Potential Probiotic Bacillus Strains with Antioxidant and Antimutagenic Activity Increased Weight Gain and Altered hsp70, cxc, tnfα, il1β, and lysC Gene Expression in Clarias gariepinus
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cell-Free Supernatants (CFSs) of Strains
2.2. Biosensor Test on Antioxidant and DNA-Protective Activity
2.3. Inducers and Protectors
2.4. Growth Conditions
2.5. Testing of CFS and Bioluminescence Measurement
2.6. Culture Fluid Antimutagenic Activity Study
2.7. Preparation and Use of Potential Probiotic Formulations
2.8. Fish Containment Condition
2.9. Evaluation of the Effects on Gene Expression Associated with Immunity and Stress
2.10. Estimation of the Growth Parameters of C. gariepinus
2.11. Statistical Analysis
3. Results
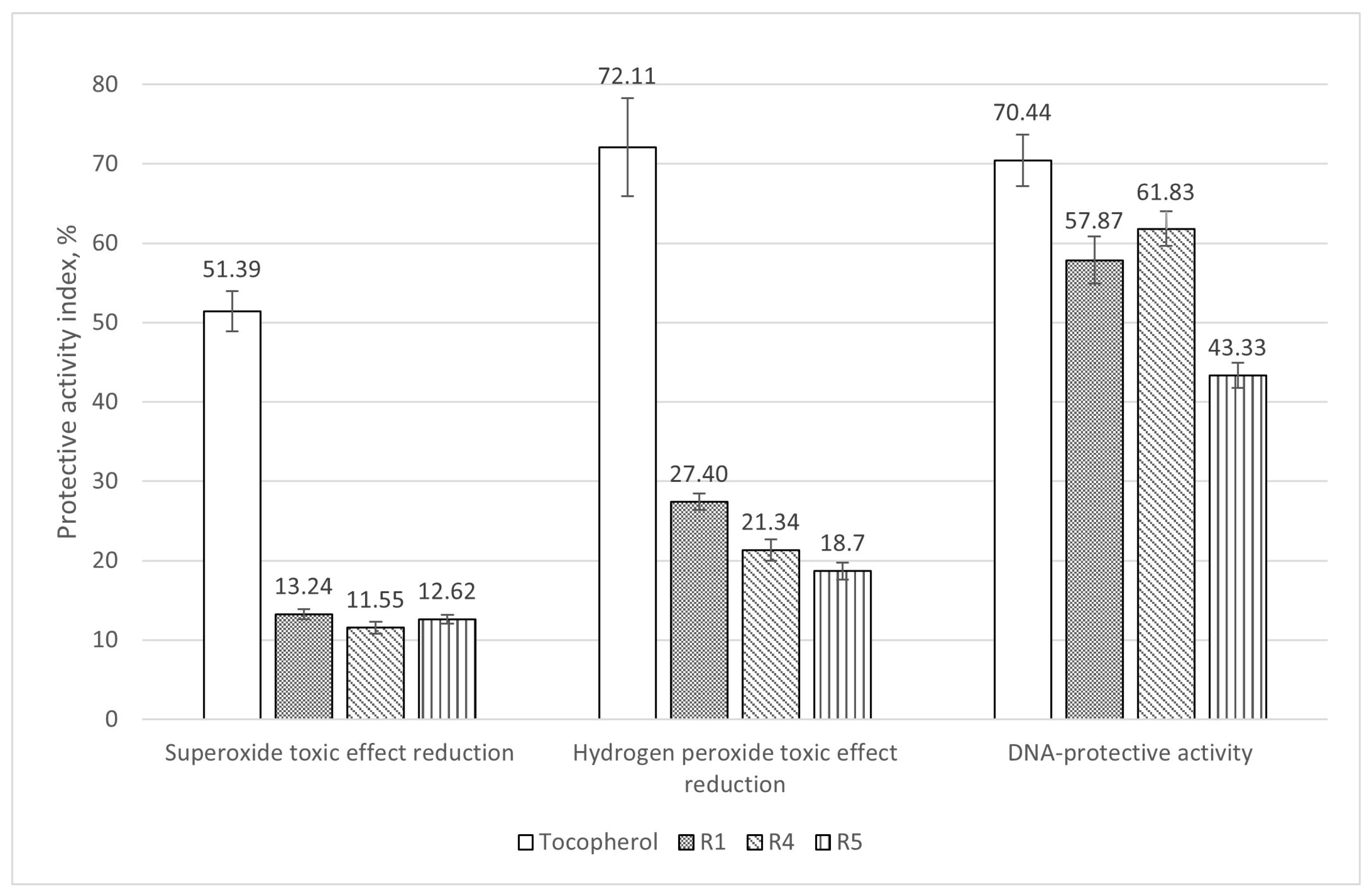
3.1. Evaluation of Antioxidant and DNA Protective Activity
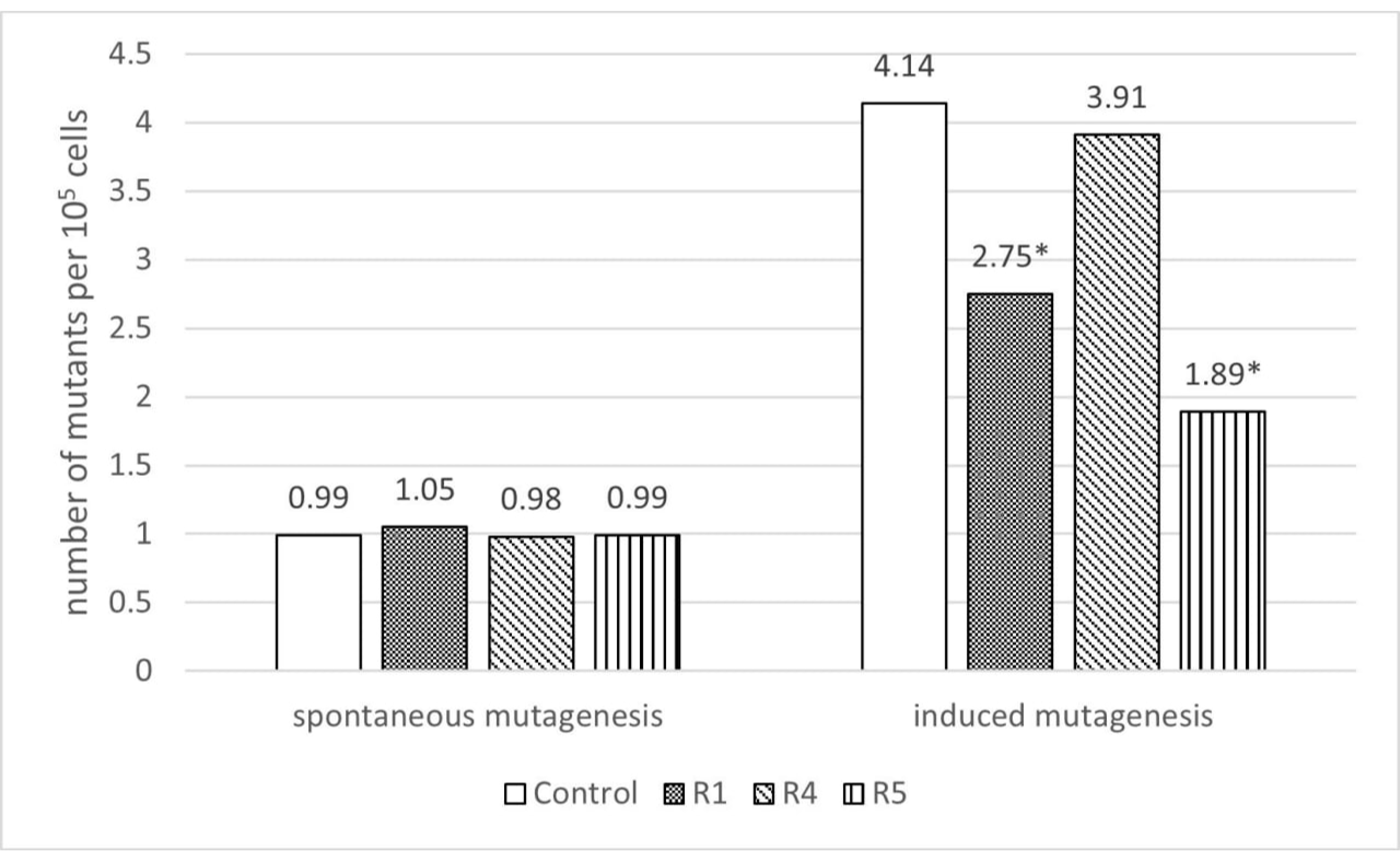
3.2. Study of the Antimutagenic Activity of CFS
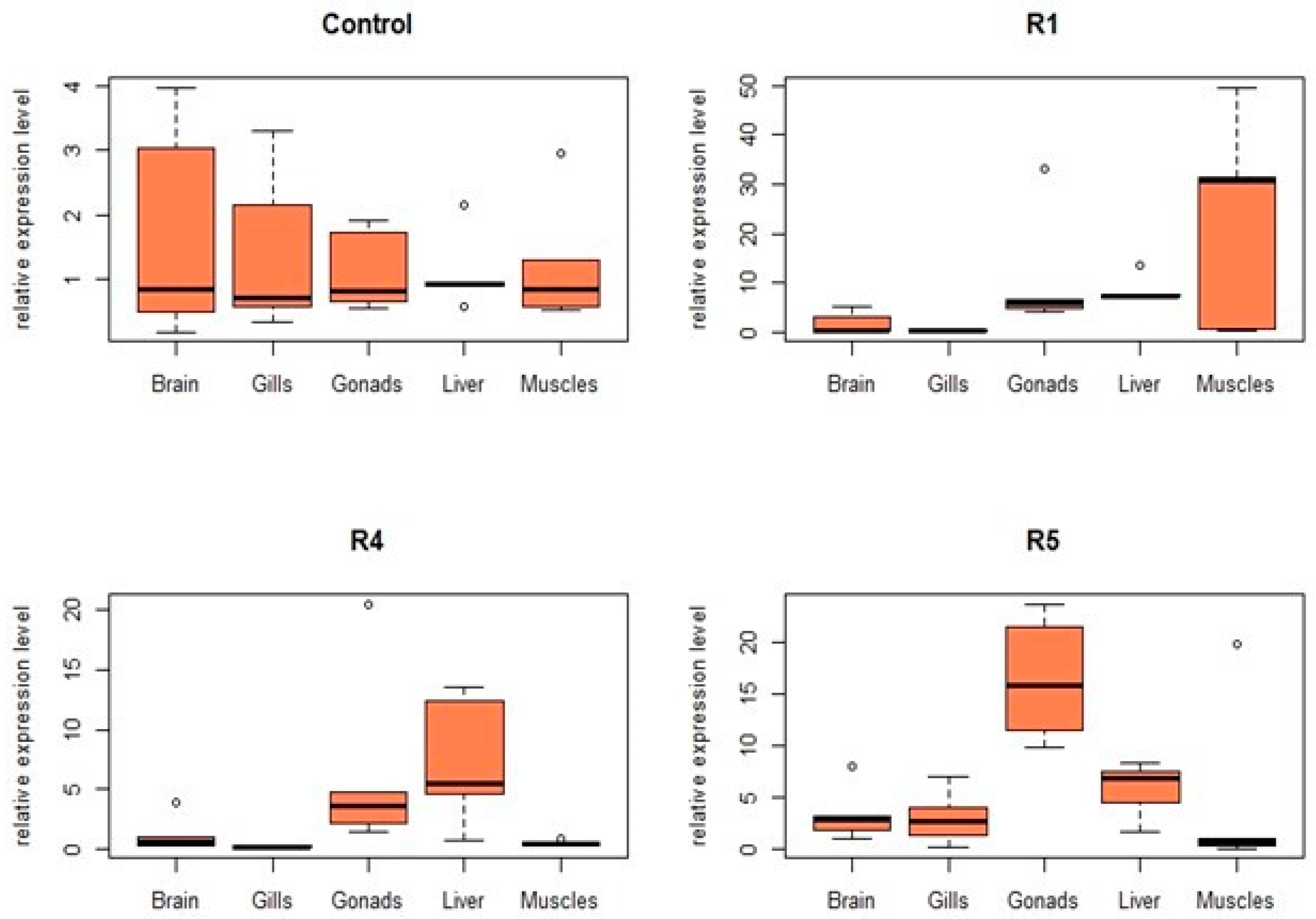
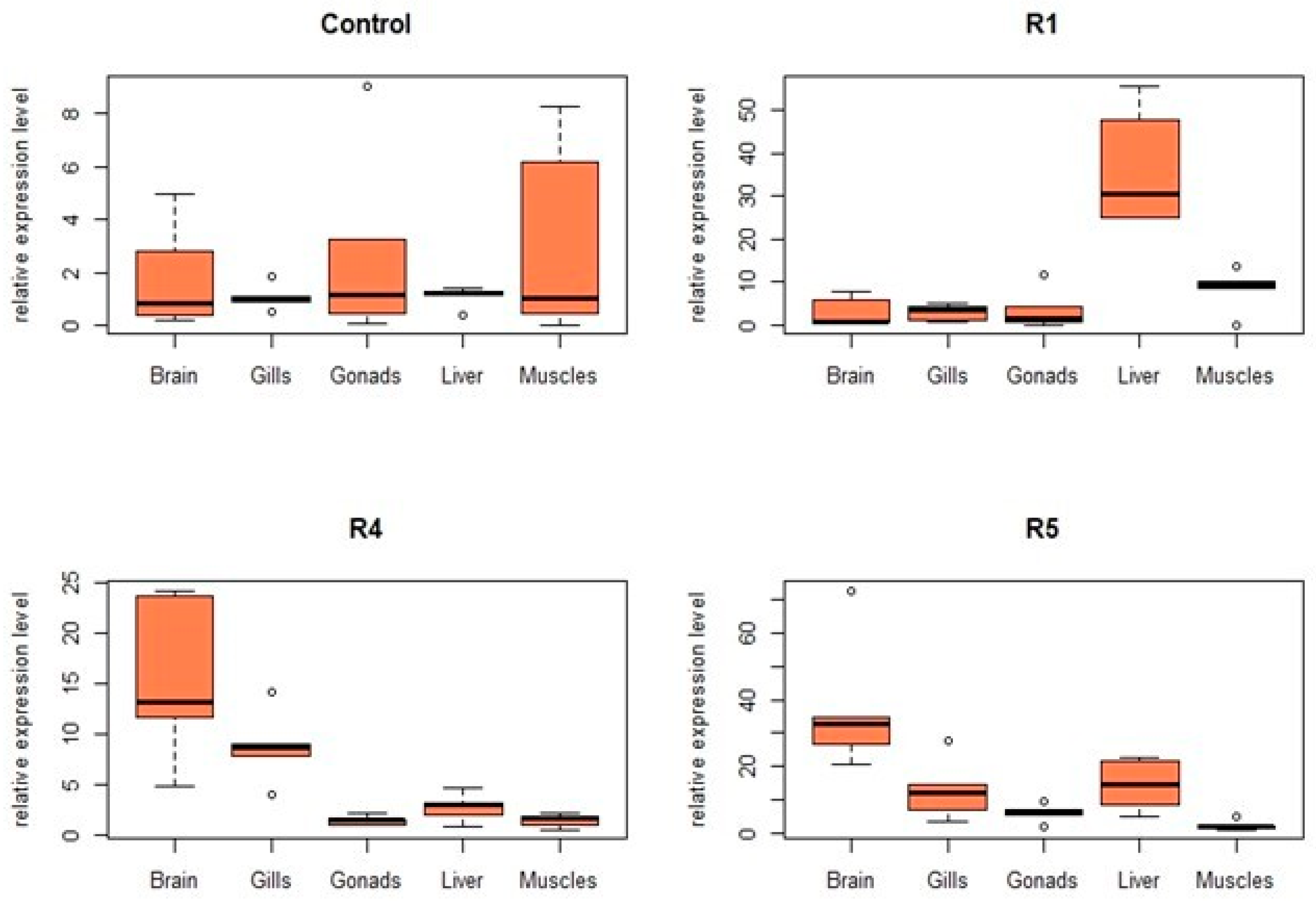
3.3. Effects on Gene Expression
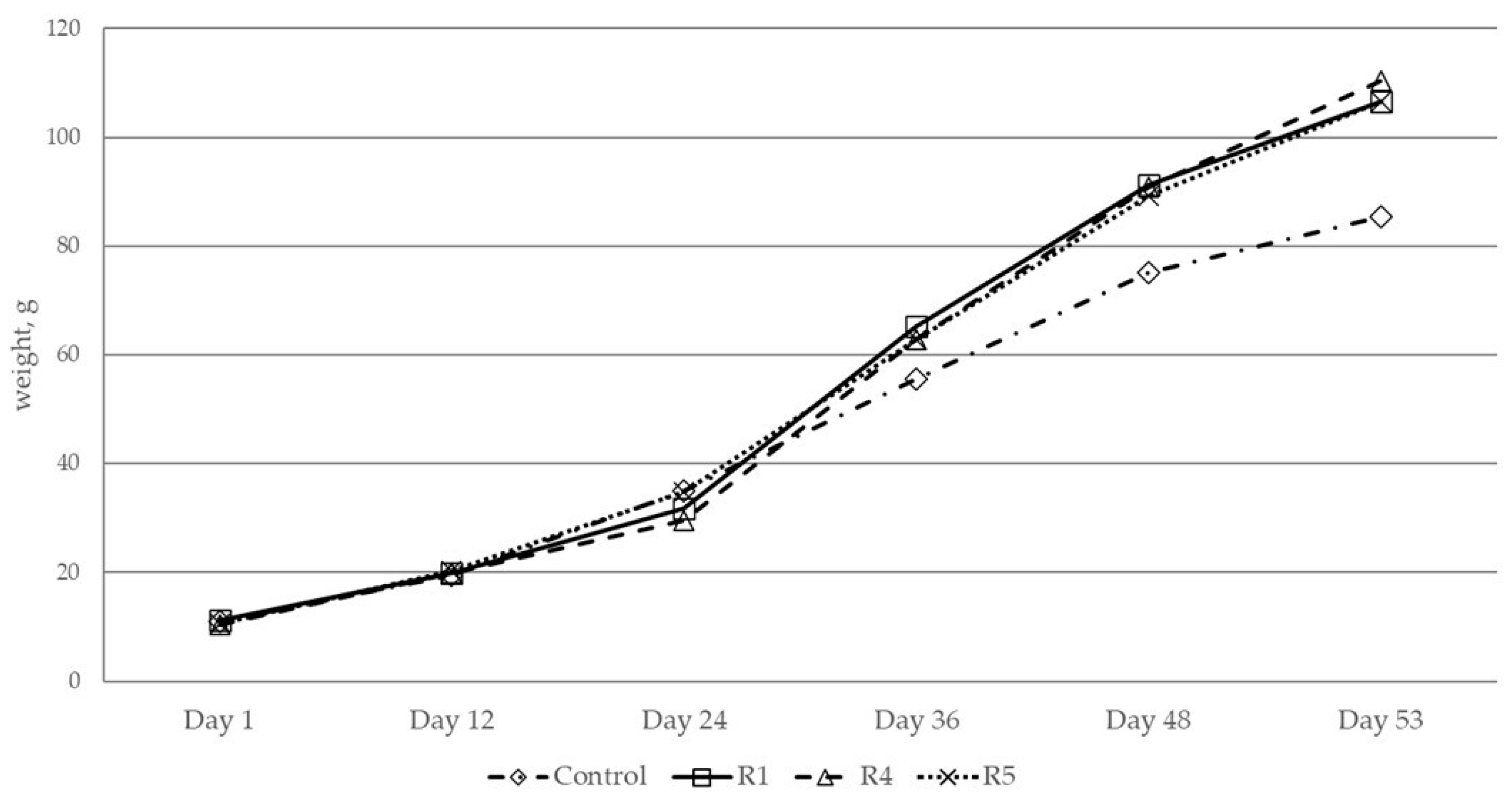
3.4. Estimating the Increase in Weight Gain of C. gariepinus
4. Discussion
4.1. Influence on the Stress and Immunity Genes
4.1.1. Influence on hsp70
4.1.2. Influence on the Immunity Genes
Influence on cxc
Influence on tnfa
Influence on il1β
Influence on lysC
4.2. Interpretation of Systemic Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eyo, V.O.; Ekane, A.P. Dietary Probiotic Supplementation; Effects on Fecundity and Relationship with Biometric Parameters of Clarias gariepinus. J. Microbes Res. 2023, 2, 2836-2187. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Lima, J.M.S.; Bucheli, J.E.V.; Popov, I.V.; Tiwari, S.K.; Chikindas, M.L. Probiotics for Aquaculture: Hope, Truth, and Reality. Probiotics Antimicrob. Proteins 2024, 16, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Parra, M.; Maisey, K.; Vargas, R.A.; Cabezas-Cruz, A.; Conzalez, A.; Tello, M.; Bermúdez-Humarán, L.G. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms 2024, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Prazdnova, E.V.; Mazanko, M.S.; Chistyakov, V.A.; Bogdanova, A.A.; Refeld, A.G.; Kharchenko, E.Y.; Chikindas, M.L. Antimutagenic Activity as a Criterion of Potential Probiotic Properties. Probiotics Antimicrob. Proteins 2022, 14, 1094–1109. [Google Scholar] [CrossRef]
- Makarenko, M.S.; Chistyakov, V.A.; Usatov, A.V.; Mazanko, M.S.; Prazdnova, E.V.; Bren, A.B.; Gorlov, I.F.; Komarova, Z.B.; Chikindas, M.L. The Impact of Bacillus subtilis KATMIRA1933 Supplementation on Telomere Length and Mitochondrial DNA Damage of Laying Hens. Probiotics Antimicrob. Proteins 2019, 11, 588–593. [Google Scholar] [CrossRef]
- Mazanko, M.S.; Gorlov, I.F.; Prazdnova, E.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Chistyakov, V.A.; Tutelyan, A.V.; Komarova, Z.B.; Mosolova, N.I.; et al. Bacillus Probiotic Supplementations Improve Laying Performance, Egg Quality, Hatching of Laying Hens, and Sperm Quality of Roosters. Probiotics Antimicrob. Proteins 2018, 10, 367–373. [Google Scholar] [CrossRef]
