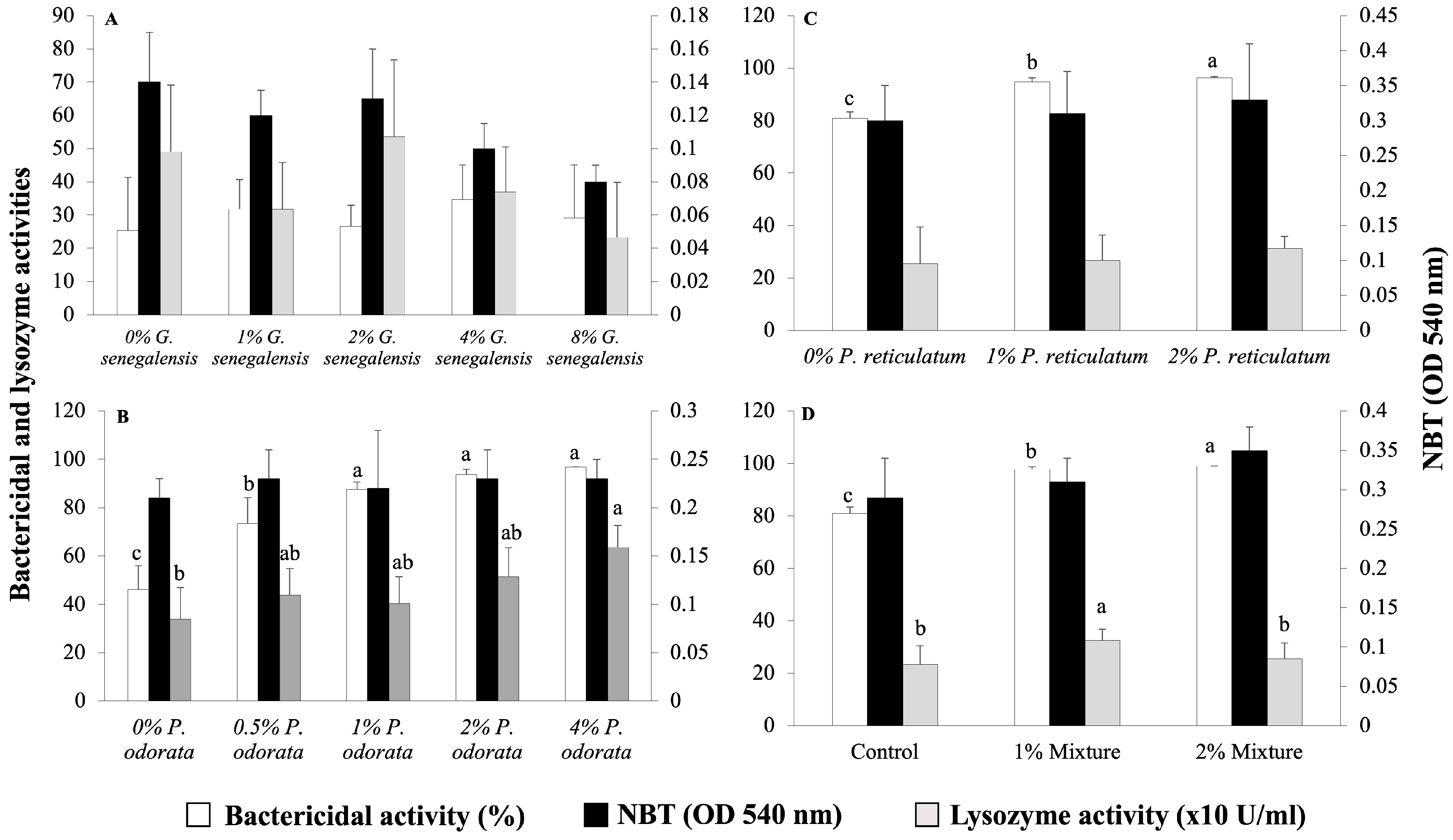
The immunological parameters of the fish from the four experiments are presented in Figure 2. None of the tested dietary inclusion levels of G. senegalensis had a significant effect on the immunological parameters of the fish (Figure 2A). Fish fed the 1, 2, and 4% P. odorata diets showed similar bactericidal activity of the plasma, which was higher than that of the groups fed the 0% and 0.5% P. odorata diets (Figure 2B). Regarding the lysozyme activity of the plasma, a significant difference was observed between the fish fed the control diet and those fed the 4% P. odorata diet (Figure 2B). However, there were no significant differences in respiratory burst activity among diets, as assessed by the NBT assay (Figure 2B). The inclusion of P. reticulatum in the diet resulted in a significant dose-dependent increase in the bactericidal activity of the plasma. On the contrary, there were no significant differences among diets in terms of plasma lysozyme and respiratory burst activities (Figure 2C). For the plant-mixture diets, the bactericidal activity of the plasma significantly increased in a dose-dependent manner (p < 0.05, Figure 2D). Plasma lysozyme activity increased significantly in the 1% mixture group compared to the control (p < 0.05, Figure 2D). However, there were no significant differences among diets in terms of respiratory burst activity (p > 0.05, Figure 2D).
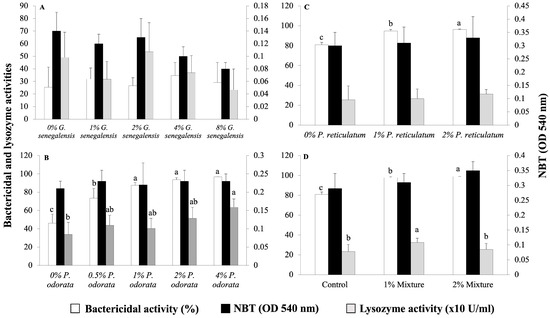
Figure 2.
Plasma bactericidal and lysozyme activities, and NBT activity in Nile tilapia juveniles fed different experimental diets. (A) Groups fed 0, 1, 2, 4, and 8% G. senegalensis dietary inclusions; (B) groups fed 0, 0.5, 1, 2, and 4% P. odorata dietary inclusions; (C) groups fed 0, 1, and 2% P. reticulatum dietary inclusions; (D) groups fed 0, 1, and 2% mixture dietary inclusions. Data are expressed as mean ± SD (n = 6). Different letters indicate statistically significant differences among dietary groups (Kruskal–Wallis test, p < 0.05).
