Abstract
In order to collect information on ichthyofauna of a deep-sea vulnerable marine ecosystem (VME) network along the Apulian margin (central Mediterranean Sea), two low-impact sampling tools were used in three VMEs characterized by cold-water corals (CWC), namely Bari Canyon (BC), off Monopoli (Mn), and off Santa Maria di Leuca (SML). Using an experimental longline, 53 deployments were carried out between a 314 and 650 m depth for a total of 217 fishing hours, whereas when using the baited lander MEMO (Marine Environment MOnitoring system), 31 deployments were carried out between 427 and 792 m, for a total of 223 h of video recordings. A total of 37 taxa were recorded, comprising 13 Chondrichthyes and 24 Osteichthyes. The similarities in species observed among the VMEs confirm the presence of a network of CWC-VMEs along the Apulian margin, whereas some differences detected are due to the different abundance of some species, such as Galeus melastomus, Helicolenus dactylopterus, and Phycis blennoides. The presence of commercial species, vulnerable/endangered cartilaginous fishes, and large and sexually mature individuals of G. melastomus, H. dactylopterus, and Pagellus bogaraveo in all the VMEs confirms that the network of CWC-VMEs along the Apulian margin can act as a network of refuge areas and an essential fish habitat (EFH) for species threatened by fishing activities.
Key Contribution:
This paper characterizes fishes distributed in the CWC-VMEs along the Apulian coast, highlighting differences among the different CWC-VMEs and also between the two different sampling tools. Information on sizes, maturity, and behaviour is also provided. The presence of vulnerable/endangered species according to the IUCN Red List category and commercial species confirms the importance of effective conservation measures.
1. Introduction
The deep sea, the part the ocean deeper than 200 m, represents the vastest ecosystem on Earth and provides ecosystem goods and services that are crucial to human wellbeing [1,2,3,4]. Among the biodiversity hotspots hosted by the deep sea ecosystem, cold-water coral (CWC) communities represent complex three-dimensional habitats that can provide reproductive areas and refuge to a large variety of valuable fish and invertebrates of commercial interest, both in the adult and juvenile stages [5,6,7,8]. CWC communities may act as a feeding area, a refuge from predators and fishing activities, and a spawning and nursery area for many fish species, and these areas generally show higher diversity and abundance than in adjacent soft bottom areas [6,8,9]. Although several studies have reported higher diversity and densities of ichthyofauna associated with CWC, it is still difficult to demonstrate whether the CWC habitat or its complexity are the attractive factor for fish species [9,10,11], Most studies, in fact, reported species that are distributed at comparable depth and common also in different types of habitats such as soft and rocky bottom areas. Although the fish fauna associated with CWC habitats may be not exclusive of this habitat, this species seems to benefit from the shelter provided by the structures built by the CWC and from the enhanced trophic conditions. A higher density of zooplankton, in fact, represents a potential trophic resource for planktivorous fish and can lead to a higher density of small invertebrates which are prey for benthic feeders and scavengers [6,8,12]. These ecosystems support a high biodiversity and high biomass, and they are impacted by commercial fishing activities causing extensive damage to CWC, resulting in productive ecosystems being transformed into coral rubble [13,14,15].
The Apulian margin (central Mediterranean) is characterized by the presence of an almost continuous belt of vulnerable marine ecosystems (VMEs) characterized by the presence of CWC communities whose real extension is still poorly understood. The Food and Agriculture Organization (FAO) indicates the following criteria for defining what constitutes a VME: (1) uniqueness or rarity; (2) functional significance of the habitat; (3) fragility; (4) life history traits of component species that make recovery difficult; and (5) structural complexity [16]. The exploration of the Apulian margin from the southern Adriatic to the northern Ionian Sea (central Mediterranean) has led to the discovery of several CWC communities distributed between 300 and 1100 m depths that can represent a network of VMEs mostly built by the colonial scleractinians Madrepora oculata and Desmophyllum pertusum [17,18,19]. CWC habitats have been described on the Gondola slide off Manfredonia, inside Bari Canyon (BC), off Monopoli, Otranto, Tricase, and up to the Santa Maria di Leuca (SML) CWC province, which is the largest living occurrence described in the Mediterranean Sea; lastly, off the Porto Cesareo marine protected area, living colonies of Dendrophyllia cornigera have also been observed down to a 217 m depth [17,18].
The exploration of fragile, heterogenous, and complex habitats like those built by CWC species requires the use of low-impact sampling techniques, such as experimental longlines or video systems, such as baited remote underwater video surveys (BRUVSs). A baited lander represents an effective method to explore sensitive habitats with complex geomorphology, such as seamount, canyon, and CWC communities, as well as their associated benthopelagic biodiversity [20,21,22,23,24,25,26]. In particular, the baited lander is a low-impact non-extractive tool for collecting data on megafauna diversity, abundance, and behaviour without damage to habitat former organisms and associated vulnerable species [10,21,22,23,24,27,28]. An experimental longline with a small number of hooks is a tool that can allow the capture of fish fauna with low impact in a heterogeneous and complex habitat, providing complementary information to that provided by BRUVSs [21,29]. Therefore, the aim of this study is to provide a further contribution to the knowledge of fishes and benthopelagic fauna distributed in the CWC-VMEs along the Apulian margin using low-impact sampling tools.
2. Materials and Methods
2.1. Area of Study
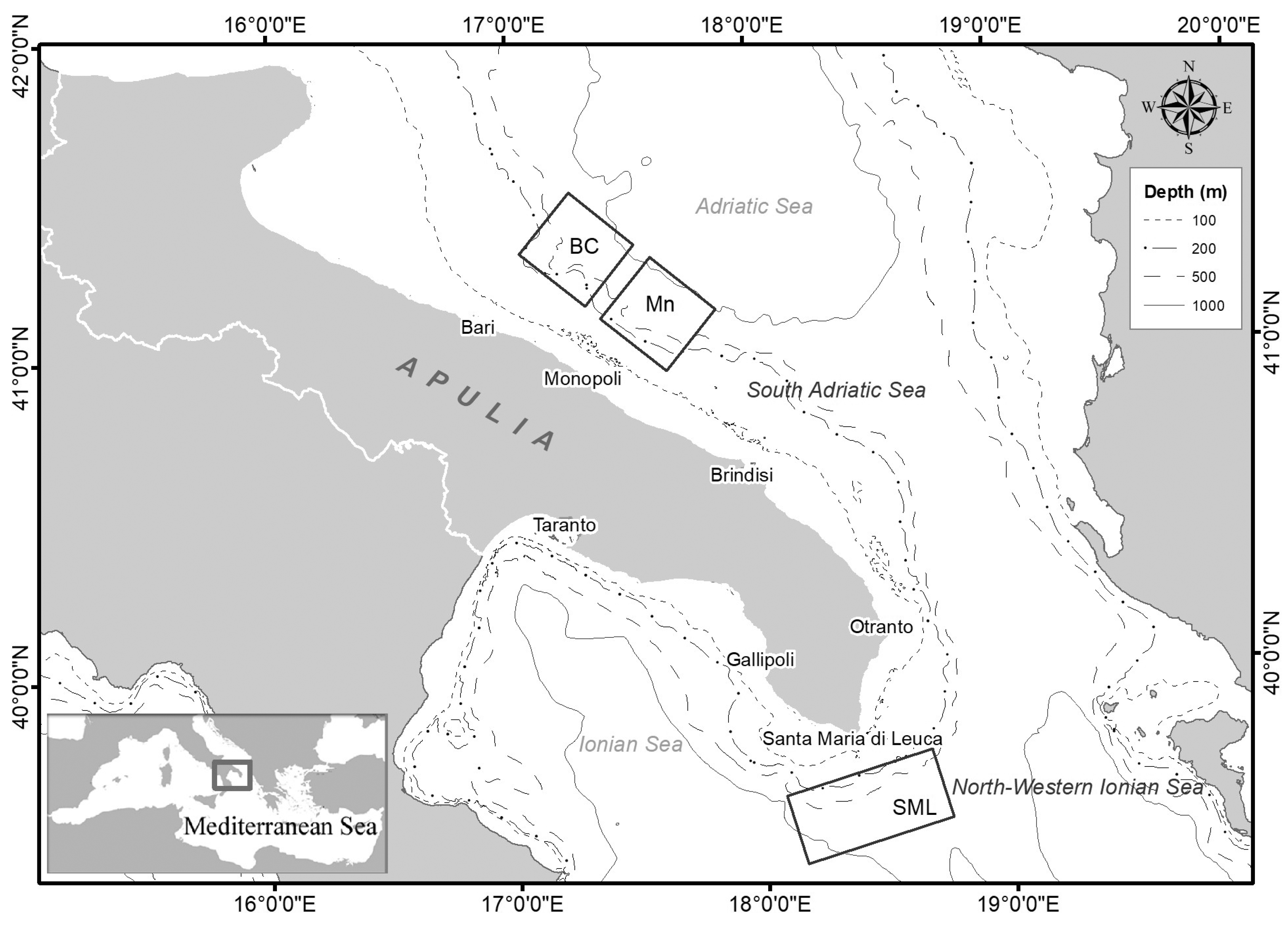
The study area is located in the central Mediterranean Sea along the Apulian margin between the southern Adriatic Sea and northern Ionian Sea (Figure 1). This area is characterized by the presence of several morphological and geological structures which incise the continental shelf and slope [30,31].
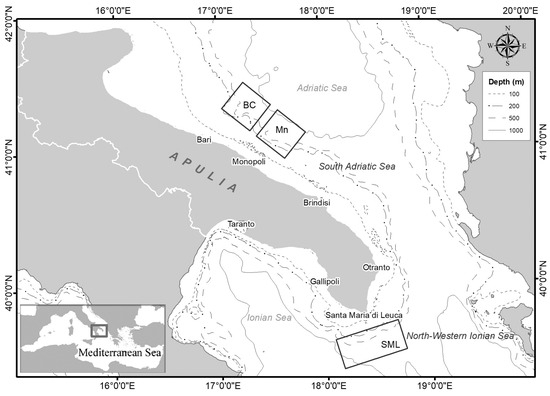
Figure 1.
Map of the study area with an indication of the areas in which the baited lander and longline were deployed (BC: Bari Canyon; Mn: Monopoli; SML: Santa Maria di Leuca).
The Bari Canyon is a complex morphological structure that breaches the southern Adriatic shelf with a west–east trend [30,31]. It is 10 km wide, 30 km long, and ranges between 200 and about 1000 m in depth. The Bari Canyon hosts a diversified community of deep-water cnidarians characterized by the presence of Madrepora oculata and Desmophyllum pertusum, together with Desmophyllum dianthus and Dendrophyllia cornigera, as well as Leiopathes glaberrima associated with sponges, serpulids, and bryozoans [19,22,23,32].
Recent explorations have revealed the existence of new CWC habitats southward of the Bari Canyon system in the area off Monopoli (Mn), where living colonies of M. oculata have been collected [18]. This area is characterized by the presence of erosive features and numerous shallowly incised and relatively straight canyons [33].
The northern Ionian Sea is characterized by the presence of the Santa Maria di Leuca CWC province, the largest occurrence of a living CWC community known in the Mediterranean [17,19]. Dead and living colonies of D. pertusum and M. oculata are distributed in an area of about 1000 km2 at a depth between 300 and 1110 m [17,34,35].
2.2. Survey Methodology and Data Analysis
Data were collected using two different tools, namely an experimental bottom longline and a baited lander in 3 VMEs characterized by the presence of CWC habitats along the Apulian coast, which were the Bari Canyon (BC) CWC province, off Monopoli (Mn), and the Santa Maria di Leuca (SML) CWC province (Figure 1). These sampling tools were used as a part of different national and international projects carried out between 2010 and 2019.
Using a longline, a total of 53 deployments were carried out between 314 and 650 m depths for a total time of about 217 fishing hours (Table 1). A commercial fishing vessel was hired and equipped with a monofilament longline with 500 hooks and baited with Sardina pilchardus as bait (see details in [18]). The soak time lasted about 4 h on average. The abundance of the species collected in each deployment was standardized as the number of individuals per hour of fishing on the seabed (N/h). Total length (TL) (mm) and sex were recorded for each specimen collected with the longline.

Table 1.
Sampling data for each area and tool, with an indication of time of the day explored and number of deployments realized in the depth strata (BC: Bari Canyon; Mn: Monopoli; SML: Santa Maria di Leuca).