- Shareef, R.H.; Sharba, Z.F.; Hameed, E.N. The Positive Role of Antioxidants on Body Immunity: An Overview. Med. J. Babylon 2021, 18, 169. [Google Scholar] [CrossRef]
- Sadeghi, J.; Chaganti, S.R.; Heath, D.D. Regulation of host gene expression by gastrointestinal tract microbiota in Chinook Salmon (Oncorhynchus tshawytscha). Mol. Ecol. 2023, 32, 4427–4446. [Google Scholar] [CrossRef]
- Gorreja, F. Gene Expression Changes as Predictors of the Immune-Modulatory Effects of Probiotics: Towards a Better Understanding of Strain-Disease Specific Interactions. NFS J. 2019, 14–15, 1–5. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Dharmasiddhi, I.P.W.; Chen, S.; Liu, Y.; Liu, H. Review of the Potential of Probiotics in Disease Treatment: Mechanisms, Engineering, and Applications. Processes 2024, 12, 316. [Google Scholar] [CrossRef]
- Choi, W.; Moniruzzaman, M.; Bae, J.; Hamidoghli, A.; Lee, S.; Choi, Y.H.; Min, T.; Bai, S.C. Evaluation of Dietary Probiotic Bacteria and Processed Yeast (GroPro-Aqua) as the Alternative of Antibiotics in Juvenile Olive Flounder Paralichthys olivaceus. Antibiotics 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Ehsannia, S.; Ahari, H.; Kakoolaki, S.; Anvar, S.A.; Yousefi, S. Effects of Probiotics on Zebrafish Model Infected with Aeromonas hydrophila: Spatial Distribution, Antimicrobial, and Histopathological Investigation. BMC Microbiol. 2022, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Noh, D.-I.; Lee, Y.-S.; Hasan, M.T.; Hur, S.W.; Lee, S.; Jeong, S.-M.; Lee, J.M.; Lee, E.-W.; Kim, K.-W.; et al. Effects of Host-Associated Low-Temperature Probiotics in Olive Flounder (Paralichthys olivaceus) aquaculture. Sci. Rep. 2024, 14, 2134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zeng, Y.; Zeng, D.; Wang, H.; Zhou, M.; Sun, N.; Xin, J.; Khalique, A.; Rajput, D.S.; Pan, K.; et al. Probiotics and MicroRNA: Their Roles in the Host–Microbe Interactions. Front. Microbiol. 2021, 11, 604462. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, M.; Ekawati, A.; Arifin, N.; Yuniarti, A.; Hariati, A. Effect of Probiotics on Survival Rate and Growth Performance of Clarias gariepinus. Nat. Environ. Pollut. Technol. 2019, 18, 313–316. [Google Scholar]
- Romanova, E.M.; Lyubomirova, V.N.; Romanov, V.V.; Mukhitova, M.E.; Shlenkina, T.M. Seasonal Studies of Caviar Production and the Growth Rate of the African Catfish (Clarias gariepinus, Burchell, 1822). Egypt. J. Aquat. Res. 2018, 44, 315–319. [Google Scholar] [CrossRef]
- Mutalib, A.; Alhassan, E.; Larbi Ayisi, C. Evaluating the Impact of Varied Probiotic Levels (Bacillus Subtilis 200) on Feed Utilization, Growth Performance, and Proximate Composition in African Catfish (Clarias gariepinus). J. Energy Nat. Resour. Manag. 2024, 9, 52–63. [Google Scholar] [CrossRef]
- Strauch, S.M.; Wenzel, L.C.; Bischoff, A.; Dellwig, O.; Klein, J.; Schüch, A.; Wasenitz, B.; Palm, H.W. Commercial African catfish (Clarias gariepinus) recirculating aquaculture systems: Assessment of element and energy pathways with special focus on the phosphorus cycle. Sustainability 2018, 10, 1805. [Google Scholar] [CrossRef]
- El-Shebly, A. Evaluation of Growth Performance, Production and Nutritive Value of the African Catfish, Clarias gariepinus. Cultured In Earthen Ponds. Egypt. J. Aquat. Biol. Fish. 2006, 10, 55–67. [Google Scholar] [CrossRef][Green Version]