3.3. Infection Challenge
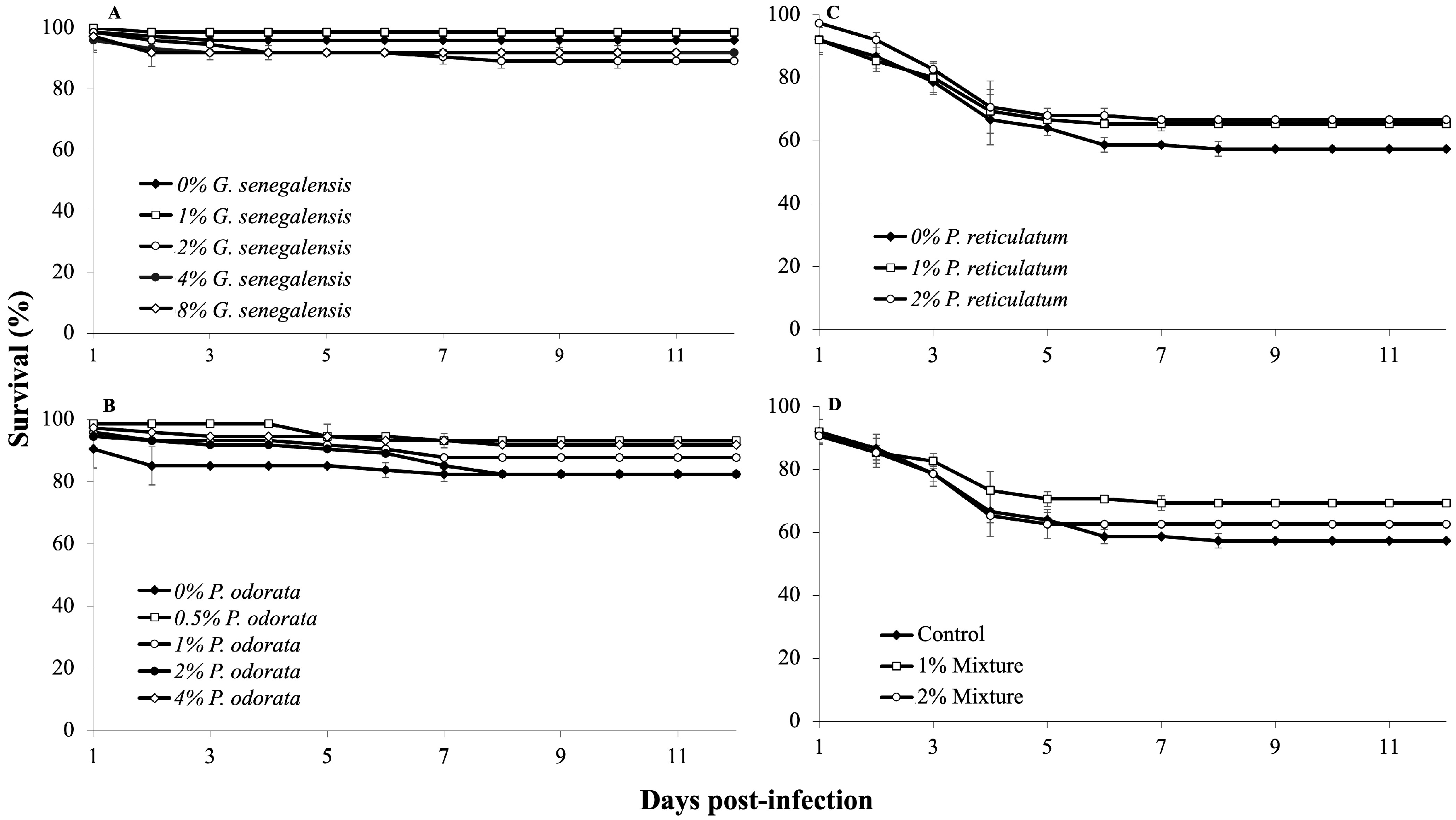
In experiment A, using G. senegalensis, the first mortalities occurred the day after infection. Although some groups exhibited varying survival rates, these differences were not statistically significant (p > 0.05, Figure 3A). In experiment B with P. odorata, the control group displayed a tendency of higher mortality rate shortly after infection, but ultimately, no significant differences emerged among the dietary treatments (p > 0.05, Figure 3B). In experiment C, which involved P. reticulatum, initial mortalities occurred on the first day after infection. Despite some groups showing resilience, the differences in survival were not statistically significant (p > 0.05, Figure 3C). Notably, some infected fish showed one or more typical signs of aeromoniasis, such as skin ulcers, reddening at the base of the fins, hemorrhages, discoloration, and abnormal swimming behavior. Finally, in experiment D, the infection challenge revealed no significant differences in survival rates among the dietary treatments after 12 days (p > 0.05). Mortalities primarily occurred during the first week after infection (Figure 3D).
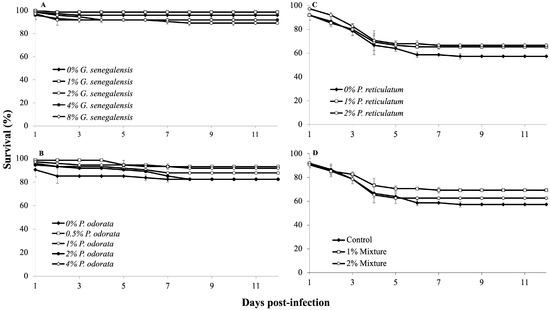
Figure 3.
Survival rates of Nile tilapia juveniles during a 12-day infection challenge with Aeromonas hydrophila (n = 3). Fish were fed different experimental diets for 28 days prior to and throughout the infection challenge. (A) Groups fed 0, 1, 2, 3, 4, and 8% G. senegalensis dietary inclusions; (B) groups fed 0, 0.5, 1, 2, and 4% P. odorata dietary inclusions; (C) groups fed 0, 1, and 2% P. reticulatum dietary inclusions; (D) groups fed 0, 1, and 2% mixture dietary inclusions.
