Insights into Decapod Sentience: Applying the General Welfare Index (GWI) for Whiteleg Shrimp (Penaeus vannamei—Boone, 1931) Reared in Aquaculture Grow-Out Ponds
Abstract
:1. Introduction
1.1. Contextualisation and Foundations
1.1.1. Welfare, Stress, and Distress
1.1.2. Nociception and Pain Perception
1.1.3. Sentience and Consciousness
1.1.4. Sentience in Decapod Crustaceans
- The scientific basis is still very controversial
- Questionable criteria for defining the experience of pain in crustaceans
- Experimental limitations and misinterpretations of data
- The use of anthropomorphic criteria leads to false equivalences with the human experience of pain
- The creation of animal welfare legislation in countries like Switzerland and the UK may reflect ethical considerations and societal pressures more than robust scientific evidence, potentially leading to unwarranted restrictions on research and the food industry
- The risk of imposing unnecessary restrictions on research and the food industry is based on a few scientific studies.
1.1.5. Shrimp Sentience
1.1.6. Sentience of Decapods and Legislation
1.1.7. The Application of Animal Welfare in Shrimp Farming
2. Materials and Methods
2.1. Systematic Review
- Mandatory presentation of indices or methodologies for estimating the degree of welfare of fish, crustaceans, or molluscs;
- The article should provide a detailed description of the mathematical logic and calculations employed to assess the welfare of the respective target animals;
- The proposed method directly applies to animals farmed commercially within aquaculture systems.
2.2. Mathematical Model and Welfare Indicators Used in the GWI
2.3. Application of the GWI for Diagnosing the Welfare Degree of P. vannamei Cultivated in Ponds
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saraswathy, R.; Muralidhar, M.; Thiagarajan, R.; Panigrahi, A.; Suvana, S.; Kumararaja, P.; Katneni, V.K.; Nagavel, A. Effect of lined and earthen farming practices on pond health in white leg shrimp, Penaeus vannamei culture. Aquacult. Res. 2022, 53, 5606–5617. [Google Scholar] [CrossRef]
- Villarreal, H. Shrimp farming advances, challenges, and opportunities. J. World Aquacult. Soc. 2023, 54, 1092–1095. [Google Scholar] [CrossRef]
- Kim, D.E.; Lim, S.S. Market Power Analysis on Shrimp Import from Tropical Asia: The Korean Case. In Proceedings of the Sustainability, Economics, Innovation, Globalisation and Organisational Psychology Conference, Singapore, 1–3 March 2023; pp. 203–214. [Google Scholar]
- GVR. Shrimp Market Size, Share & Trends Analysis Report by Species (L. Vannamei, P. Chinensis), by Source (Wild, Aquaculture), by Form, by Distribution Channel, by Region, And Segment Forecasts, 2023–2030; Grand View Research (GVR): San Francisco, CA, USA, 2023; p. 164. [Google Scholar]
- Globefish. FAO. 2023. GLOBEFISH Highlights—International Markets for Fisheries and Aquaculture Products–Second Issue 2023, with January–December 2022 Statistics. GLOBEFISH Highlights, 2023, 2. Rome. Available online: https://openknowledge.fao.org/items/b92f2b03-0e0a-48ff-9619-ec6e0cc34759 (accessed on 4 April 2023).
- Knibb, W.; Giang, C.T.; Premachandra, H.K.A.; Ninh, N.H.; Domínguez, B.C. Feasible options to restore genetic variation in hatchery stocks of the globally important farmed shrimp species, Litopenaeus vannamei. Aquaculture 2020, 518, 734823. [Google Scholar] [CrossRef]
- Jory, D. Annual Farmed Shrimp Production Survey: A Slight Decrease in Production Reduction in 2023 with Hopes for Renewed Growth in 2024. Available online: https://encurtador.com.br/azT04 (accessed on 29 January 2024).
- Boyd, C.E.; Davis, R.P.; McNevin, A.A. Comparison of resource use for farmed shrimp in Ecuador, India, Indonesia, Thailand, and Vietnam. Aquac. Fish Fish. 2021, 1, 3–15. [Google Scholar] [CrossRef]
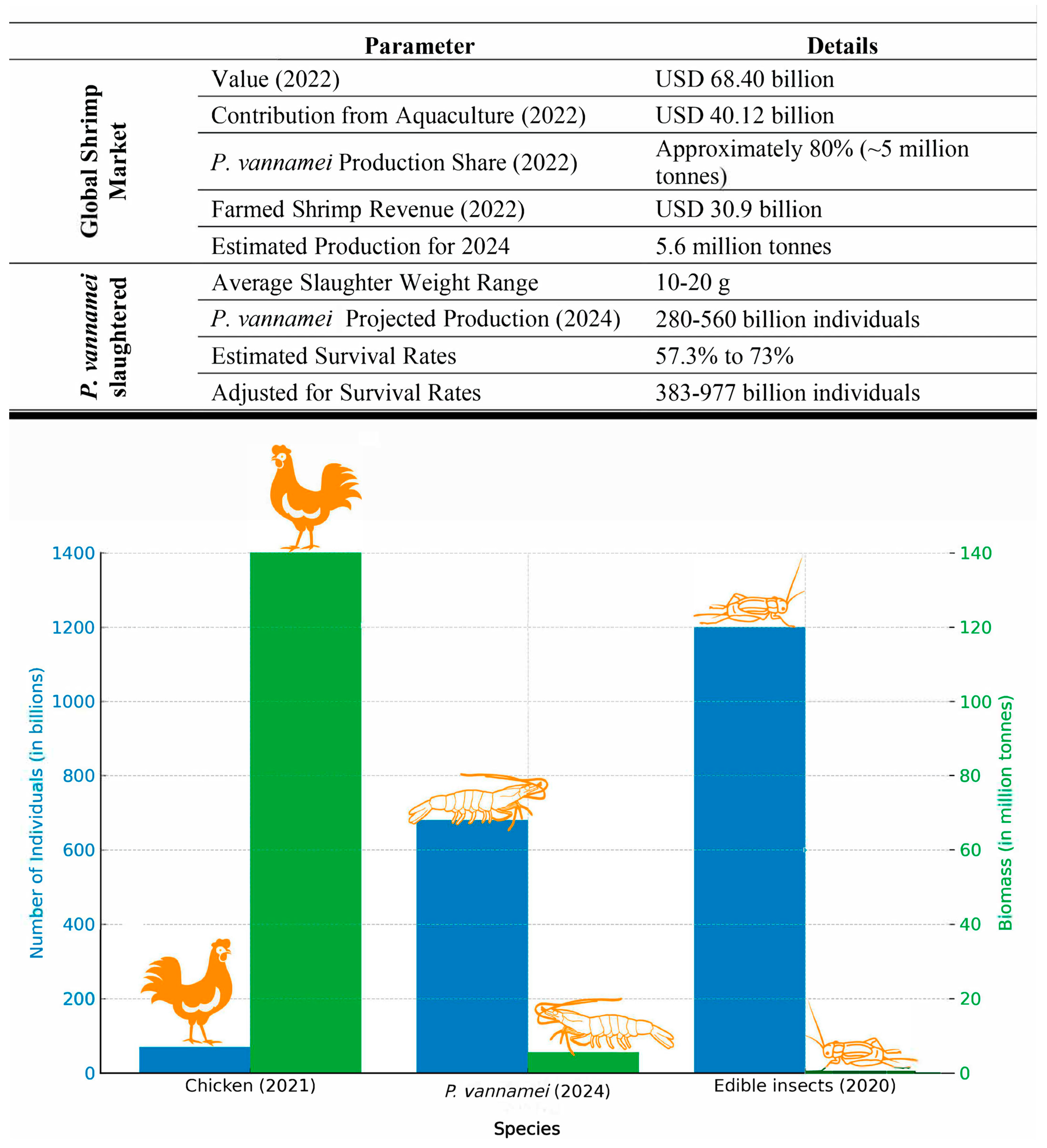
- Waldhorn, D.R.; Autric, E. Shrimp: The animals most commonly used and killed for food production. Rethink. Priorities 2023, 37. [Google Scholar] [CrossRef]
- CFET. Poultry Industry Statistics (2023): Meat & Egg Production. Available online: https://encurtador.com.br/wCHLM (accessed on 29 January 2024).
- Rowe, A. Insects Farmed for Food and Feed—Global Scale, Practices, and Policy. Available online: https://osf.io/nh6k3/download (accessed on 1 February 2024).
- Comstock, G. Pain in Pleocyemata, but not in Dendrobranchiata? Anim. Sentience 2022, 7, 13. [Google Scholar] [CrossRef]
- Pedrazzani, A.S.; Tavares, C.P.d.S.; Quintiliano, M.; Cozer, N.; Ostrensky, A. New indices for the diagnosis of fish welfare and their application to the grass carp (Ctenopharyngodon idella) reared in earthen ponds. Aquacult. Res. 2022, 53, 5825–5845. [Google Scholar] [CrossRef]
- Pedrazzani, A.S.; Cozer, N.; Quintiliano, M.H.; dos Santos Tavares, C.P.; Biernaski, V.; Ostrensky, A. From egg to slaughter: Monitoring the welfare of Nile tilapia, Oreochromis niloticus, throughout their entire life cycle in aquaculture. Front. Vet. Sci. 2023, 10, 1268396. [Google Scholar] [CrossRef]
- Farm Animal Welfare Council (FAWC). Five Freedoms First Written Report. 5 December 1979. First Report on the Five Freedoms. UK Government. Available online: https://www.gov.uk/government/groups/farm-animal-welfare-committee-fawc (accessed on 13 March 2023).
