Abstract
The vasa gene, encoding an ATP-dependent RNA helicase, and the nanos-2 gene, an RNA-binding protein, are essential for germ cell origination, migration, maintenance, and development in vertebrates and invertebrates. The expression levels of the vasa and nanos-2 genes have not yet been investigated or reported in crappie species. These two genes were partially sequenced and characterized, and their expression patterns were analyzed using reverse-transcription quantitative polymerase chain reaction (RT-qPCR) according to age and sex. The vasa sequences of white crappie (WC) females and males showed significant similarity with the vasa homologs of largemouth bass (Micropterus salmoides; 93.1–93.98%) and smallmouth bass (M. dolomieu; 91.95–92.77%), indicating its conserved nature within the Family Centrarchidae. The vasa sequence of black crappie (BC) females showed significant similarity with the vasa homologs of white crappie (91.67%), largemouth bass (96.10%), smallmouth bass (96.10%), spotted scat (Scatophagus argus; 97.37%), mandarin fish (Siniperca chutasi; 96.15%), Japanese sea bass (Lateolabrax japonicus; 94.87%), lumpfish (Cyclopterus lumpus; 91.95%), southern bluefin tuna (Thunnus maccoyii; 94.74%), large yellow croaker (Larimichthys crocea; 92.21%), and Nile tilapia (Oreochromis niloticus; 92.21%). The nanos-2 sequences of WC females, WC males, and BC females showed significant similarity with the nanos-2 of largemouth bass (92.92–96.36%), smallmouth bass (92.92–96.36%), and mandarin fish (92.66–94.34%). The expression of vasa in BC females was significantly higher at age-2 than at age-1, while WC males and females presented no significant age-related differences. Neither species had a significant difference in nanos-2 gene expression with age. The expression levels of vasa and nanos-2 were significantly higher in WC males than females.
Keywords:
vasa; nanos-2; gene expression; crappie species; germ cell transplantation; fish reproduction; DNA sequencing Key Contribution:
This study provides the first report of the sequences of the important germ cell genes vasa and nanos-2 in white crappie (WC) and black crappie (BC), two popular sportfish species in the United States. The findings of this study provide a foundational step for further research on germ cell transplantation, germplasm conservation, and the creation of hybrid crappie using germ cell transplantation techniques.
1. Introduction
The vasa and nanos-2 genes play important roles in the development and function of germ cells in both the ovary and testis of different fish species. The vasa gene, which encodes an ATP-dependent RNA helicase of the DEAD (Asp-Glu-Ala-Asp) box family, was the first germ cell gene to be identified in fish species [1]. It plays an essential role in germ cell origination and migration and is maintained throughout the development of germ cells in fish species [2,3,4,5]. The vasa gene has been used to identify fish germlines in various species such as Atlantic cod (Gadus morhua) [6], black rockfish (Sebastes schlegelii) [5], and rainbow trout (Oncorhynchus mykiss) [7]. A study on zebrafish (Danio rerio) highlighted that the vasa gene is essential during the female meiosis, differentiation, and maintenance of germline stem cells, and that the disruption of the vasa gene results in sterility in males [8]. The nanos gene family encodes an RNA-binding protein and is essential for germ cell maintenance and development in vertebrates [9,10] and invertebrates [11]. Several nanos homolog genes, such as nanos-1a, nanos-1b, nanos-2, and nanos-3, have been reported in a wide variety of vertebrate and invertebrate species [9,12,13,14,15]. Our gene of interest, the nanos-2 gene, has been identified as a specific marker of germline stem cells in zebrafish, and it plays an essential role in the survival and maintenance of germline stem cells [9].
The germ cell transplantation technique can be utilized for several applications, such as the production of live fish from cryopreserved germ cells, the conservation of endangered fish species, and the production of hybrid fish [16,17]. Therefore, the identification and characterization of these two genes are essential for the success of germ cell transplantation in fish, as these genes serve as molecular markers of germline stem cells. The combined use of these two genes is expected to support the efficient detection of donor germline stem cells for successful germ cell transplantation and the production of surrogate broodstock [16,17].
The species of interest, white crappie (WC; Pomoxis annularis) and black crappie (BC; P. nigromaculatus), which are members of Family Centrarchidae, are two popular sportfish species in lakes and reservoirs throughout the USA, and are a significant source of income in local, regional, and state economies in the southeastern US [18,19,20,21]. The vasa and nanos-2 genes have yet to be investigated or reported in these crappie species, and complete or partial nucleotide sequences for the vasa and nanos-2 genes are not yet available in the National Center for Biotechnology Information (NCBI) GenBank database. Identifying and characterizing these genes in crappie species can have significant implications for germplasm conservation and the production of hybrid crappie using novel germ cell transplantation techniques.
In this study, we identified and sequenced two germ cell genes—vasa and nanos-2—from white crappie and black crappie gonads and evaluated their expression levels at different ages and sexes. The results provide a basis for further studies on germ cell transplantation, germplasm conservation, and gonadal development in crappie species. The technique used to identify germ cell genes in this study can also be utilized to identify germ cell genes in other species.
2. Materials and Methods
2.1. Ethics
All procedures for animal management, euthanasia, and dissection were conducted according to the University of Arkansas at Pine Bluff (UAPB) Institutional Animal Care and Use Committees (IACUC) protocols and guidelines.
2.2. Fish Selection and Its Parameters
The WC and BC were obtained from a local aquaculture producer in Arkansas, USA, and stocked in separate ponds at the University of Arkansas at Pine Bluff (UAPB) Aquaculture Research Center. Fish were collected from the ponds using a seine net during the spring season to conduct this research. The fish were categorized into age-1 and age-2 groups based on their size and weight. The total length, body weight, gonad weight, and gonadosomatic index (GSI) for each category of fish are listed in Table 1. Each value represents the mean ± standard deviation (SD) for three fish per category.

Table 1.
Morphometric data and gonadosomatic index (GSI) for different age groups and sexes of white crappie (WC) and black crappie (BC). Data are presented as mean ± standard deviation (SD) for three fish per category.