3.4. Histological Analyses
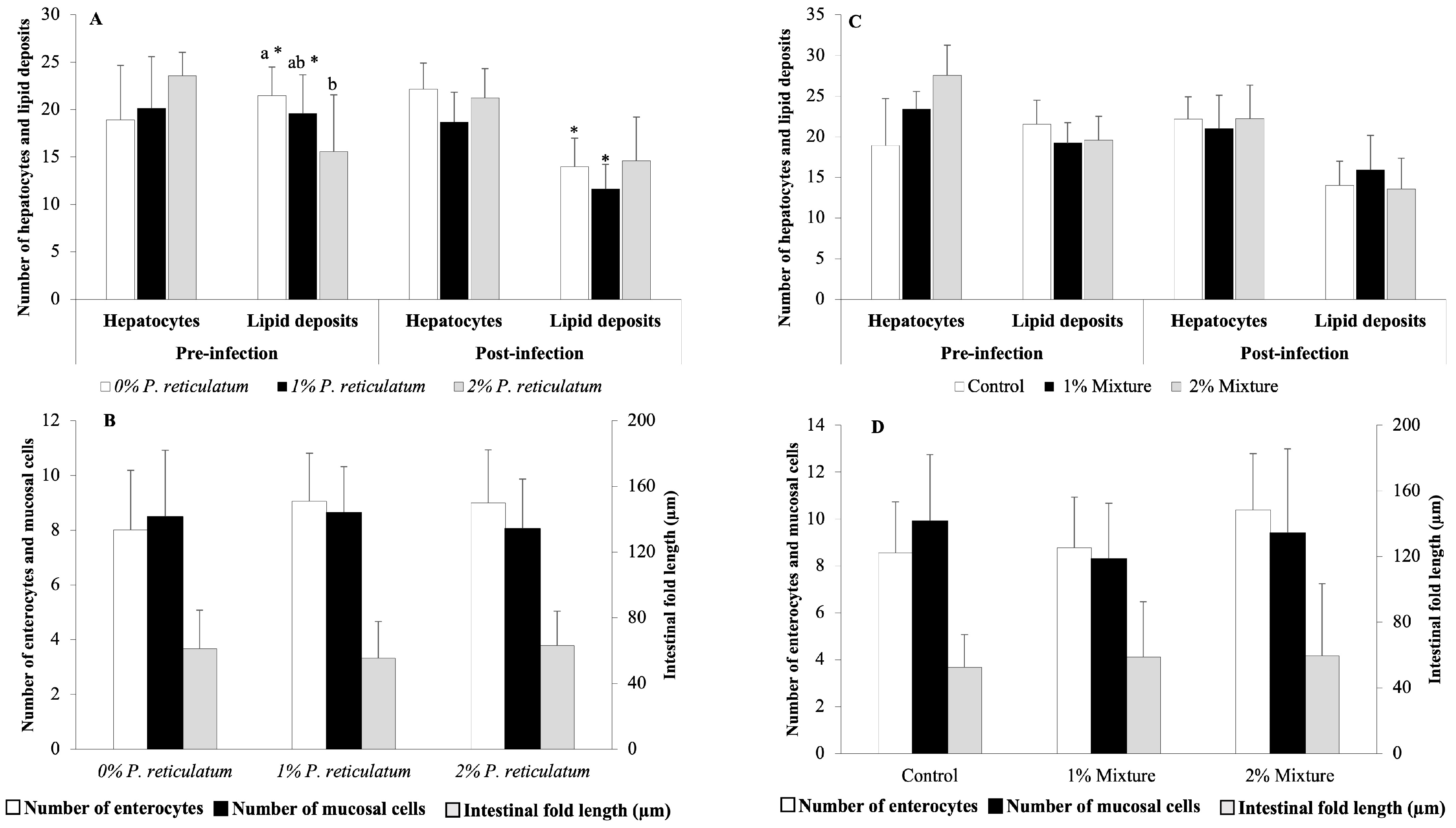
The results of the histological analyses performed in experiments C and D are shown in Figure 4. Following the 28-day feeding trials with P. reticulatum and the plant mixtures, none of the evaluated histological features was significantly affected by the medicinal plants (p > 0.05, Figure 4B,D), except for the 2% P. reticulatum dietary group, which exhibited a lower number of hepatic lipid deposits than the control group (p < 0.05, Figure 4A). After the infection challenge, the groups fed the control and 1% P. reticulatum diets showed a decreased number of hepatic lipid deposits (Figure 4C). There were no significant differences in the remaining histological features between the pre- and post-infection challenges in any of the dietary groups tested (p > 0.05, Figure 4A,C). After the infection challenge in experiment D, histological sections of the liver from fish fed the control diet showed congestion of leukocytes, hepatocyte degeneration, a decreased number of hepatocytes, increased lipid deposits, and degeneration of blood vessels (Figure 5A). Fish fed the 1 and 2% mixture diets displayed lesions including vessel degeneration, congestion, and degenerated hepatocytes (Figure 5B,C). Sections of the intestines from the control group revealed mucosal cell degeneration, leukocyte infiltration, degeneration of the intestinal epithelium, leukocyte congestion, and edema. In contrast, the groups fed the 1 and 2% mixture exhibited healthier intestinal mucosa with some signs of edema and mucosal cell degeneration (Figure 5A’–C’).
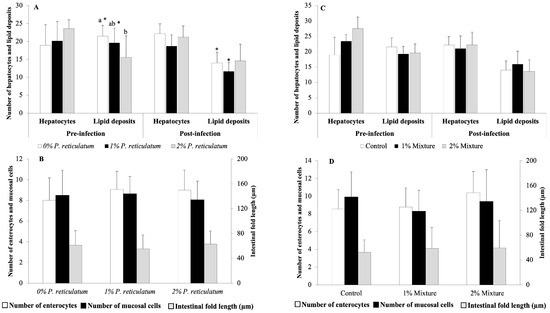
Figure 4.
Histological analyses of the livers and intestines of Nile tilapia juveniles at the end of a 28-day feeding period with different experimental diets and at the end of a 12-day infection challenge with Aeromonas hydrophila. (A) Number of hepatocytes and hepatic lipid deposits in fish fed 0, 1, and 2% P. reticulatum diets for 28 days and at 12 days post-infection; (B) number of enterocytes and mucous cells and length of intestinal folds in fish fed 0, 1, and 2% P. reticulatum diets for 28 days; (C) number of hepatocytes and hepatic lipid deposits in fish fed 0, 1, and 2% mixture diets for 28 days and at 12 days post-infection; (D) number of enterocytes and mucous cells and length of intestinal folds in fish fed 0, 1, and 2% mixture diets for 28 days. Data are expressed as mean ± SD (n = 6). Different letters indicate statistically significant differences among experimental groups (Mann–Whitney U test, p < 0.05), and asterisks denote differences in histological features compared to those before the infection challenge within the same dietary group (Mann–Whitney U test, p < 0.05).