- Skelton, P. A Complete Guide to the Freshwater Fishes of Southern Africa; Halfway House: Southern Book Publishers Ltd.: Cape Town, South Africa, 2015. [Google Scholar]
- Dienye, H.E.; Olopade, O.A.; Obi, C.O. Socio-Economic and Cost Benefits of Catfish (Clarias gariepinus) Marketing in Obio-Akpor Local Government Area, Rivers State, Nigeria. J. Limnol. Freshw. Fish. Res. 2021, 7, 40–48. [Google Scholar] [CrossRef]
- Nasrullah, H.; Layinatuzzain, L.; Soelistiyowati, D.T.; Widanarni, W.; Alimuddin, A. Heat-Shock Protein 70: Sequence and Expression Analysis in African Catfish Clarias gariepinus after Bacterial Infection and Stress Exposures. Genet. Aquat. Org. 2022, 6, GA509. [Google Scholar] [CrossRef]
- Elmowalid, G.A.; Ghonimi, W.A.M.; Abd Allah, H.M.; Abdallah, H.; El-Murr, A.; Abdelwahab, A.M. β-1,3-Glucan Improved the Health and Immunity of Juvenile African Catfish (Clarias gariepinus) and Neutralized the Histological Changes Caused by Lead and Fipronil Pollutants. BMC Vet. Res. 2023, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Popoola, O.M.; Oyelade, A.M.; Torhukerıjho, S.T. Expression of Immune-Related Gene from African Mud Catfish Clarias gariepinus Reared in Bioflocs Systems after Aeromonas hydrophilia Infection. Biotech Stud. 2022, 31, 17–27. [Google Scholar] [CrossRef]
- Zavilgelsky, G.B.; Kotova, V.Y.; Manukhov, I.V. Action of 1,1-Dimethylhydrazine on Bacterial Cells is Determined by Hydrogen Peroxide. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2007, 634, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mazanko, M.S.; Chistyakov, V.A.; Prazdnova, E.V.; Pokudina, I.O.; Churilov, M.N.; Chmyhalo, V.K.; Batyushin, M.M. Dioxidine Induces Bacterial Resistance to Antibiotics. Mol. Genet. Microbiol. Virol. 2016, 31, 227–232. [Google Scholar] [CrossRef]
- Chistyakov, V.; Melnikov, V.; Chikindas, M.L.; Khutsishvili, M.; Chagelishvili, A.; Bren, A.; Kostina, N.; Cavera, V.; Elisashvili, V. Poultry-Beneficial Solid-State Bacillus amyloliquefaciens B-1895 Fermented Soybean Formulation. Biosci. Microbiota Food Health 2015, 34, 25–28. [Google Scholar] [CrossRef]
- Swaleh, S.B.; Banday, U.Z.; Asadi, M.-A.; Usmani, N. Biochemical Profile and Gene Expression of Clarias gariepinus as a Signature of Heavy Metal Stress. Environ. Pollut. 2020, 264, 114693. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Dawood, M.A.O.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Mat, K.; Ismail, T.A.; et al. Effect of Fish Meal Substitution with Fermented Soy Pulp on Growth Performance, Digestive Enzyme, Amino Acid Profile, and Immune-Related Gene Expression of African Catfish (Clarias gariepinus). Aquaculture 2022, 546, 737418. [Google Scholar] [CrossRef]