Using the baited lander MEMO (Marine Environment MOnitoring system), 31 deployments were carried out between 427 and 792 m, for a total of 223 h of video recordings (Table 1). The MEMO lander consisted of a stainless-steel frame (ø 2.15 m; h 1.65 m) equipped with 2 video cameras (HD Multi Sea Cam) with two white LED lights, an electronic compass, inclinometer, and altimeter. There was a multiparametric probe for the measurement of pressure, temperature, conductivity, oxygen, pH, and turbidity, as well as a Doppler current meter, 4 Deep-Sea batteries (12 V-80 Ah), an acoustic modem, and an electronic control unit (Communication Technology, Srl, Cesena, Italy) Ltd.) capable of managing the entire system. On the seabed, the lander was linked by a zinc-coated steel cable to buoys, which kept the cable under tension (back-up buoys), and then to a surface floating buoy. The system was deployed to the seabed by winch and the surface buoy remained connected for recovery [24]. During each deployment, the MEMO lander was baited with fresh specimens of Scomber scombrus. The bait produces an odour plume that attracts the benthopelagic fauna in the field of video cameras. The videos recorded during each deployment were analyzed using Adobe Premier Pro software (version 8.1) and the different species recorded by the baited lander MEMO were identified to the lower taxonomical level using morphological characteristics. The taxonomic determination was based on the comparison with the World Register of Marine Species (WoRMS) [36]. For each species, MaxN was recorded as the maximum number of individuals of the same species recorded at the same time in the field of the camera. MaxN per hour was used to standardize the abundance as N/h in each deployment [29].
Non-metric multidimensional scaling (nMDS) ordination, based on a Bray–Curtis dissimilarity matrix derived from the fourth root transformation of the data, was applied to reveal multivariate patterns in the species assemblages [37]. In order to detect differences in the mean abundances of species amongst the three VMEs explored with the two sampling tools, a permutational multivariate analysis of variance (PERMANOVA) based on the Bray–Curtis dissimilarity matrix was conducted. PERMANOVA is a geometric partitioning of variation across a multivariate data cloud, defined explicitly in the space of a chosen dissimilarity measure in response to one or more factors in an analysis of variance design [38]. PERMANOVA is used to test the simultaneous response of one or more variables to one or more factors in an analysis of variance experimental design on the basis of any resemblance measure using permutation methods. PERMANOVA provides a useful statistical tool for the analysis of multivariate data on the basis of dissimilarity measures, allowing for a rigorous meaningful analysis of high-dimensional systems [38,39].
Similarity percentage analysis (SIMPER) was used to determine which species are responsible for the dissimilarity among the CWC-VMEs [37,40]. SIMPER analysis is a method used for comparisons among levels of a categorical variable, where the response matrix is expressed as a distance matrix, particularly the Bray–Curtis dissimilarity measure [36,40]. It is an effective method for assessing which taxa are primarily responsible for an observed difference between groups of samples. Statistical analyses were performed using the software PRIMER 7 [41].
Length–frequency distributions were computed for the fish species Galeus melastomus, Helicolenus dactylopterus, and Pagellus bogaraveo collected inside the CWC-VMEs using the longline.
Using the lander MEMO, for the shark species, the presence of scars or distinctive signs on the skin allowed us to discern different specimens. Therefore, for some identified specimens, the number of returns of each of them and the time interval between each return were counted and evaluated in some stations of the three VMEs. For all the specimens returning in the field of the video cameras, the behaviour toward the bait was recorded as no interaction (NI) toward the bait, exploring the bait (E), and feeding on the bait (F).
3. Results
3.1. Species Distribution and Abundance
A total of 37 taxa were recorded as follows (Table 2): 13 Chondrichthyes (seven species observed with the MEMO lander and 11 collected with the longline) and 24 Osteichthyes (16 recorded by the baited lander and 15 sampled with the longline); this last category was observed with a higher frequency in all three VMEs using both the longline and baited lander. The taxa observed in each VME with two sampling tools with an indication of depth range are reported in Table 2. Using the lander MEMO, among sharks, Etmopterus spinax showed a wide bathymetric range in all the VMEs, whereas Hexanchus griseus was observed across a wide depth range in BC and SML. Concerning bony fishes, Conger conger and Helicolenus dactylopterus showed a wide bathymetric distribution both in BC and SML, whereas Pagellus bogaraveo was observed in a wide bathymetric range both in BC and Mn. Of the 34 taxa recorded at the species level, only the species Hoplostetus mediterraneus is not assessed in the IUCN Mediterranean Red List (Table 2). The species assessed as Least Concern represented 60% of the species recorded by the two sampling tools, whereas 12% of the species are classified as Critically Endangered (Table 2). Elasmobranch were the taxonomic group with the highest number of species classified in the most critical level of IUCN Red Lists. Chimaera monstrosa and Dipturus oxyrinchus are classified as Near Threatened, Dalatias licha as Vulnerable, and four species of cartilaginous fishes are classified as Critically Endangered, namely Centrophorus granulosus, Leucoraja circularis, Leucoraja fullonica, and Prionace glauca. Somniosus rostratus is classified as Data Deficient (Table 2). Concerning the teleost fishes, Xiphias gladius and Merluccius merluccius are classified as Near Threatened and Vulnerable, respectively, and three species are classified as Data Deficient, namely Brama brama, Molva macrophthalma, and Polyprion americanus.

Table 2.
Taxa recorded in each CWC-VME (BC: Bari Canyon; Mn: Monopoli; SML: Santa Maria di Leuca) with lander MEMO and longline, with indication of depth range and conservation status according to IUCN Mediterranean Red List (CR: Critically Endangered; VU: Vulnerable; NT: Near Threatened; LC: Least Concern; DD: Data Deficient).
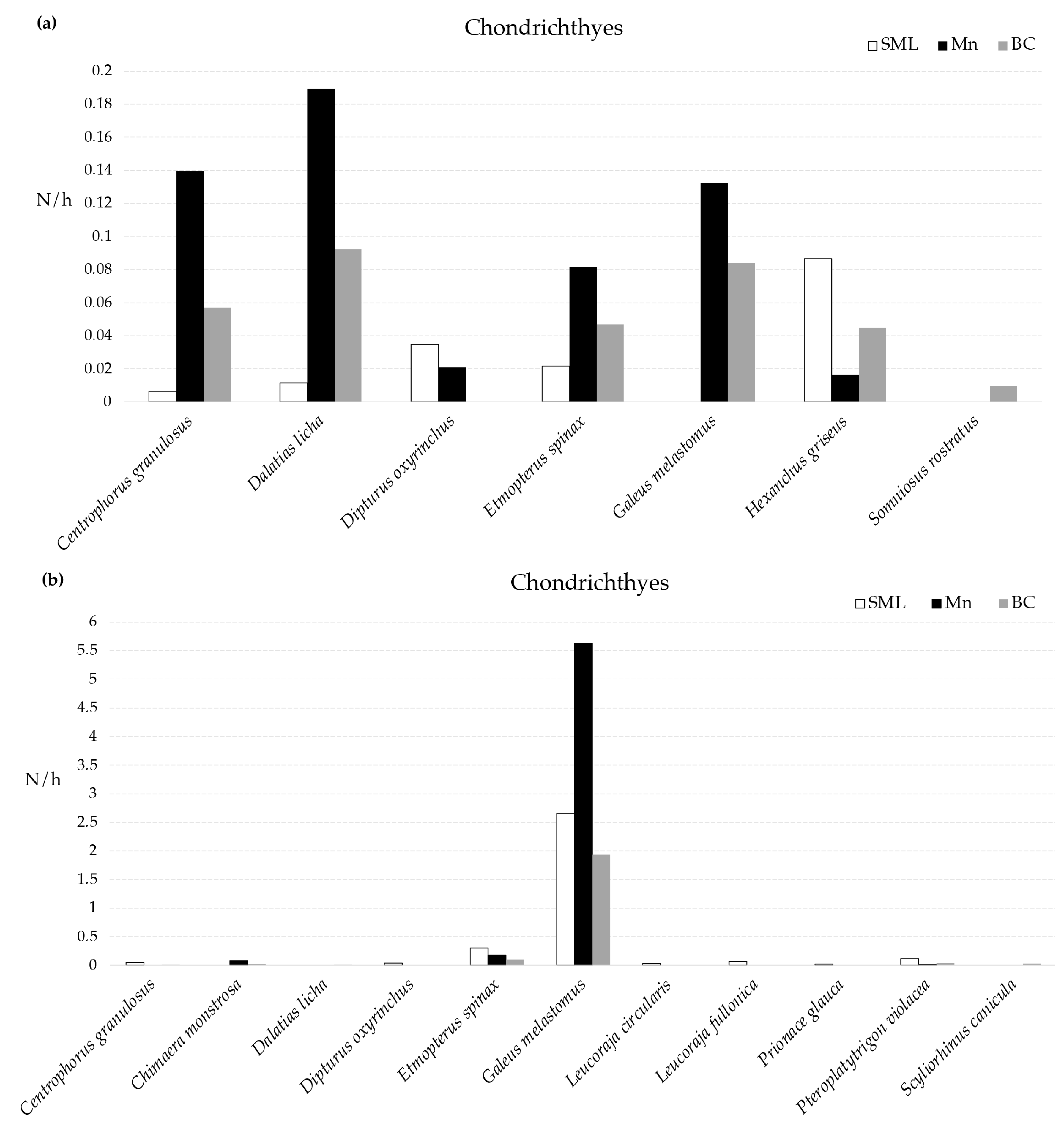
With regard to the abundance (N/h) of the taxa, using the MEMO lander, a total of four chondrichthyes were observed in all the VMEs, namely C. granulosus, D. licha, E. spinax, and H. griseus (Figure 2); D. licha was the most abundant species in both BC and Mn, whereas the most abundant species in SML was H. griseus (Figure 2). Using the longline, E. spinax, G. melastomus, and Pteroplatytrigon violacea were the only species collected in all VMEs, with G. melastomus being the most abundant species recorded in all the VMEs (Figure 2).
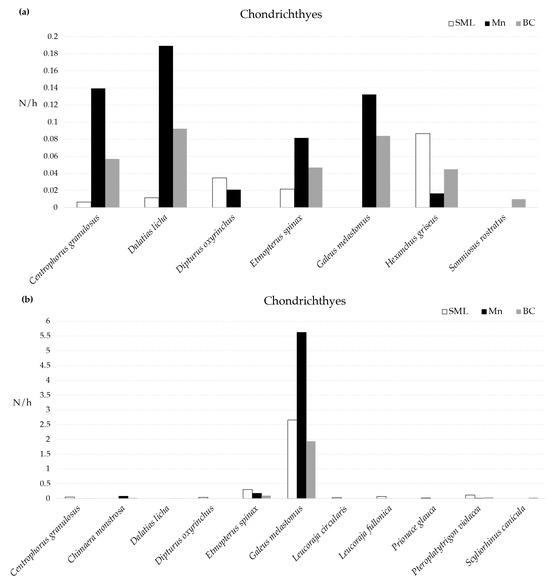
Figure 2.
Mean abundance (N/h) of cartilaginous fishes recorded in three VMEs using MEMO baited lander (a) and longline (b).