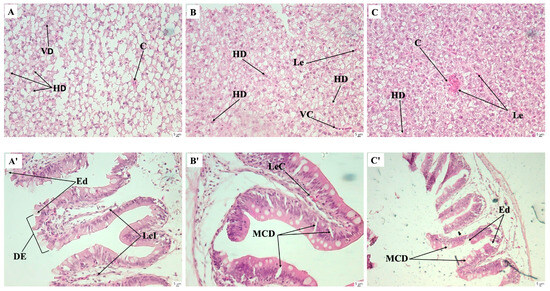
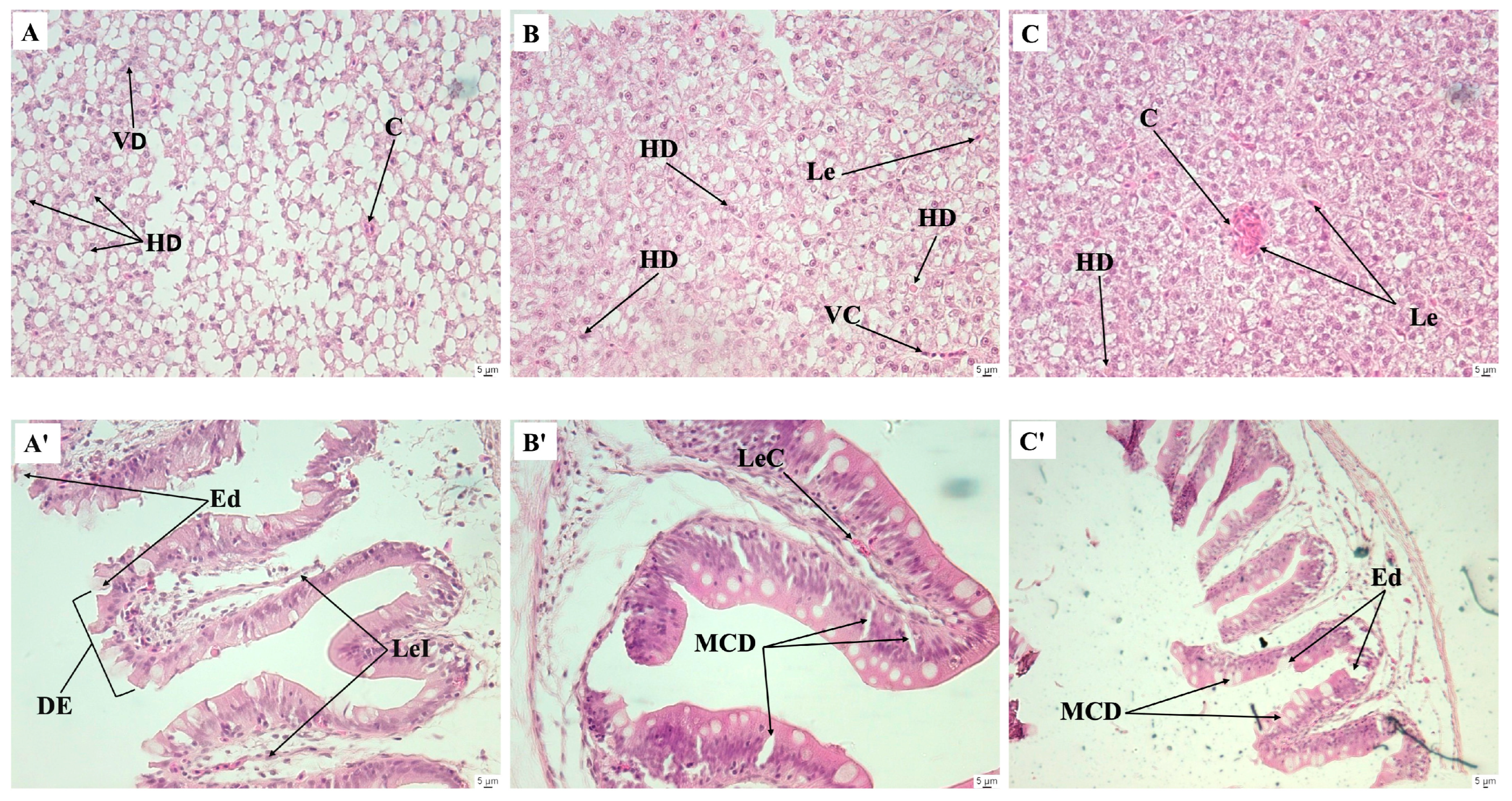
Figure 5.
Histological sections of livers (A–C) and intestines (A’–C’) of juvenile Nile tilapia fed 0, 1, and 2% mixture diets, at 12-day post-infection challenge with Aeromonas hydrophila. (A) Group fed 0% mixture diet; (B) group fed 1% mixture diet; (C) group fed 2% mixture diet. Hematoxylin-eosin staining, ×40. VD, vessel degeneration; C, congestion; DH, hepatocyte degeneration; Le, leucocyte; MCD, mucosal cell degeneration; LeI, leukocyte infiltration; DE, degeneration of the intestinal epithelium; LeC, leukocyte congestion; Ed, edema.
4. Discussion
The present study revealed that survival rates during the 28-day feeding trial were not significantly affected by the inclusion of any tested medicinal plants when compared to the control. A meta-analysis examining the treatment duration of plant-enriched diets, which ranged from 1 to 16 weeks, and their impacts on various fish health parameters (weight gain, specific growth rate, feeding conversion ratio, haemoglobin, serum total protein, immunoglobulin, lysozyme, complement activity, phagocytic activity, and disease survival) indicated that the most common duration was 4 weeks. This analysis also demonstrated that shorter supplementation periods (e.g., 2–4 weeks) were as effective as longer durations (≥8 weeks) in enhancing fish health and performance [32].