- Hamid, N.H.; Daud, H.M.; Kayansamruaj, P.; Hassim, H.A.; Mohd Yusoff, M.S.; Abu Bakar, S.N.; Srisapoome, P. Short- and Long-Term Probiotic Effects of Enterococcus hirae Isolated from Fermented Vegetable Wastes on the Growth, Immune Responses, and Disease Resistance of Hybrid Catfish (Clarias gariepinus × Clarias macrocephalus). Fish Shellfish Immunol. 2021, 114, 1–19. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Y.L.; Xu, X.; Huang, Y.; Cui, Z.W.; Yu, D.Y.; Li, W.F. Modulatory effects of Bacillus subtilis BS02 on viability and immune responses of RAW 264.7 murine macrophages. J. Anim. Vet. Adv. 2012, 11, 1934–1938. [Google Scholar]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y.; Hlordzi, V.; Sakyi, M.E.; Afriyie, G.; Wang, Z.; Li, Y.; Xie, C.X. Mechanisms and the Role of Probiotic Bacillus in Mitigating Fish Pathogens in Aquaculture. Fish Physiol. Biochem. 2020, 46, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Vazirzadeh, A.; Roosta, H.; Masoumi, H.; Farhadi, A.; Jeffs, A. Long-Term Effects of Three Probiotics, Singular or Combined, on Serum Innate Immune Parameters and Expressions of Cytokine Genes in Rainbow Trout during Grow-Out. Fish Shellfish Immunol. 2020, 98, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Nakharuthai, C.; Boonanuntanasarn, S.; Kaewda, J.; Manassila, P. Isolation of Potential Probiotic Bacillus spp. from the Intestine of Nile Tilapia to Construct Recombinant Probiotic Expressing CC Chemokine and Its Effectiveness on Innate Immune Responses in Nile Tilapia. Animals 2023, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative Induction of Inflammatory Responses by Dectin-1 and Toll-like Receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef]
- Ji, Z.; Lu, X.; Xue, M.; Fan, Y.; Tian, J.; Dong, L.; Zhu, C.; Wen, H.; Jiang, M. The Probiotic Effects of Host-Associated Bacillus velezensis in Diets for Hybrid Yellow Catfish (Pelteobagrus fulvidraco ♀ × Pelteobagrus vachelli ♂). Anim. Nutr. 2023, 15, 114–125. [Google Scholar] [CrossRef]
- Zhao, C.; Men, X.; Dang, Y.; Zhou, Y.; Ren, Y. Probiotics mediate intestinal microbiome and microbiota-derived metabolites regulating the growth and immunity of rainbow trout (Oncorhynchus mykiss). Microbiol. Spectr. 2023, 11, e03980-22. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Saputra, F.; Chen, Y.-C.; Hu, S.-Y. Dietary Administration of Bacillus amyloliquefaciens R8 Reduces Hepatic Oxidative Stress and Enhances Nutrient Metabolism and Immunity against Aeromonas hydrophila and Streptococcus agalactiae in Zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 86, 410–419. [Google Scholar] [CrossRef]
- Cámara-Ruiz, M.; Balebona, M.C.; Moriñigo, M.Á.; Esteban, M.Á. Probiotic Shewanella putrefaciens (SpPdp11) as a Fish Health Modulator: A Review. Microorganisms 2020, 8, 1990. [Google Scholar] [CrossRef]
- Reda, R.M.; El-Hady, M.A.; Selim, K.M.; El-Sayed, H.M. Comparative Study of Three Predominant Gut Bacillus Strains and a Commercial B. amyloliquefaciens as Probiotics on the Performance of Clarias gariepinus. Fish Shellfish Immunol. 2018, 80, 416–425. [Google Scholar] [CrossRef]
- Salinas, I.; Cuesta, A.; Esteban, M.Á.; Meseguer, J. Dietary Administration of Lactobacillus delbrüeckii and Bacillus subtilis, Single or Combined, on Gilthead Seabream Cellular Innate Immune Responses. Fish Shellfish Immunol. 2005, 19, 67–77. [Google Scholar] [CrossRef]
