Cultivation and Growth Dynamics of Capelin (Mallotus villosus) from Hatch to Adulthood
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Collection
2.2. Incubation
2.3. Rearing Conditions
Group B
2.4. Measurements and Data Analysis
Growth Comparison Between Cultivated and Wild Icelandic Capelin
2.5. Statistical Analysis
3. Results
3.1. Hatching
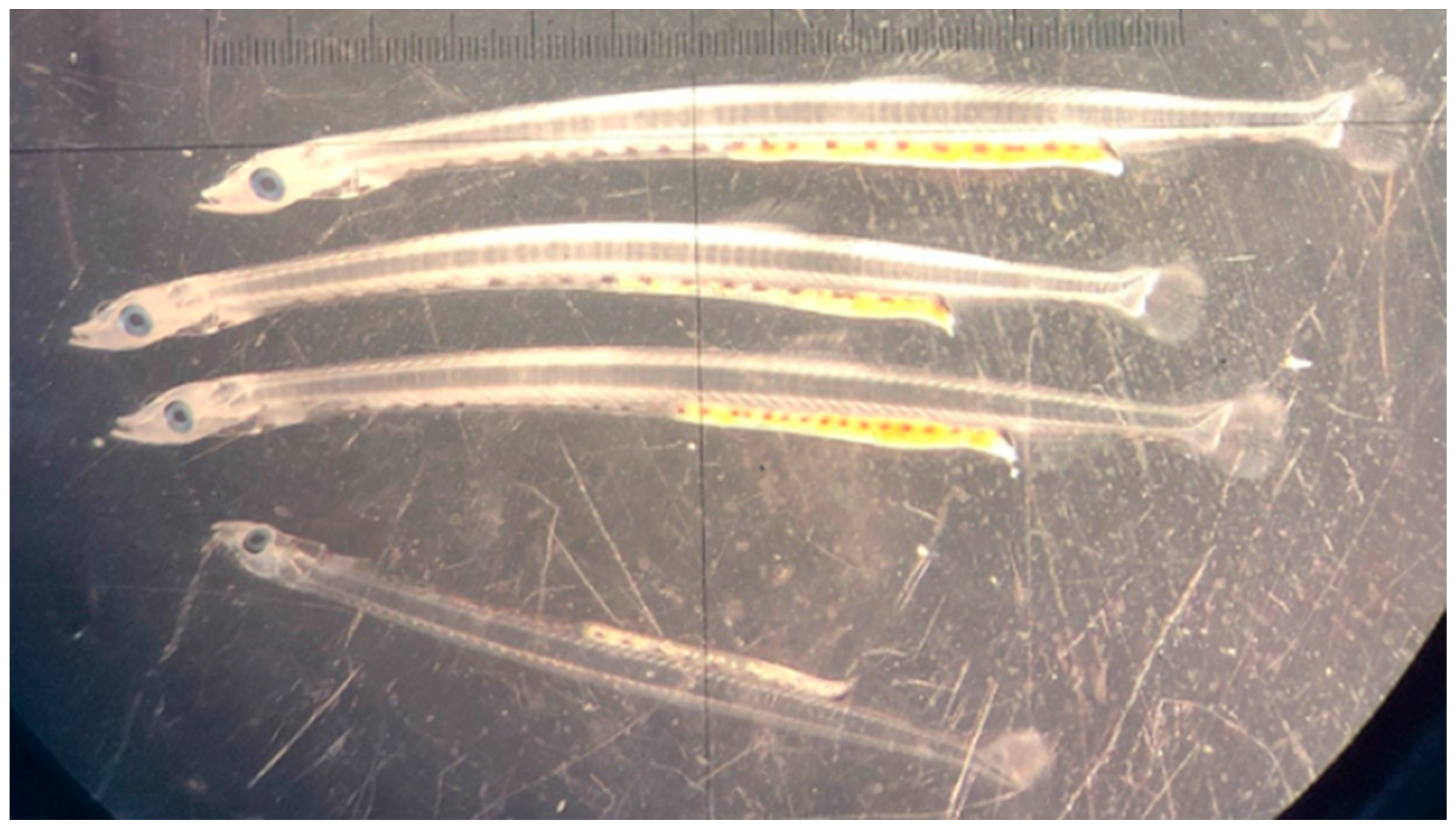
3.2. Feeding
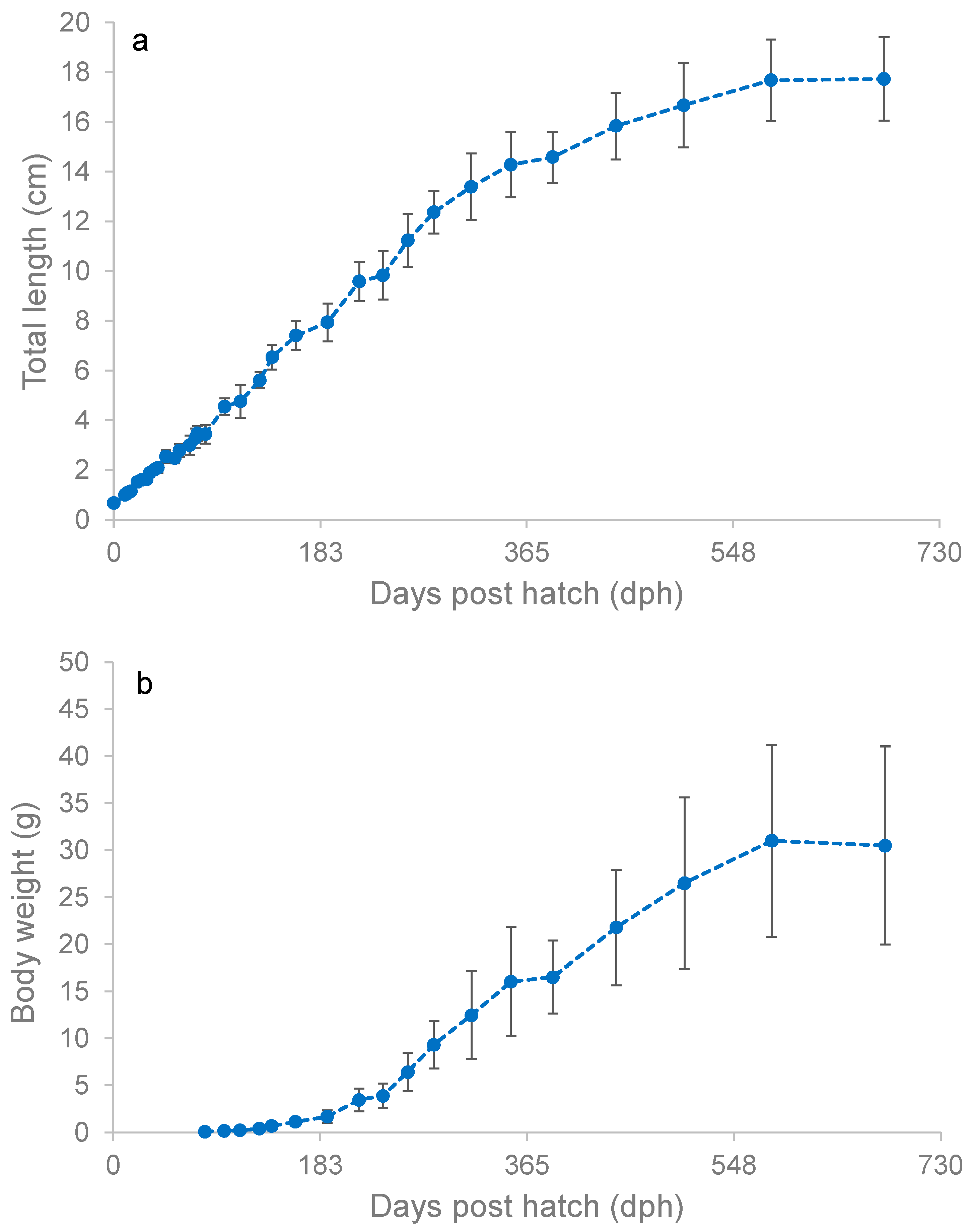
3.3. Early Growth Performance
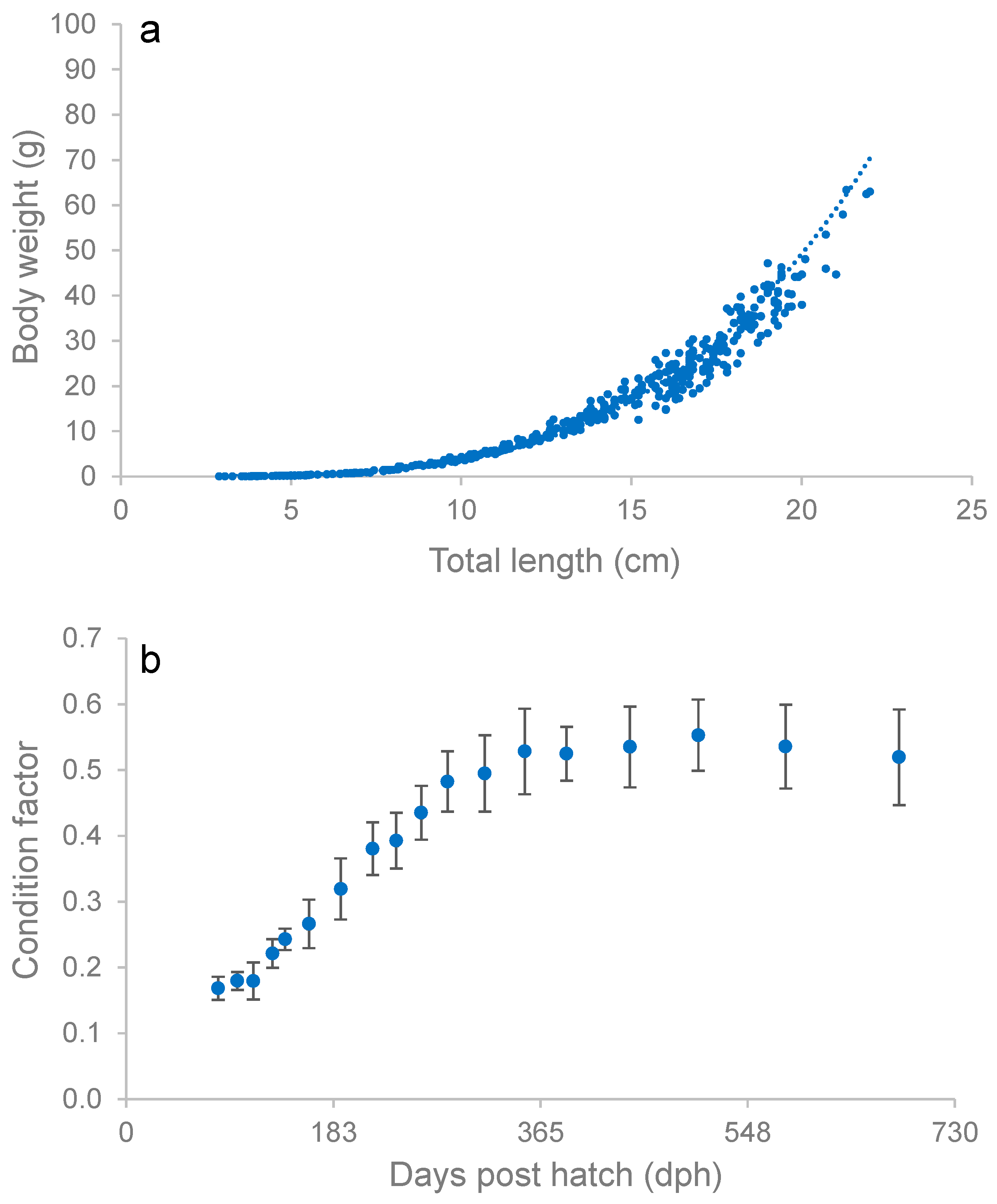
3.4. Adult Growth Performance
3.5. Sexual Size Dimorphism
3.6. Body Condition
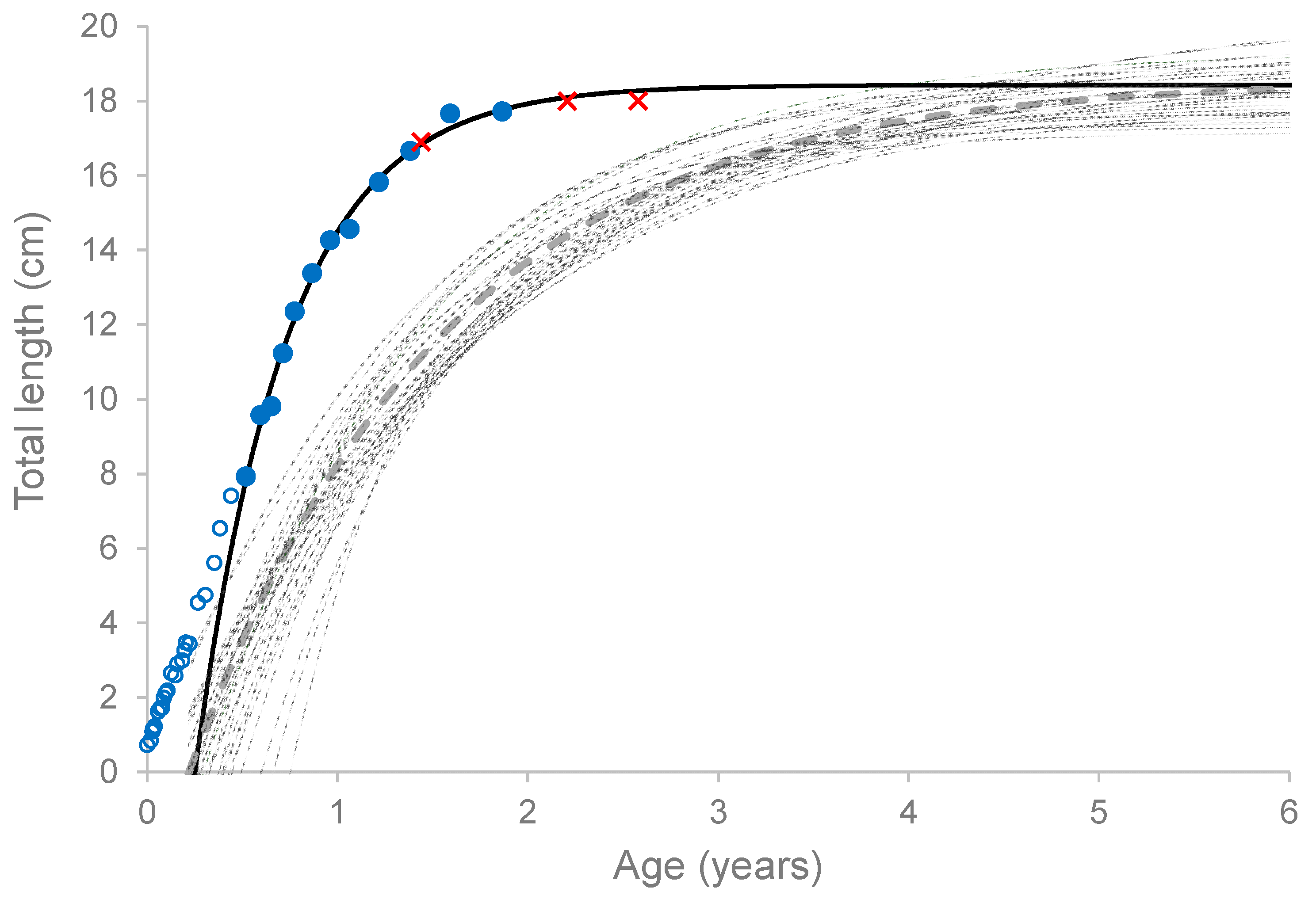
3.7. Wild Stock Comparison
3.8. Behavior, Survival, and Deformity
4. Discussion
4.1. Cultivation of Capelin
4.2. Growth Dynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carscadden, J.E.; Vilhjálmsson, H. Capelin—What Are They Good For? Introduction. ICES J. Mar. Sci. 2002, 59, 863–869. [Google Scholar] [CrossRef]
- Præbel, K.; Westgaard, J.I.; Fevolden, S.E.; Christiansen, J.S. Circumpolar Genetic Population Structure of Capelin Mallotus villosus. Mar. Ecol. Prog. Ser. 2008, 360, 189–199. [Google Scholar] [CrossRef]
- Vilhjálmsson, H. The Icelandic Capelin Stock. Capelin, Mallotus villosus (Müller) in Iceland–Greenland–Jan Mayen Area; Rit Fiskideildar; Marine Research Institute: Reykjavík, Iceland, 1994; Volume XIII, 281p. [Google Scholar]
- Christiansen, J.S.; Præbel, K.; Siikavuopio, S.I.; Carscadden, J. Facultative Semelparity in Capelin Mallotus villosus (Osmeridae)—An Experimental Test of a Life History Phenomenon in a Sub-Arctic Fish. J. Exp. Mar. Biol. Ecol. 2008, 360, 47–55. [Google Scholar] [CrossRef]