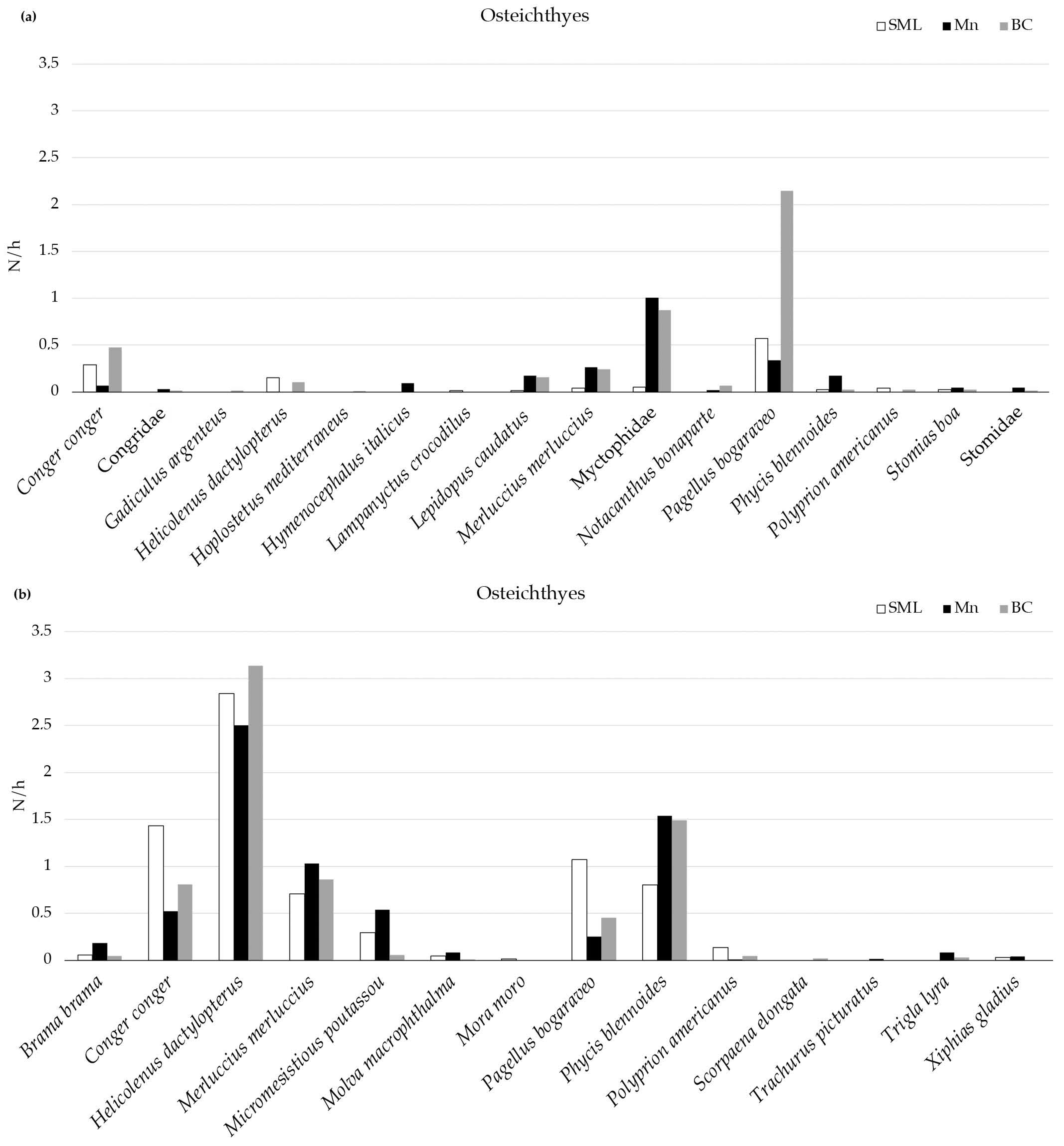
Among the teleost, a total of six species (C. conger, Lepidopus caudatus, M. merluccius, P. bogaraveo, P. blennoides, and Stomias boa) and the family Myctophidae were observed in all the VMEs. P. bogaraveo was the most abundant species in all the VMEs, followed by C. conger in BC and SML and M. merluccius in Mn (Figure 3). Using the longline, a total of nine species (Brama brama, C. conger, H. dactylopterus, M. merluccius, Micromesistious poutassou, Molva macrophthalma, P. bogaraveo, P. blennoides, and Polyprion americanus) were collected in all the VMEs, and H. dactylopterus was the most abundant in each area.
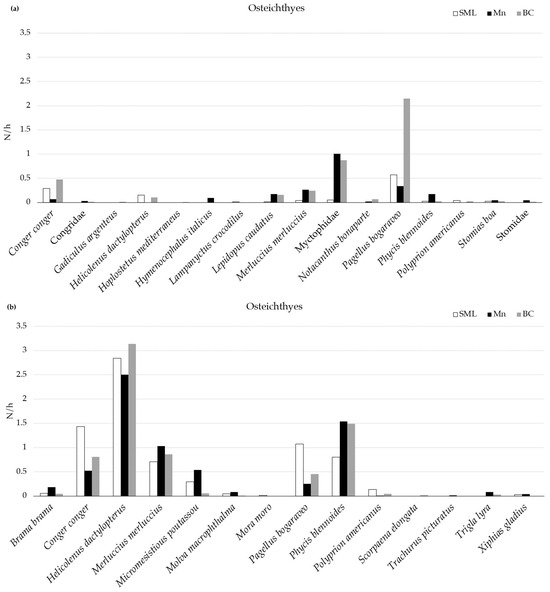
Figure 3.
Mean abundance (N/h) of Osteichthyes recorded in three VMEs using MEMO baited lander (a) and longline (b).
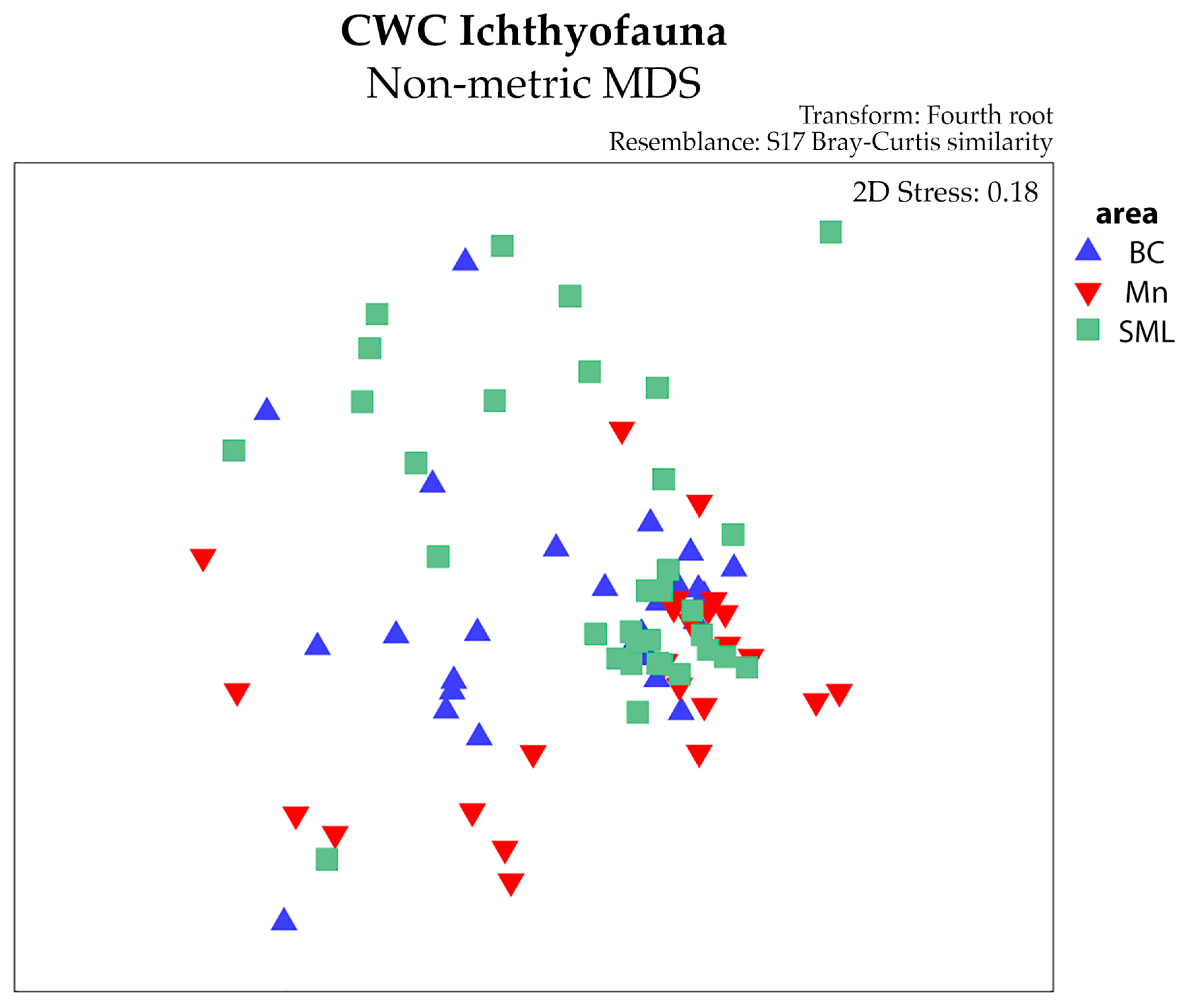
Although there was overlap between the areas observed using nMDS (Figure 4), PERMANOVA detected significant differences among the different CWC-VMEs and between the two different sampling tools (Table 3). This latter difference can be obvious due to the differences between the tools, but it is not the objective of the study. Regarding the CWC-VMEs, highly significant differences were found between BC and SML and between Mn and SML using both the MEMO lander and the longline (Table 4).
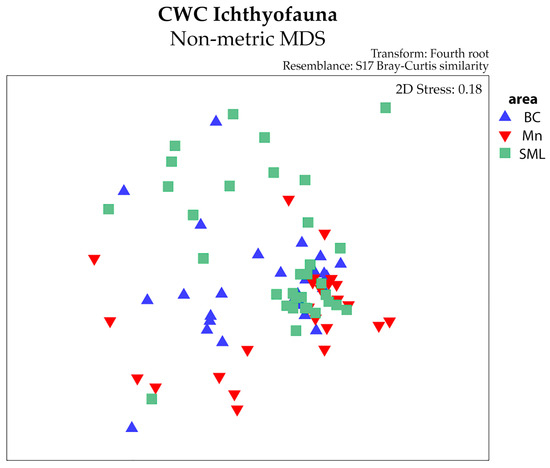
Figure 4.
Non-metric multidimensional scaling (nMDS) plot of abundance, distinguishing among the VMEs.

Table 3.
Results of the permutational analysis of variance (PERMANOVA) investigating differences between geographic areas and between sampling tools, based on Bray–Curtis dissimilarity matrix of distances of abundance (N/h) of species (9999 permutations).

Table 4.
Pairwise comparison of geographic area effect on the abundance indices of species (N/h) collected with MEMO lander and longline based on non-parametric permutation test (9999 permutations) (* = p < 0.05).
The SIMPER analysis highlighted that G. melastomus, H. dactylopterus, and P. blennoides mostly contributed to the dissimilarity between the two sampling tools (Table 5). The species that contribute to the dissimilarity between BC and Mn were G. melastomus, P. bogaraveo, and C. conger, whereas the dissimilarity between BC an SML is due to P. bogaraveo, M. merluccius, and G. melastomus (Table 6); lastly, the species P. bogaraveo, P. blenoides, and G. melastomus mostly contribute to the difference between Mn and SML (Table 6).

Table 5.
Results of similarity percentage (SIMPER) analysis of differences in species composition between MEMO lander (MEMO) and longline (LL).

Table 6.
Results of similarity percentage (SIMPER) analysis of differences in species composition between VMEs.