2.3. Conserved Region Identification and Primer Selection
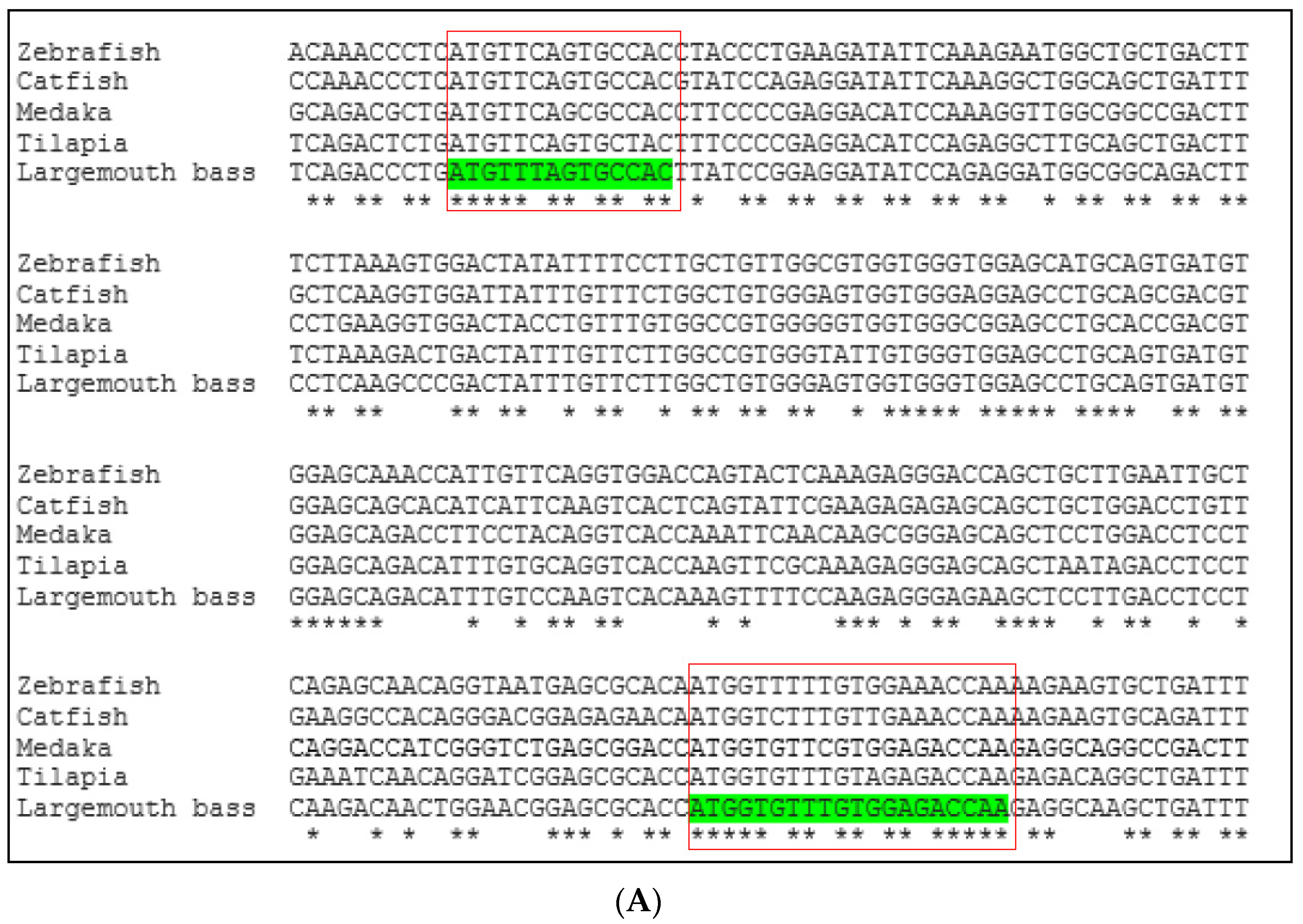
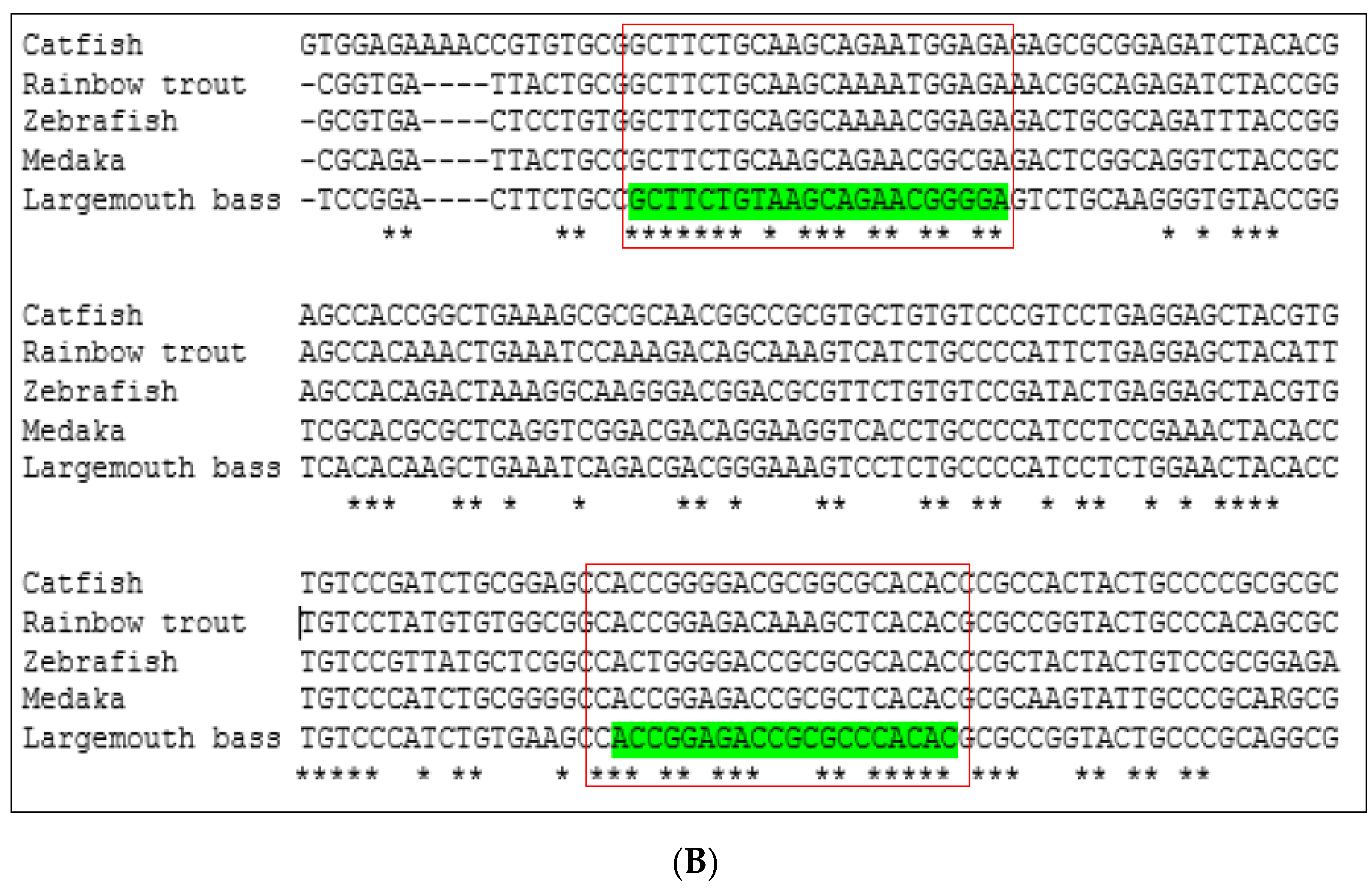
Conserved sequences of these two genes were identified by comparing available DNA sequences from the NCBI database of six fish species: largemouth bass (M. salmoides), channel catfish (Ictalurus punctatus), Nile tilapia (Oreochromis niloticus), medaka (Oryzias latipes), rainbow trout (Oncorhynchus mykiss), and zebrafish (Danio rerio). The Clustal Omega program [22] was used to align multiple vasa and nanos-2 DNA sequences (Figure 1). The Integrated DNA Technologies (IDT) OligoAnalyzerTM tool was used to design forward and reverse primers from the conserved region. The forward and reverse primers are listed in Table 2.
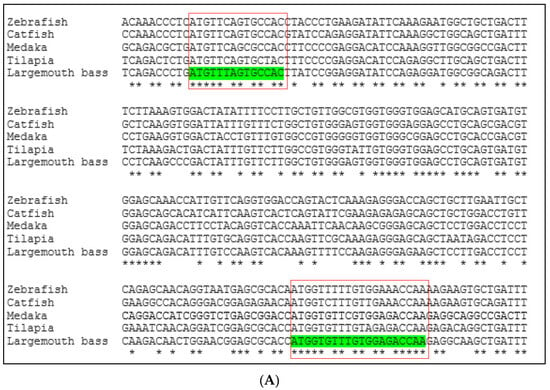
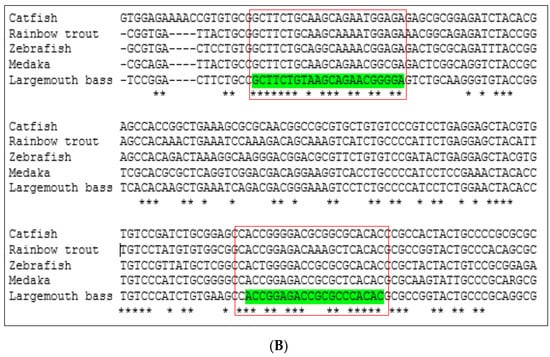
Figure 1.