- Carscadden, J.E.; Frank, K.T.; Miller, D.S. Capelin (Mallotus villosus) Spawning on the Southeast Shoal: Influence of Physical Factors Past and Present. Can. J. Fish. Aquat. Sci. 1989, 46, 1743–1754. [Google Scholar] [CrossRef]
- Vandeperre, F.; Methven, D.A. Do Bigger Fish Arrive and Spawn at the Spawning Grounds Before Smaller Fish: Cod (Gadus morhua) Predation on Beach Spawning Capelin (Mallotus villosus) from Coastal Newfoundland. Estuar. Coast. Shelf Sci. 2007, 71, 391–400. [Google Scholar] [CrossRef]
- Baulier, L.; Heino, M.; Gjøsæter, H. Temporal Stability of the Maturation Schedule of Capelin Mallotus villosus in the Barents Sea. Aquat. Living Resour. 2012, 25, 151–161. [Google Scholar] [CrossRef]
- ICES. Arctic Fisheries Working Group (AFWG); ICES Scientific Reports; ICES: Copenhagen, Denmark, 2021; Volume 3, 817p. [Google Scholar] [CrossRef]
- Jourdain, N.O.A.S.; Fuglebakk, E.; Subbey, S. Maturation in the Barents Sea Capelin–Contrasting Length- and Gonad-Based Metrics. Fish. Res. 2021, 237, 105880. [Google Scholar] [CrossRef]
- Gjøsæter, H. The Population Biology and Exploitation of Capelin (Mallotus villosus) in the Barents Sea. Sarsia 1998, 83, 453–496. [Google Scholar] [CrossRef]
- Hedeholm, R.; Grønkjær, P.; Rosing-Asvid, A.; Rysgaard, S. Variation in Size and Growth of West Greenland Capelin (Mallotus villosus) Along Latitudinal Gradients. ICES J. Mar. Sci. 2010, 67, 1128–1137. [Google Scholar] [CrossRef][Green Version]