The effects on growth varied depending on the plant species, dietary inclusion level, and whether the plants were included individually or in combination. Inclusion levels of G. senegalensis and P. odorata up to 4%, as well as P. reticulatum up to 1%, had no negative effect on growth. However, higher inclusion levels of these plants or their combination at 1 or 2% led to increased FCR and decreased SGR and growth. These findings suggest that the combination of the three plants reduced the nutritional quality of the feed, potentially due to the overall higher inclusion levels of medicinal plants (3 and 6%, respectively), interactions between components of the three plants, and/or increased presence of antinutritional factors [36]. Anti-nutritional factors such as phytate reduce mineral bioavailability and protein digestibility by forming phytic acid–protein complexes that inhibit nutrient absorption [37]. Studies conducted on Nile tilapia with other medicinal plants at various inclusion levels, such as Lagenaria siceraria seed powder up to 1%, Salvadora persica or Centella asiatica leaf powders up to 2%, Portulaca oleracea leaf powder up to 3%, Moringa oleifera leaf powder up to 5%, or a combination of Stachytarpheta jamaicensis and Garcinia kola at 3.5% inclusion levels each, have also shown no significant impact on growth parameters such as weight gain or SGR [38,39,40,41,42,43]. Reduced growth at higher inclusion levels of several medicinal plants has also been reported in Nile tilapia and other fish species, likely associated with the increased content of phytochemicals [20,44,45,46,47]. However, the literature indicates that powdered plants remain the most used material for fish feed supplementation due to their low costs, higher accessibility, easy use, and relative safety compared to other extracts, such as ethanolic and methanolic extracts or essential oils [48].
The immune response of fish varied depending on the specific medicinal plant and level of dietary inclusion, which can be attributed to the varying amounts and nature of the active compounds present in each plant. In fish, lysozyme acts as the first line of defense against bacteria and is considered an important biomarker of the immune system [49]. It catalyzes the β-1,4 linked glycoside bonds found in both Gram-positive and Gram-negative bacterial cell wall peptidoglycans, leading to the breakdown and lysis of the bacterial cell wall. Among the three tested medicinal plants, only P. odorata stimulated lysozyme activity in fish when included at a 4% dietary level. This stimulatory effect was also observed in fish fed the combination of the three plants, which was most likely attributed to the presence of P. odorata. Similarly, in Nile tilapia, juveniles of similar weights exhibited significantly improved lysozyme activity when fed licorice (Glycyrrhiza glabra) root powder at dietary inclusion levels ranging from 0.5 to 2% [13]. Licorice root powder also increased respiratory burst activity in fish at the same inclusion levels [13]. Conversely, none of the three medicinal plants tested in the present study influenced the respiratory burst activity. The response of respiratory burst activity has been shown to vary depending on factors such as the type and level of dietary inclusion of medicinal plants, fish species, and development stage [20,50,51]. Plasma bactericidal activity is a lysin mechanism known for its role in killing and eliminating pathogenic organisms in fish [52]. Similar to lysozyme activity, plasma bactericidal activity increased in fish fed P. odorata at all tested inclusion levels and with the mixture of the three plants, indicating that these dietary supplements enhanced humoral immune elements in the serum. The enhancement of bactericidal activity observed in this study has been previously reported for other plant and fish species [53,54,55,56,57].
The histology of the digestive system is a reliable marker for assessing the nutritional condition in fish and the adequacy of experimental diets [58,59]. Additionally, histopathological examination is widely employed to study pathological alterations caused by chemicals or biological infectious agents [60]. It is desirable for medicinal plants to increase growth performance, biochemical parameters, and immunological responses in fish without adversely affecting the gastrointestinal tract at histological level [45,61,62]. In the present study, none of the histological features analyzed in the liver and intestine of the fish were affected by the tested medicinal plants, except for the 2% inclusion level of P. reticulatum, which showed a decrease in the number of hepatic lipid deposits compared to the control group. Research indicates that flavonoid-rich plant extracts can modulate fat deposition in rats and fish by affecting energy and lipid metabolism [63,64,65]. In wild grass carp (Ctenopharyngodon idellus), lotus leaf extract has been found to reduce fat accumulation in the liver and muscle by inhibiting fatty acid synthesis and promoting lipid breakdown and export in a dose-dependent manner [65]. Given the significant phenolic content of P. reticulatum [66], it is suggested that it may inhibit liver lipid accumulation in fish at a 2% dietary inclusion level. Regarding the infection challenge with A. hydrophyla, none of the tested diets showed a favorable effect on the survival rate after infection compared to the control group. Similar results have been reported in juvenile Clarias gariepinus fed P. betle, Psidium guajava, and Tithonia diversifolia at 8% inclusion levels each [20]. Nonetheless, the histological results evidenced the observed bactericidal effect of the medicinal plants. Intracellular infectious bacteria depend on host lipid deposits to acquire nutrients and lipids for immune evasion [67]. Following infection, the control group exhibited fusion of hepatic lipid deposits due to hepatocyte degeneration, most likely caused by the bacteria. The control group also showed hyperplasia of mucosal cells, along with degeneration of the epithelium, leukocyte infiltration, and atrophy of enterocytes upon infection with A. hydrophila. Similar histopathological changes in the liver of Nile tilapia following A. hydrophila infection have been reported previously [68]. In contrast, the groups fed the mixture diets, especially at 1% inclusion, mitigated intestinal and hepatic damage, showing healthier intestines and livers with reduced cell swelling and normal appearance of the lipid deposits compared to the control group.
5. Conclusions
The findings of this study highlight the beneficial effects of incorporating 4% P. odorata and a 1% mixture of the studied plants into the diets of juvenile Nile tilapia. Introducing these medicinal plants contributes to sustainable improvements in aquaculture by enhancing immune responses in fish, thereby improving welfare and increasing resistance to bacterial infections through enhanced protection of the intestinal mucosa. Utilizing plant-enriched diets offers a cost-effective and adaptable alternative to chemicals, suitable for a range of aquacultural practices from small-scale to intensive production systems. This approach also capitalizes on local biodiversity, as these plants are readily available in Senegal. Further research is required to improve knowledge of locally available plant species and their potential use in fish diets, which could reduce the reliance on antibiotics and support the One Health approach—a crucial element for improving health and sustainability in aquatic food systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9100390/s1, Table S1: Composition of plant leaves of Guiera senegalensis, Pluchea odorata, and Piliostigma reticulatum.