Multiple DNA sequence alignment of (A) vasa gene and (B) nanos-2 gene by comparing available DNA sequences from a database of different fish species using Clustal Omega program. The red box indicates the highly conserved portion of the sequences. Asterisks (*) indicate sequence conservation among all input sequences.

Table 2.
Primers used for amplification of germ cell genes in crappie species. Primers for vasa and nanos-2 genes were designed from the conserved region using the Integrated DNA Technologies (IDT) OligoAnalyzerTM tool.
2.4. RNA Isolation and Gene Expression
Following the manufacturer’s instructions, total RNA was extracted from ovaries and testis using a TRIzolTM Plus RNA isolation kit (InvitrogenTM by Thermo Fisher Scientific, Waltham, MA, USA). The quality and concentration of RNA were checked using a NanoDropTM 2000 spectrophotometer(Thermo Fisher Scientific, Waltham, MA, USA). The RNA from each sample (1 µg) was reverse transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied BiosystemsTM by Thermo Fisher Scientific, Waltham, MA, USA). The complementary DNA (cDNA) was subjected to real-time quantitative PCR (RT-qPCR) analysis using a QuantStudioTM 3 RT-qPCR system (Applied BiosystemsTM by Thermo Fisher Scientific, Waltham, MA, USA) with PowerUp SYBR Green Master Mix (Applied BiosystemsTM by Thermo Fisher Scientific, Waltham, MA, USA) and specific primers for Vasa and Nanos-2 genes (as listed in Table 2). GAPDH was used as an internal housekeeping control, as its expression remained stable among all the samples. The reaction mixture for all samples subjected to RT-qPCR was as follows: 1.0 μL of each primer (10 μM), 5.0 μL SYBR Green, 2.0 μL RNase/DNase free water, and 1.0 μL cDNA (200 ng/μL). The 2−∆∆Ct method was used to calculate the relative gene expression, where Ct is the threshold cycle for target amplification, ∆Ct = Ct target gene − Ct internal reference (GAPDH), and ∆∆CT = ∆Ct sample − ∆Ct calibrator [23]. The relative expression levels of the vasa and nanos-2 genes were analyzed through several comparisons: age-1 WC females versus age-2 WC females, age-1 BC females versus age-2 BC females, age-1 WC males versus age-2 WC males, and age-2 WC females versus age 2- WC males.
2.5. Sequence Verification