- McNicholl, D.G.; Davoren, G.K.; Reist, J.D. Life History Variation Across Latitudes: Observation Between Capelin (Mallotus villosus) from Newfoundland and the Eastern Canadian Arctic. Polar Biol. 2018, 41, 643–651. [Google Scholar] [CrossRef]
- Magnaye, M.; Rideout, R.M.; Davoren, G. Irregular Growth Patterns in the Otoliths of a Short-Lived Forage Fish Do Not Reliably Indicate Reproductive History. Fish. Res. 2019, 218, 120–126. [Google Scholar] [CrossRef]
- Berg, F.; Shirajee, S.; Folkvord, A.; Godiksen, J.A.; Skaret, G.; Slotte, A. Early Life Growth Is Affecting Timing of Spawning in the Semelparous Barents Sea Capelin (Mallotus villosus). Prog. Oceanogr. 2021, 196, 102614. [Google Scholar] [CrossRef]
- Winters, G.H. Life History and Geographical Patterns of Growth in Capelin, Mallotus villosus, of the Labrador and Newfoundland Areas. J. Northwest Atl. Fish. Sci. 1982, 3, 105–114. [Google Scholar] [CrossRef]
- Friis-Rødel, E.; Kanneworff, P. A Review of Capelin (Mallotus villosus) in Greenland Waters. ICES J. Mar. Sci. 2002, 59, 890–896. [Google Scholar] [CrossRef]
- Hamre, J.; Johnsen, E.; Hamre, K. A New Model for Simulating Growth in Fish. PeerJ 2014, 2, e244. [Google Scholar] [CrossRef]
- Winters, G.H. Record Size and Age of Atlantic Capelin, Mallotus villosus. J. Fish. Res. Board Can. 1970, 27, 393–394. [Google Scholar] [CrossRef]
- Peck, M.A.; Alheit, J.; Bertrand, A.; Catalán, I.A.; Garrido, S.; Moyano, M.; Rykaczewski, R.R.; Takasuka, A.; van der Lingen, C.D. Small Pelagic Fish in the New Millennium: A Bottom-Up View of Global Research Effort. Prog. Oceanogr. 2021, 191, 102494. [Google Scholar] [CrossRef]