Author Contributions
Conceptualization: J.F., M.J.D., D.C., M.D. and S.S.; data curation: P.M.N.; formal analysis: P.M.N. and S.S.; investigation: P.M.N., M.C., E.P., S.H. and S.K.L.F.; methodology: P.M.N., J.F., M.J.D., D.C., M.D. and S.S.; project administration: S.S.; resources: M.J.D. and S.S.; supervision: J.F., M.J.D. and S.S.; validation: P.M.N. and S.S.; visualization: P.M.N.; writing—original draft: P.M.N.; writing—review and editing: J.F., M.J.D., D.C., M.D. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Institutional Review Board Statement
The experiments were conducted in compliance with the Guidelines of the European Union Council (2010/63/EU) on the protection of animals used for scientific purposes and received approval from the French Ministry of Higher Education and Research (project authorization APAFIS#28283-2020112412125734 v2).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available either within the paper or from the corresponding author upon reasonable request.
Acknowledgments
Paul M. Ndour benefited from a research fellowship from the “Service de Coopération et d’Action Culturelle” of the French Embassy in Dakar, Senegal. We would like to thank CIRAD for their warm welcome and support provided at the experimental facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Bastos, G.; Hutson, K.S.; Domingos, J.A.; Chung, C.; Hayward, S.; Miller, T.L.; Jerry, D.R. Use of environmental DNA (eDNA) and water quality data to predict protozoan parasites outbreaks in fish farms. Aquaculture 2017, 479, 467–473. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef] [PubMed]
- Allameh, S.K.; Yusoff, F.M.; Ringø, E.; Daud, H.M.; Saad, C.R.; Ideris, A. Effects of dietary mono-and multiprobiotic strains on growth performance, gut bacteria and body composition of Javanese carp (Puntius gonionotus, B leeker 1850). Aquac. Nutr. 2016, 22, 367–373. [Google Scholar] [CrossRef]
- Kari, Z.A.; Wee, W.; Sukri, S.; Harun, H.C.; Reduan, M.F.H.; Khoo, M.I.; Van Doan, H.; Goh, K.W.; Wei, L.S. Role of phytobiotics in relieving the impacts of Aeromonas hydrophila infection on aquatic animals: A mini-review. Front. Vet. Sci. 2022, 6, 1023784–1023795. [Google Scholar] [CrossRef]
- Jiofack, T.; Fokunang, C.; Guedje, N.M.; Kemeuze, V.; Fongnzossie, E.; Nkongmeneck, B.A.; Mapongmetsem, P.M.; Tsabang, N. Ethnobotanical uses of medicinal plants of two ethnoecological regions of Cameroon. Int. J. Med. Med. Sci. 2010, 2, 60–79. [Google Scholar]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Che, C.-T.; Wan, Z.J.; Chow, M.S.S.; Lam, C.W.K. Herb-herb combination for therapeutic enhancement and advancement: Theory, practice and future perspectives. Molecules 2013, 18, 5125–5141. [Google Scholar] [CrossRef]
- Caruso, D.; Lusiastuti, A.M.; Slembrouck, J.; Komarudin, O.; Legendre, M. Traditional pharmacopeia in small scale freshwater fish farms in West Java, Indonesia: An ethnoveterinary approach. Aquaculture 2013, 416–417, 334–345. [Google Scholar] [CrossRef]
- Bulfon, C.; Volpatti, D.; Galeotti, M. Current research on the use of plant-derived products in farmed fish. Aquac. Res. 2015, 46, 513–551. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, J.; Paichha, M.; Chakrabarti, R. Achyranthes aspera (Prickly chaff flower) leaves- and seeds-supplemented diets regulate growth, innate immunity, and oxidative stress in Aeromonas hydrophila-challenged Labeo rohita. J. Appl. Aquac. 2020, 32, 250–267. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; El-Araby, D.A. Immune and antioxidative effects of dietary licorice (Glycyrrhiza glabra L.) on performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to Aeromonas hydrophila infection. Aquaculture 2021, 530, 735828. [Google Scholar] [CrossRef]
- Romana-Eguia, M.R.R.; Eguia, R.V.; Pakingking, R.V. Tilapia Culture: The Basics; Aquaculture Department, Southeast Asian Fisheries Development Center: Iloilo, Philippines, 2020. [Google Scholar]
- Pang, M.; Jiang, J.; Xie, X.; Wu, Y.; Dong, Y.; Kwok, A.H.Y.; Zhang, W.; Yao, H.; Lu, C.; Leung, F.C.; et al. Novel insights into the pathogenicity of epidemic Aeromonas hydrophila ST251 clones from comparative genomics. Sci. Rep. 2015, 5, 9833. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yan, H.; Li, S.; Bai, L.; Lv, Q. Effects of novel polyhedral oligomeric silsesquioxane containing hydroxyl group and epoxy group on the dicyclopentadiene bisphenol dicyanate ester composites. Polym. Test. 2017, 59, 316–327. [Google Scholar] [CrossRef]
- Sheikhlar, A.; Meng, G.Y.; Alimon, R.; Romano, N.; Ebrahimi, M. Dietary Euphorbia hirta Extract Improved the Resistance of Sharptooth Catfish Clarias gariepinus to Aeromonas hydrophila. J. Aquat. Anim. Health 2017, 29, 225–235. [Google Scholar] [CrossRef]
- Kuebutornye, F.; Abarike, E. The contribution of medicinal plants to tilapia aquaculture: A review. Aquac. Int. 2020, 28, 965–983. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Caruso, D.; Lebrun, M.; Nguyen, N.T.; Trinh, T.T.; Meile, J.-C.; Chu-Ky, S.; Sarter, S. Antibacterial activity of Litsea cubeba (Lauraceae, May Chang) and its effects on the biological response of common carp Cyprinus carpio challenged with Aeromonas hydrophila. J. Appl. Microbiol. 2016, 121, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Nafiqoh, N.; Sukenda, S.; Zairin, M.J.; Alimuddin, A.; Lusiastuti, A.; Sarter, S.; Caruso, D.; Avarre, J.C. Antimicrobial properties against Aeromonas hydrophila and immunostimulant effect on Clarias gariepinus of Piper betle, Psidium guajava, and Tithonia diversifolia plants. Aquac. Int. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Maiti, S.; Saha, S.; Jana, P.; Chowdhury, A.; Khatua, S.; Ghosh, T.K. Effect of dietary Andrographis paniculata leaf extract on growth, immunity, and disease resistance against Aeromonas hydrophila in Pangasianodon hypopthalmus. J. Appl. Aquac. 2023, 35, 305–329. [Google Scholar] [CrossRef]