The amplified RT-qPCR products were verified by 1% agarose gel electrophoresis (Figure S1). Eurofins Genomics, LLC (Louisville, KY, USA) purified and sequenced the RT-qPCR product for each gene (Figures S2–S7).
2.6. NCBI BLAST Analysis
The obtained sequences were analyzed using the Basic Local Alignment Search Tool (BLAST) available from the National Center for Biotechnology Information (NCBI). The sequences were uploaded to the NCBI BLAST website (https://blast.ncbi.nlm.nih.gov/Blast.cgi; accessed on 27 July 2024). The species and sequences showing the highest similarity to our query sequences were identified.
2.7. Statistical Analysis
All the RT-qPCR data are presented as mean ± S.E. (n = 3). A t-test was performed using the R-Studio (Version 2023.12.1+402) software to analyze the differential expression levels of the vasa and nanos-2 genes by age and sex of WC and age of BC. Statistical significance was determined at p < 0.05.
3. Results
3.1. Sequence Identification and NCBI BLAST Analysis
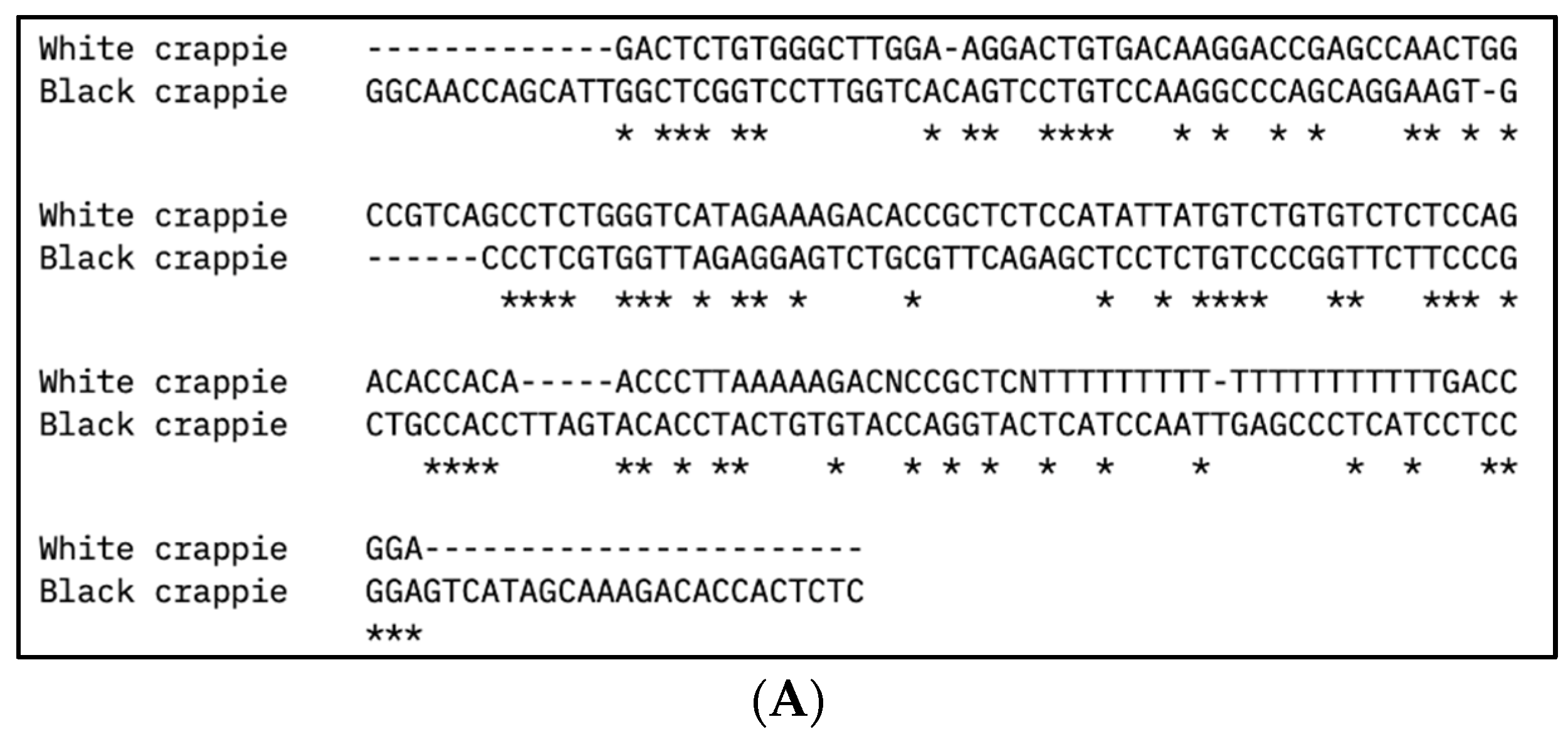
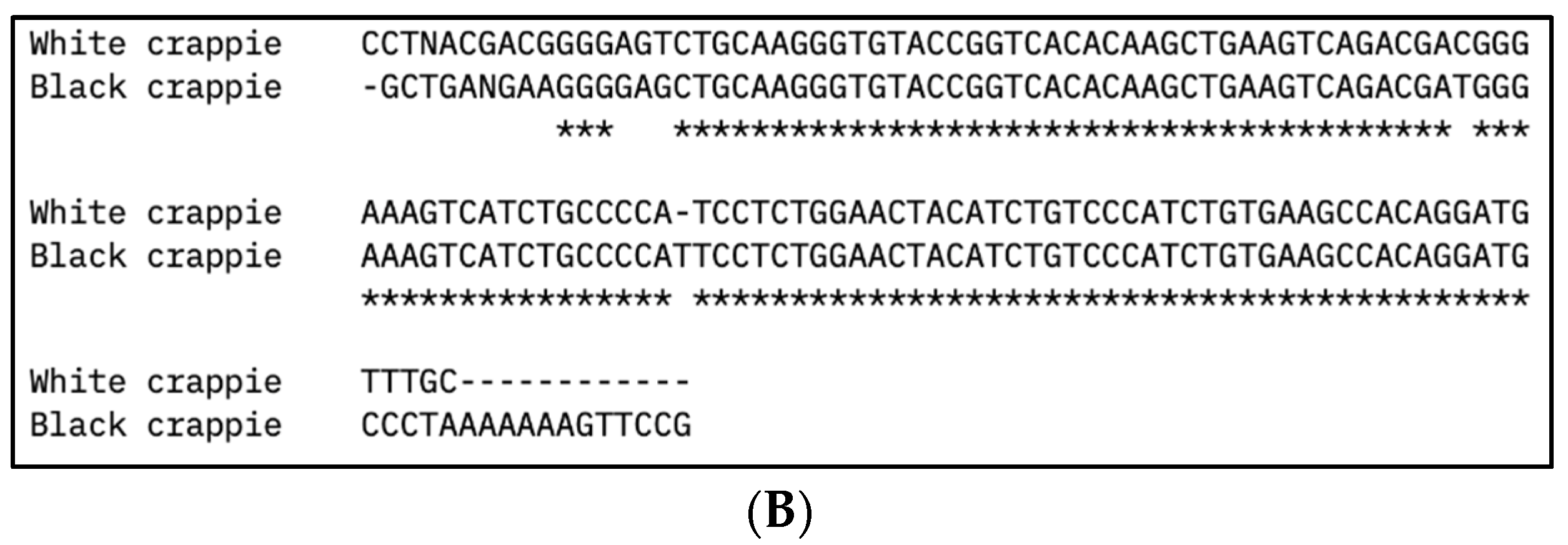
The partial DNA sequences of the vasa and nanos-2 genes for WC females, WC males, and BC females were identified, as presented in Table 3. The DNA sequence alignment of vasa and nanos-2 genes from WC and BC is presented in Figure 2. The vasa gene sequences of WC females and WC males showed significant similarity with the vasa homologs of largemouth bass (M. salmoides; 93.1–93.98%) and smallmouth bass (M. dolomieu; 91.95–92.77%). The vasa gene sequence of BC females showed significant similarity with the vasa homologs of white crappie (91.67%), largemouth bass (96.10%), smallmouth bass (96.10%), spotted scat (Scatophagus argus; 97.37%), mandarin fish (Siniperca chutasi; 96.15%), Japanese sea bass (Lateolabrax japonicus; 94.87%), lumpfish (Cyclopterus lumpus; 91.95%), southern bluefin tuna (Thunnus maccoyii; 94.74%), large yellow croaker (Larimichthys crocea; 92.21%), and Nile tilapia (Oreochromis niloticus; 92.21%). The nanos-2 gene sequences of WC females, WC males, and BC females showed significant similarity with the nanos-2 genes of largemouth bass (92.92–96.36%), smallmouth bass (92.92–96.36%), and mandarin fish (Siniperca chutasi; 92.66–94.34%). In addition, the nanos-2 gene sequences of WC females and WC males also showed sequence similarity with the nanos-2 gene sequences of large yellow croaker (Larimichthys crocea; 94.39–95.28%), lumpfish (Cyclopterus lumpus; 93.46–94.34%), and striped bass (Morone sexatilis; 93.46–94.34%).