- Dorval, E.; Appel, P.; Human, M.H.; Macewicz, B.J.; Watson, W. Rearing and Inducing Spawning in Captive Pacific Sardine (Sardinops sagax). Calif. Coop. Ocean. Fish. 2019, 60, 123–134. [Google Scholar]
- Garrido, S.; Saiz, E.; Peters, J.; Ré, P.; Alvarez, P.; Cotano, U.; Herrero, D.L.; Martínez de Murguía, A.; Irigoien, X. Effects of Food Type and Concentration on Growth and Fatty Acid Composition of Early Larvae of the Anchovy. J. Exp. Mar. Biol. Ecol. 2012, 434, 16–24. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Yoneda, M.; Sakai, N.; Nakashima, K.; Kitano, H.; Matsuyama, M. Comprehensive Experimental System for a Promising Model Organism Candidate for Marine Teleosts. Sci. Rep. 2019, 9, 4948. [Google Scholar] [CrossRef]
- Pozdnyakov, Y.F. Spawning of Capelin in the Aquarium. Izv. Karel. Kolsk. Fil. SSSR 1959, 3, 145–147. (In Russian) [Google Scholar]
- Pozdnyakov, Y.F. Materiali o Razvitii Moivi Barentsevo Morja. Tr. Murm. Morsk Biol. Ins. 1960, 2, 211–225. (In Russian) [Google Scholar]
- Friðgeirsson, E. Observations on Spawning Behaviour and Embryonic Development of the Icelandic Capelin; Rit Fiskideildar; Hafrannsóknastofnunin: Reykjavík, Iceland, 1976; Volume 4, 35p. [Google Scholar]
- Moksness, E. Food Uptake, Growth and Survival of Capelin Larvae (Mallotus villosus Müller) in an Outdoor Constructed Basin. Fisk. Dir. Skr. Ser. HavUnders. 1982, 17, 267–285. [Google Scholar]
- Zenzerov, V.S. Morphofunctional Changes in the Thyroid Gland of the Capelin, Mallotus villosus villosus, Under Experimental Conditions. Vopr. Ihtiol. 1982, 22, 93–96. [Google Scholar]