- Mellor, D.J.; Reid, C. Concepts of animal well-being and predicting the impact of procedures on experimental animals. Anim. Welf. 1994, 3–18. [Google Scholar]
- Mellor, D.; Stafford, K. Integrating practical, regulatory and ethical strategies for enhancing farm animal welfare. Aust. Vet. J. 2001, 79, 762–768. [Google Scholar] [CrossRef]
- Mellor, D.; Patterson-Kane, E.; Stafford, K. Animal welfare, grading compromise and mitigating suffering. Sci. Anim. Welf. 2009, 72–94. [Google Scholar]
- Mellor, D.J. Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 five domains model: Including human–animal interactions in assessments of animal welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Stott, G. What is animal stress and how is it measured? J. Anim. Sci. 1981, 52, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G. When Stress Becomes Distress; CABI Publishing: Wallingford, UK, 2000; pp. 11–12. [Google Scholar]
- Bayne, B.L. Aspects of physiological condition in Mytilus edulis L. with respect of the effects of oxygen tension and salinity. Proc. Ninth Eur. Mar. Biol. 1975, 213–238. [Google Scholar]
- Morton, D.B. Distress in Animals: Its Recognition and a Hypothesis for Its Assessment; WellBeing International: Potomac, MD, USA, 2009; Volume 14, p. 10. [Google Scholar]
- Wuertz, S.; Bierbach, D.; Bögner, M. Welfare of Decapod Crustaceans with Special Emphasis on Stress Physiology. Aquacult. Res. 2023, 2023, 1307684. [Google Scholar] [CrossRef]
- FAWC. Farm Animal Welfare in Great Britain: Past, Present and Future. Available online: https://encurtador.com.br/ekS08 (accessed on 30 January 2024).
- Green, T.; Mellor, D.J. Extending ideas about animal welfare assessment to include ‘quality of life’ and related concepts. N. Z. Vet. J. 2011, 59, 263–271. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Stokes, J.E.; Mullan, S.; Takahashi, T.; Monte, F.; Main, D.C. Economic and welfare impacts of providing good life opportunities to farm animals. Animals 2020, 10, 610. [Google Scholar] [CrossRef]
- Diener, E.; Sandvik, E.; Pavot, W. Happiness is the frequency, not the intensity, of positive versus negative affect. In Assessing Well-Being: The Collected Works of Ed Diener; Springer: Berlin/Heidelberg, Germany, 2009; pp. 213–231. [Google Scholar]
- Espinosa, R.; Treich, N. The Animal-Welfare Levy; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- WOAH. Terrestrial Animal Health Code; World Organisation for Animal Health (WOAH): Paris, France, 2016; Volume 2. [Google Scholar]
- Rowe, A. Should scientific research involving decapod crustaceans require ethical review? J. Agric. Environ. Ethics 2018, 31, 625–634. [Google Scholar] [CrossRef]
- Zimmerman, M. Physiological mechanisms of pain and its treatment. Klin. Anaesthesiol. Intensiv. 1986, 32, 1–19. [Google Scholar]
- Hu, L.; Iannetti, G. Neural indicators of perceptual variability of pain across species. Proc. Natl. Acad. Sci. USA 2019, 116, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Passantino, A.; Elwood, R.W.; Coluccio, P. Why protect decapod crustaceans used as models in biomedical research and in ecotoxicology? Ethical and legislative considerations. Animals 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Elwood, R.W. Behavioural Indicators of Pain and Suffering in Arthropods and Might Pain Bite Back? Animals 2023, 13, 2602. [Google Scholar] [CrossRef]
- Nijs, J.; De Baets, L.; Hodges, P. Phenotyping nociceptive, neuropathic, and nociplastic pain: Who, how, & why? Braz. J. Phys. Ther. 2023, 27, 100537. [Google Scholar]
- McKune, C.M.; Murrell, J.C.; Nolan, A.M.; White, K.L.; Wright, B.D. Nociception and pain. In Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; Wiley: Hoboken, NJ, USA, 2015; pp. 584–623. [Google Scholar]
- Pace, M.C.; Passavanti, M.B.; De Nardis, L.; Bosco, F.; Sansone, P.; Pota, V.; Barbarisi, M.; Palagiano, A.; Iannotti, F.A.; Panza, E. Nociceptor plasticity: A closer look. J. Cell. Physiol. 2018, 233, 2824–2838. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell. Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B 2020, 287, 20201309. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Browning, H.; Schnell, A.; Burn, C.; Birch, J. Sentience in decapod crustaceans: A general framework and review of the evidence. Anim. Sentience 2022, 7, 1. [Google Scholar] [CrossRef]
- Puri, S.; Faulkes, Z. Can crayfish take the heat? Procambarus clarkii show nociceptive behaviour to high temperature stimuli, but not low temperature or chemical stimuli. Biol. Open 2015, 4, 441–448. [Google Scholar] [CrossRef]
- Seth, A.K.; Bayne, T. Theories of consciousness. Nat. Rev. Neurosci. 2022, 23, 439–452. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. How does the non-conscious become conscious? Curr. Biol. 2020, 30, R196–R199. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Thomas, N. Self-Awareness and Selfhood in Animals. In Animal Ethics and the Autonomous Animal Self; Springer: Berlin/Heidelberg, Germany, 2016; pp. 37–67. [Google Scholar]
- Fabbro, F.; Aglioti, S.M.; Bergamasco, M.; Clarici, A.; Panksepp, J. Evolutionary aspects of self- and world consciousness in vertebrates. Front. Hum. Neurosci. 2015, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Dung, L.; Newen, A. Profiles of animal consciousness: A species-sensitive, two-tier account to quality and distribution. Cognition 2023, 235, 105409. [Google Scholar] [CrossRef]
- Correll, J. Descartes’ Dualism and Its Influence on Our Medical System. SUURJ Seattle Univ. Undergrad. Res. J. 2022, 6, 11. [Google Scholar]
- Kraus, P.A. Mens Humana: Res Cogitans and the Doctrine of Faculties in Descartes’ Meditationes. Int. Stud. Philos. 1986, 18, 1–18. [Google Scholar] [CrossRef]
- Brentari, C. How to Think About Human-Animal Differences in Thinking: Two Cases of Marginal Analogy in the Philosophical Explication of Animal Cognition. In Thinking: Bioengineering of Science and Art; Springer: Berlin/Heidelberg, Germany, 2022; pp. 73–93. [Google Scholar]
- Lashley, K.S. Persistent problems in the evolution of mind. Q. Rev. Biol. 1949, 24, 28–42. [Google Scholar] [CrossRef]
- Baranzke, H.; Ingensiep, H. Sentientism–for whose sake? Ethics, sciences, and crypto-teleological fact-value bridges, illustrated by the research about sentience in invertebrates. Animal 2023, 17, 100875. [Google Scholar] [CrossRef]
- Harrison, R. Animal Machines; Stuart (Vincent) & J.M.Watkins Ltd.: London, UK, 1964; p. 186. [Google Scholar]
- Broom, D.M. Animal welfare concepts. In Routledge Handbook of Animal Welfare, 1st ed.; Routledge: London, UK; New York, NY, USA, 2022. [Google Scholar]
- Browning, H.; Birch, J. Animal sentience. Philos. Compass 2022, 17, e12822. [Google Scholar] [CrossRef]
- Epstein, Y.; Bernet Kempers, E. Animals and Nature as Rights Holders in the European Union. Mod. Law Rev. 2023, 86, 1336–1357. [Google Scholar] [CrossRef]
- Peters, A. Rights of Nature Include Rights of Domesticated Animals. In Der Schutz des Individuums Durch das Recht: Festschrift für Rainer Hofmann zum 70. Geburtstag; Springer: Berlin/Heidelberg, Germany, 2023; pp. 15–30. [Google Scholar]
- Wilkie, R. Animals as sentient commodities. In The Oxford Handbook of Animal Studies; Oxford University Press: Oxford, UK, 2017; pp. 279–301. [Google Scholar]
- Cox, J.; Bridgers, J. Why Is Animal Welfare Important for Sustainable Consumption and Production? UN Environment: Nairobi, Kenya, 2019. [Google Scholar]
- Orth, D.J. Pain, Sentience, and Animal Welfare. In Fish, Fishing, and Conservation; Virginia Tech Publishing: Blacksburg, VA, USA, 2023. [Google Scholar]
- Fragoso, A.A.H.; Capilé, K.; Taconeli, C.A.; de Almeida, G.C.; de Freitas, P.P.; Molento, C.F.M. Animal Welfare Science: Why and for Whom? Animals 2023, 13, 1833. [Google Scholar] [CrossRef] [PubMed]
- Block, N.J. On a confusion about the function of consciousness. Behav. Brain Sci. 1995, 18, 227–247. [Google Scholar] [CrossRef]