Table 3.
Partial DNA sequences of the vasa and nanos-2 genes obtained from female and male white crappie (Pomoxis annularis) and female black crappie (P. nigromaculatus). Eurofins Genomics, LLC (Louisville, KY, USA) purified and sequenced the RT-qPCR product for each gene.
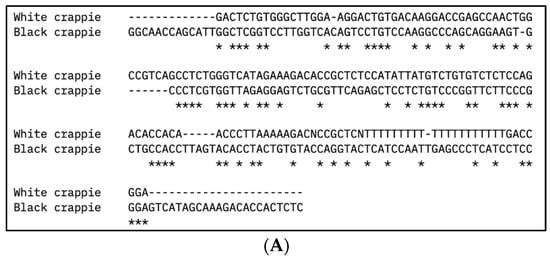
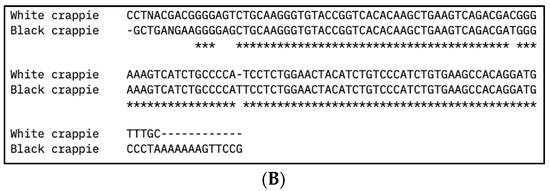
Figure 2.
Multiple DNA sequence alignment of (A) vasa and (B) nanos-2 gene sequences from white crappie (Pomoxis annularis) and black crappie (P. nigromaculatus) using Clustal Omega program. Asterisks (*) indicate sequence conservation between two species.
3.2. Gene Expression by Age
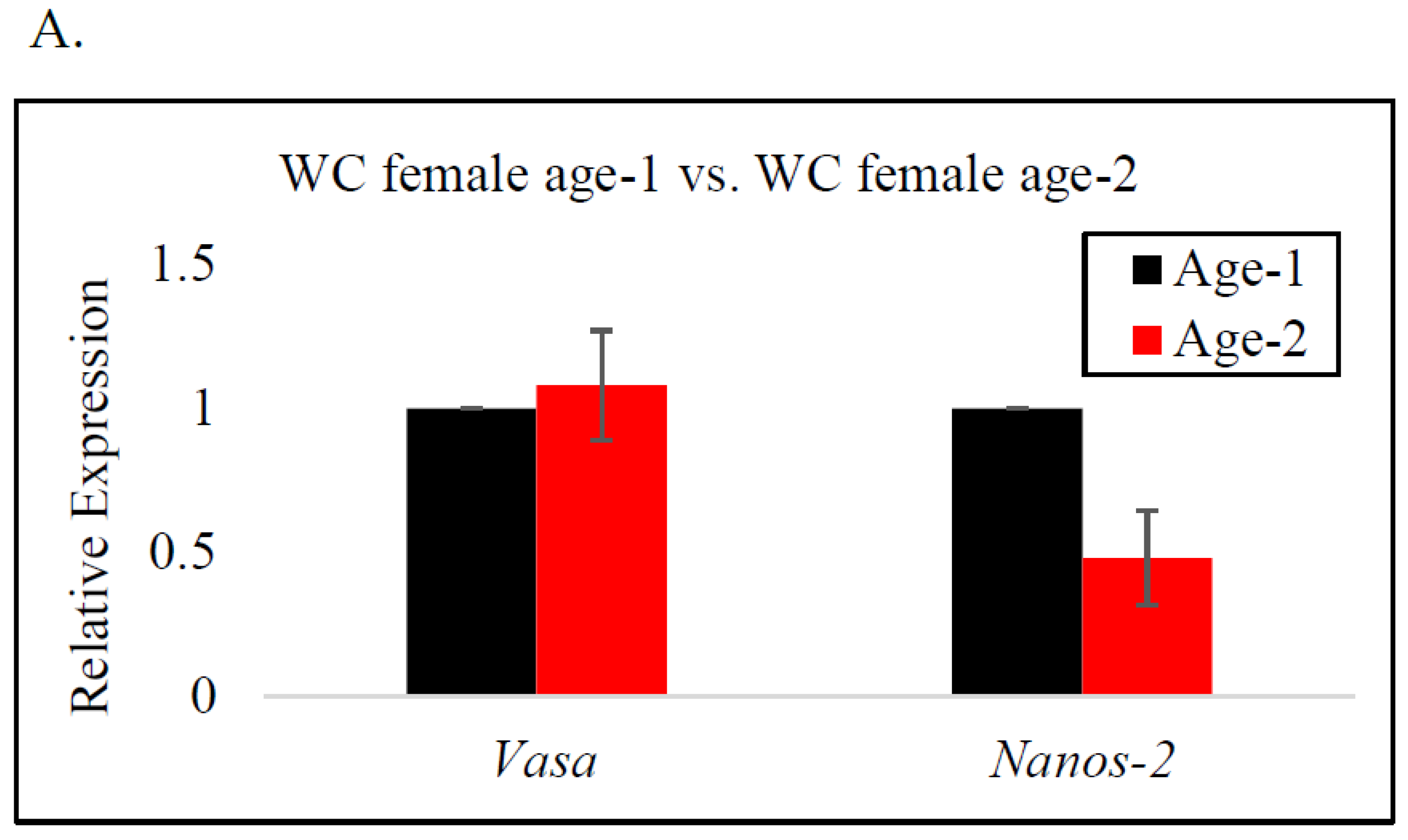
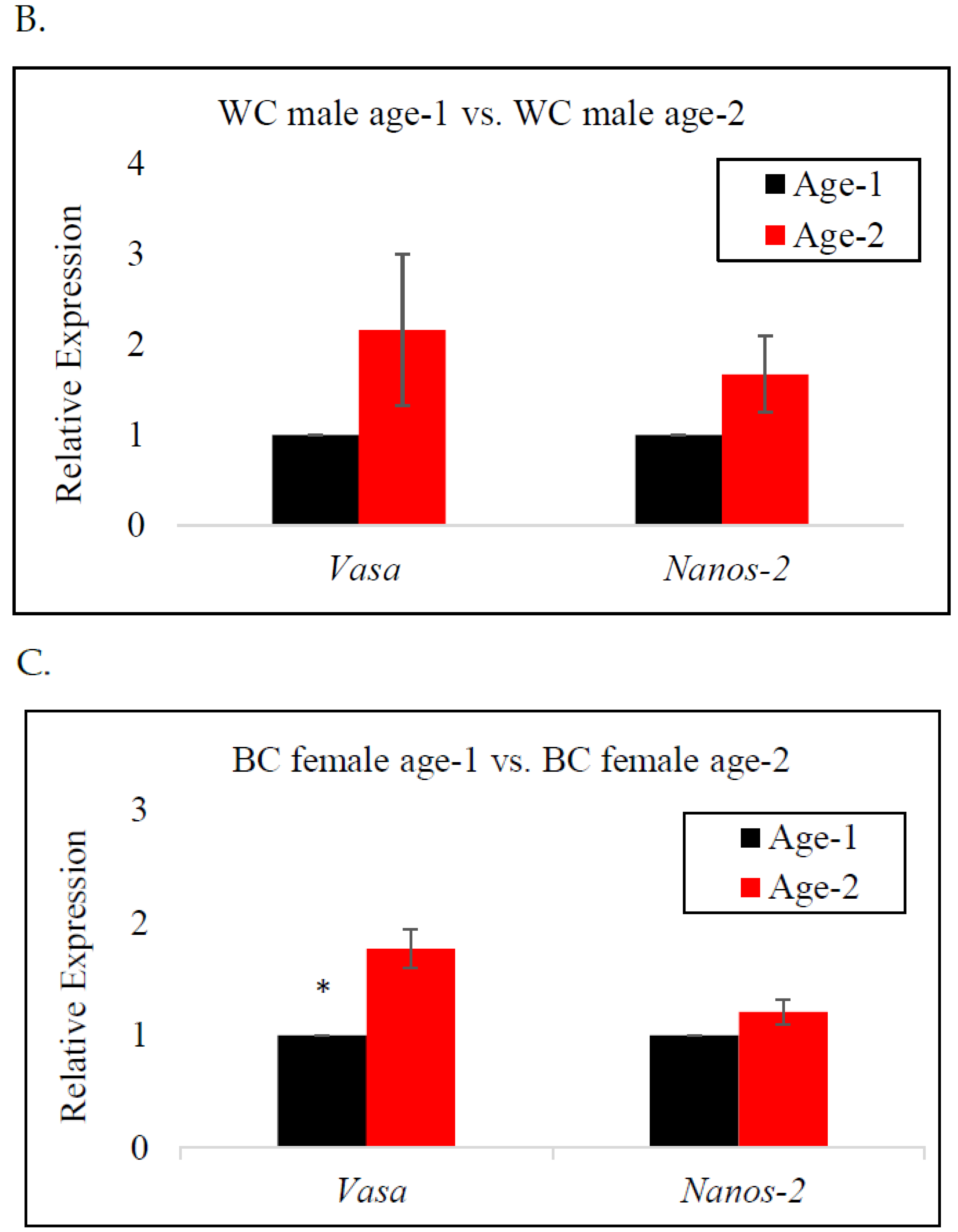
In female BC, vasa gene expression was significantly higher at age-2 than at age-1, indicating a significant upregulation of the vasa gene as the fish neared reproductive age (Figure 3C). A similar trend was observed for WC males and females; however, there was no significant difference in the expression of the vasa gene by age (Figure 3A,B). The expression of the nanos-2 gene in BC females and WC males indicated an increase from age-1 to age-2. In contrast, nanos-2 gene expression in WC females demonstrated a different pattern, with lower expression at age-2, indicating the downregulation of the gene. Nonetheless, neither species presented a significant difference in nanos-2 gene expression with age.
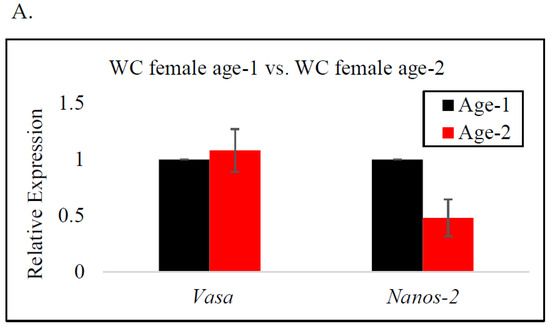
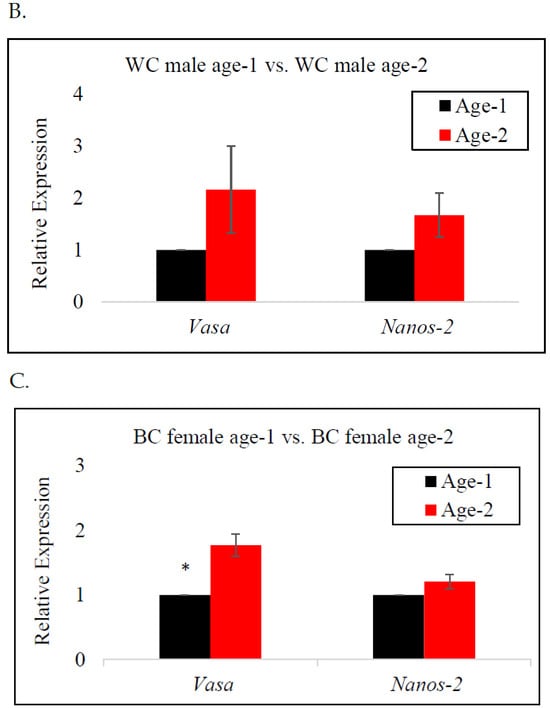
Figure 3.
RT-qPCR analysis of vasa and nanos-2 gene expression in age-1 vs. age-2: (A) female white crappie, (B) male white crappie, and (C) female black crappie. GAPDH was used as the control. The expression levels of vasa and nanos-2 for the age-1 group were set to 1. Each bar represents the mean ± standard error (SE) from three individuals. Asterisk (*) indicates significant differences (p ≤ 0.05) between age-1 and age-2 female BC, determined via t-test.