- Gjøsæter, J.; Monstad, T. Primary Growth Rings in Otoliths of the Barents Sea Capelin. Fisk. Dir. Skr. Ser. HavUnders. 1985, 17, 521–528. [Google Scholar]
- Frank, K.T.; Leggett, W.C. Effect of Prey Abundance and Size on the Growth and Survival of Larval Fish: An Experimental Study Employing Large Volume Enclosures. Mar. Ecol. Prog. Ser. 1986, 34, 11–22. [Google Scholar] [CrossRef]
- Gjøsæter, H.; Gjøsæter, J. Observations on the Embryonic Development of Capelin (Mallotus villosus Müller) from the Barents Sea. Fisk. Dir. Skr. Ser. HavUnders. 1986, 18, 59–68. [Google Scholar]
- Davenport, J.; Stene, A. Freezing Resistance, Temperature and Salinity Tolerance in Eggs, Larvae and Adult Capelin, Mallotus villosus, from Balsfjord. J. Mar. Biol. Assoc. 1986, 66, 145–157. [Google Scholar] [CrossRef]
- Davenport, J. The Effects of Salinity and Low Temperature on Eggs of the Icelandic Capelin Mallotus villosus. J. Mar. Biol. Assoc. 1989, 69, 1–9. [Google Scholar] [CrossRef]
- Paine, M.D.; Leggett, W.C.; McRuer, J.K.; Frank, K.T. Effects of Hibernia Crude Oil on Capelin (Mallotus villosus) Embryos and Larvae. Mar. Environ. Res. 1992, 33, 159–187. [Google Scholar] [CrossRef]
- Morgan, M.J.; Anderson, J.T.; Brown, J.A. Early Development of Shoaling Behavior in Larval Capelin (Mallotus villosus). Mar. Behav. Physiol. 1994, 24, 197–206. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Siikavuopio, S.I. Survival and Growth of Post-Spawning Capelin (Mallotus villosus)—An Introductory Report from a Laboratory Study. ICES CM, 1998, CC, 1–11. Available online: https://www.ices.dk/sites/pub/CM%20Doccuments/1998/CC/CC0898.pdf (accessed on 10 November 2024).