- Nagel, T. What is it like to be a bat? Philos. Rev. 1974, 83, 435–450. [Google Scholar] [CrossRef]
- DeGrazia, D. Taking Animals Seriously: Mental Life and Moral Status; Cambridge University Press: Washington, DC, USA, 1996; p. 316. [Google Scholar]
- Jones, R.C. Science, sentience, and animal welfare. Biol. Philos. 2013, 28, 1–30. [Google Scholar] [CrossRef]
- Proctor, H.S.; Carder, G.; Cornish, A.R. Searching for animal sentience: A systematic review of the scientific literature. Animals 2013, 3, 882–906. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare and legislation. Food Saf. Assur. Vet. Public Health 2009, 5, 339–350. [Google Scholar]
- Birch, J.; Broom, D.M.; Browning, H.; Crump, A.; Ginsburg, S.; Halina, M.; Harrison, D.; Jablonka, E.; Lee, A.Y.; Kammerer, F. How should we study animal consciousness scientifically? J. Conscious. Stud. 2022, 29, 8–28. [Google Scholar] [CrossRef]
- Birch, J. Should animal welfare be defined in terms of consciousness? Philos. Sci. 2022, 89, 1114–1123. [Google Scholar] [CrossRef]
- Eliasen, K.; Patursson, E.J.; McAdam, B.J.; Pino, E.; Morro, B.; Betancor, M.; Baily, J.; Rey, S. Liver colour scoring index, carotenoids and lipid content assessment as a proxy for lumpfish (Cyclopterus lumpus L.) health and welfare condition. Sci. Rep. 2020, 10, 8927. [Google Scholar] [CrossRef]
- Robertson, I.; Goldsworthy, D. Recognising and defining animal sentience in legislation: A framework for importing positive animal welfare through the five domains model. Monash Univ. Law Rev. 2022, 48, 244–271. [Google Scholar]
- Budaev, S.; Kristiansen, T.S.; Giske, J.; Eliassen, S. Computational animal welfare: Towards cognitive architecture models of animal sentience, emotion and wellbeing. R. Soc. Open Sci. 2020, 7, 201886. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.; Mikhalevich, I. Affective sentience and moral protection. Anim. Sentience 2020, 29, 1–20. [Google Scholar] [CrossRef]
- Shriver, A. The Unpleasantness of Pain for Humans and Other Animals. In Philosophy of Pain; Bain, D., Brady, M., Corns, J., Eds.; Routledge: London, UK, 2018; pp. 147–162. [Google Scholar]
- Tague, I.H. The history of emotional attachment to animals. In The Routledge Companion to Animal-Human History; Routledge: London, UK, 2018; pp. 345–366. [Google Scholar]
- Birch, J.; Read, E. Animal sentience and the capabilities approach to justice. Biol. Philos. 2023, 38, 26. [Google Scholar]
- Broom, D.M. Concepts and Interrelationships of Awareness, Consciousness, Sentience, and Welfare. J. Conscious. Stud. 2022, 29, 129–149. [Google Scholar] [CrossRef]
- Schönfeld, M. Animal consciousness: Paradigm change in the life sciences. Perspect. Sci. 2006, 14, 354–381. [Google Scholar] [CrossRef]
- Provencio, J.J.; Hemphill, J.C.; Claassen, J.; Edlow, B.L.; Helbok, R.; Vespa, P.M.; Diringer, M.N.; Polizzotto, L.; Shutter, L.; Suarez, J.I. The curing coma campaign: Framing initial scientific challenges—Proceedings of the first curing coma campaign scientific advisory council meeting. In Proceedings of the Neurocritical Care, Online, 22–25 September 2020; pp. 1–12. [Google Scholar]
- Andreotta, A.J. The hard problem of AI rights. AI Soc. 2021, 36, 19–32. [Google Scholar] [CrossRef]
- Dawkins, M. Animal welfare and the paradox of animal consciousness. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 2015; Volume 47, pp. 5–38. [Google Scholar]
- Veit, W. A Philosophy for the Science of Animal Consciousness; Taylor & Francis: Abingdon, UK, 2023. [Google Scholar]
- Qureshi, Q.A. Bridging Philosophy and Neuroscience: How Behavioral Experiments Inform a Recent Theory of Animal Consciousness. 2023. Cognitive Science Senior Theses. 2. Available online: https://digitalcommons.dartmouth.edu/cognitive-science_senior_theses/2 (accessed on 4 July 2023).
- Walters, E.T. Strong inferences about pain in invertebrates require stronger evidence. Anim. Sentience 2022, 7, 14. [Google Scholar] [CrossRef]
- Diggles, B.K. Review of some scientific issues related to crustacean welfare. ICES J. Mar. Sci. 2019, 76, 66–81. [Google Scholar] [CrossRef]
- Diggles, B.K.; Arlinghaus, R.; Browman, H.I.; Cooke, S.J.; Cooper, R.L.; Cowx, I.G.; Derby, C.D.; Derbyshire, S.W.; Hart, P.J.; Jones, B. Reasons to be skeptical about sentience and pain in fishes and aquatic invertebrates. Rev. Fish. Sci. Aquac. 2024, 32, 127–150. [Google Scholar] [CrossRef]
- Reber, A.S.; Baluska, F.; Miller Jr, W.B. Of course crustaceans are sentient: But there’s more to the story. Anim. Sentience 2022, 7, 3. [Google Scholar] [CrossRef]
- Andrews, K. All animals are conscious: Shifting the null hypothesis in consciousness science. Mind Lang. 2024, 39, 415–433. [Google Scholar] [CrossRef]
- Browning, H.; Veit, W. Studying animal feelings: Integrating sentience research and welfare science. J. Conscious. Stud. 2023, 30, 196–222. [Google Scholar] [CrossRef]
- Ng, Y.K. No need for certainty in animal sentience. Anim. Sentience 2022, 7, 6. [Google Scholar] [CrossRef]
- Deckha, M. Animals as Legal Beings: Contesting Anthropocentric Legal Orders; University of Toronto Press: Toronto, ON, Canada, 2021. [Google Scholar]
- Fieber, L.A. Neurotransmitters and neuropeptides of invertebrates. In The Oxford Handbook of Invertebrate Neurobiology; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Greenberg, M.; Price, D. Invertebrate neuropeptides: Native and naturalized. Annu. Rev. Physiol. 1983, 45, 271–288. [Google Scholar] [CrossRef]
- Tong, R.; Pan, L.; Zhang, X.; Li, Y. Neuroendocrine-immune regulation mechanism in crustaceans: A review. Rev. Aquac. 2022, 14, 378–398. [Google Scholar] [CrossRef]
- Strausfeld, N.J.; Wolff, G.H.; Sayre, M.E. Mushroom body evolution demonstrates homology and divergence across Pancrustacea. Elife 2020, 9, e52411. [Google Scholar] [CrossRef]
- Tomina, Y.; Takahata, M. A behavioral analysis of force-controlled operant tasks in American lobster. Physiol. Behav. 2010, 101, 108–116. [Google Scholar] [CrossRef]
- Elwood, R.W.; McClean, A.; Webb, L. The development of shell preferences by the hermit crab Pagurus bernhardus. Anim. Behav. 1979, 27, 940–946. [Google Scholar] [CrossRef]
- Okada, S.; Hirano, N.; Abe, T.; Nagayama, T. Aversive operant conditioning alters the phototactic orientation of the marbled crayfish. J. Exp. Biol. 2021, 224, jeb242180. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Elwood, R.W.; Adamo, S.A.; Leach, M.C. Defining and assessing animal pain. Anim. Behav. 2014, 97, 201–212. [Google Scholar] [CrossRef]
- Elwood, R.W. Assessing the potential for pain in crustaceans and other invertebrates. Welf. Invertebr. Anim. 2019, 18, 147–177. [Google Scholar]
- Dyuizen, I.V.; Kotsyuba, E.P.; Lamash, N.E. Changes in the nitric oxide system in the shore crab Hemigrapsus sanguineus (Crustacea, Decapoda) CNS induced by a nociceptive stimulus. J. Exp. Biol. 2012, 215, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Elwood, R.W.; Barr, S.; Patterson, L. Pain and stress in crustaceans? Appl. Anim. Behav. Sci. 2009, 118, 128–136. [Google Scholar] [CrossRef]
- Pushpalatha, E.; Ramesh, P.; Sudhakar, S. Response to autotomy in anesthetized freshwater crab, Paratelphusa hydrodromous (Herbst). J. Adv. Lab. Res. Biol. 2014, 5, 27–28. [Google Scholar]
- McCambridge, C.; Dick, J.T.; Elwood, R.W. Effects of autotomy compared to manual declawing on contests between males for females in the edible crab cancer pagurus: Implications for fishery practice and animal welfare. J. Shellfish Res. 2016, 35, 1037–1044. [Google Scholar] [CrossRef]
- Fossat, P.; Bacqué-Cazenave, J.; De Deurwaerdère, P.; Delbecque, J.-P.; Cattaert, D. Anxiety-like behavior in crayfish is controlled by serotonin. Science 2014, 344, 1293–1297. [Google Scholar] [CrossRef]
- Maza, F.J.; Urbano, F.J.; Delorenzi, A. Aversive memory conditioning induces fluoxetine-dependent anxiety-like states in the crab Neohelice granulata. J. Exp. Biol. 2023, 226, jeb245590. [Google Scholar] [CrossRef]