3.3. WC Gene Expression by Sex
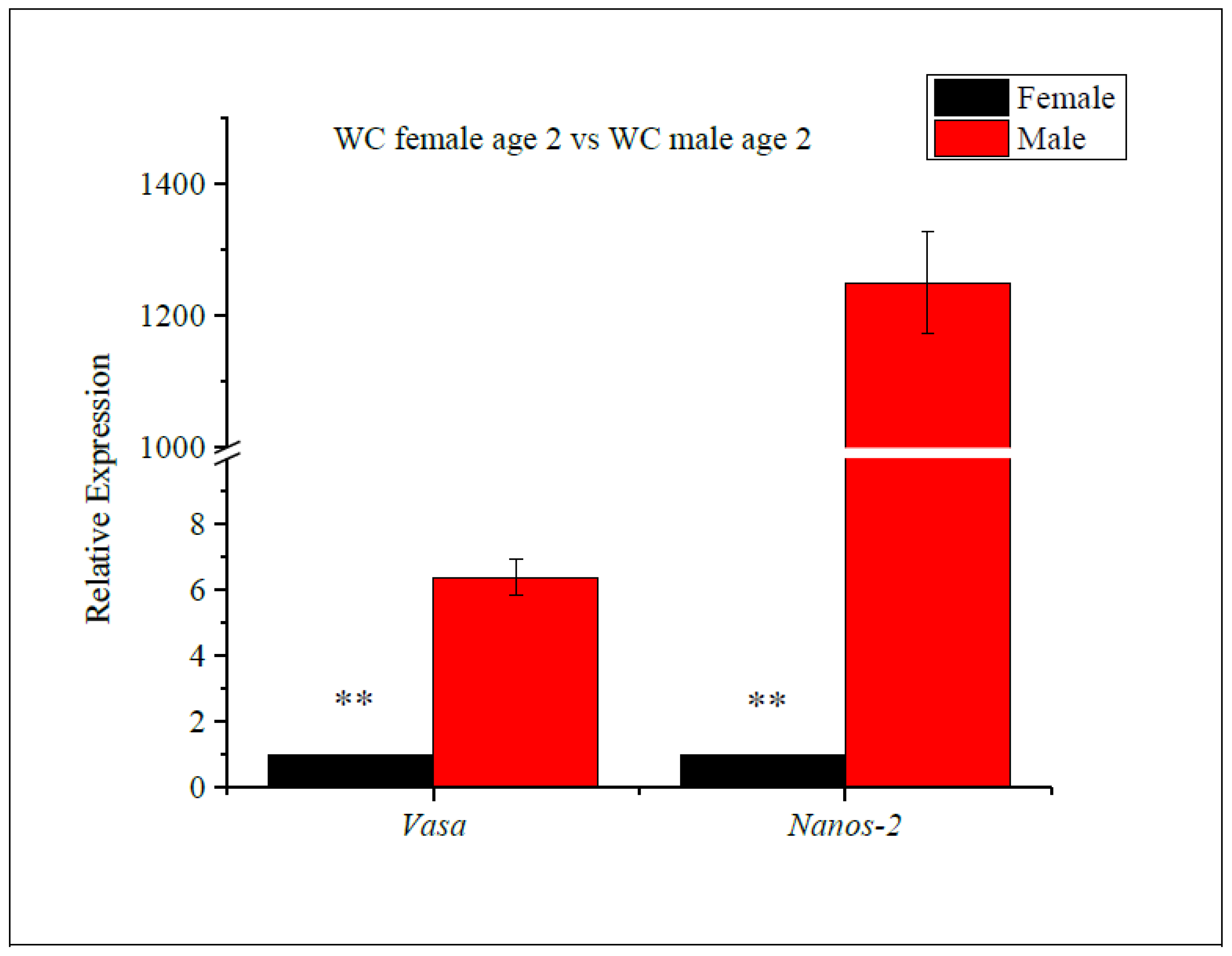
The expression levels of the vasa and nanos-2 genes were evaluated in age-2 WC females and age-2 WC males (Figure 4), and vasa and nanos-2 gene expression was found to significantly differ between the age-2 females and males. Comparing vasa gene expression, the fold change in males was upregulated by 6.4-fold compared to females, while the expression of the nanos-2 gene was upregulated by 1250-fold in males compared to females. Both genes were significantly upregulated in WC males, compared to females. These findings suggest that the sex of the WC influences the expression of the vasa and nanos-2 genes.
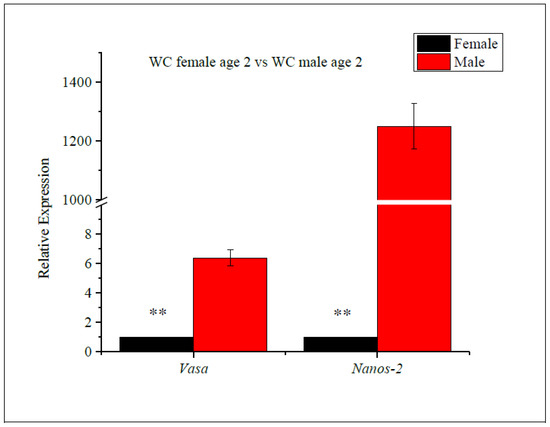
Figure 4.
RT-qPCR analysis of vasa and nanos-2 gene expression levels in female vs. male white crappie. GAPDH was used as the control. The expression levels of vasa and nanos-2 for females were set to 1. Each bar represents the mean ± standard error (SE) from three individuals. Asterisk (**) indicates significant differences (p ≤ 0.01) between age-2 females vs. age-2 males, determined via t-test.
4. Discussion
The key findings of this study are as follows: (1) The sequences of the vasa and nanos-2 genes in the considered species are conserved and significantly similar to fish species within the same and different families; (2) The expression of the vasa and nanos-2 genes does not significantly differ between age-1 WC and age-2 WC for both sexes; (3) The expression of vasa and nanos-2 genes is significantly higher in WC males than females; and (4) Partial sequences of the vasa and nanos-2 genes were identified and reported.
Several studies have reported sequence similarity among fish species within the same family, as well as among different vertebrate species. For example, the vasa amino acid sequence in cobia (Rachycentron canadum) was shown to have 86% sequence similarity with Seriola quinqueradiata, indicating a high percentage of conservation within the Rachycentridae family [24]. Similarly, the grass carp (Ctenopharyngodon idella) vasa homolog, CiVasa, showed significant similarity to the vasa homologs of medaka (75%) and zebrafish (78%), indicating its conserved nature among fish species within the same and different families [25]. Although the literature does not provide the exact percentage of sequence alignment for the nanos-2 gene across different fish species, the presence of nanos gene homologs and their roles in germ cell development and differentiation have been reported in various fish species, including zebrafish [26], medaka [27], rainbow trout [28], and orange-spotted grouper (Epinephelus coioides) [13]. In this study, the partial vasa gene sequences obtained from WC females and males showed significant similarity to the vasa homologs of largemouth bass (M. salmoides; 93.1–93.98%) and smallmouth bass (M. dolomieu; 91.95–92.77%), indicating its conserved nature within Family Centrarchidae. Further, the vasa gene sequence obtained from BC females showed high similarity with those of species of different families, including Scatophagidae (spotted scat), Lateolabracidae (Japanese sea bass), Cyclopteridae (lumpfish), Scombridae (southern bluefin tuna), Sciaenidae (large yellow croaker), and Cichlidae (Nile tilapia). Similarly, the nanos-2 gene sequences of WC females, WC males, and BC females showed significant similarity with the nanos-2 gene sequences of largemouth bass (92.92–96.36%) and smallmouth bass (92.92–96.36%). In addition, the nanos-2 gene sequences obtained from WC females and males also showed significant sequence similarity with the nanos-2 sequences of fish species belonging to different families, including Sciaenidae (large yellow croaker), Cyclopteridae (lumpfish), Sinipercidae (mandarian fish), and Moronidae (striped bass). Our findings support results in the existing literature regarding the conserved nature of vasa and nanos-2 genes among fish species within the same and different families.