- Burton, M.P.M.; Flynn, S.R. Differential Postspawning Mortality Among Male and Female Capelin (Mallotus villosus Müller) in Captivity. Can. J. Zool. 1998, 76, 588–592. [Google Scholar] [CrossRef]
- Karamushko, L.I.; Christiansen, J.S. Aerobic Scaling and Resting Metabolism in Oviferous and Post-Spawning Barents Sea Capelin Mallotus villosus villosus (Müller, 1776). J. Exp. Mar. Biol. Ecol. 2002, 269, 1–8. [Google Scholar] [CrossRef]
- Behrens, J.W.; Præbel, K.; Steffensen, J.F. Swimming Energetics of the Barents Sea Capelin (Mallotus villosus) During the Spawning Migration Period. J. Exp. Mar. Biol. Ecol. 2006, 331, 208–216. [Google Scholar] [CrossRef]
- Karamushko, O.V.; Christiansen, J.S. Some Aspects of the Feeding and Behavior of Larvae of the Barents Sea Capelin Mallotus villosus villosus (Salmoniformes, Osmeridae) in Experimental Conditions. J. Ichthyol. 2006, 46, 322–327. [Google Scholar] [CrossRef]
- Ivarjord, T.; Pedersen, T.; Moksness, E. Effects of Growth Rates on the Otolith Increments Deposition Rate in Capelin Larvae (Mallotus villosus). J. Exp. Mar. Biol. Ecol. 2008, 358, 170–177. [Google Scholar] [CrossRef]
- Frantzen, M.; Falk-Petersen, I.B.; Nahrgang, J.; Smith, T.J.; Olsen, G.H.; Hangstad, T.A.; Camus, L. Toxicity of Crude Oil and Pyrene to the Embryos of Beach Spawning Capelin (Mallotus villosus). Aquat. Toxicol. 2012, 108, 42–52. [Google Scholar] [CrossRef]
- Penton, P.M.; Davoren, G.K. A Common Garden Experiment on Capelin (Mallotus villosus) Early Life History Stages to Examine Use of Beach and Deep-Water Spawning Habitats. J. Exp. Mar. Biol. Ecol. 2013, 439, 54–60. [Google Scholar] [CrossRef]
- Præbel, K.; Christiansen, J.S.; Kettunen-Præbel, A.; Fevolden, S.-E. Thermohaline Tolerance and Embryonic Development in Capelin Eggs (Mallotus villosus) from the Northeast Atlantic Ocean. Environ. Biol. Fish 2013, 96, 753–761. [Google Scholar] [CrossRef]
- Beirão, J.; Baillon, L.; Litt, M.A.; Langlois, V.S.; Purchase, C.F. Impact of Crude Oil and the Dispersant Corexit™ EC95004 on Capelin (Mallotus villosus) Embryo Development. Mar. Environ. Res. 2019, 147, 90–100. [Google Scholar] [CrossRef]
- Tairova, Z.; Frantzen, M.; Mosbech, A.; Arukwe, A.; Gustavson, K. Effects of Water Accommodated Fraction of Physically and Chemically Dispersed Heavy Fuel Oil on Beach Spawning Capelin (Mallotus villosus). Mar. Environ. Res. 2019, 147, 62–71. [Google Scholar] [CrossRef]
- Shadrin, A.M.; Makhotin, V.V.; Eriksen, E. Incubation Temperature Effect on Qualitative and Quantitative Composition of Abnormalities and Mortality Rate in Embryogenesis of the Barents Sea Capelin Mallotus villosus (Osmeridae). J. Ichthyol. 2020, 60, 79–89. [Google Scholar] [CrossRef]
- Nahrgang, J.; Granlund, C.; Bender, M.L.; Sørensen, L.; Greenacre, M.; Frantzen, M. No Observed Developmental Effects in Early Life Stages of Capelin (Mallotus villosus) Exposed to a Water-Soluble Fraction of Crude Oil During Embryonic Development. J. Toxicol. Environ. Health A 2023, 86, 404–419. [Google Scholar] [CrossRef]
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods; R Package Version 0.9.4. 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 2 February 2024).