3.2. Size and Maturity
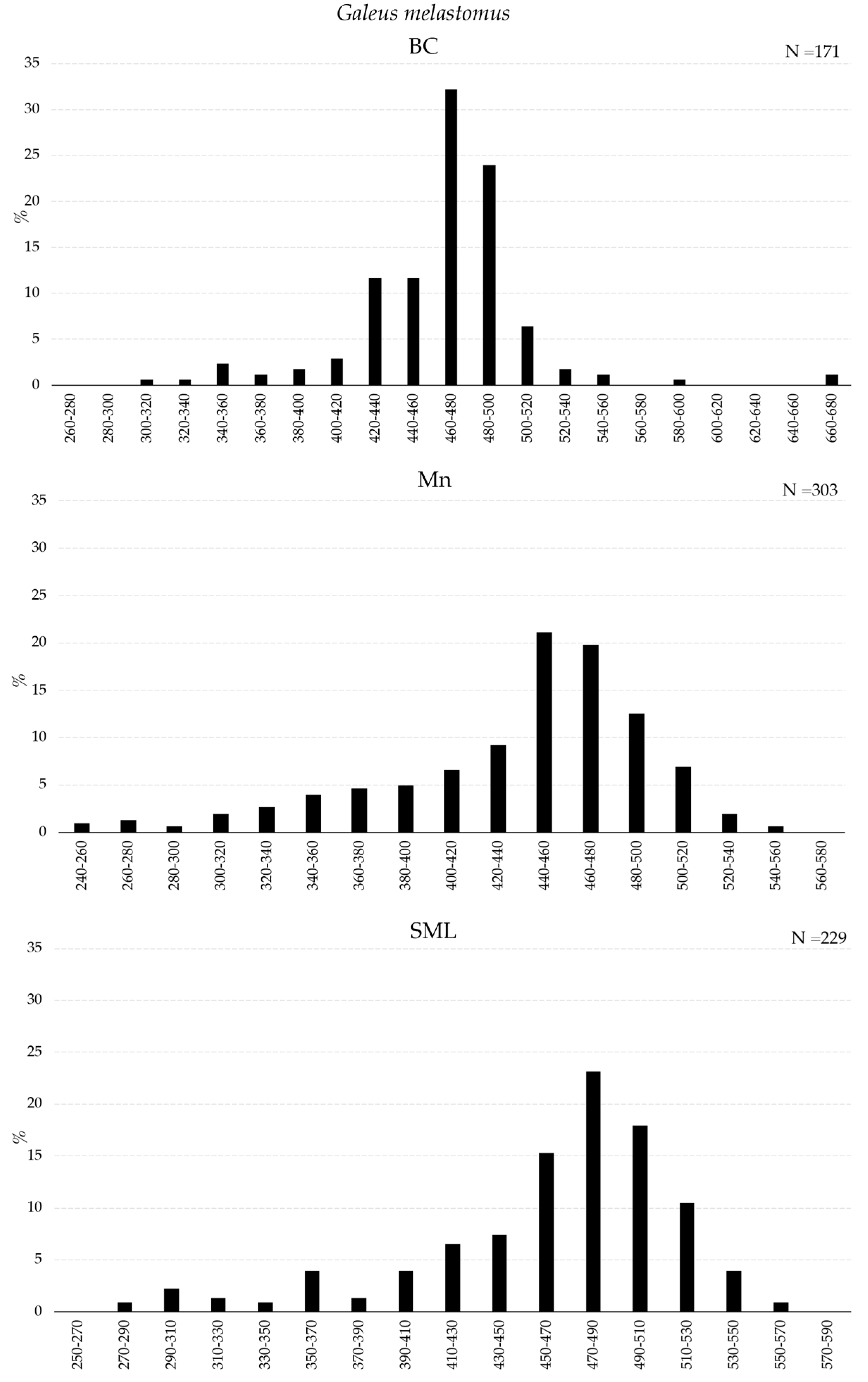
The size distributions of G. melastomus, H. dactyloptierus, and P. bogaraveo collected inside BC, Mn, and SML VMEs with the longline are presented in Figure 5 and Figure 6. G. melastomus showed a wide size range in all VMEs, with a greater fraction of individuals smaller than 400 mm in Mn and SML (Figure 5).
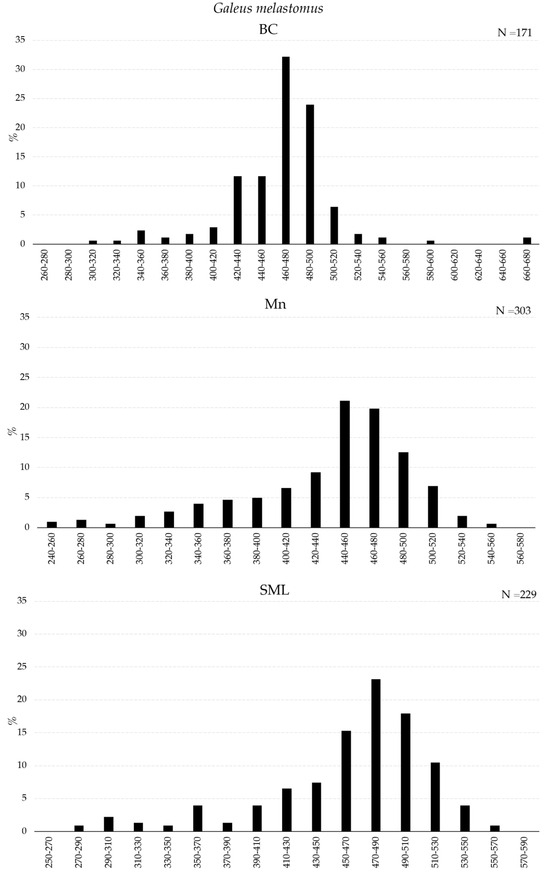
Figure 5.
Length–frequency distribution of Galeus melastomus collected with longline inside VMEs.
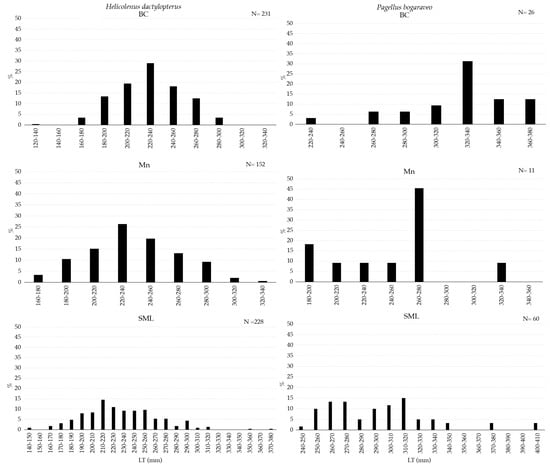
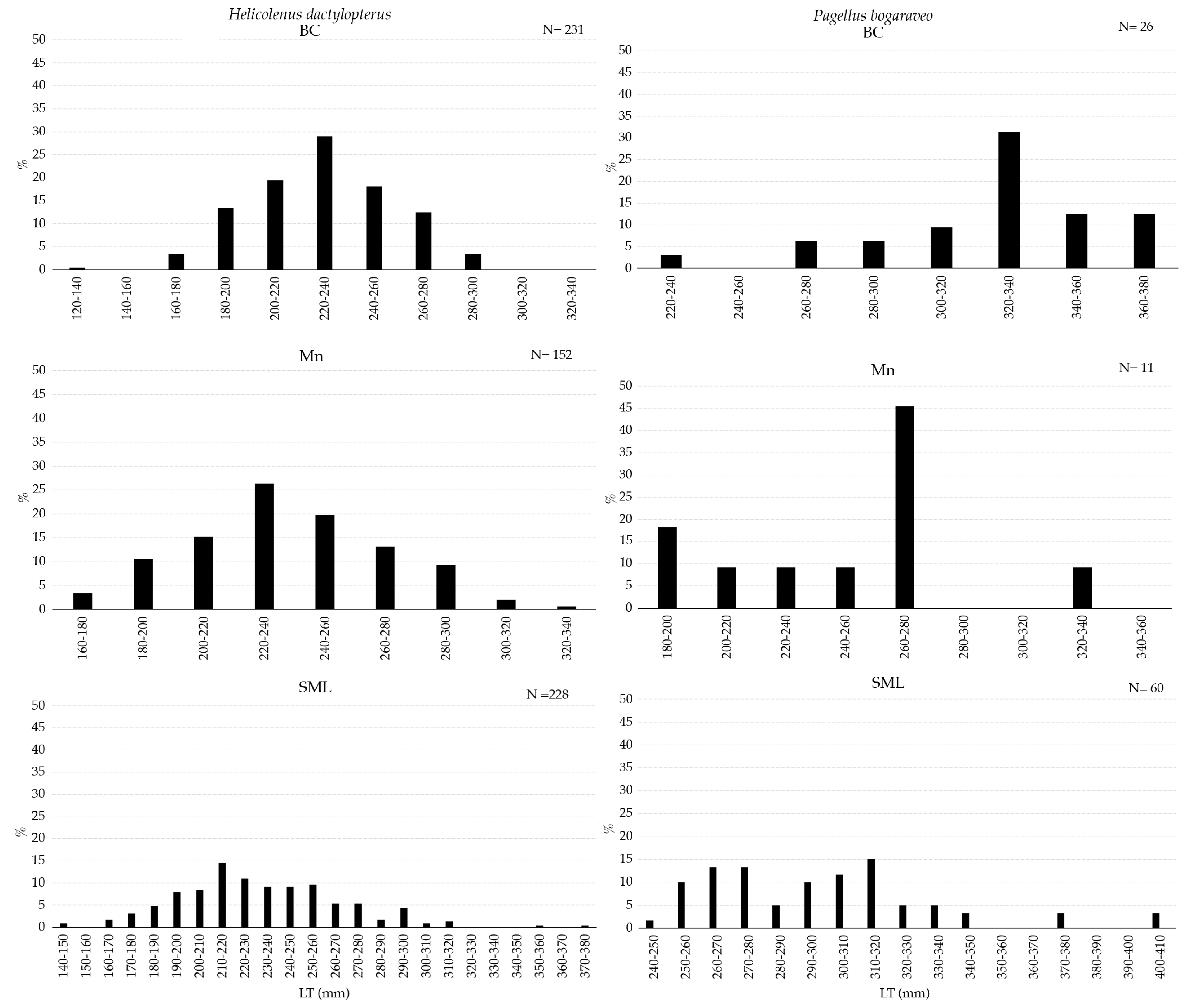
Figure 6.
Length–frequency distribution of Helicolenus dactylopterus (left) and Pagellus bogaraveo (right) collected with longline inside VMEs.
H. dactylopterus was sampled with the widest size range in the SML VME. However, the most abundant fraction of individuals was between 200 and 260 mm in all three areas (Figure 6). P. bogaraveo was collected in a greater number of individuals, mostly smaller than 320 mm, in SML (Figure 6).