In this study, the expression levels of vasa and nanos-2 were evaluated in age-1 and age-2 WC females and males, as well as BC females, in order to identify the appropriate age of donor fish for the cryopreservation of germ cells and germ cell transplantation to create xenogenic fish. Although the expression of the vasa gene in age-2 BC females was significantly higher than that in age-1 BC females, there was no significant difference in the expression of the vasa gene between age-1 and age-2 WC females and males. There was also no significant difference in nanos-2 gene expression between age-1 and age-2 WC females, WC males, and BC females. These results suggest that age-1 and age-2 WC females, WC males, and BC females can all be considered suitable donors for germ cell isolation and transplantation. In the available literature, the expression patterns of vasa and nanos-2 genes have been studied during early germ cell stages. For example, in the marbled goby (Oxyeleotris marmorata), vasa mRNA was strongly expressed in spermatogonia and was undetectable in somatic cells [29]. Similarly, in cobia (Rachycentron canadum), vasa mRNA was strongly expressed in spermatogonia and spermatids, as well as in early primary oocytes in the ovary [24]. In the marbled goby, the Vasa gene was not detected in the spermatids, and, in the cobia, the vasa gene was weakly expressed in spermatids and spermatozoa. The nanos-2 gene was specifically expressed in the germline stem cells in both the ovary and testis of zebrafish [30]. The expression of both vasa and nanos-2 genes was observed in the gonadal development of Nile tilapia (Oreochromis niloticus) testes [31]. Similarly, in Japanese sea bass (Lateolabrax japonicus), the vasa gene was highly expressed during early spermatogenesis and was regulated by external sex hormones, highlighting its importance in testicular development [32]. These studies highlight the functions of vasa and nanos-2 genes in developing and identifying germ cells in different fish species and provide an understanding of their conserved function among different fish species. As previously discussed, the literature suggests that expression of the vasa gene during early developmental stages varies between species. Similarly, in our study, vasa gene expression was significantly higher in age-2 BC females compared to age-1 BC females. In contrast, there was no significant difference in vasa gene expression between age-1 and age-2 WC females and males.
The findings of this study will be utilized in future studies on germ cell cryopreservation and germ cell transplantation to create a hybrid crappie using a xenogenic method. This research was focused on the testis and ovary of WC and only the ovary of BC due to specific future research needs—for example, both the testis and ovary of WC were considered, as it will be used as a donor species for future cryopreservation and germ cell transplantation experiments. As such, the identification of an appropriate donor WC will be beneficial for future studies. Meanwhile, the primary goal for BC will be cryopreserving its ovarian germ cells.
In this study, the expression levels of vasa and nanos-2 genes were significantly higher in the WC testis, compared to the WC ovary. A similar result has been reported in rock bream (Oplegnathus fasciatus), where the expression of the nanos-2 gene was significantly higher in testis than in the ovaries [33]. The predominant expression of nanos-2 has also been reported in mice (Mus musculus) testis [34]; this study further highlighted that eliminating the nanos-2 gene in mice results in a complete loss of spermatogonia, which comprises the early stage of sperm cells, indicating the essential function of the nanos-2 gene for male germ cell development. Therefore, the higher expression of vasa and nanos-2 genes in the testis compared to the ovary may be due to their important role in spermatogenesis, the maintenance of spermatogonial stem cells, and the continuous production of germ cells in male fish [32,35]. Therefore, in this study, we speculated that the nanos-2 gene was actively involved in the self-renewal and proliferation of spermatogonia in the WC testis.
In conclusion, this study provides the first report of the sequences of the important germ cell genes vasa and nanos-2 in white crappie (WC) and black crappie (BC), two popular sportfish species in the United States. Our study enhances the understanding of the expression levels of these genes with respect to age and sex in crappie species. The findings from this study provide a foundational step for further research on germ cell transplantation, germplasm conservation, and the creation of hybrid crappie using xenogenic techniques.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9100394/s1. Figure S1: Gel electrophoresis result, where 1 = BC ovary vasa, 2 = BC ovary nanos-2, 3 = WC ovary vasa, 4 = WC ovary nanos-2, 5 = WC testis vasa, 6 = WC testis nanos-2. Figure S2: Partial DNA sequence of white crappie (Pomoxis annularis) ovary vasa gene. Figure S3: Partial DNA sequence of the white crappie (Pomoxis annularis) ovary nanos-2 gene. Figure S4: Partial DNA sequence of white crappie (Pomoxis annularis) testis vasa gene. Figure S5: Partial DNA sequence of white crappie (Pomoxis annularis) testis nanos-2 gene. Figure S6: Partial DNA sequence of black crappie (Pomoxis nigromaculatus) ovary vasa gene. Figure S7: Partial DNA sequence of black crappie (Pomoxis nigromaculatus) ovary nanos-2 gene.
Author Contributions
Conceptualization, Methodology, Investigation, Formal Analysis, and Writing—Original Draft Preparation, S.B.; Funding Acquisition and Writing—Review and Editing, N.N.R. and A.M.K.; Conceptualization, Supervision, Project Administration, Funding Acquisition, and Writing—Review and Editing, D.A.P.; Methodology, Validation, and Writing—Review and Editing, S.J.; Writing—Review and Editing, A.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
U.S. Department of Agriculture’s National Institute of Food and Agriculture, Grant/Award Number: 2019-38821-29048.
Institutional Review Board Statement
The study conducted was approved by the University of Arkansas at Pine Bluff (UAPB) Institutional Animal Care and Use Committee (IACUC) (Protocol number: UAPB2018-10, Approval date: 17 October 2018).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
This material is based upon work supported by the U.S. Department of Agriculture’s National Institute of Food and Agriculture (Capacity Building Grant—2019-38821-29048). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture. Additionally, any mention of trade names, commercial practices, or organization does not imply endorsement by the USDA. We thank Emmanuel Asiamah, PhD for allowing us to use his Animal Biotechnology laboratory to conduct the research.
Conflicts of Interest
There are no conflicts of interest declared in this article.