- Bailey, R.F.J.; Able, K.W.; Leggett, W.C. Evidence for the Presence of a Metamorphic Check in Capelin (Mallotus villosus) in Otoliths and Implications for Age Determination. J. Fish. Res. Board Can. 1977, 34, 2008–2014. [Google Scholar] [CrossRef]
- Vesin, J.-P.; Leggett, W.C.; Able, K.W. Feeding Ecology of Capelin (Mallotus villosus) in the Estuary and Western Gulf of St. Lawrence and Its Multispecies Implications. Can. J. Fish. Aquat. Sci. 1981, 38, 257–267. [Google Scholar] [CrossRef]
- Royston, P. Remark AS R94: A Remark on Algorithm AS 181: The W Test for Normality. J. R. Stat. Soc. Ser. C Appl. Stat. 1995, 44, 547–551. [Google Scholar] [CrossRef]
- Árnason, T.; Bárðarson, B.; Steinarsson, A. Cultivation of Capelin in the Aquaculture Research Station in Grindavík. In Capelin in a Changing Environment; Haf-og vatnarannsóknir, HV 2023-43; Singh, W., Ólafsdóttir, A.H., Jónsson, S.Þ., Óskarsson, G.J., Eds.; Marine and Freshwater Research Institute: Hafnarfjörður, Iceland, 2023; pp. 27–30. [Google Scholar]
- Frank, K.T.; Leggett, W.C. Wind Regulation of Emergence Times and Early Larval Survival in Capelin (Mallotus villosus). Can. J. Fish. Aquat. Sci. 1981, 38, 215–223. [Google Scholar] [CrossRef]
- Yúfera, M.; Darias, M.J. The Onset of Exogenous Feeding in Marine Fish Larvae. Aquaculture 2007, 268, 53–63. [Google Scholar] [CrossRef]
- Fossheim, M.; Tande, K.S.; Semenova, T.; Timonin, A. Capelin Larvae (Mallotus villosus) and Community Structure of Zooplankton of the Coast of Northern Norway. J. Plankton Res. 2006, 28, 585–595. [Google Scholar] [CrossRef]
- Muller-Feuga, A.; Robert, R.; Cahu, C.; Robin, J.; Divernach, P. Use of Microalgae in Aquaculture. In Live Feeds in Marine Aquaculture; Støttrup, J.A., McEvoy, L.A., Eds.; Blackwell Publishing: Oxford, UK, 2003; pp. 253–299. [Google Scholar]
- van der Meeren, T.; Mangor-Jensen, A.; Pickova, J. The Effect of Green Water and Light Intensity on Survival, Growth, and Lipid Composition in Atlantic Cod (Gadus morhua) During Intensive Larval Rearing. Aquaculture 2007, 265, 206–217. [Google Scholar] [CrossRef]
- Downing, G.; Litvak, M.K. The Effect of Photoperiod, Tank Color, and Light Intensity on Growth of Larval Haddock. Aquacult. Int. 2000, 7, 369–382. [Google Scholar] [CrossRef]
- McLean, E.; Cotter, P.; Thain, C.; King, N. Tank Color Impacts Performance of Cultured Fish. Ribarstvo 2008, 66, 43–54. [Google Scholar]
- Bera, A.; Kailasam, M.; Mandaal, B.; Sukumaran, K.; Makesh, M.; Hussain, T.; Sivaramakrishnan, T.; Subburaj, T.; Thiagarajan, G.; Vijayan, K.K. Effect of Tank Color on Foraging Capacity, Growth, and Survival of Milkfish (Chanos chanos) Larvae. Aquaculture 2019, 515, 734347. [Google Scholar] [CrossRef]
- Planas, M.; Cunha, I. Larviculture of Marine Fish: Problems and Perspectives. Aquaculture 1999, 177, 171–190. [Google Scholar] [CrossRef]
- Suuronen, P.; Erickson, D.L.; Orrensalo, A. Mortality of Herring Escaping from Pelagic Trawl Codends. Fish. Res. 1996, 25, 305–321. [Google Scholar] [CrossRef]
- Suuronen, P.; Peres-Comas, J.; Lehtonen, E.; Tschernij, V. Size-Related Mortality of Herring (Clupea harengus L.) Escaping Through a Rigid Sorting Grid and Trawl Codend Meshes. ICES J. Mar. Sci. 1996, 53, 691–700. [Google Scholar] [CrossRef]
- Berillis, P. Factors that can lead to the development of skeletal deformities in fishes: A review. J. Fish. Sci. 2015, 9, 17–23. [Google Scholar]