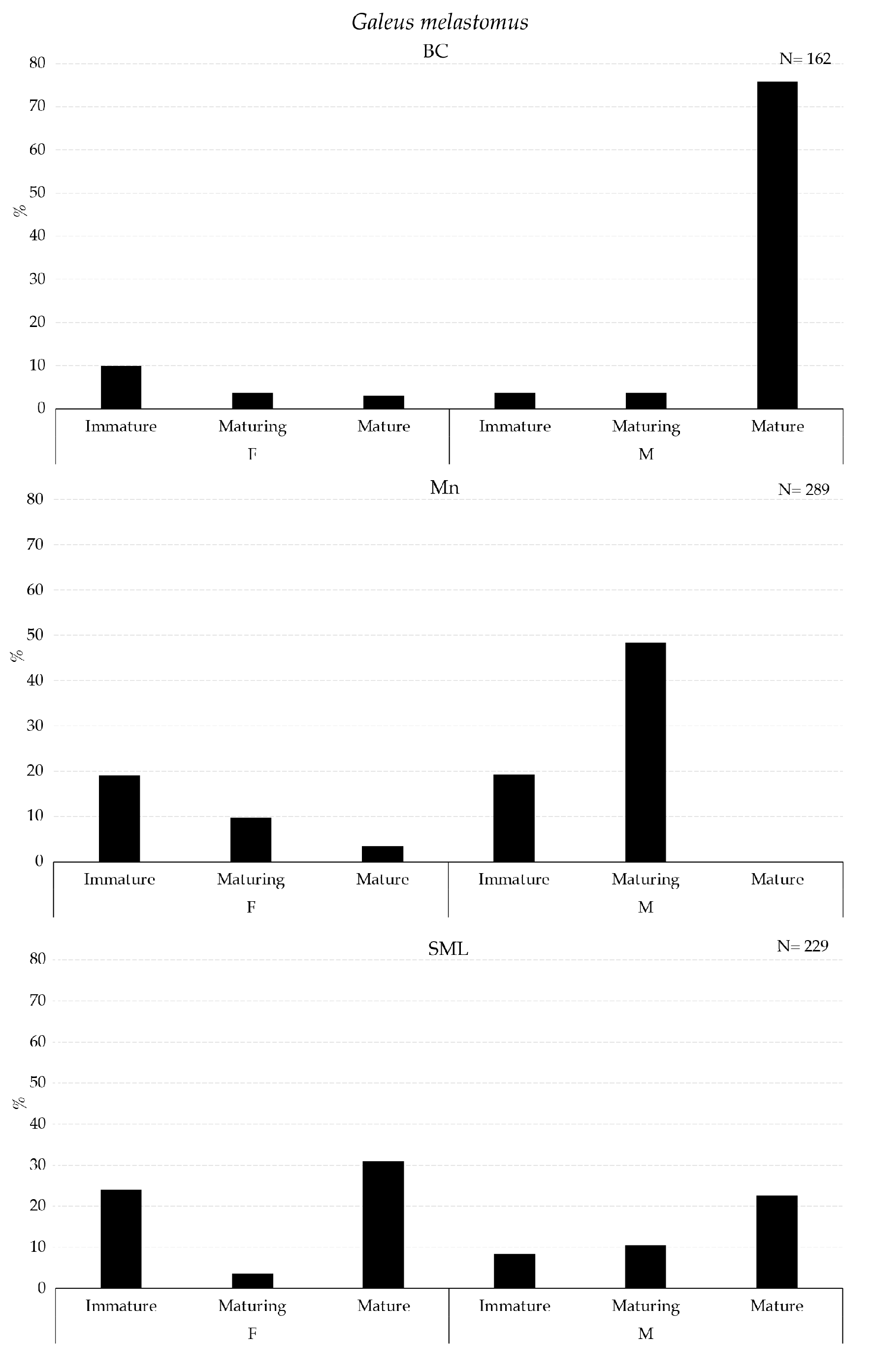
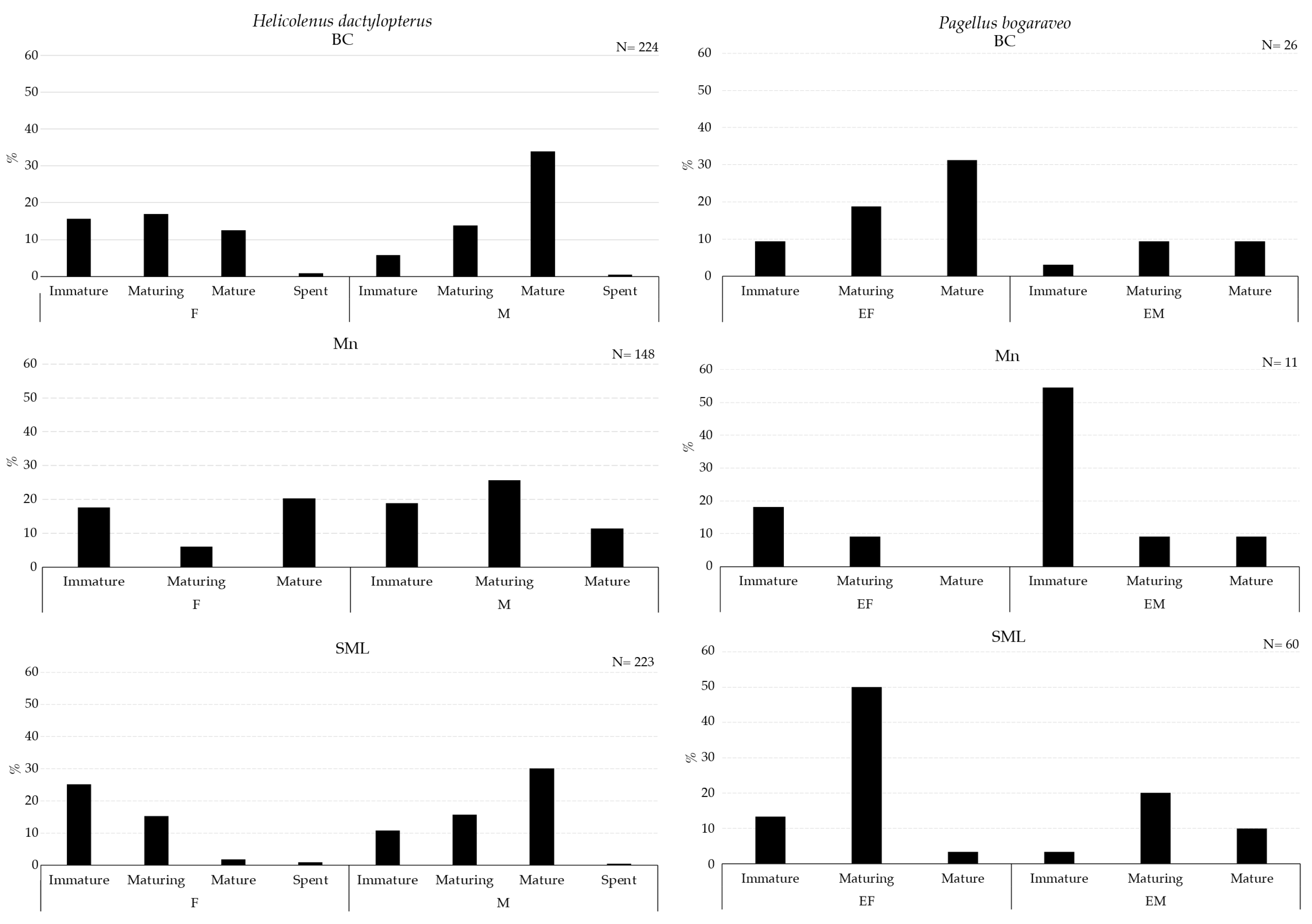
The maturity stage of the gonads of Galeus melastomus, Helicolenus dactylopterus, and Pagellus bogaraveo are presented in Figure 7 and Figure 8.
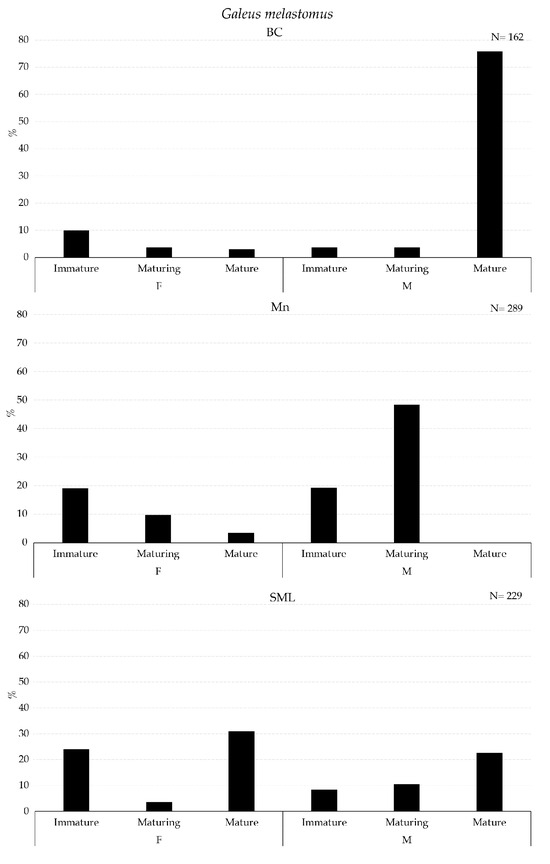
Figure 7.
Maturity stages of gonad for females (F) and males (M) of Galeus melastomus collected with longline inside VMEs.
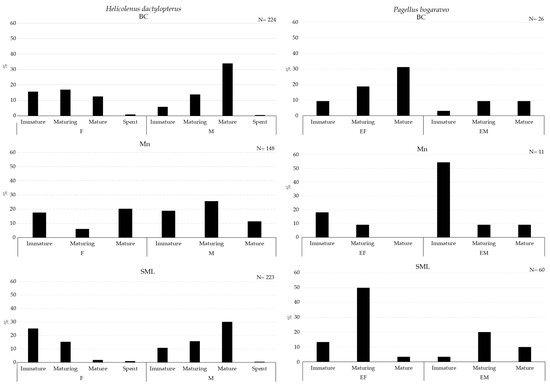
Figure 8.
Maturity stages of gonad for females (F) and males (M) of Helicolenus dactylopterus (left) and Pagellus bogaraveo (right) collected with longline inside VMEs.
A higher abundance of mature males of G. melastomus were collected in BC (Figure 7). Mature males of H. dactylopterus were collected inside BC and SML, whereas in both areas, immature and maturing females were collected in all three VMEs; moreover, spent individuals of both sexes were collected in BC and SML VMEs (Figure 8). Mature individuals of both females and males of P. bogaraveo were collected inside BC and SML VMEs (Figure 8).
3.3. Behaviour
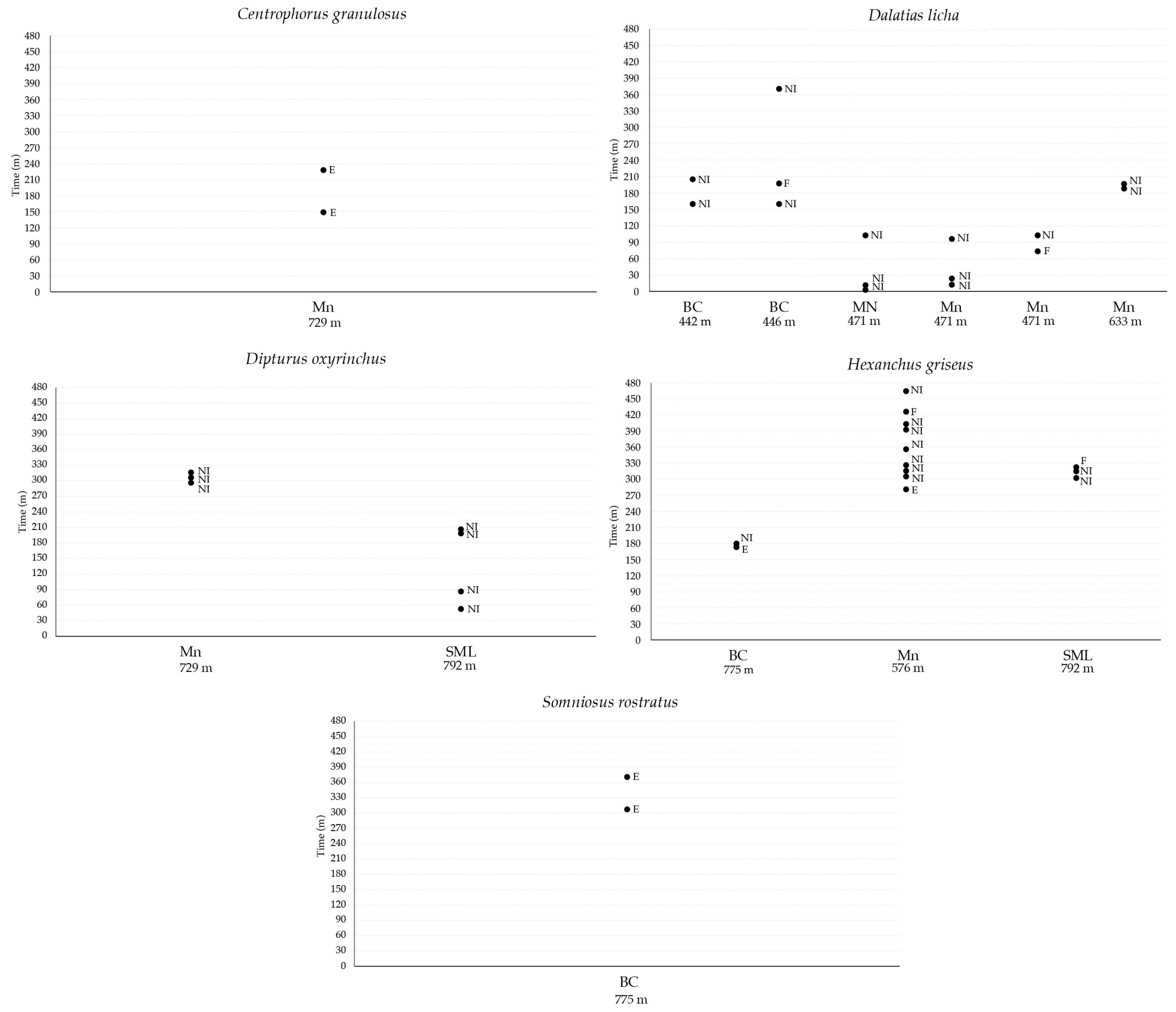
Observing the presence of scars or distinctive signs on the skin, it was possible to discern different specimens of cartilaginous fish returning to the field of the video camera during the same deployment (Table 7, Figure 9). Of the total 10 specimens of C. granulosus observed in all the VMEs, only one, a male characterized by the presence of a white dot on the head, was observed returning to explore the bait after 78 min in the deployment realized in Mn at a 729 m depth; moreover, the same specimen was already recorded in the deployment realized one year before in the same VME at a 633 m depth, suggesting fidelity to the area. A total of nine specimens of D. licha were recorded in all the deployments and six were observed returning during the deployments. In the deployment realized in BC at a 442 m depth, a female was observed returning after 45 min of video recording without interaction with the bait, whereas in the deployment at a 446 m depth, a male was observed returning two times after 60 min to feed on the bait and after 173 min of video recording without interaction with the bait. In Mn, in the deployment realized at a 471 m depth, all the specimens of D. licha returned in the field of view of the video camera. In particular, two females with different patterns of marks on their skin returned two times without interaction with the bait, whereas the male returned only one time 29 min after feeding on the bait; lastly, in the station realized at a 633 m depth, a male of D. licha was observed returning after 7 min without interaction with the bait. A total of four specimens of D. oxyinchus were recorded in all the VMEs, and two of them were observed returning without interaction with the bait during the deployments realized in Mn and SML at 729 and 792 m depths, respectively. In particular, in Mn, a female was observed returning two times after 6 min in both cases, whereas in SML, a male was observed returning three times after 24, 108, and 5 min of video recording. A total of 11 specimens of H. griseus were recorded in all the VMEs, and three were observed returning during the deployments. In BC at a 775 m depth, a specimen was observed returning one time after 6 min of video recording and a feeding event on the bait, whereas in the deployment realized in Mn at a 576 m depth, a big female was observed returning eight times during the same deployment; in these returns, the specimen was observed exploring the bait during the first arrival and then in the six subsequent returns, no interaction with the bait was recorded until the penultimate return, in which the female fed on the bait. Lastly, in SML at a 792 m depth, a specimen was observed to return twice after 8 min in both cases, and in the last return, it was observed to be feeding on bait. Somniosus rostratus was recorded only in BC at a 775 m depth, and it was observed returning only one time after 53 min of video recording to explore the bait.