- Guèye, F. Médecine Traditionnelle du Sénégal Exemples de Quelques Plantes Médicinales de la Pharmacopée Sénégalaise Traditionnelle. Ph.D. Dissertation, Aix-Marseille University, Marseille, France, 2019. [Google Scholar]
- Perera, W.H.C.; González, L.; Payo, A.; Nogueiras, C.; Oquendo, M.; Sarduy, R. Antimicrobial activity of crude extracts and flavonoids from leaves of Pluchea carolinensis (Jacq.) G. Don. Pharmacologyonline 2006, 3, 757–761. [Google Scholar]
- Perez, C.; Balcinde, Y.; Suarez, C.; Hernandez, V.; Falero, A.; Hung, B.R. Ensayo de la actividad antimicrobiana de Pluchea carolinensis (salvia de playa). Rev. Cenic. Cienc. Biolόgicas 2007, 38, 150–154. [Google Scholar]
- Biabiany, M.; Roumy, V.; Hennebelle, T.; Francois, N.; Sendid, B.; Pottier, M.; Aliouat, E.; Rouaud, I.; Lohezic-Le, D.F.; Joseph, H.; et al. Antifungal Activity of 10 Guadeloupean Plants. Phytother. Res. 2013, 27, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.; Torres, M. Evaluation of Pluchea carolinensis extracts as antioxidants by the epinephrine oxidation method. Fitoterapia 2006, 77, 221–226. [Google Scholar] [CrossRef]
- Perera, W.H.C.; Wauters, J.N.; Kevers, C.; Frédérich, M.; Dommes, J. Antioxidant fractions and phenolic constituents from leaves of Pluchea carolinensis and Pluchea rosea. Free Radic. Antioxid. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Garcia, M.; Perera, W.H.; Scull, R.; Monzote, L. Antileishmanial assessment of leaf extracts from Pluchea carolinensis, Pluchea odorata and Pluchea rosea. Asian Pac. J. Trop. Med. 2011, 4, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Yelemou, B.; Bationo, B.A.; Yameogo, G.; Rasolodimby, J.M. Gestion traditionnelle et usages de Piliostigma reticulatum sur le plateau central du Burkina Faso. Bois Forêts Des Trop. 2007, 291, 55–66. [Google Scholar] [CrossRef]
- Babajide, O.J.; Babajide, O.O.; Daramola, A.O.; Mabusela, W.T. Flavonols and an oxychromonol from Piliostigma reticulatum. Phytochemistry 2008, 69, 2245–2250. [Google Scholar] [CrossRef]
- Arbonnier, M. Arbres, Arbustes et Lianes des Zones Seches d’Afrique de l’Ouest; MNHN-QUAE: Versailles, France, 2009. [Google Scholar]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards more sustainable aquaculture practices: A meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquac. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Leaño, M.E.; Ju, G.J.; Chang, S.L.; Liao, C.I. Levamisole enchances non-specific immune response of cobia, Rachycentron canadum, fingerlings. J. Fish. Soc. Taiwan 2003, 30, 321–330. [Google Scholar]
- Caruso, D.; Lazard, J. Subordination stress in Nile tilapia and its effect on plasma lysozyme activity. J. Fish Biol. 1999, 55, 451–454. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ali, H.A.; Ali, J.A.; Musthafa, S.M.; Kumar, M.A.; Naveed, S.M.; Mehrajuddin, W.; Altaff, K. Impact of formulated diets on the growth and survival of ornamental fish Pterophyllum scalare (Angel Fish). J. Aquac. Res. Dev. 2016, 7, 1–4. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Ekanem, S.B.; Eyo, V.O.; Okon, E.E. The effects of Brazilian tea (Stachytarpheta jamaicensis) and Bitter kola (Garcinia kola) seed meal on the growth and gonad development of the African catfish Clarias gariepinus (Burchell, 1822). Ege J. Fish. Aquat. Sci. 2017, 34, 179–185. [Google Scholar] [CrossRef][Green Version]
- Abdel-Razek, N.; Awad, S.M.; Abdel-Tawwab, M. Effect of dietary purslane (Portulaca oleracea L.) leaves powder on growth, immunostimulation, and protection of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Fish Physiol. Biochem. 2019, 45, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Lebda, M.A.; El-Hawarry, W.N.; Shourbela, R.M.; El-Far, A.H.; Shewita, R.S.; Mousa, S.A. Miswak (Salvadora persica) dietary supplementation improves antioxidant status and nonspecific immunity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 88, 619–626. [Google Scholar] [CrossRef]
- Abd El-Gawad, E.A.; El Asely, A.M.; Soror, E.I.; Abbass, A.A.; Austin, B. Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquac. Int. 2020, 28, 389–402. [Google Scholar] [CrossRef]
- Srichaiyo, N.; Tongsiri, S.; Hoseinifar, S.H.; Dawood, M.A.O.; Jaturasitha, S.; Esteban, M.A.; Ringø, E.; Van Doan, H. The effects gotu kola (Centella asiatica) powder on growth performance, skin mucus, and serum immunity of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Rep. 2020, 16, 100239. [Google Scholar] [CrossRef]
- Radwan, M.; Abbas, M.M.M.; Mohammadein, A.; Al Malki, J.S.; Elraey, S.M.A.; Magdy, M. Growth performance, immune response, antioxidative status, and antiparasitic and antibacterial capacity of the Nile tilapia (Oreochromis niloticus) after dietary supplementation with Bottle gourd (Lagenaria siceraria, Molina) seed powder. Front. Mar. Sci. 2022, 9, 901439. [Google Scholar] [CrossRef]
- Dada, A.A.; Ikuerowo, M. Effect of ethanoic extracts of Garcinia kola seeds on growth and haematology of catfish (Clarias gariepinus) broodstock. Afr. J. Agric. Res. 2009, 4, 344–347. [Google Scholar]
- Yilmaz, E. Effects of dietary anthocyanin on innate immune parameters, gene expression responses, and ammonia resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 93, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Ergun, S.; Kaya, H.; Gurkan, M. Influence of Tribulus terrestris extract on the survival and histopathology of Oreochromis mossambicus (Peters, 1852) fry before and after Streptococcus iniae infection. J. Appl. Ichthyol. 2014, 30, 994–1000. [Google Scholar] [CrossRef]