- Cobcroft, J.M.; Battaglene, S. Jaw Malformation in Striped Trumpeter (Latris lineata) Larvae Linked to Walling Behavior and Tank Color. Aquaculture 2009, 289, 274–282. [Google Scholar] [CrossRef]
- Noble, C.; Cañon Jones, H.A.; Damsgård, B.; Flood, M.J.; Midling, K.Ø.; Roque, A.; Sæther, B.-S.; Cottee, S.Y. Injuries and Deformities in Fish: Their Potential Impacts Upon Aquacultural Production and Welfare. Fish Physiol. Biochem. 2012, 38, 61–83. [Google Scholar] [CrossRef]
- Huse, G. Sex-Specific Life History Strategies in Capelin (Mallotus villosus)? Can. J. Fish. Aquat. Sci. 1998, 55, 631–638. [Google Scholar] [CrossRef]
- Ólafsdóttir, A.H.; Anderson, T. Growth and Survival of Icelandic Capelin Mallotus villosus Larvae. Mar. Ecol. Prog. Ser. 2010, 403, 231–241. [Google Scholar] [CrossRef][Green Version]
- Jacquaz, B.; Able, K.W.; Leggett, W.C. Seasonal Distribution, Abundance, and Growth of Larval Capelin (Mallotus villosus) in the St. Lawrence Estuary and Northwestern Gulf of St. Lawrence. J. Fish. Res. Board Can. 1977, 34, 2015–2029. [Google Scholar] [CrossRef]
- Shikon, V.; Pepin, P.; Schneider, D.C.; Castonguay, M.; Robert, D. Spatiotemporal Variability in Newfoundland Capelin (Mallotus villosus) Larval Abundance and Growth: Implications for Recruitment. Fish. Res. 2019, 218, 237–245. [Google Scholar] [CrossRef]
- Jakobsen, R.A.; Pedersen, T.; Moksness, E. Growth Rates and Age Distribution of Capelin (Mallotus villosus) Larvae in the Barents Sea Investigated by Otolith Increment Analysis. ICES CM 2004, DD:08, 1–17. Available online: https://www.ices.dk/sites/pub/CM%20Doccuments/2004/DD/DD0804.pdf (accessed on 10 February 2024).


| dph | T °C | Light Intensity (Lux) | Flow Rate (L/min) | Algae Paste (mL) | S-Rotifer (m) | L-Rotifer (m) | Artemia Nauplii (m) | Enriched Artemia (m) | Dry Feed (g) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 7.2 | 150 | 5 | 2 × 30 | 2 × 15 | ||||
| 10 | 7.2 | 350 | 5 | 2 × 30 | 2 × 20 | ||||
| 17 | 7.2 | 420 | 7 | 2 × 30 | 2 × 10 | 2 × 10 | |||
| 24 | 7.2 | 650 | 7 | 2 × 30 | 2 × 13 | 2 × 13 | |||
| 31 | 7.2 | 650 | 10 | 2 × 50 | 2 × 13 | 2 × 13 | |||
| 38 | 7.2 | 650 | 10 | 2 × 50 | 2 × 25 | 2 × 15 | 2 × 3 | ||
| 45 | 7.2 | 650 | 15 | 2 × 50 | 2 × 23 | 2 × 5 | |||
| 52 | 7.2 | 650 | 15 | 2 × 50 | 2 × 7.5 | 5 | |||
| 59 | 7.2 | 650 | 15 | 2 × 30 | 2 × 9 | 15 | |||
| 66 | 7.2 | 650 | 15 | 2 × 30 | 2 × 8 | 20 | |||
| 73 | 7.2 | 650 | 25 | 2 × 30 | 2 × 8 | 25 | |||
| 80 | 7.3 | 650 | 25 | 2 × 30 | 2 × 5 | 40 | |||
| 87 | 7.3 | 650 | 25 | 2 × 30 | 2 × 2.5 | 60 | |||
| 94 | 7.3 | 650 | 30 | 2 × 30 | 2 × 1 | 70 | |||
| 101 | 7.3 | 650 | 30 | 2 × 30 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Árnason, T.; Bárðarson, B.; Steinarsson, A. Cultivation and Growth Dynamics of Capelin (Mallotus villosus) from Hatch to Adulthood. Fishes 2024, 9, 460. https://doi.org/10.3390/fishes9110460
Árnason T, Bárðarson B, Steinarsson A. Cultivation and Growth Dynamics of Capelin (Mallotus villosus) from Hatch to Adulthood. Fishes. 2024; 9(11):460. https://doi.org/10.3390/fishes9110460
Chicago/Turabian StyleÁrnason, Tómas, Birkir Bárðarson, and Agnar Steinarsson. 2024. "Cultivation and Growth Dynamics of Capelin (Mallotus villosus) from Hatch to Adulthood" Fishes 9, no. 11: 460. https://doi.org/10.3390/fishes9110460
APA StyleÁrnason, T., Bárðarson, B., & Steinarsson, A. (2024). Cultivation and Growth Dynamics of Capelin (Mallotus villosus) from Hatch to Adulthood. Fishes, 9(11), 460. https://doi.org/10.3390/fishes9110460