Table 7.
Shark specimen identification with indication of deployment characteristics, number of returns of each specimen during deployment, and time interval between each return.
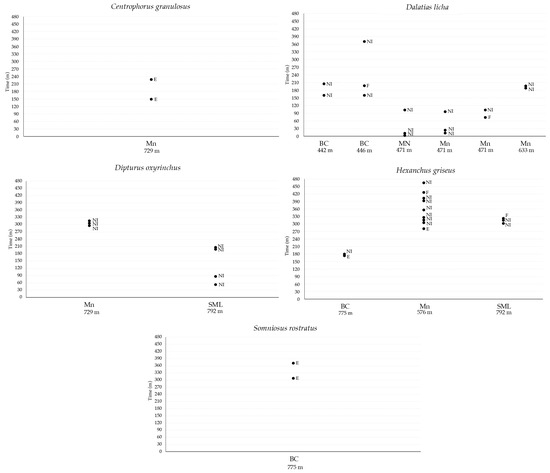
Figure 9.
Returning times of single specimens of C.granulosus, D. licha, D. oxyrinchus. H. griseus, and S. rostratus with indication of behaviour observed toward the bait recorded as no Interaction (NI), exploring the bait (E), and feeding on bait (F).
4. Discussion
The utilization of two low-impact sampling techniques allowed for the collection of new information on the distribution and abundance, size, and maturity of fishes associated with deep CWC-VMEs along the Apulian margin. The use of a baited lander allowed for the detection of species which can be barely sampled with traditional sampling tools and provided observations on the behaviour of some cartilaginous fishes.
The faunal assemblages of the VMEs are representative of the deep Mediterranean Sea and are also related to the presence of scavenger species attracted by the odour plume produced by the baits. Although the presence of several species in common in all the VMEs, differences among the VMEs could be explained by the different densities of some species. The highly significant difference between BC and SML and between Mn and SML detected by the MEMO lander can be explained with a higher abundance of sharks recorded both in BC and Mn and by the absence of G. melastomus in the video recorded with the MEMO lander in SML.
Although a wide period of sampling (2010–2019) could be subjected to environmental changes, the samplings for this study were conducted in deep sea habitats characterized by stable environmental conditions and small fluctuations in the sea bottom temperature [42]. The VMEs explored are also characterized by occasional fishing pressure due to the presence of conservation measures such as the FRAs instituted in BC and SML and the presence of an irregular morphology of the seabed. Moreover, information on the species assemblages of the trawlable muddy bottom of the northwestern Ionian Sea was collected by Maiorano et al. [43], who found no significant variations in the distribution and abundance of the fish species collected inside VMEs during the present study. Lastly, the species observed with the MEMO lander and collected using the experimental longline are characterized by high longevity that could minimizes the effects of a long period of sampling.
The most abundant teleost fish in all the VMEs explored with the MEMO lander was P. bogaraveo, whereas in the same VMEs, using the experimental longline, the most abundant species was H. dactylopterus. This could be due to the different depths and period of the day explored with the two different sampling tools and the vertical migratory behaviour of P. bogaraveo, which shows a shallower distribution during the daytime and a deeper distribution due to migration during dusk and early nighttime [44,45]. Capezzuto et al. [46] detected a relevant device effect for H. dactylopterus, showing higher abundances for a longline than for a baited lander. This species is a typical sit-and-wait ambush predator, feeding mainly on benthic crustaceans and fishes, as well as on planktonic organisms [47]. H. dactylopterus is frequently associated with submarine canyons and CWC habitats [6,26,48,49]. This species, in fact, is the most abundant species collected close to corals in the eastern Ionian Sea [50], in the northwestern Ionian Sea CWC province [8], in the Quirra Canyon (Tyrrhenian Sea) [51], and in French Mediterranean submarine canyons [52]. This habitat preference can be explained by the enhanced availability of zooplankton and small crustaceans, which are suitable prey for H. dactylopterus [47,49].
Although M. merluccius is one of the most abundant species collected using the longline in all the VMEs, its recorded abundances with the MEMO lander in the same VMEs were low. This could be explained with the daily vertical migration carried out by this species and the different period of the day explored with the two different sampling tools. M. merluccius, in fact, feeds in mid-water or near the surface during the night and spends extended periods of time near the seabed in the daytime [53,54]. Using both sampling techniques, C. conger was recorded in all the VMEs explored in this study. This species is a large opportunistic feeder, feeding mainly on bethopelagic and benthic prey, with fishes as main prey group [55]. This species shows a preferential distribution in complex habitats such as rocky bottom areas and those built by CWC [7,8,55].
Phycis blennoides was also observed with lower densities than those detected using the longline in the same VMEs. This highlights the importance of using different low-impact tools to have more complete information on the biodiversity of the megafauna in heterogenous and complex deep sea sensitive habitats. The two sampling tools, in fact, have a different efficacy and selectivity. The dimension of the hook used in the longline can be selective in terms of species composition and dimension of the individuals, whereas the presence of the lights on the baited lander could attract or lead to light avoidance in some species. Given these differences, these sampling tools should be used simultaneously to obtain reliable information on ichthyofauna biodiversity. However, the comparison of the two tools is not an objective of this study.
P. bogaraveo showed a higher abundance of mature females in BC, whereas a higher abundance of immature and maturing specimens was collected in Mn and SML. This species seems to prefer complex habitats such as CWC, canyons, and seamounts, where usually larger individuals are more abundant [8,44,56]. P. bogaraveo shows an ontogenetic habitat shift with juveniles up to 180 mm in TL mainly distributed in shallower waters and muddy bottoms, and larger individuals distributed in deeper water preferably characterized by a three-dimensional habitat [56,57]. The residency of sub-adults and adults of P. bogaraveo at the Condor seamount (Azores, mid-north Atlantic) was confirmed through acoustic telemetry [44]. The strict association of this species with CWC habitats was also confirmed by D’Onghia et al. [8], comparing megafauna distribution in coral versus non coral habitats; this species, in fact, was collected exclusively in coral habitats. P. bogaraveo, moreover, was exclusively collected inside BC using the longline [58]. These findings suggest that this species is more abundant in habitats less accessible to trawling such as CWC habitats and canyons.
The fact that a male specimen of C. granulosus was recorded after one year in the same VME off Monopoli is of crucial importance, since this can give an indication of some form of site fidelity that this shark shows to the VME, in which it can find trophic resources or protection from fishing activities carried out on surrounding muddy bottoms. C. granulosus is a large deep-water shark that lives in the outer continental shelf and upper slope of the Mediterranean Sea from 100 to 1200 m in depth and is classified as Critically Endangered in this basin. It is a very active feeder and usually preys on teleost and squids [59,60]. D. licha, a shark classified as Vulnerable in the Mediterranean, was the species that was observed returning most often in the same deployment, especially in Mn. This could be due to the very slow swimming speed measured for this species [61], leading to very short and limited movement from the MEMO lander and indicating probable fidelity to the area. This may also be true for other species, such as H. griseus and D. oxyrinchus, but only a greater number of observations could confirm the fidelity to the area.
The presence of commercial species and cartilaginous fishes and the presence of large and sexually mature individuals of G. melastomus, H. dactylopterus, and P. bogaraveo in all the VMEs confirm that the network of CWC-VMEs along the Apulian margin can act as a network of refuge areas for some species exploited during fishing activities in the surrounding muddy bottoms [5,7,8,18,58,62,63]. CWC communities and submarine canyons indeed represent suitable areas in which these species can spend crucial phases of the life cycle such as reproduction and spawning, thus providing an essential fish habitat (EFH) for species threatened by anthropogenic impacts such as fishing activities carried out on the seabeds surrounding the VMEs. Bottom trawling is one of the most important anthropogenic threats to CWC ecosystems [6,13,64,65,66] but the indirect impact of habitat destruction on the demersal resources is still poorly explored.
The main impact of trawling on CWC communities is mechanical damage and the destruction of the three-dimensional structures of the colonies. The impact of bottom trawling on CWC habitats has been widely documented in the Atlantic and in the Mediterranean Sea [13,14,15,35,67,68,69,70,71]. In SML, Savini et al. [35] recorded trawling traces using an ROV and D’Onghia et al. [13] observed the presence of longlines entangled in corals and trawl scars using towed cameras. In northwestern Sicily (southern Tyrrhenian Sea), the most important anthropogenic impact on CWC habitats, observed using an ROV, was represented mainly by longlines and ropes entangled with or hanging between rocks and organisms, causing heavy impacts on the community [65]. In addition to the direct destruction caused by fishing activities, bottom trawling alters the sedimentary condition through the resuspension of large amount of sediment due to the mechanical effects of the fishing gear [69,70].
The governance of VMEs requires information regarding biodiversity and the presence of endangered species and the identification and protection of habitats that can act as spawning and nursery areas for these species [6,72]; this necessitates the utilization of low-impact sampling methods in order to propose new and more effective conservation measures. A baited lander is a low impact non-extractive sampling method, and its non-destructive nature allows for its deployment in structurally complex habitats, such as CWC communities and submarine canyons. A BRUVS is a passive sampling tool that enables the investigation of the abundance and behaviour of rare and threatened species and the exploration of sensitive and vulnerable habitats that could be damaged by traditional sampling tools [21,24,73]. BRUVSs allow for the direct observation of species behaviour and enable the analysis of macro- and megafauna interaction and feeding behaviour [23,27,74]. Lastly, the videos recorded by the benthic lander can be examined by different observers, allowing for impartial and repeatable data collection [24,27,73]. However, some difficulties should be considered when using a baited lander. The precise positioning of a benthic lander can be difficult, especially in complex habitats in which a free-falling lander could miss the target. Moreover, a baited lander attracts principally scavenging species due to the presence of the bait, which make a BRUVS a selective sampling tool [24]. Lastly, although the videos are permanent and can be analyzed several times, the correct identification of ichthyofauna to the species level can be difficult.
Currently, the conservation measures adopted for the CWC ecosystems along Apulian coasts are the fishery-restricted areas (FRAs) established by the General Fishery Commission for the Mediterranean (GFCM). In particular, the SML CWC province FRA was established in 2007, whereas the BC CWC province FRA was established more recently in 2021. Although CWC ecosystems are included in the list of VMEs and in Annex I of the Habitat Directive, none of the CWC areas along the Apulian margin have been designated as a Natura 2000 site, which is an important European conservation tool based on the Habitat (92/43/EEC) and Birds (2009/147/EEC) Directives [75,76]. The institution of a network of high-sea marine protected areas and offshore Natura 2000 sites could represent a more effective conservation measure that can ensure the protection of vulnerable ecosystems and species in combination with the management of deep-water fishery resources.
5. Conclusions
In this study, two low-impact sampling tools were used to gain new insights into ichthyofauna diversity and abundance in a network of CWC communities along the Apulian margin. Using an experimental longline, H. dactylopterus was the most abundant species in all the VMEs explored, whereas using a baited lander MEMO, P. bogaraveo was the most abundant species. The presence of vulnerable/endangered and commercial species and the large and sexually mature individuals of G. melastomus, H. dactylopterus, and P. bogaraveo in all the VMEs confirm that the network of CWC-VMEs along the Apulian margin can act as a network of refuge areas for some species exploited during fishing activities in the surrounding muddy bottoms. These findings highlight the importance of effective surveillance and the enhancement of conservation measures for deep sea ecosystems in order to better protect CWC-VMEs.