- Fossat, P.; Bacqué-Cazenave, J.; De Deurwaerdère, P.; Cattaert, D.; Delbecque, J.-P. Serotonin, but not dopamine, controls the stress response and anxiety-like behavior in the crayfish Procambarus clarkii. J. Exp. Biol. 2015, 218, 2745–2752. [Google Scholar] [CrossRef]
- Conneely, E.A.; Coates, C.J. Meta-analytic assessment of physiological markers for decapod crustacean welfare. Fish Fish. 2024, 25, 134–150. [Google Scholar] [CrossRef]
- Albalat, A.; Gornik, S.G.; Atkinson, R.J.; Coombs, G.H.; Neil, D.M. Effect of capture method on the physiology and nucleotide breakdown products in the Norway lobster (Nephrops norvegicus). Mar. Biol. Res. 2009, 5, 441–450. [Google Scholar] [CrossRef]
- Xu, D.; Wu, J.; Sun, L.; Qin, X.; Fan, X.; Zheng, X. Energy metabolism response of Litopenaeus vannamei to combined stress of acute cold exposure and waterless duration: Implications for physiological regulation and waterless live transport. J. Therm. Biol. 2022, 104, 103149. [Google Scholar] [CrossRef]
- Jimenez, A.G.; Kinsey, S.T. Energetics and metabolic regulation. Nat. Hist. Crustac. 2015, 4, 391–419. [Google Scholar]
- Stoner, A.W. Assessing stress and predicting mortality in economically significant crustaceans. Rev. Fish. Sci. 2012, 20, 111–135. [Google Scholar] [CrossRef]
- Madeira, C.; Leal, M.C.; Diniz, M.S.; Cabral, H.N.; Vinagre, C. Thermal stress and energy metabolism in two circumtropical decapod crustaceans: Responses to acute temperature events. Mar. Environ. Res. 2018, 141, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Stein, W.; Harzsch, S. The Neurobiology of Ocean Change–insights from decapod crustaceans. Zoology 2021, 144, 125887. [Google Scholar] [CrossRef]
- Rotllant, G.; Llonch, P.; García del Arco, J.A.; Chic, Ò.; Flecknell, P.; Sneddon, L.U. Methods to induce analgesia and anesthesia in crustaceans: A supportive decision tool. Biology 2023, 12, 387. [Google Scholar] [CrossRef]
- McKay, H.; McAuliffe, W.; Waldhorn, D.R. Welfare Considerations for Farmed Shrimp; Rethink Priorities: San Francisco, CA, USA, 2023. [Google Scholar]
- Arnott, S.A.; Neil, D.M.; Ansell, A.D. Tail-flip mechanism and size-dependent kinematics of escape swimming in the brown shrimp Crangon crangon. J. Exp. Biol. 1998, 201, 1771–1784. [Google Scholar] [CrossRef]
- Weineck, K.; Ray, A.J.; Fleckenstein, L.J.; Medley, M.; Dzubuk, N.; Piana, E.; Cooper, R.L. Physiological changes as a measure of crustacean welfare under different standardized stunning techniques: Cooling and electroshock. Animals 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Vinatea, L.; Ozorio, R.; Schuweitzer, R.; Andreatta, E. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture 2004, 233, 173–179. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Song, Z.; Wang, G. Automatic monitoring of relevant behaviors for crustacean production in aquaculture: A review. Animals 2021, 11, 2709. [Google Scholar] [CrossRef]
- Prunier, A.; Mounier, L.; Le Neindre, P.; Leterrier, C.; Mormède, P.; Paulmier, V.; Prunet, P.; Terlouw, C.; Guatteo, R. Identifying and monitoring pain in farm animals: A review. Animal 2013, 7, 998–1010. [Google Scholar] [CrossRef]
- Birch, J.; Burn, C.; Schnell, A.; Browning, H.; Crump, A. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans; LSE Consulting; LSE Enterprise Ltd.; The London School of Economics and Political Science: London, UK, 2021; p. 107. [Google Scholar]
- da Costa, F.P.; Gomes, B.S.F.d.F.; Pereira, S.D.d.N.A.; de Fátima Arruda, M. Influence of stocking density on the behaviour of juvenile Litopenaeus vannamei (Boone, 1931). Aquacult. Res. 2016, 47, 912–924. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Cannibalism of decapod crustaceans and implications for their aquaculture: A review of its prevalence, influencing factors, and mitigating methods. Rev. Fish. Sci. Aquac. 2017, 25, 42–69. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, X.; Huang, G.; Li, J. Effects of limited dissolved oxygen supply on the growth and energy allocation of juvenile Chinese shrimp, Fenneropenaeus chinensis. J. World Aquacult. Soc. 2009, 40, 483–492. [Google Scholar] [CrossRef]
- Wu, J.; Namikoshi, A.; Nishizawa, T.; Mushiake, K.; Teruya, K.; Muroga, K. Effects of shrimp density on transmission of penaeid acute viremia in Penaeus japonicus by cannibalism and the waterborne route. Dis. Aquat. Org. 2001, 47, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Robles-Romo, A.; Zenteno-Savín, T.; Racotta, I.S. Bioenergetic status and oxidative stress during escape response until exhaustion in whiteleg shrimp Litopenaeus vannamei. J. Exp. Mar. Biol. Ecol. 2016, 478, 16–23. [Google Scholar] [CrossRef]
- Paterson, B.D. Respiration rate of the kuruma prawn, Penaeus japonicus Bate, is not increased by handling at low temperature (12 C). Aquaculture 1993, 114, 229–235. [Google Scholar] [CrossRef]
- de Souza Valente, C. Anaesthesia of decapod crustaceans. Vet. Anim. Sci. 2022, 16, 100252. [Google Scholar] [CrossRef]
- Albalat, A.; Zacarias, S.; Coates, C.; Neil, D.; Planellas, S. Welfare in farmed decapod crustaceans, with particular reference to Penaeus vannamei. Front. Mar. Sci. 2022, 9, 886024. [Google Scholar] [CrossRef]
- Coates, C.J.; Rowley, A.F.; Smith, L.C.; Whitten, M.M. Host defences of invertebrates to pathogens and parasites. Invertebr. Pathol. 2022, 1, 3–40. [Google Scholar]
- Gong, Y.; Zhang, X. RNAi-based antiviral immunity of shrimp. Dev. Comp. Immunol. 2021, 115, 103907. [Google Scholar] [CrossRef] [PubMed]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Dev. Comp. Immunol. 2018, 80, 81–93. [Google Scholar] [CrossRef]
- Freire, C.A.; Cuenca, A.L.; Leite, R.D.; Prado, A.C.; Rios, L.P.; Stakowian, N.; Sampaio, F.D. Biomarkers of homeostasis, allostasis, and allostatic overload in decapod crustaceans of distinct habitats and osmoregulatory strategies: An empirical approach. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 248, 110750. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An important tool to assess the welfare of aquatic animals. Biology 2021, 10, 61. [Google Scholar] [CrossRef]
- Sandeman, D.C.; Kenning, M.; Harzsch, S. Adaptive trends in malacostracan brain form and function related to behavior. Nerv. Syst. Control Behav. 2014, 3, 11–45. [Google Scholar]
- Chung, J.S.; Zmora, N.; Katayama, H.; Tsutsui, N. Crustacean hyperglycemic hormone (CHH) neuropeptides family: Functions, titer, and binding to target tissues. Gen. Comp. Endocrinol. 2010, 166, 447–454. [Google Scholar] [CrossRef]
- Santos, E.A.; Keller, R. Crustacean hyperglycemic hormone (CHH) and the regulation of carbohydrate metabolism: Current perspectives. Comp. Biochem. Physiol. Part A Physiol. 1993, 106, 405–411. [Google Scholar] [CrossRef]
- Xu, L.; Pan, L.; Zhang, X.; Wei, C. Effects of crustacean hyperglycemic hormone (CHH) on regulation of hemocyte intracellular signaling pathways and phagocytosis in white shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2019, 93, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Mellor, D.J. Welfare-aligned sentience: Enhanced capacities to experience, interact, anticipate, choose and survive. Animals 2019, 9, 440. [Google Scholar] [CrossRef]
- Blattner, C.E. The recognition of animal sentience by the law. J. Anim. Ethics 2019, 9, 121–136. [Google Scholar] [CrossRef]
- Vitale, A.; Pollo, S.; Aaltola, E. Human/Animal Relationships in Transformation: Scientific, Moral and Legal Perspectives; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Nussbaum, M.C. Justice for Animals: Our Collective Responsibility; Simon and Schuster: New York, NY, USA, 2023. [Google Scholar]
- New Zealand. Animal Welfare Act 1999; Ministry for Primary Industries: Wellington, New Zealand, 1999.
- New Zealand. Animal Welfare (Care and Procedures) Regulations 2018 Animal Welfare (Care and Procedures) Regulations; Ministry for Primary Industries: Wellington, New Zealand, 2018.