- Ahmed, H.; Bakry, K.A.; Abdeen, A.; El bagny, H.E.K.; Abdo, M.; Imbrea, F.; Fericean, L.; Elshemy, M.A.; Ibrahim, S.F.; Shukry, M.; et al. The involvement of antioxidant, stress, and immune-related genes in the responsive mechanisms of common carp (Cyprinus carpio) to hypersalinity exposure. Front. Mar. Sci. 2023, 10, 1195016. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Zanuzzo, F.S.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The Atlantic salmon’s stress- and immune-related transcriptional responses to moderate hypoxia, an incremental temperature increase, and these challenges combined. G3 2021, 11, jkab102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Thorat, S.T.; Gunaware, M.A.; Kumar, P.; Reddy, K.S. Unraveling gene regulation mechanisms in fish: Insights into multistress responses and mitigation through iron nanoparticles. Front. Immunol. 2024, 15, 1410150. [Google Scholar] [CrossRef]
- Guo, H.; Dixon, B. Understanding acute stress-mediated immunity in teleost fish. Fish Shellfish Immunol. Rep. 2021, 12, 100010. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Eissa, E.A.; El-Sayed, T.I.; Attia, A.A.; Rashed, M.E.; Refaat, H.M. Proinflammatory cytokines in plasma of patients with typhoid fever and resistance to therapy. Egypt. J. Microbiol. 2018, 53, 141–149. [Google Scholar] [CrossRef]
- Shukry, M.; Abd El-Kader, M.F.; Hendam, B.M.; Dawood, M.A.O.; Farrag, F.A.; Aboelenin, S.M.; Soliman, M.M.; Abdel-Latif, H.M.R. Dietary Aspergillus oryzae Modulates Serum Biochemical Indices, Immune Responses, Oxidative Stress, and Transcription of HSP70 and Cytokine Genes in Nile Tilapia Exposed to Salinity Stress. Animals 2021, 11, 1621. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Farahat, E.M. Probiotic Effects of Aspergillus oryzae on the Oxidative Status, Heat Shock Protein, and Immune Related Gene Expression of Nile Tilapia (Oreochromis niloticus) under Hypoxia Challenge. Aquaculture 2020, 520, 734669. [Google Scholar] [CrossRef]
- Arciuch-Rutkowska, M.; Nowosad, J.; Gil, Ł.; Czarnik, U.; Kucharczyk, D. Synergistic Effect of Dietary Supplementation with Sodium Butyrate, β-Glucan and Vitamins on Growth Performance, Cortisol Level, Intestinal Microbiome and Expression of Immune-Related Genes in Juvenile African Catfish (Clarias gariepinus). Int. J. Mol. Sci. 2024, 25, 4619. [Google Scholar] [CrossRef]
- Omar, A.A.; Gado, M.S.; Kandel, H.E.; Farrag, F.A.; Shukry, M. Probiotic Efficacy in Aquaculture: The Role of Technospore® (Bacillus coagulans) in Improving Nile Tilapia (Oreochromis niloticus) Performance and Disease Resistance: A Study on Gut Health, Immunological Response, and Gene Expression. Probiotics Antimicrob. Proteins 2024, 1, 1–18. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Zanuzzo, F.S.; Koch, J.F.A.; de Oliveira, C.A.F.; Sima, P.; Vetvicka, V. Development of Fish Immunity and the Role of β-Glucan in Immune Responses. Molecules 2020, 25, 5378. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qiu, H.; Yan, J.; Shen, X.; Wei, X.; Duan, M.; Yang, J. The Involvement of TNF-α and TNF-β as Proinflammatory Cytokines in Lymphocyte-Mediated Adaptive Immunity of Nile Tilapia by Initiating Apoptosis. Dev. Comp. Immunol. 2021, 115, 103884. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.A. The Probiotic Paradox: Live and Dead Cells Are Biological Response Modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Jang, W.J.; Lee, J.M.; Hur, S.W.; Lim, S.G.; Kim, K.W.; Han, H.S.; Kong, I.S. Effects of Immunostimulants, Prebiotics, Probiotics, Synbiotics, and Potentially Immunoreactive Feed Additives on Olive Flounder (Paralichthys olivaceus): A Review. Rev. Fish. Sci. Aquac. 2019, 27, 417–437. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and Prebiotics Associated with Aquaculture: A Review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef]