- Kapinga, I.B.; Limbu, S.M.; Madalla, N.A.; Kimaro, W.H.; Tamatamah, R.A. Aspilia mossambicensis and Azadirachta indica medicinal leaf powders modulate physiological parameters of Nile tilapia (Oreochromis niloticus). Int. J. Vet. Sci. Med. 2018, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, O.; Olaifa, F.; Emikpe, B.; Ogunbanwo, S. Effects of dietary tamarind (Tamarindus indica L.) leaves extract on growth performance, nutrient utilization, gut physiology, and susceptibility to Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus L.). Int. Aquat. Res. 2021, 13, 37–51. [Google Scholar] [CrossRef]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.-C.; Combe, M.; Pepey, E.; Gozlan, R.E. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Basha, K.A.; Raman, R.P.; Prasad, K.P.; Kumar, K.; Nilavan, E.; Kumar, S. Effect of dietary supplemented andrographolide on growth, non-specific immune parameters and resistance against Aeromonas hydrophila in Labeo rohita (Hamilton). Fish Shellfish Immunol. 2013, 35, 1433–1441. [Google Scholar] [CrossRef]
- Adeshina, I.; Adewale, Y.A.; Tiamiyu, L.O. Growth performance and innate immune response of Clarias gariepinus infected with Aeromonas hydrophila fed diets fortified with Curcuma longa leaf. West Afr. J. Appl. Ecol. 2017, 25, 87–99. [Google Scholar]
- Ellis, A.E. Innate host defence mechanism of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Ahmad, M.; Seden, M.; Sakr, S. Use of green tea, Camellia sinensis L., in practical diet for growth and protection of Nile tilapia, Oreochromis niloticus (L.), against Aeromonas hydrophila infection. J. World Aquac. Soc. 2010, 41, 203–213. [Google Scholar] [CrossRef]
- Awad, E.; Austin, B. Use of lupin, Lupinus perennis, mango, Mangifera indica, and stinging nettle, Urtica dioica, as feed additives to prevent Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2010, 33, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.; Abasali, H. Effect of plant extracts supplemented diets on immunity and resistance to Aeromonas hydrophila in common carp (Cyprinus carpio). Agric. J. 2010, 5, 119–127. [Google Scholar]
- Talpur, A.D.; Ikhwanuddin, M. Azadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol. 2013, 34, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, C.C.; Oyoo-Okoth, E.; Mugo-Bundi, J.; Orina, P.S.; Chemoiwa, E.J.; Aloo, P.A. Effects of dietary administration of stinging nettle (Urtica dioica) on the growth performance, biochemical, hematological and immunological parameters in juvenile and adult Victoria Labeo (Labeo victorianus) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 44, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ruiz, D.; Andree, K.B.; Magris, J.; Fernandez-Mendez, C.; Garcia-Davila, C.; Gisbert, E.; Darias, M.J. DHA-enrichment of live and compound feeds influences the incidence of cannibalism, digestive function, and growth in the neotropical catfish Pseudoplatystoma punctifer (Castelnau, 1855) during early life stages. Aquaculture 2022, 561, 738667. [Google Scholar] [CrossRef]
- Castro-Ruiz, D.; Andree, K.B.; Solovyev, M.M.; Fernandez-Mendez, C.; Garcia-Davila, C.; Gisbert, E.; Darias, M.J. The digestive function of Pseudoplatystoma punctifer early juveniles is differentially modulated by dietary protein, lipid and carbohydrate content and their ratios. Animals 2021, 11, 369. [Google Scholar] [CrossRef]
- Camargo, M.M.P.; Martinez, C.B.R. Histopathology of gills, kidney, and liver of a neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef]
- Nofouzia, K.; Aghapoura, M.; Ezazia, A.; Sheikhzadehb, N.; Tukmechic, A.; Khordadmehra, M.; Akbari, M.; Tahapour, K.; Mousavi, M. Effects of Verbascum speciosum on growth performance, intestinal histology, immune system and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. Aquat. Sci. 2017, 17, 145–152. [Google Scholar] [CrossRef]
- Abdel Rahman, A.; Elhady, M.; Hassanin, M.; Mohamed, A. Alleviative effects of dietary Indian lotus leaves on heavy metals-induced hepato-renal toxicity, oxidative stress, and histopathological alterations in Nile tilapia, Oreochromis niloticus (L.). Aquaculture 2019, 509, 198–208. [Google Scholar] [CrossRef]
- Song, H.; Han, W.; Yan, F.; Xu, D.; Chu, Q.; Zheng, X. Dietary Phaseolus vulgaris extract alleviated diet-induced obesity, insulin resistance and hepatic steatosis and alters gut microbiota composition in mice. J. Funct. Foods 2016, 20, 236–244. [Google Scholar] [CrossRef]
- Kim, M.; Pichiah, P.B.T.; Kim, D.K.; Cha, Y.S. Black adzuki bean (Vigna angularis) extract exerts phenotypic effects on white adipose tissue and reverses liver steatosis in diet-induced obese mice: Antiobesity and antisteatotic effects of black adzuki beans. J. Food Biochem. 2017, 41, e12333. [Google Scholar] [CrossRef]
- Yao, J.; Hu, P.; Zhu, Y.; Xu, Y.; Tan, Q.; Liang, X. Lipid-lowering effects of lotus leaf alcoholic extract on serum, hepatopancreas, and muscle of juvenile grass carp via gene expression. Front. Physiol. 2020, 11, 584782. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.O.; Santos, V.G.D.; Pezzato, L.E.; Carvalho, P.L.P.F.D.; Teixeira, C.P.; Freitas, J.M.A.; Padovani, C.R.; Sartori, M.M.P.; Barros, M.M. Activity of Brazilian propolis against Aeromonas hydrophila and its effect on Nile tilapia growth, hematological and non-specific immune response under bacterial infection. An. Acad. Bras. Ciências 2017, 89, 1785–1799. [Google Scholar] [CrossRef] [PubMed]
- Libbing, C.L.; McDevitt, A.R.; Azcueta, R.P.; Ahila, A.; Mulye, M. Lipid droplets: A significant but understudied contributor of host bacterial interactions. Cells 2019, 8, 354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).