- Federal Ministry Republic of Austria. The Federal Act on Animal Welfare (Tierschutzgesetz-TSchG). Austria, 2005, 2004/118. Available online: https://info.bml.gv.at/en/topics/agriculture/agriculture-in-austria/animal-production-in-austria/animal-welfare-act.html (accessed on 15 February 2023).
- Johnston, C.; Jungalwalla, P. Aquatic Animal Welfare Guidelines: Guidelines on Welfare of Fish and Crustaceans in Aquaculture and/or in Live Holding Systems for Human Consumption; National Aquaculture Council: Tallahassee, FL, USA, 2005. [Google Scholar]
- United Kingdom. Animal Welfare (Sentience) Act 2022; Parliament of the United Kingdom: London, UK, 2022.
- Norway. Animal Welfare Act 2009. Norwegian Ministry of Agriculture and Food. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC100049 (accessed on 23 March 2023).
- Switzerland. Animal Protection Ordinance-Protection of Nature, Landscape and Animals 2018. Federal Food Safety and Veterinary Office (FSVO). Available online: https://www.blv.admin.ch/dam/blv/en/dokumente/tiere/rechts-und-vollzugsgrundlagen/tschv-en.pdf.download.pdf/Animal%20Protection%20Ordinance%20455.1.pdf (accessed on 25 May 2023).
- ASC. FAQ Shrimp Health & Welfare. Available online: https://asc-aqua.org/wp-content/uploads/2023/09/EXTERNAL-FAQ-Shrimp-Health-and-Welfare.pdf (accessed on 31 January 2024).
- Krause, G.; Brugere, C.; Diedrich, A.; Ebeling, M.W.; Ferse, S.C.; Mikkelsen, E.; Agúndez, J.A.P.; Stead, S.M.; Stybel, N.; Troell, M. A revolution without people? Closing the people–policy gap in aquaculture development. Aquaculture 2015, 447, 44–55. [Google Scholar] [CrossRef]
- Nikolik, G. Global Shrimp Aquaculture Prodution Survey and Forecast; Rabobank: Hong Kong, China, 2022; p. 27. [Google Scholar]
- Waite, R.; Beveridge, M.; Brummett, R.; Castine, S.; Chaiyawannakarn, N.; Kaushik, S.; Mungkung, R.; Nawapakpilai, S.; Phillips, M. Improving Productivity and Environmental Performance of Aquaculture; WorldFish: Penang, Malaysia, 2014. [Google Scholar]
- Emerenciano, M.G.; Rombenso, A.N.; Vieira, F.d.N.; Martins, M.A.; Coman, G.J.; Truong, H.H.; Noble, T.H.; Simon, C.J. Intensification of penaeid shrimp culture: An applied review of advances in production systems, nutrition and breeding. Animals 2022, 12, 236. [Google Scholar] [CrossRef]
- Alday-Sanz, V. The Shrimp Book II; 5m Books Ltd.: Essex, UK, 2022. [Google Scholar]
- Kasper, S.; Adeyemo, O.K.; Becker, T.; Scarfe, D.; Tepper, J. Aquatic Environment and Life Support Systems. In Fundamentals of Aquatic Veterinary Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Darodes, J.B.d.T.; Keitel, J.; Owen, M.A.; Alcaraz-Calero, J.M.; Alexander, M.E.; Sloman, K.A. Monitoring methods of feeding behaviour to answer key questions in penaeid shrimp feeding. Rev. Aquac. 2021, 13, 1828–1843. [Google Scholar] [CrossRef]
- de Oliveira, G.B.; Griczinski, P.; Pedrazzani, A.S.; Quintiliano, M.H.; Molento, C.F.M. Brazilians’ perception of shrimp sentience and welfare. J. Vet. Behav. 2023, 71, 41–56. [Google Scholar] [CrossRef]
- Xuan, B.B.; Sandorf, E.D.; Ngoc, Q.T.K. Stakeholder perceptions towards sustainable shrimp aquaculture in Vietnam. J. Environ. Manag. 2021, 290, 112585. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: A review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Asche, F.; Anderson, J.L.; Botta, R.; Kumar, G.; Abrahamsen, E.B.; Nguyen, L.T.; Valderrama, D. The economics of shrimp disease. J. Invertebr. Pathol. 2021, 186, 107397. [Google Scholar] [CrossRef] [PubMed]
- Phong, T.N.; Tat Thang, V.; Nguyen Trong, H. The effect of sustainability labels on farmed-shrimp preferences: Insights from a discrete choice experiment in Vietnam. Aquacult. Econ. Manag. 2023, 27, 468–497. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, 71. [Google Scholar] [CrossRef]
- Pedrazzani, A.S.; Cozer, N.; Quintiliano, M.H.; Tavares, C.P.d.S.; da Silva, U.d.A.T.; Ostrensky, A. Non-invasive methods for assessing the welfare of farmed White-leg Shrimp (Penaeus vannamei). Animals 2023, 13, 807. [Google Scholar] [CrossRef] [PubMed]
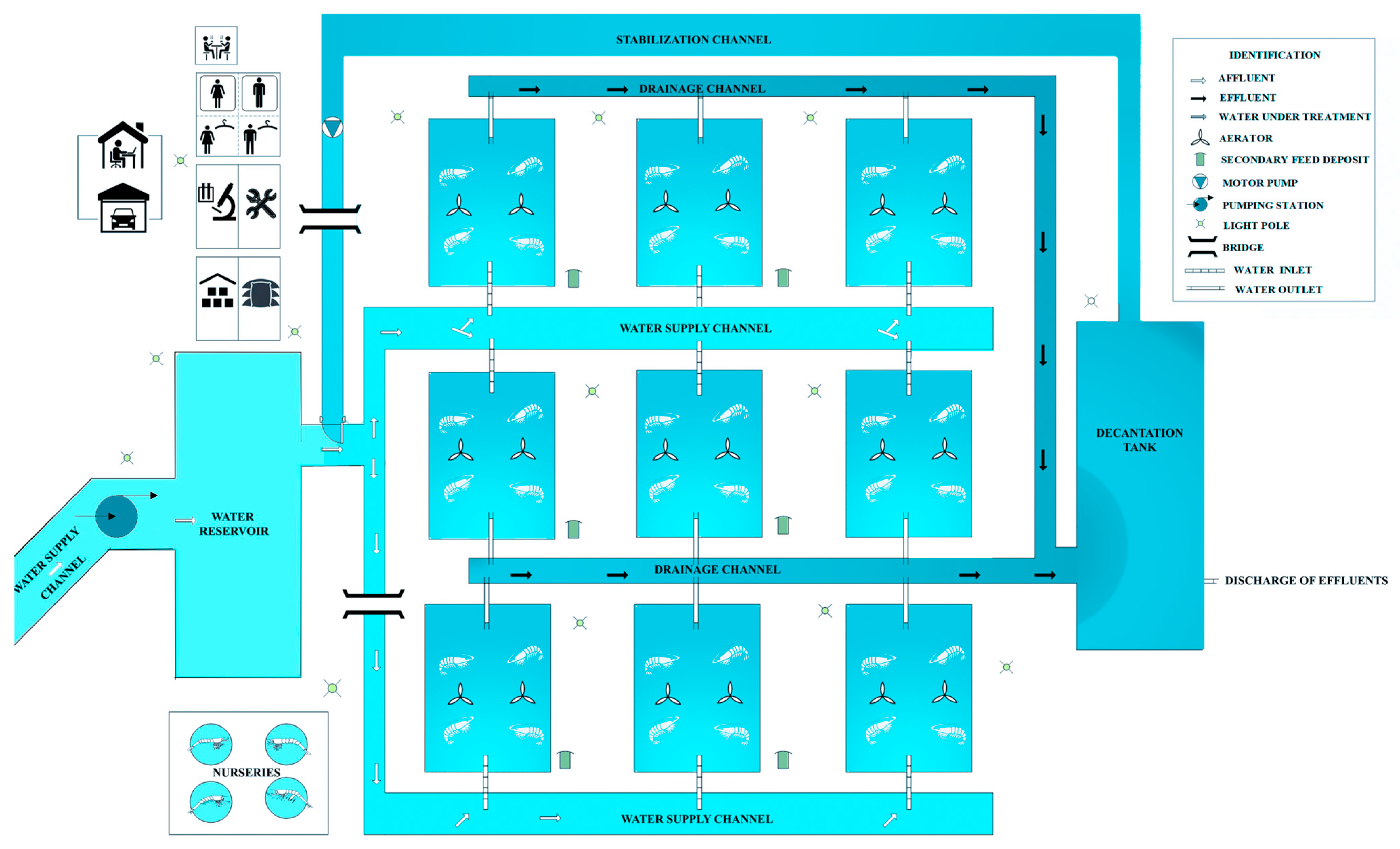
- Cozer, N.; Pont, G.D.; Horodesky, A.; Ostrensky, A. Infrastructure, management and energy efficiency in a hypothetical semi-intensive shrimp model farm in Brazil: A systematic review and meta-analysis. Rev. Aquac. 2020, 12, 1072–1089. [Google Scholar] [CrossRef]
- ABCC. Apostila Técnica de Boas Práticas de Manejo Para a Capacitação de Pequenos Produtores. Associação Brasileira de Criadores de Camarão 2010, 1, 330. [Google Scholar]
- Costa, F.P.d. Influência da Densidade de Estocagem Sobre o Crescimento, Ciclo de Muda e o Comportamento em Juvenis do Camarão Marinho Litopenaeus Vannamei; Universidade Federal do Rio Grande do Norte: Natal, Brazil, 2012. [Google Scholar]
- ABCC. Levantamento da Infraestrutura Produtiva e dos aspectos Tecnológicos, Econômicos, Sociais e Ambientais da Carcinicultura Marinha no Brasil em 2011. Assoc. Bras. Criadores Camarão 2013, 82, 8–77. [Google Scholar]
- Wahltinez, S.J.; Stacy, N.I.; Hadfield, C.A.; Harms, C.A.; Lewbart, G.A.; Newton, A.L.; Nunamaker, E.A. Perspective: Opportunities for advancing aquatic invertebrate welfare. Front. Vet. Sci. 2022, 9, 973376. [Google Scholar] [CrossRef]
- He, J.; Shi, H.; Xu, W.; Su, Z. Research progress on the cannibalistic behavior of Aquatic Animals and The Screening of Cannibalism-Preventing Shelters. Isr. J. Aquac.-Bamidgeh 2020, 72, 21024. [Google Scholar] [CrossRef]
- Abdussamad, E. Cannibalism in the Tiger Prawn Penaeus monodon fabricius in nursery rearing phase. J. Aqua. Trop. 1994, 9, 67–75. [Google Scholar]
- Bardera, G.; Owen, M.A.; Façanha, F.N.; Alcaraz-Calero, J.M.; Alexander, M.E.; Sloman, K.A. The influence of density and dominance on Pacific white shrimp (Litopenaeus vannamei) feeding behaviour. Aquaculture 2021, 531, 735949. [Google Scholar] [CrossRef]