- Montalban-Arques, A.; De Schryver, P.; Bossier, P.; Gorkiewicz, G.; Mulero, V.; Gatlin, D.M.; Galindo-Villegas, J. Selective Manipulation of the Gut Microbiota Improves Immune Status in Vertebrates. Front. Immunol. 2015, 6, 512. [Google Scholar] [CrossRef]
- Sommer, F.; Torraca, V.; Meijer, A.H. Chemokine Receptors and Phagocyte Biology in Zebrafish. Front. Immunol. 2020, 11, 325. [Google Scholar] [CrossRef]
- DeVries, M.E.; Kelvin, A.A.; Xu, L.; Ran, L.; Robinson, J.; Kelvin, D.J. Defining the Origins and Evolution of the Chemokine/Chemokine Receptor System1. J. Immunol. 2006, 176, 401–415. [Google Scholar] [CrossRef]
- Sun, X.; Fang, Z.; Yu, H.; Zhao, H.; Wang, Y.; Zhou, F.; Zhao, L.; Sun, J.; Tian, Y. Effects of Enterococcus faecium (R8a) on Nonspecific Immune Gene Expression, Immunity and Intestinal Flora of Giant Tiger Shrimp (Penaeus monodon). Sci. Rep. 2024, 14, 1823. [Google Scholar] [CrossRef]
- Waiyamitra, P.; Zoral, M.A.; Saengtienchai, A.; Luengnaruemitchai, A.; Decamp, O.; Gorgoglione, B.; Surachetpong, W. Probiotics modulate tilapia resistance and immune response against tilapia lake virus infection. Pathogens 2020, 9, 919. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, T.; Zou, J. Fish TNF and TNF Receptors. Sci. China Life Sci. 2021, 64, 196–220. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Aini, N.; Putri, D.S.Y.R.; Achhlam, D.H.; Fatimah, F.; Andriyono, S.; Hariani, D.; Do, H.D.K.; Wahyuningsih, S.P.A. Supplementation of Bacillus subtilis and Lactobacillus casei to increase growth performance and immune system of catfish (Clarias gariepinus) due to Aeromonas hydrophila infection. Vet. World 2024, 17, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Hooshyar, Y.; Kenari, A.A.; Hamed, P.; Gandomi, H. Effects of Lactobacillus rhamnosus ATCC 7469 on different parameters related to health status of rainbow trout (Oncorhynchus mykiss) and the protection against Yersinia ruckeri. Probiotics Antimicrob. Proteins 2020, 12, 1370–1384. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhu, J.H.; Cui, Z.; He, J.; Jia, W.H. The association between the polymorphisms of TNF-α and non-Hodgkin lymphoma: A meta-analysis. Tumor Biol. 2014, 35, 12509–12517. [Google Scholar] [CrossRef]
- Gioacchini, G.; Giorgini, E.; Olivotto, I.; Maradonna, F.; Merrifield, D.L.; Carnevali, O. The Influence of Probiotics on Zebrafish Danio Rerio Innate Immunity and Hepatic Stress. Zebrafish 2014, 11, 98–106. [Google Scholar] [CrossRef]
- Nandi, A.; Banerjee, G.; Dan, S.K.; Ghosh, K.; Ray, A.K. Evaluation of in vivo probiotic efficiency of Bacillus amyloliquefaciens in Labeo rohita challenged by pathogenic strain of Aeromonas hydrophila MTCC 1739. Probiotics Antimicrob. Proteins 2017, 10, 391–398. [Google Scholar] [CrossRef]