References
- Yoon, C.; Kawakami, K.; Hopkins, N. Zebrafish Vasa Homologue RNA Is Localized to the Cleavage Planes of 2- and 4-Cell-Stage Embryos and Is Expressed in the Primordial Germ Cells. Development 1997, 124, 3157–3165. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kajiura-Kobayashi, H.; Nagahama, Y. Differential Expression of Vasa Homologue Gene in the Germ Cells during Oogenesis and Spermatogenesis in a Teleost Fish, Tilapia, Oreochromis niloticus. Mech. Dev. 2000, 99, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Liu, Q.; Li, M.; Li, Z.; Hong, N.; Li, J.; Hong, Y. Transient and Stable GFP Expression in Germ Cells by the Vasa Regulatory Sequences from the Red Seabream (Pagrus major). Int. J. Biol. Sci. 2012, 8, 882–890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, H.; Yue, H.-M.; Yang, X.-G.; Li, C.-J.; Wei, Q.-W. Identification and Sexually Dimorphic Expression of Vasa Isoforms in Dabry’s Sturgeon (Acipenser dabryanus), and Functional Analysis of Vasa 3′-Untranslated Region. Cell Tissue Res. 2016, 366, 203–218. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Du, S.; Wang, Y.; Zhao, H.; Du, T.; Yu, J.; Wu, L.; Song, Z.; Liu, Q.; et al. Germline Specific Expression of a Vasa Homologue Gene in the Viviparous Fish Black Rockfish (Sebastes schlegelii) and Functional Analysis of the Vasa 3′ Untranslated Region. Front. Cell Dev. Biol. 2020, 8, 575788. [Google Scholar] [CrossRef] [PubMed]
- Presslauer, C.; Nagasawa, K.; Fernandes, J.M.O.; Babiak, I. Expression of Vasa and Nanos3 during Primordial Germ Cell Formation and Migration in Atlantic Cod (Gadus morhua L.). Theriogenology 2012, 78, 1262–1277. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Tago, Y.; Takeuchi, Y.; Sawatari, E.; Kobayashi, T.; Takeuchi, T. Green Fluorescent Protein Labeling of Primordial Germ Cells Using a Nontransgenic Method and Its Application for Germ Cell Transplantation in Salmonidae. Biol. Reprod. 2005, 73, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hartung, O.; Forbes, M.M.; Marlow, F.L. Zebrafish Vasa Is Required for Germ-cell Differentiation and Maintenance. Mol. Reprod. Dev. 2014, 81, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.L.; Draper, B.W. Nanos3 Maintains Germline Stem Cells and Expression of the Conserved Germline Stem Cell Gene Nanos2 in the Zebrafish Ovary. Dev. Biol. 2013, 374, 308–318. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Shao, C.; Wang, N.; Chen, S. Identification, Characterization and Functional Analysis of Regulatory Region of Nanos Gene from Half-Smooth Tongue Sole (Cynoglossus semilaevis). Gene 2017, 617, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Asaoka-Taguchi, M.; Yamada, M.; Nakamura, A.; Hanyu, K.; Kobayashi, S. Maternal Pumilio Acts Together with Nanos in Germline Development in Drosophila Embryos. Nat. Cell Biol. 1999, 1, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Chen, S.; Cai, M.; Jiang, Y.; Zhang, Z.; Wang, Y. Nanos3 Not Nanos1 and Nanos2 Is a Germ Cell Marker Gene in Large Yellow Croaker during Embryogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 218, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-H.; Wang, Y.; Lu, W.-J.; Li, Z.; Liu, X.-C.; Li, S.-S.; Zhou, L.; Gui, J.-F. Divergent Expression Patterns and Function Implications of Four Nanos Genes in a Hermaphroditic Fish, Epinephelus coioides. Int. J. Mol. Sci. 2017, 18, 685. [Google Scholar] [CrossRef] [PubMed]
- De Keuckelaere, E.; Hulpiau, P.; Saeys, Y.; Berx, G.; Van Roy, F. Nanos Genes and Their Role in Development and Beyond. Cell. Mol. Life Sci. 2018, 75, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, X.; Wang, J.; Liu, B.-Z.; Wei, J.-L.; Zhang, W.-J.; Sun, Z.-H.; Chang, Y.-Q. Molecular Cloning and Sexually Dimorphic Expression Analysis of Nanos2 in the Sea Urchin, Mesocentrotus nudus. Int. J. Mol. Sci. 2019, 20, 2705. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.M.S.N.; Costa, G.M.J.; Campos-Junior, P.H.A.; Segatelli, T.M.; Yazawa, R.; Takeuchi, Y.; Morita, T.; Yoshizaki, G.; França, L.R. Germ Cell Transplantation as a Potential Biotechnological Approach to Fish Reproduction. Fish Physiol. Biochem. 2013, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G. Germ Cell Transplantation in Fish. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 764–768. [Google Scholar] [CrossRef]
- Culpepper, C.M.; Allen, P.J. Aquaculture Techniques for Crappie, Pomoxis spp., Culture. J. World Aquac. Soc. 2016, 47, 314–326. [Google Scholar] [CrossRef]
- Hwang, J.; Bi, X.; Morales, N.; Camp, E.V. The Economic Value of Freshwater Fisheries in Florida: An Application of the Travel Cost Method for Black Crappie Fishing Trips. Fish. Res. 2021, 233, 105754. [Google Scholar] [CrossRef]
- Hutt, C.P.; Hunt, K.M.; Steffen, S.F.; Grado, S.C.; Miranda, L.E. Economic Values and Regional Economic Impacts of Recreational Fisheries in Mississippi Reservoirs. N. Am. J. Fish. Manag. 2013, 33, 44–55. [Google Scholar] [CrossRef]
- Bhattarai, S.; Jones, J.H.; Renukdas, N.N.; Kelly, A.M.; Perera, D.A. Isolation, in Vitro Culture, and Characterization of Black Crappie, Pomoxis nigromaculatus and White Crappie, P. annularis Ovarian Tissue Primary Cells. J. World Aquac. Soc. 2023, 54, 1534–1545. [Google Scholar] [CrossRef]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ma, Q.; Kuang, J.; Chen, G.; Zhang, J.; Huang, J.; Mao, F.; Zhou, Q. Cloning and Expression Profiling of the Gene Vasa during First Annual Gonadal Development of Cobia (Rachycentron canadum). Fishes 2022, 7, 60. [Google Scholar] [CrossRef]
- Li, C.-J.; Liu, L.; Chen, X.-H.; Zhang, T.; Gan, F.; Cheng, B.-L. Identification of a Vasa Homologue Gene in Grass Carp and Its Expression Pattern in Tissues and during Embryogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Köprunner, M.; Thisse, C.; Thisse, B.; Raz, E. A Zebrafish Nanos -Related Gene Is Essential for the Development of Primordial Germ Cells. Genes Dev. 2001, 15, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Nakamura, S.; Ishikawa, Y.; Tanaka, M. Expression and Syntenic Analyses of Four Nanos Genes in Medaka. Zoolog. Sci. 2009, 26, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Bellaiche, J.; Lareyre, J.-J.; Cauty, C.; Yano, A.; Allemand, I.; Le Gac, F. Spermatogonial Stem Cell Quest: Nanos2, Marker of a Subpopulation of Undifferentiated A Spermatogonia in Trout Testis. Biol. Reprod. 2014, 90, 79. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Xiang, Y.; Jia, K.; Luo, M.; Yi, M. Molecular Characterization of Vasa Homologue in Marbled Goby, Oxyeleotris Marmorata: Transcription and Localization Analysis during Gametogenesis and Embryogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 229, 42–50. [Google Scholar] [CrossRef]
- Draper, B.W. Identification of Germ-Line Stem Cells in Zebrafish. In Germline Stem Cells; Buszczak, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1463, pp. 103–113. [Google Scholar] [CrossRef]
- Melo, L.H.; Melo, R.M.C.; Luz, R.K.; Bazzoli, N.; Rizzo, E. Expression of Vasa, Nanos2 and Sox9 during Initial Testicular Development in Nile Tilapia (Oreochromis niloticus) Submitted to Sex Reversal. Reprod. Fertil. Dev. 2019, 31, 1637. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Wen, H.; Ni, M.; Qian, K.; Zhang, P.; Chai, S. The Characteristics of Vasa Gene from Japanese Sea Bass (Lateolabrax japonicas) and Its Response to the External Hormones. J. Ocean Univ. China 2015, 14, 717–723. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Q.; Chen, R.; Liu, M.; Xu, D. Identification and Characterization of Dimorphic Expression of Sex-Related Genes in Rock Bream, a Fish With Multiple Sex Chromosomes. Front. Genet. 2021, 12, 791179. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Sasaoka, Y.; Kiso, M.; Abe, K.; Haraguchi, S.; Kobayashi, S.; Saga, Y. Conserved Role of Nanos Proteins in Germ Cell Development. Science 2003, 301, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Wen, H.; He, F.; Li, J.; Liu, M.; Ma, R.; Zhang, Y.; Hu, J.; Qi, B. Cloning and Expression Analysis of Vasa during the Reproductive Cycle of Korean Rockfish, Sebastes schlegeli. J. Ocean Univ. China 2013, 12, 115–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).