Author Contributions
Conceptualization, A.C. and G.D.; methodology, A.C. and P.M.; validation, F.C., P.M. and L.S.; formal analysis, A.C.; investigation, G.D., P.M., L.S. and F.C.; writing—original draft preparation, A.C.; supervision, G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EU_7FP CoralFISH, OBAMA_PRIN, and RITMARE projects funded by the Italian Ministry of University and Research (MIUR) and the Marine Strategy Framework Directive monitoring project, funded by the Italian Ministry of the Environment (MATTM).
Institutional Review Board Statement
Ethical review and approval were not necessary for this study because all animals sampled were already dead when arriving on board. Therefore, the scientific activity in the context of this study is not subject to the European Commission recommendations (directive 2010/63/EU of the European Parliament and of the Council, 22 September 2010) or to Italian national law (decree law no. 26 of 4 March 2014) regarding the protection of animals used for scientific experiments.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Danovaro, R.; Fanelli, E.; Aguzzi, J.; Billett, D.; Carugati, L.; Corinaldesi, C.; Dell’Anno, A.; Gjerde, K.; Jamieson, A.J.; Kark, S.; et al. Ecological Variables for Developing a Global Deep-Ocean Monitoring and Conservation Strategy. Nat. Ecol. Evol. 2020, 4, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Folkersen, M.V.; Fleming, C.M.; Hasan, S. The Economic Value of the Deep Sea: A Systematic Review and Meta-Analysis. Mar. Policy 2018, 94, 71–80. [Google Scholar] [CrossRef]
- Mejjad, N.; Rovere, M. Understanding the Impacts of Blue Economy Growth on Deep-Sea Ecosystem Services. Sustainability 2021, 13, 12478. [Google Scholar] [CrossRef]
- Thurber, A.R.; Sweetman, A.K.; Narayanaswamy, B.E.; Jones, D.O.B.; Ingels, J.; Hansman, R.L. Ecosystem Function and Services Provided by the Deep Sea. Biogeosciences 2014, 11, 3941–3963. [Google Scholar] [CrossRef]
- Capezzuto, F.; Sion, L.; Ancona, F.; Carlucci, R.; Carluccio, A.; Cornacchia, L.; Maiorano, P.; Ricci, P.; Tursi, A.; D’Onghia, G. Cold-Water Coral Habitats and Canyons as Essential Fish Habitats in the Southern Adriatic and Northern Ionian Sea (Central Mediterranean). Ecol. Quest. 2018, 29, 9–23. [Google Scholar] [CrossRef]
- D’Onghia, G. Cold-Water Corals as Shelter, Feeding and Life-History Critical Habitats for Fish Species: Ecological Interactions and Fishing Impact. In Mediterranean Cold-Water Corals: Past, Present and Future: Understanding the Deep-Sea Realms of Coral; Springer: Berlin/Heidelberg, Germany, 2019; pp. 335–356. [Google Scholar]
- D’Onghia, G.; Maiorano, P.; Sion, L.; Giove, A.; Capezzuto, F.; Carlucci, R.; Tursi, A. Effects of Deep-Water Coral Banks on the Abundance and Size Structure of the Megafauna in the Mediterranean Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 397–411. [Google Scholar] [CrossRef]
- D’Onghia, G.; Maiorano, P.; Carlucci, R.; Capezzuto, F.; Carluccio, A.; Tursi, A.; Sion, L. Comparing Deep-Sea Fish Fauna between Coral and Non-Coral “Megahabitats” in the Santa Maria Di Leuca Cold-Water Coral Province (Mediterranean Sea). PLoS ONE 2012, 7, e44509. [Google Scholar] [CrossRef]
- Rueda, J.L.; Urra, J.; Aguilar, R.; Angeletti, L.; Bo, M.; García-Ruiz, C.; González-Duarte, M.M.; López, E.; Madurell, T.; Maldonado, M. Cold-Water Coral Associated Fauna in the Mediterranean Sea and Adjacent Areas. In Mediterranean Cold-Water Corals: Past, Present. and Future: Understanding the Deep-Sea Realms of Coral; Springer: Berlin/Heidelberg, Germany, 2019; pp. 295–333. [Google Scholar]
- Söffker, M.; Sloman, K.A.; Hall-Spencer, J.M. In Situ Observations of Fish Associated with Coral Reefs Off Ireland. Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 818–825. [Google Scholar] [CrossRef]
- Kutti, T.; Bergstad, O.A.; Fosså, J.H.; Helle, K. Cold-Water Coral Mounds and Sponge-Beds as Habitats for Demersal Fish on the Norwegian Shelf. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 122–133. [Google Scholar] [CrossRef]
- Mastrototaro, F.; D’Onghia, G.; Corriero, G.; Matarrese, A.; Maiorano, P.; Panetta, P.; Gherardi, M.; Longo, C.; Rosso, A.; Sciuto, F. Biodiversity of the White Coral Bank off Cape Santa Maria Di Leuca (Mediterranean Sea): An Update. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 412–430. [Google Scholar] [CrossRef]
- D’Onghia, G.; Calculli, C.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Grehan, A.; Indennidate, A.; Maiorano, P.; Mastrototaro, F.; Pollice, A.; et al. Anthropogenic Impact in the Santa Maria Di Leuca Cold-Water Coral Province (Mediterranean Sea): Observations and Conservation Straits. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 87–101. [Google Scholar] [CrossRef]
- Hall-Spencer, J.; Allain, V.; Fosså, J.H. Trawling Damage to Northeast Atlantic Ancient Coral Reefs. Proc. R. Soc. B Biol. Sci. 2002, 269, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Hinz, H. Impact of Bottom Fishing on Animal Forests: Science, Conservation, and Fisheries Management. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1041–1059. [Google Scholar]
- FAO. Report of the Technical Consultation on International Guidelines for the Management of Deep-Sea Fisheries in the High Seas; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Chimienti, G.; Bo, M.; Taviani, M.; Mastrototaro, F. Occurrence and Biogeography of Mediterranean Cold-Water Corals. In Mediterranean Cold-Water Corals: Past, Present and Future: Understanding the Deep-Sea Realms of Coral; Orejas, C., Jiménez, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 213–243. ISBN 978-3-319-91608-8. [Google Scholar]
- D’Onghia, G.; Calculli, C.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Maiorano, P.; Pollice, A.; Ricci, P.; Sion, L.; Tursi, A. New Records of Cold-Water Coral Sites and Fish Fauna Characterization of a Potential Network Existing in the Mediterranean Sea. Mar. Ecol. 2016, 37, 1398–1422. [Google Scholar] [CrossRef]
- Freiwald, A.; Beuck, L.; Rüggeberg, A.; Taviani, M.; Hebbeln, D. R/V Meteor Cruise M70-1 Participants. The White Coral Community in the Central Mediterranean Sea Revealed by ROV Surveys. Oceanography 2009, 22, 58–74. [Google Scholar] [CrossRef]
- Bailey, D.M.; King, N.J.; Priede, I.G. Cameras and Carcasses: Historical and Current Methods for Using Artificial Food Falls to Study Deep-Water Animals. Mar. Ecol. Prog. Ser. 2007, 350, 179–191. [Google Scholar] [CrossRef]
- Carluccio, A.; Capezzuto, F.; Maiorano, P.; Sion, L.; D’Onghia, G. Deep-Water Cartilaginous Fishes in the Central Mediterranean Sea: Comparison between Geographic Areas with Two Low Impact Tools for Sampling. J. Mar. Sci. Eng. 2021, 9, 686. [Google Scholar] [CrossRef]
- D’Onghia, G.; Capezzuto, F.; Cardone, F.; Carlucci, R.; Carluccio, A.; Chimienti, G.; Corriero, G.; Longo, C.; Maiorano, P.; Mastrototaro, F.; et al. Macro- and Megafauna Recorded in the Submarine Bari Canyon (Southern Adriatic, Mediterranean Sea) Using Different Tools. Mediterr. Mar. Sci. 2015, 16, 180–196. [Google Scholar] [CrossRef]
- D’Onghia, G.; Capezzuto, F.; Carluccio, A.; Carlucci, R.; Giove, A.; Mastrototaro, F.; Panza, M.; Sion, L.; Tursi, A.; Maiorano, P. Exploring Composition and Behaviour of Fish Fauna by in Situ Observations in the Bari Canyon (Southern Adriatic Sea, Central Mediterranean). Mar. Ecol. 2015, 36, 541–556. [Google Scholar] [CrossRef]
- D’Onghia, G.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Maiorano, P.; Panza, M.; Ricci, P.; Sion, L.; Tursi, A. Using a benthic lander to explore and monitor vulnerable ecosystems in the Mediterranean Sea. ACTA IMEKO 2018, 7, 45. [Google Scholar] [CrossRef]
- Fontes, J.; Menezes, G. Baited Image Lander for Deep Water Fish Counts and Biodiversity Studies-a Tool for MPA Science and Fisheries Management. In CONDOR Observatory for Long-Term Study and Monitoring of Azorean Seamount Ecosystems–Final Project Report; Giacomello, E., Menezes, G., Eds.; (Horta: Arquivos do DOP, Série Estudos 1/2012); University of the Azores: Ponta Delgada, Portugal, 2011; pp. 17–23. [Google Scholar]
- Linley, T.D.; Lavaleye, M.; Maiorano, P.; Bergman, M.; Capezzuto, F.; Cousins, N.J.; D’Onghia, G.; Duineveld, G.; Shields, M.A.; Sion, L.; et al. Effects of Cold-water Corals on Fish Diversity and Density (European Continental Margin: Arctic, NE Atlantic and Mediterranean Sea): Data from Three Baited Lander Systems. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 8–21. [Google Scholar] [CrossRef]
- Capezzuto, F.; Maiorano, P.; Panza, M.; Indennidate, A.; Sion, L.; D’Onghia, G. Occurrence and Behaviour of Paromola cuvieri (Crustacea, Decapoda) in the Santa Maria Di Leuca Cold-Water Coral Community (Mediterranean Sea). Deep Sea Res. Part I Oceanogr. Res. Pap. 2012, 59, 1–7. [Google Scholar] [CrossRef]
- Yeh, J.; Drazen, J.C. Baited-Camera Observations of Deep-Sea Megafaunal Scavenger Ecology on the California Slope. Mar. Ecol. Prog. Ser. 2011, 424, 145–156. [Google Scholar] [CrossRef]
- McLean, D.L.; Green, M.; Harvey, E.S.; Williams, A.; Daley, R.; Graham, K.J. Comparison of Baited Longlines and Baited Underwater Cameras for Assessing the Composition of Continental Slope Deepwater Fish Assemblages off Southeast Australia. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 98, 10–20. [Google Scholar] [CrossRef]
- Ridente, D.; Foglini, F.; Minisini, D.; Trincardi, F.; Verdicchio, G. Shelf-Edge Erosion, Sediment Failure and Inception of Bari Canyon on the Southwestern Adriatic Margin (Central Mediterranean). Mar. Geol. 2007, 246, 193–207. [Google Scholar] [CrossRef]
- Trincardi, F.; Foglini, F.; Verdicchio, G.; Asioli, A.; Correggiari, A.; Minisini, D.; Piva, A.; Remia, A.; Ridente, D.; Taviani, M. The Impact of Cascading Currents on the Bari Canyon System, SW-Adriatic Margin (Central Mediterranean). Mar. Geol. 2007, 246, 208–230. [Google Scholar] [CrossRef]
- Angeletti, L.; Taviani, M.; Canese, S.; Foglini, F.; Mastrototaro, F.; Argnani, A.; Trincardi, F.; Bakran-Petricioli, T.; Ceregato, A.; Chimienti, G.; et al. New Deep-Water Cnidarian Sites in the Southern Adriatic Sea. Mediterr. Mar. Sci. 2013, 15, 263–273. [Google Scholar] [CrossRef]
- Foglini, F.; Campiani, E.; Trincardi, F. The Reshaping of the South West Adriatic Margin by Cascading of Dense Shelf Waters. Mar. Geol. 2016, 375, 64–81. [Google Scholar] [CrossRef]
- Bargain, A.; Marchese, F.; Savini, A.; Taviani, M.; Fabri, M.C. Santa Maria Di Leuca Province (Mediterranean Sea): Identification of Suitable Mounds for Cold-Water Coral Settlement Using Geomorphometric Proxies and Maxent Methods. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Savini, A.; Vertino, A.; Marchese, F.; Beuck, L.; Freiwald, A. Mapping Cold-Water Coral Habitats at Different Scales within the Northern Ionian Sea (Central Mediterranean): An Assessment of Coral Coverage and Associated Vulnerability. PLoS ONE 2014, 9, e87108. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. 2024. Available online: https://www.marinespecies.org (accessed on 10 October 2024).