- Panigrahi, A.; Kiron, V.; Satoh, S.; Hirono, I.; Kobayashi, T.; Sugita, H.; Puangkaew, J.; Aoki, T. Immune modulation and expression of cytokine genes in rainbow trout Oncorhynchus mykiss upon probiotic feeding. Dev. Comp. Immunol. 2007, 31, 372–382. [Google Scholar] [CrossRef]
- Li, L.; Cardoso, J.C.R.; Félix, R.C.; Mateus, A.P.; Canário, A.V.M.; Power, D.M. Fish lysozyme gene family evolution and divergent function in early development. Dev. Comp. Immunol. 2021, 114, 103772. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Song, T.Y.; Ge, J.Q. Expression Profiles of Zebrafish (Danio rerio) Lysozymes and Preparation of c-Type Lysozyme with High Bacteriolytic Activity against Vibrio vulnificus. Antibiotics 2022, 11, 1803. [Google Scholar] [CrossRef]
- Zhu, L.; Kong, Y.; Chang, X.; Feng, J.; Wang, X.; Hou, L.; Zhaou, X.; Pei, C.; Kong, X. Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture 2023, 562, 738765. [Google Scholar] [CrossRef]
- El-Kady, A.A.; Magouz, F.I.; Mahmoud, S.A.; Abdel-Rahim, M.M. The Effects of Some Commercial Probiotics as Water Additive on Water Quality, Fish Performance, Blood Biochemical Parameters, Expression of Growth and Immune-Related Genes, and Histology of Nile Tilapia (Oreochromis Niloticus). Aquaculture 2022, 546, 737249. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Chi, C.; Kim, H.J.; Yun, S.; Park, S.C.; Sukumaran, V. Effect of cellular products of potential probiotic bacteria on the immune response of Labeo rohita and susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2015, 46, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Long, W.; He, J.; Liu, Y.; Si, Y.; Tian, L. Effects of Dietary Bacillus Licheniformis on Growth Performance, Immunological Parameters, Intestinal Morphology and Resistance of Juvenile Nile Tilapia (Oreochromis Niloticus) to Challenge Infections. Fish Shellfish Immunol. 2015, 46, 225–231. [Google Scholar] [CrossRef]
- Ashouri, G.; Mahboobi Soofiani, N.; Hoseinifar, S.H.; Jalali, S.A.H.; Morshedi, V.; Valinassab, T.; Bagheri, D.; Doan, H.V.; Mozanzadeh, M.T.; Carnevali, O. Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass (Lates calcarifer) juveniles. Aquaculture 2020, 518, 734638. [Google Scholar] [CrossRef]
- Mohammadi, G.; Hafezieh, M.; Karimi, A.A.; Azra, M.N.; Doan, H.V.; Tapingkae, W.; Abdelrahman, H.A.; Dawood, M.A.O. The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 120, 304–313. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Grudniewska, J.; Andriichuk, A. Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis. Fish Physiol. Biochem. 2014, 40, 1289–1300. [Google Scholar] [CrossRef]




| Component | Mass Content, % |
|---|---|
| Probiotic preparation (experimental groups) | 1 |
| Non-inoculated soybean preparation (control group) | 1 |
| Protein, total | 53 |
| Fat, total | 12 |
| Fiber, total | 1 |
| Ash, total | 33 |
| Gene | Forward Primer Sequence, 5′-3′ | Reverse Primes Sequence, 5′-3′ |
|---|---|---|
| actβ | GTTGGGCACAAGGCATCCTA | GGACTCCATACCCAGGAAAGATGG |
| hsp70 | GTTTCAGGCAAGCACGTGAG | GTTCCCTGAGGCTGTTCGAT |
| cxc | AGATCACCGGGAACTGTGAC | GTCCTCACTTCAGCTTGCCT |