- Ariadi, H.; Fadjar, M.; Mahmudi, M. The relationships between water quality parameters and the growth rate of white shrimp (Litopenaeus vannamei) in intensive ponds. Aquac. Aquar. Conserv. Legis. 2019, 12, 2103–2116. [Google Scholar]
- Zhang, P.; Zhang, X.; Li, J.; Huang, G. The effects of body weight, temperature, salinity, pH, light intensity and feeding condition on lethal DO levels of whiteleg shrimp, Litopenaeus vannamei (Boone, 1931). Aquaculture 2006, 256, 579–587. [Google Scholar] [CrossRef]
- Duan, Y.; Li, M.; Sun, M.; Wang, A.; Chai, Y.; Dong, J.; Chen, F.; Yu, Z.; Zhang, X. Effects of Salinity and Dissolved Oxygen Concentration on the Tail-Flip Speed and Physiologic Response of Whiteleg Shrimp, Litopenaeus vannamei. Sustainability 2022, 14, 15413. [Google Scholar] [CrossRef]
- Xu, C.; Animali, E.; Initiative, F.W. Key Aquatic Animal Welfare Recommendations for Aquaculture; Aquatic Animal Alliance: New York, NY, USA, 2020. [Google Scholar]
- Santos, A.D.A.; López-Olmeda, J.F.; Sánchez-Vázquez, F.J.; Fortes-Silva, R. Synchronization to light and mealtime of the circadian rhythms of self-feeding behavior and locomotor activity of white shrimps (Litopenaeus vannamei). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 199, 54–61. [Google Scholar] [CrossRef]
- Silva, P.F.; Medeiros, M.d.S.; Silva, H.P.A.; Arruda, M.d.F. A study of feeding in the shrimp Farfantepenaeus subtilis indicates the value of species level behavioral data for optimizing culture management. Mar. Freshwat. Behav. Physiol. 2012, 45, 121–134. [Google Scholar] [CrossRef]
- Bardera, G.; Usman, N.; Owen, M.; Pountney, D.; Sloman, K.A.; Alexander, M.E. The importance of behaviour in improving the production of shrimp in aquaculture. Rev. Aquac. 2019, 11, 1104–1132. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.K.; Singh, S.P.; Awasthi, A. Immunostimulants for shrimp aquaculture: Paving pathway towards shrimp sustainability. Environ. Sci. Pollut. Res. 2023, 30, 25325–25343. [Google Scholar] [CrossRef]
- Dai, W.-F.; Zhang, J.-J.; Qiu, Q.-F.; Chen, J.; Yang, W.; Ni, S.; Xiong, J.-B. Starvation stress affects the interplay among shrimp gut microbiota, digestion and immune activities. Fish Shellfish Immunol. 2018, 80, 191–199. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, L.; Wang, Y.; Lin, J. Alterations of protein expression in response to crowding in the Chinese shrimp (Fenneropenaeus chinensis). Aquaculture 2014, 428, 135–140. [Google Scholar] [CrossRef]
- Sung, Y.Y.; MacRae, T.H.; Sorgeloos, P.; Bossier, P. Stress response for disease control in aquaculture. Rev. Aquac. 2011, 3, 120–137. [Google Scholar] [CrossRef]
- Lemos, D.; Weissman, D. Moulting in the grow-out of farmed shrimp: A review. Rev. Aquac. 2021, 13, 5–17. [Google Scholar] [CrossRef]
- Parnes, S.; Raviv, S.; Sagi, A. Male and female reproduction in penaeid shrimps. In Reproductive Biology of Crustaceans. Case Studies of Decapod Crustaceans; CRC Press: Boca Raton, FL, USA, 2008; pp. 427–455. [Google Scholar]
- Conan, G.Y. Periodicity and phasing of molting. In Crustacean Issues 3; Routledge: London, UK, 2017; pp. 73–99. [Google Scholar]
- Molina-Poveda, C.; Escobar, V.; Gamboa-Delgado, J.; Cadena, E.; Orellana, F.; Piña, P. Estrategia de alimentación de acuerdo a la demanda fisiológica del juvenil Litopenaeus vannamei (Boone). Av. En Nutr. Acuicola 2002. Cancún, Quintana Roo, México. Available online: https://nutricionacuicola.uanl.mx/index.php/acu/article/view/231 (accessed on 15 January 2023).
- Ren, X.; Wang, Q.; Shao, H.; Xu, Y.; Liu, P.; Li, J. Effects of low temperature on shrimp and crab physiology, behavior, and growth: A review. Front. Mar. Sci. 2021, 8, 746177. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.; Wang, F.; Dong, S. Effects of periodical salinity fluctuation on the growth, molting, energy homeostasis and molting-related gene expression of Litopenaeus vannamei. J. Ocean Univ. China 2016, 15, 911–917. [Google Scholar] [CrossRef]
- Stumpf, L.; Timpanaro, S.; Battista, A.; López Greco, L. Effects of intermittent starvation on the survival, growth, and nutritional status of the freshwater prawn Macrobrachium borellii Nobili, 1896 (Decapoda: Caridea: Palaemonidae). J. Crustac. Biol. 2020, 40, 489–497. [Google Scholar] [CrossRef]
- Wu, L.; Dong, S. The effects of repetitive’starvation-and-refeeding’cycles on the compensatory growth response in Chinese shrimp, Fenneropenaeus chinensis (Osbeck, 1765) (Decapoda, Penaeidae). Crustaceana 2001, 74, 1225–1239. [Google Scholar]
- Briffa, M.; Weiss, A. Animal personality. Curr. Biol. 2010, 20, R912–R914. [Google Scholar] [CrossRef]
- Gherardi, F.; Aquiloni, L.; Tricarico, E. Behavioral plasticity, behavioral syndromes and animal personality in crustacean decapods: An imperfect map is better than no map. Curr. Zool. 2012, 58, 567–579. [Google Scholar] [CrossRef]
- Tidwell, J.; Bratvold, D. 15 Utility of Added Substrates in Shrimp Culture. In Periphyton: Ecology, Exploitation and Management; CABI: Wallingford, UK, 2005; p. 247. [Google Scholar]
- Thapa, H.; Rafiquzzaman, S.; Rahman, M.; Alam, M. Effects of Artifical Substrate on Rearing of Macrobrachium Rosenbergii Post-larvae in Pond Net Cage. Ann. Bangladesh Agric. 2020, 24, 95–106. [Google Scholar] [CrossRef]
- Browning, H. Assessing measures of animal welfare. Biol. Philos. 2022, 37, 36. [Google Scholar] [CrossRef]
- Nilsson, J.; Stien, L.H.; Iversen, M.H.; Kristiansen, T.S.; Torgersen, T.; Oppedal, F.; Folkedal, O.; Hvas, M.; Gismervik, K.; Ellingsen, K. Welfare Indicators for farmed Atlantic salmon–Part A. Knowledge and theoretical background. Welf. Indic. Farmed Atl. Salmon Tools Assess. Fish Welf. 2018, 351, 10–145. [Google Scholar]
- Wolfensohn, S.; Sharpe, S.; Hall, I.; Lawrence, S.; Kitchen, S.; Dennis, M. Refinement of welfare through development of a quantitative system for assessment of lifetime experience. Anim. Welf. 2015, 24, 139–149. [Google Scholar] [CrossRef]