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley Statsref: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Getting Started with PRIMER V7. PRIMER-E Plymouth Plymouth Mar. Lab. 2015, 20, 296. [Google Scholar]
- Sion, L.; Pollice, A.; Maiorano, P.; Calculli, C.; Capezzuto, F.; Carluccio, A.; Ricci, P.; D’Onghia, G. Chondrichthyes in the North-Western Ionian Sea (Central Mediterranean): Species Diversity, Abundance and Spatio-Temporal Changes. Fish. Res. 2024, 274, 106977. [Google Scholar] [CrossRef]
- Maiorano, P.; Ricci, P.; Chimienti, G.; Calculli, C.; Mastrototaro, F.; D’Onghia, G. Deep-Water Species Assemblages on the Trawlable Bottoms of the Central Mediterranean: Changes or Not over Time? Front. Mar. Sci. 2022, 9, 1007671. [Google Scholar] [CrossRef]
- Afonso, P.; Graça, G.; Berke, G.; Fontes, J. First Observations on Seamount Habitat Use of Blackspot Seabream (Pagellus bogaraveo) Using Acoustic Telemetry. J. Exp. Mar. Biol. Ecol. 2012, 436–437, 1–10. [Google Scholar] [CrossRef]
- Afonso, P.; McGinty, N.; Graça, G.; Fontes, J.; Inácio, M.; Totland, A.; Menezes, G. Vertical Migrations of a Deep-Sea Fish and Its Prey. PLoS ONE 2014, 9, e97884. [Google Scholar] [CrossRef]
- Capezzuto, F.; Calculli, C.; Carlucci, R.; Carluccio, A.; Maiorano, P.; Pollice, A.; Sion, L.; Tursi, A.; D’Onghia, G. Revealing the Coral Habitat Effect on Benthopelagic Fauna Diversity in the Santa Maria Di Leuca Cold-Water Coral Province Using Different Devices and Bayesian Hierarchical Modelling. Aquat. Conserv. 2019, 29, 1608–1622. [Google Scholar] [CrossRef]
- Capezzuto, F.; Ancona, F.; Calculli, C.; Sion, L.; Maiorano, P.; D’Onghia, G. Feeding of the Deep-Water Fish Helicolenus dactylopterus (Delaroche, 1809) in Different Habitats: From Muddy Bottoms to Cold-Water Coral Habitats. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 159, 103252. [Google Scholar] [CrossRef]
- Linley, T.D.; Craig, J.; Jamieson, A.J.; Priede, I.G. Bathyal and Abyssal Demersal Bait-Attending Fauna of the Eastern Mediterranean Sea. Mar. Biol. 2018, 165, 159. [Google Scholar] [CrossRef]
- El Vadhel, H.; Buhl-Mortensen, L.; Babou, D.A.; Dridi, A.; Balde, B.S.; Bouzouma, M.E.M.; Psomadakis, P.N. Do Cold Water Corals Provide an Essential Habitat for Helicolenus Dactylopterus (Delaroche, 1809) in the Northwest Africa? Mar. Environ. Res. 2024, 198, 106538. [Google Scholar] [CrossRef] [PubMed]
- Mytilineou, C.; Smith, C.J.; Anastasopoulou, A.; Papadopoulou, K.N.; Christidis, G.; Bekas, P.; Kavadas, S.; Dokos, J. New Cold-Water Coral Occurrences in the Eastern Ionian Sea: Results from Experimental Long Line Fishing. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 146–157. [Google Scholar] [CrossRef]
- Sabatini, A.; Follesa, M.C.; Locci, I.; Pendugiu, A.A.; Pesci, P.; Cau, A. Assemblages in a Submarine Canyon: Influence of Depth and Time. In Proceedings of the Biodiversity in Enclosed Seas and Artificial Marine Habitats: Proceedings of the 39th European Marine Biology Symposium, Genoa, Italy, 21–24 July 2004; Springer: Berlin/Heidelberg, Germany, 2007; pp. 265–271. [Google Scholar]
- Fabri, M.-C.; Pedel, L.; Beuck, L.; Galgani, F.; Hebbeln, D.; Freiwald, A. Megafauna of Vulnerable Marine Ecosystems in French Mediterranean Submarine Canyons: Spatial Distribution and Anthropogenic Impacts. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 104, 184–207. [Google Scholar] [CrossRef]
- Carpentieri, P.; Colloca, F.; Cardinale, M.; Belluscio, A.; Ardizzone, G.D. Feeding Habits of European Hake (Merluccius merluccius) in the Central Mediterranean Sea. Fish B-NOAA 2005, 103, 411–416. [Google Scholar]
- De Pontual, H.; Jolivet, A.; Bertignac, M.; Fablet, R. Diel Vertical Migration of European Hake Merluccius merluccius and Associated Temperature Histories: Insights from a Pilot Data-Storage Tagging (DST) Experiment. J. Fish Biol. 2012, 81, 728–734. [Google Scholar] [CrossRef]
- Xavier, J.C.; Cherel, Y.; Assis, C.A.; Sendão, J.; Borges, T.C. Feeding Ecology of Conger Eels (Conger conger) in North-East Atlantic Waters. J. Mar. Biol. Assoc. UK 2010, 90, 493–501. [Google Scholar] [CrossRef]
- Mytilineou, C.; Tsagarakis, K.; Bekas, P.; Anastasopoulou, A.; Kavadas, S.; Machias, A.; Haralabous, J.; Smith, C.J.; Petrakis, G.; Dokos, J. Spatial Distribution and Life-history Aspects of Blackspot Seabream Pagellus bogaraveo (Osteichthyes: Sparidae). J. Fish Biol. 2013, 83, 1551–1575. [Google Scholar] [CrossRef]
- Capezzuto, F.; Ancona, F.; Calculli, C.; Carlucci, R.; Sion, L.; Maiorano, P.; D’Onghia, G. Comparison of Trophic Spectrum in the Blackspot Seabream, Pagellus bogaraveo (Brünnich, 1768), between Cold-Water Coral Habitats and Muddy Bottoms in the Central Mediterranean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2021, 169, 103474. [Google Scholar] [CrossRef]
- Sion, L.; Calculli, C.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Cornacchia, L.; Maiorano, P.; Pollice, A.; Ricci, P.; Tursi, A.; et al. Does the Bari Canyon (Central Mediterranean) Influence the Fish Distribution and Abundance? Prog. Oceanogr. 2019, 170, 81–92. [Google Scholar] [CrossRef]
- Megalofonou, P.; Chatzispyrou, A. Sexual Maturity and Feeding of the Gulper Shark, Centrophorus granulosus, from the Eastern Mediterranean Sea. Cybium 2006, 30, 67–74. [Google Scholar]
- Scacco, U.; la Mesa, G.; Vacchi, M. Morfometría Corporal, Diversidad Natatoria y Nicho de Los Tiburones Demersales: Estudio Comparativo En El Mar Mediterráneo. Sci. Mar. 2010, 74, 37–53. [Google Scholar] [CrossRef]
- Pinte, N.; Parisot, P.; Martin, U.; Zintzen, V.; De Vleeschouwer, C.; Roberts, C.D.; Mallefet, J. Ecological Features and Swimming Capabilities of Deep-Sea Sharks from New Zealand. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 156, 103187. [Google Scholar] [CrossRef]
- Capezzuto, F.; Ancona, F.; Carlucci, R.; Carluccio, A.; Cornacchia, L.; Maiorano, P.; Ricci, P.; Sion, L.; Tursi, A.; D’Onghia, G. Cold-Water Coral Communities in the Central Mediterranean: Aspects on Megafauna Diversity, Fishery Resources and Conservation Perspectives. Rend. Lincei 2018, 29, 589–597. [Google Scholar] [CrossRef]
- Maiorano, P.; Capezzuto, F.; Carluccio, A.; Calculli, C.; Cipriano, G.; Carlucci, R.; Ricci, P.; Sion, L.; Tursi, A.; D’Onghia, G. Food from the Depths of the Mediterranean: The Role of Habitats, Changes in the Sea-Bottom Temperature and Fishing Pressure. Foods 2022, 11, 1420. [Google Scholar] [CrossRef]
- Althaus, F.; Williams, A.; Schlacher, T.A.; Kloser, R.J.; Green, M.A.; Barker, B.A.; Bax, N.J.; Brodie, P.; Schlacher-Hoenlinger, M.A. Impacts of Bottom Trawling on Deep-Coral Ecosystems of Seamounts Are Long-Lasting. Mar. Ecol. Prog. Ser. 2009, 397, 279–294. [Google Scholar] [CrossRef]
- Angiolillo, M.; La Mesa, G.; Giusti, M.; Salvati, E.; Di Lorenzo, B.; Rossi, L.; Canese, S.; Tunesi, L. New Records of Scleractinian Cold-Water Coral (CWC) Assemblages in the Southern Tyrrhenian Sea (Western Mediterranean Sea): Human Impacts and Conservation Prospects. Prog. Oceanogr. 2021, 197, 102656. [Google Scholar] [CrossRef]
- Roberts, S.; Hirshfield, M. Deep-Sea Corals: Out of Sight, but No Longer out of Mind. Front. Ecol. Environ. 2004, 2, 123–130. [Google Scholar] [CrossRef]
- Fosså, J.H.; Mortensen, P.B.; Furevik, D.M. The Deep-Water Coral Lophelia pertusa in Norwegian Waters: Distribution and Fishery Impacts. Hydrobiologia 2002, 471, 1–12. [Google Scholar] [CrossRef]
- Fanelli, E.; Delbono, I.; Ivaldi, R.; Pratellesi, M.; Cocito, S.; Peirano, A. Cold-water Coral Madrepora oculata in the Eastern Ligurian Sea (NW Mediterranean): Historical and Recent Findings. Aquat. Conserv. 2017, 27, 965–975. [Google Scholar] [CrossRef]
- Bilan, M.; Gori, A.; Grinyó, J.; Biel-Cabanelas, M.; Puigcerver-Segarra, X.; Santín, A.; Piraino, S.; Rossi, S.; Puig, P. Vulnerability of Six Cold-Water Corals to Sediment Resuspension from Bottom Trawling Fishing. Mar. Pollut. Bull. 2023, 196, 115423. [Google Scholar] [CrossRef]
- Lastras, G.; Canals, M.; Ballesteros, E.; Gili, J.-M.; Sanchez-Vidal, A. Cold-Water Corals and Anthropogenic Impacts in La Fonera Submarine Canyon Head, Northwestern Mediterranean Sea. PLoS ONE 2016, 11, e0155729. [Google Scholar] [CrossRef] [PubMed]
- Orejas, C.; Gori, A.; Lo Iacono, C.; Puig, P.; Gili, J.-M.; Dale, M.R.T. Cold-Water Corals in the Cap de Creus Canyon, Northwestern Mediterranean: Spatial Distribution, Density and Anthropogenic Impact. Mar. Ecol. Prog. Ser. 2009, 397, 37–51. [Google Scholar] [CrossRef]
- Angeletti, L.; D’Onghia, G.; Otero, M.D.M.; Settanni, A.; Spedicato, M.T.; Taviani, M. A Perspective for Best Governance of the Bari Canyon Deep-Sea Ecosystems. Water 2021, 13, 1646. [Google Scholar] [CrossRef]
- White, J.; Simpfendorfer, C.A.; Tobin, A.J.; Heupel, M.R. Application of Baited Remote Underwater Video Surveys to Quantify Spatial Distribution of Elasmobranchs at an Ecosystem Scale. J. Exp. Mar. Biol. Ecol. 2013, 448, 281–288. [Google Scholar] [CrossRef]
- Carluccio, A.; Capezzuto, F.; Maiorano, P.; Sion, L.; D’Onghia, G. Feeding Behaviour of Deep-Water Scavengers in the Central Mediterranean: In Situ Observations Using a Baited Lander. In Proceedings of the 2022 IEEE International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), Milazzo, Italy, 3–5 October 2022; IEEE: New York, NJ, USA, 2022; pp. 530–534. [Google Scholar]
- EC. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities Legis. 1992, 35, 7–50. [Google Scholar]
- EC. Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the conservation of wild birds. Off. J. Eur. Communities Legis. 2009, 53, 7–25. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).