| tnfα | TCTCAGGTCAATACAACCCGC | GAGGCCTTTGCGGAAAATCTTG |
| il1β | TGCAGTGAATCCAAGAGCTACAGC | CCACCTTTCAGAGTGAATGCCAGC |
| lysC | TGCTAAACAGTATGATCGGTGTGA | TATCTGGAAAATGCCGTAGTCTGT |
| Group | FCR |
|---|---|
| Control | 1.34 |
| R1 | 1.04 |
| R4 | 1.02 |
| R5 | 1.08 |
| Strain/Effect | DNA Protection, % | Antimu-Tagenic, % | Weight Gain Increase, % | Gene | Expression Rates Increase (Compared to Control) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Brain | Gills | Gonads | Liver | Muscle | |||||
| R1 | 57.87 | 33.58 | 24.83 | hsp70 | - | +4.0 | +10.5 | +15.2 | - |
| cxc | +0.8 | - | +9.9 | +7.5 | +21.6 | ||||
| tnfα | +1.9 | +1.8 | +2.6 | +35.8 | +7.3 | ||||
| il1β | +9.4 | +8.3 | +13.9 | +10.8 | +1.0 | ||||
| lysC | - | +7.2 | +31.9 | +35.6 | +0.4 | ||||
| R4 | 61.83 | 0 | 24.60 | hsp70 | - | +2.6 | +6.8 | +7.1 | +10.4 |
| cxc | 0.2 | - | +5.5 | +6.3 | - | ||||
| tnfα | +14.4 | +7.7 | +0.4 | +1.7 | +0.4 | ||||
| il1β | +2.0 | +49.7 | +0.6 | - | - | ||||
| lysC | +1.7 | +52.9 | +55.8 | +23.9 | +3.6 | ||||
| R5 | 45.33 | 54.35 | 29.16 | hsp70 | - | +0.9 | +2.6 | +11.2 | +6.6 |
| cxc | +2.4 | +2.0 | +15.6 | +4.8 | +3.4 | ||||
| tnfα | +36.4 | +12.0 | +5.2 | +13.5 | +1.4 | ||||
| il1β | +10.0 | +13.6 | +7.7 | +9.2 | +0.7 | ||||
| lysC | +5.3 | +31.9 | +45.4 | +26.2 | +43.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skripnichenko, R.V.; Chelombitskaya, D.S.; Prazdnova, E.V.; Kulikov, M.P.; Neurov, A.M.; Zaikina, A.A.; Grigoryev, V.A.; Sorokina, M.N.; Chistyakov, V.A.; Chikindas, M.L.; et al. Potential Probiotic Bacillus Strains with Antioxidant and Antimutagenic Activity Increased Weight Gain and Altered hsp70, cxc, tnfα, il1β, and lysC Gene Expression in Clarias gariepinus. Fishes 2024, 9, 476. https://doi.org/10.3390/fishes9120476
Skripnichenko RV, Chelombitskaya DS, Prazdnova EV, Kulikov MP, Neurov AM, Zaikina AA, Grigoryev VA, Sorokina MN, Chistyakov VA, Chikindas ML, et al. Potential Probiotic Bacillus Strains with Antioxidant and Antimutagenic Activity Increased Weight Gain and Altered hsp70, cxc, tnfα, il1β, and lysC Gene Expression in Clarias gariepinus. Fishes. 2024; 9(12):476. https://doi.org/10.3390/fishes9120476
Chicago/Turabian StyleSkripnichenko, Radomir Viktorovich, Daria Sergeevna Chelombitskaya, Evgeniya Valer’evna Prazdnova, Maxim Pavlovich Kulikov, Alexey Mikhailovich Neurov, Anna Andreevna Zaikina, Vadim Alekseevich Grigoryev, Marina Nikolaevna Sorokina, Vladimir Anatolievich Chistyakov, Michael Leonidas Chikindas, and et al. 2024. "Potential Probiotic Bacillus Strains with Antioxidant and Antimutagenic Activity Increased Weight Gain and Altered hsp70, cxc, tnfα, il1β, and lysC Gene Expression in Clarias gariepinus" Fishes 9, no. 12: 476. https://doi.org/10.3390/fishes9120476
APA StyleSkripnichenko, R. V., Chelombitskaya, D. S., Prazdnova, E. V., Kulikov, M. P., Neurov, A. M., Zaikina, A. A., Grigoryev, V. A., Sorokina, M. N., Chistyakov, V. A., Chikindas, M. L., & Rudoy, D. V. (2024). Potential Probiotic Bacillus Strains with Antioxidant and Antimutagenic Activity Increased Weight Gain and Altered hsp70, cxc, tnfα, il1β, and lysC Gene Expression in Clarias gariepinus. Fishes, 9(12), 476. https://doi.org/10.3390/fishes9120476