- Narshi, T.M.; Free, D.; Justice, W.S.; Smith, S.J.; Wolfensohn, S. Welfare assessment of invertebrates: Adapting the animal welfare assessment grid (AWAG) for zoo decapods and cephalopods. Animals 2022, 12, 1675. [Google Scholar] [CrossRef]
- Stien, L.H.; Gytre, T.; Torgersen, T.; Sagen, H.; Kristiansen, T.S. A System for Online Assessment of Fish Welfare in Aquaculture; ICES: Toronto, ON, Canada, 2008. [Google Scholar]
- Stien, L.H.; Bracke, M.B.; Folkedal, O.; Nilsson, J.; Oppedal, F.; Torgersen, T.; Kittilsen, S.; Midtlyng, P.J.; Vindas, M.A.; Øverli, Ø. Salmon Welfare Index Model (SWIM 1.0): A semantic model for overall welfare assessment of caged Atlantic salmon: Review of the selected welfare indicators and model presentation. Rev. Aquac. 2013, 5, 33–57. [Google Scholar] [CrossRef]
- Pettersen, J.M.; Bracke, M.B.; Midtlyng, P.J.; Folkedal, O.; Stien, L.H.; Steffenak, H.; Kristiansen, T.S. Salmon welfare index model 2.0: An extended model for overall welfare assessment of caged Atlantic salmon, based on a review of selected welfare indicators and intended for fish health professionals. Rev. Aquac. 2014, 6, 162–179. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Arechavala-Lopez, P.; Castanheira, M.F.; Volstorf, J.; Heinzpeter Studer, B. A global assessment of welfare in farmed fishes: The FishEthoBase. Fishes 2019, 4, 30. [Google Scholar] [CrossRef]
- Weirup, L.; Schulz, C.; Seibel, H. Fish welfare evaluation index (fWEI) based on external morphological damage for rainbow trout (Oncorhynchus mykiss) in flow through systems. Aquaculture 2022, 556, 738270. [Google Scholar] [CrossRef]
- Toomey, L.; Gesto, M.; Alfonso, S.; Lund, I.; Jokumsen, A.; Lembo, G.; Carbonara, P. Monitoring welfare indicators of rainbow trout (Oncorhynchus mykiss) in a commercial organic farm: Effects of an innovative diet and accelerometer tag implantation. Aquaculture 2024, 582, 740549. [Google Scholar] [CrossRef]
- Lertwanakarn, T.; Purimayata, T.; Luengyosluechakul, T.; Grimalt, P.B.; Pedrazzani, A.S.; Quintiliano, M.H.; Surachetpong, W. Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand. Animals 2023, 13, 2498. [Google Scholar] [CrossRef]
- Gismervik, K.; Turnbull, J.F.; Nielsen, K.V.; Iversen, M.H.; Nilsson, J.; Espmark, Å.M.; Mejdell, C.M.; Sæther, B.-S.; Stien, L.H.; Izquierdo-Gomez, D. Welfare Indicators for farmed Atlantic salmon: Part C–fit for purpose OWIs for different routines and operations. In Welfare Indicators for Farmed Atlantic Salmon: Tools for Assessing Fish Welfare; Noble, C., Gismervik, K., Iversen, M.H., Kolarevic, J., Nilsson, J., Stien, L.H., Turnbull, J.F., Eds.; Nord University: Bodø, Norway, 2018; Volume 351, pp. 238–351. [Google Scholar]
- Noble, C.; Gismervik, K.; Iversen, M.H.; Kolarevic, J.; Nilsson, J.; Stien, L.H.; Turnbull, J.F. Welfare Indicators for Farmed Atlantic Salmon: Tools for Assessing Fish Welfare; Nord university: Bodø, Norway, 2018. [Google Scholar]
| Group | Combinations |
|---|---|
| I | “shrimp welfare”, “aquaculture”, AND “INDEX” AND “measure” |
| II | (“aquaculture” OR “fish farming”) AND (“well-being index” OR “welfare index” OR “welfare assessment” OR “welfare metric”) AND (“mathematical model” OR “quantitative formula” OR “evaluation index”) AND (“crustaceans” OR “fish” OR “shellfish” OR “aquatic organisms” OR Decapod) |
| III | “aquaculture” AND (“shrimp” OR “decapod” OR “Shellfish” OR “Crustacea” OR “Fish”) AND (“well-being assessment” OR “welfare assessment” OR “welfare Index” OR “well-being Index”) |
| Phase 1: Pre-Identification | Number of Documents |
|---|---|
| Number of identified documents | 1510 |
| Documents from not academic sources (manuals, technical standards, scientific dissemination articles) | 40 |
| Total number of identified documents | 1550 |
| Duplicate documents | 453 |
| Phase 2: Selection | Number of documents |
| Documents selected, excluding duplicates | 1097 |
| Documents excluded for not meeting the defined criteria | 961 |
| Phase 3: Eligibility | Number of documents |
| Documents assessed for eligibility | 136 |
| Documents excluded for not meeting the defined criteria | 76 |
| Documents evaluated through full reading | 60 |
| Documents excluded for not meeting the defined criteria | 50 |
| Result: Total number of included documents | 10 |
| Welfare Rating | PWIx and GWI | CLx and GCL |
|---|---|---|
| Critical | 0 | - |
| Low | >0 and ≤0.50 | >0 and ≤0.50 |
| Medium | >0.50 and <0.75 | >0.50 and <0.70 |
| High | ≥0.75 | ≥0.70 |
| Item | Description/Value | Unit |
|---|---|---|
| Water surface area | 9 | ha |
| Operating system | Biphase | - |
| Production regime | Semi-intensive | - |
| Post-larvae (PL20) | Specific pathogen-free (SPF) | - |
| Stocking density | 43 | shrimps/m2 |
| Biometry | 1 | time/week |
| Diet composition | Natural feed + pellets | - |
| Feeding frequency | 4 | times/day |
| Feed quantity | 2.0–5.0 | % biomass |
| Use of feeders | 35 | feeders/ha |
| Feed size | 1.0–3.0 | mm |
| Crude protein in feed | 35–40 | % |
| Apparent Feed Conversion rate | 1.5 | - |
| Stunning during slaughter | Ice | seconds |
| Method for controlling aquatic predators | Screens | - |
| Final shrimp weight | 12 | g |
| Cycle duration | 90 | days |
| Survival | 72 | % |
| Parameter | Value | Unit |
|---|---|---|
| Temperature | 25–32 | °C |
| pH | 6.5–7.5 | - |
| Transparency | 30.0–35.0 | cm |
| Alkalinity | 120.0–200.0 | mg/L CaCO3 |
| Ammonia | 0.00–0.12 | mg/L NH3 |
| Dissolved Oxygen | 68.0 | % saturation |
| Nitrite | 0.0–0.5 | mg/L NO2− |
| Salinity | 10.0–40.0 | PSU |
| Domain | Keyword | Number of Documents (n) | Weight (Y) |
|---|---|---|---|
| Environmental | “pH” “Temperature” “Salinity” “Stocking density” “Ammonia” “Dissolved oxygen” “Nitrite” “Alkalinity” “Terrestrial” AND “predator” OR “competitor” “Transparency” | 25,700 | 10 |
| 24,700 | 10 | ||
| 19,000 | 10 | ||
| 16,660 | 10 | ||
| 14,200 | 10 | ||
| 13,200 | 9 | ||
| 7590 | 9 | ||
| 2850 | 8 | ||
| 1730 | 7 | ||
| 1550 | 7 | ||
| “Aquatic” AND “predator” OR “competitor” | 778 | 7 | |
| Health | “Mortality” | 16,100 | 10 |
| “Hepatopancreas” | 11,100 | 9 | |
| “Gills” | 7800 | 9 | |
| “Eyes “ | 3950 | 8 | |
| “Exoskeleton” | 2750 | 8 | |
| “Motor appendages” | 2290 | 8 | |
| “Musculature” | 1620 | 7 | |
| “Rostrum “ | 1230 | 7 | |
| “Antennae” | 781 | 7 | |
| Nutritional | “Frequency food” | 26,100 | 10 |
| “Apparent feed conversion rate” | 12,660 | 9 | |
| “Crude protein” | 9970 | 9 | |
| “Use of trays” | 1960 | 8 | |
| “Distribution food” | 119 | 5 | |
| “Size food “ | 164 | 5 | |
| “Amount of initial food” | 147 | 5 | |
| “Digestive tract filling index” | 7 | 2 | |
| Behavioural | “Swimming behaviour” | 258 | 6 |
| “Escape behaviour” | 153 | 5 | |
| “Stunning” | 132 | 5 |
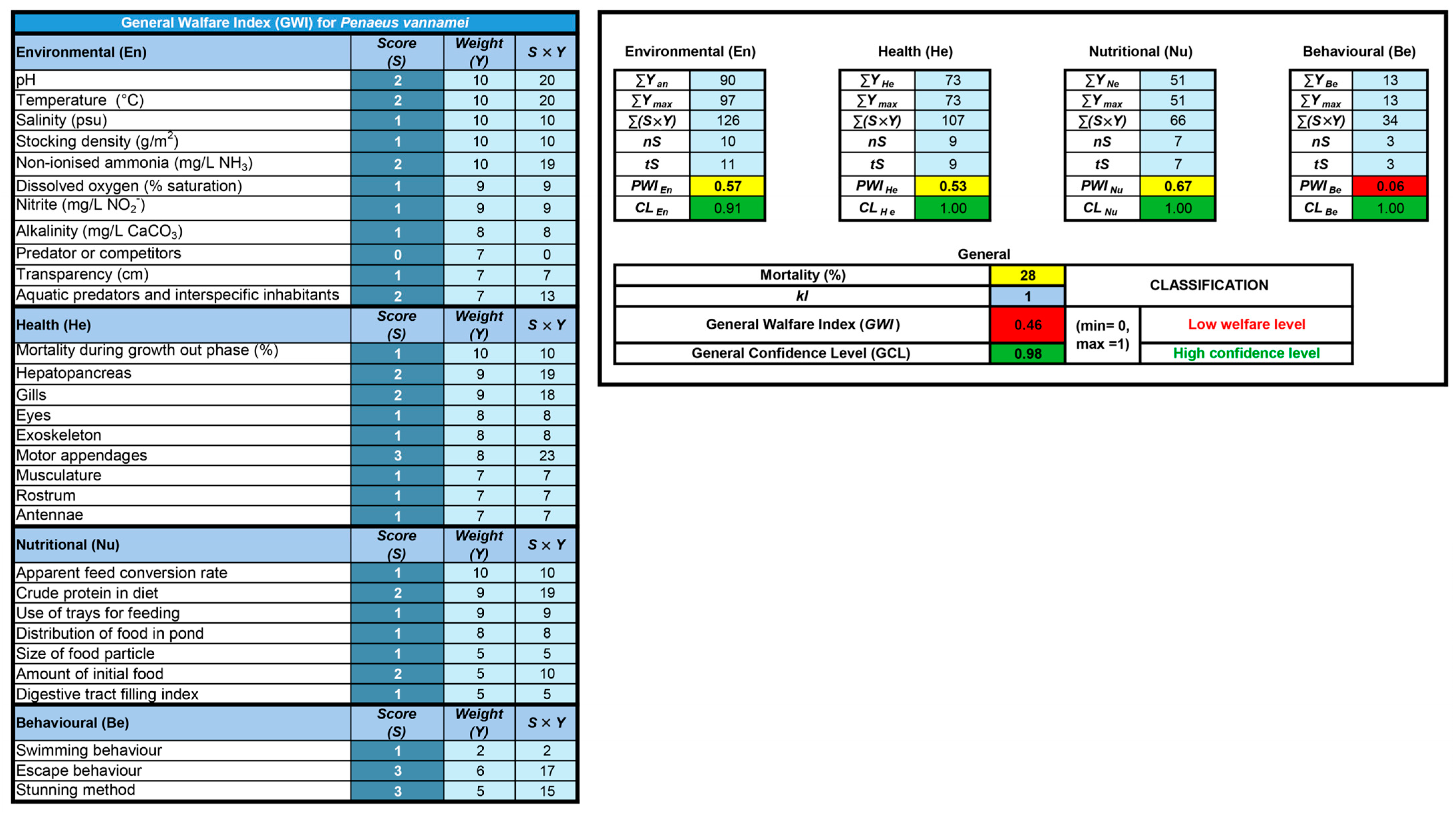
| Domain | Indicator | Value or Criteria Described at the Hypothetical Farm | Value or Criteria Considered for Scoring | Scores Obtained in Hypothetical Farm * |
|---|---|---|---|---|
| Environmental | Temperature | 25.0–32.0 | 25.0–32.0 | 1 |
| pH | 6.5–7.5 | 6.5–8.5 | 1 | |
| Transparency | 30.0–35.0 | 35.0–50.0 | 2 | |
| Alkalinity | 120.0–200.0 | 100.0–140.0 | 2 | |
| Ammonia (NH3) | 0.00–0.12 | 0.00–0.10 | 2 | |
| Dissolved Oxygen | 68.0 | ≥65.0 | 1 | |
| Nitrite | 0.0–0.5 | 0.0–0.6 | 1 | |
| Salinity | 40.0 | 10.0–40.9 | 1 | |
| Stocking density | 43.0 | ≤40.0 | 2 | |
| Aquatic Predators | Screen 500 um−1 mm | Controlled presence | 2 | |
| Behavioural | Swimming behaviour | Figure S1 | Few animals on the pond surface or irregular swimming | 1 |
| Escape behaviour | Figure S2 | Few jumping shrimps, but with high frequency and/or intensity during harvesting | 3 | |
| Stunning at slaughter–clinical reflexes | Figure S3 | Slaughter using water and ice. Progressive loss of response to external stimuli; balance; movement of pleopods and pereiopods within >30 seconds | 3 | |
| Nutritional | Size of food | 1.0–3.0 | 2.1–3.0 | 1 |
| Amount of food (% biomass) | 2.0–5.0 | 2.0–3.9 | 2 | |
| Feeding frequency (times/day) | 4.0 | ≥2 | 1 | |
| Crude Protein (%) | 32.0–40.0 | ≥32.0 | 1 | |
| FCR | 1.5 | ≤1.5 | 1 | |
| Distribution of feed (% of pond surface) | >75 | >75 | 1 | |
| Use of feeders (no./ha) ** | 35.0 | ≥20.0 | 1 | |
| Digestive tract filling index | 46% full | Full | 1 | |
| Health | Antennae | Figure S4 | Focal lesion, shortening, or darkening | 2 |
| Rostrum | Figure S5 | Mild injury, erosion, or necrosis | 2 | |
| Eyes | Figure S5 | Healthy appearance, no changes | 1 | |
| Gills | Figure S6 | Healthy appearance, no changes | 1 | |
| Hepatopancreas | Figure S4 | Healthy appearance, no changes | 1 | |
| Motor appendages | Figure S7 | Focal absence or erosions | 2 | |
| Exoskeleton | Figure S7 | Slight lesion or focal darkening, presence of debris | 2 | |
| Musculature | Figure S7 | Healthy appearance, no changes | 1 | |
| Mortality (%) | 28.0 | ≥26.0 | 3 |
| INDEX | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | GWI 1 | AWAG 2 | Welfare Meter 3 | SWIN 1.0 4 | SWIN 2.0 5 | FishEthoScores 6 | fWEI 7 | MyFishCheck 8 | Not Named 9 | FISHWELL 10 |
| Application | Aquaculture organisms | Decapods and cephalopods in zoo and aquarium | Caged Salmon | Caged salmon | Caged salmon | Farmed fish | Farmed trout | Farmed fish | Farmed tilapia | Farmed salmon and trout |
| Is it already applied to shrimps? | Yes | No | No | No | No | No | No | No | No | No |
| Domains of welfare directly addressed | 4/5 | 1/5 | 2/5 | 4/5 | 2/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 |
| The number of welfare indicators | 30 | 19 | 7 | 18 | 10 | 10 | 12 | 19 | 25 | 23 |
| Time required for measurement of indicators | Medium | Long | Automatic | Medium | Short | Long | Short | Long | Medium | Medium |
| Invasiveness of the indicators | Low | Low | Low | Low | Low | Low | Low | High | Low | High |
| Does it use factor weighting for the indicators? | Yes | No | No | Yes | Yes | No | Yes | No | No | No |
| Number of scores for each indicator | 3 | 10 | Not applied | 2–6 | 3–7 | 3 | 4 | Not applied | 4 | 4 |
| Ease of field measurement of indicators | Moderate | Moderate | Easy | Moderate | Moderate | Moderate | Easy | Difficult | Moderate | Difficult |
| Is there a calculation of a quantitative welfare index? | Yes | No | Yes | Yes | Yes | No | Yes | No | No | No |
| Is it calculated the confidence interval of the indices? | Yes | No | No | No | No | No | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrazzani, A.S.; Cozer, N.; Quintiliano, M.H.; Ostrensky, A. Insights into Decapod Sentience: Applying the General Welfare Index (GWI) for Whiteleg Shrimp (Penaeus vannamei—Boone, 1931) Reared in Aquaculture Grow-Out Ponds. Fishes 2024, 9, 440. https://doi.org/10.3390/fishes9110440
Pedrazzani AS, Cozer N, Quintiliano MH, Ostrensky A. Insights into Decapod Sentience: Applying the General Welfare Index (GWI) for Whiteleg Shrimp (Penaeus vannamei—Boone, 1931) Reared in Aquaculture Grow-Out Ponds. Fishes. 2024; 9(11):440. https://doi.org/10.3390/fishes9110440
Chicago/Turabian StylePedrazzani, Ana Silvia, Nathieli Cozer, Murilo Henrique Quintiliano, and Antonio Ostrensky. 2024. "Insights into Decapod Sentience: Applying the General Welfare Index (GWI) for Whiteleg Shrimp (Penaeus vannamei—Boone, 1931) Reared in Aquaculture Grow-Out Ponds" Fishes 9, no. 11: 440. https://doi.org/10.3390/fishes9110440
APA StylePedrazzani, A. S., Cozer, N., Quintiliano, M. H., & Ostrensky, A. (2024). Insights into Decapod Sentience: Applying the General Welfare Index (GWI) for Whiteleg Shrimp (Penaeus vannamei—Boone, 1931) Reared in Aquaculture Grow-Out Ponds. Fishes, 9(11), 440. https://doi.org/10.3390/fishes9110440







