Effect of Calanus finmarchicus Hydrolysate Inclusion on Diet Attractiveness for Whiteleg Shrimp (Litopenaeus vannamei)
Abstract
:1. Introduction
2. Materials and Methods
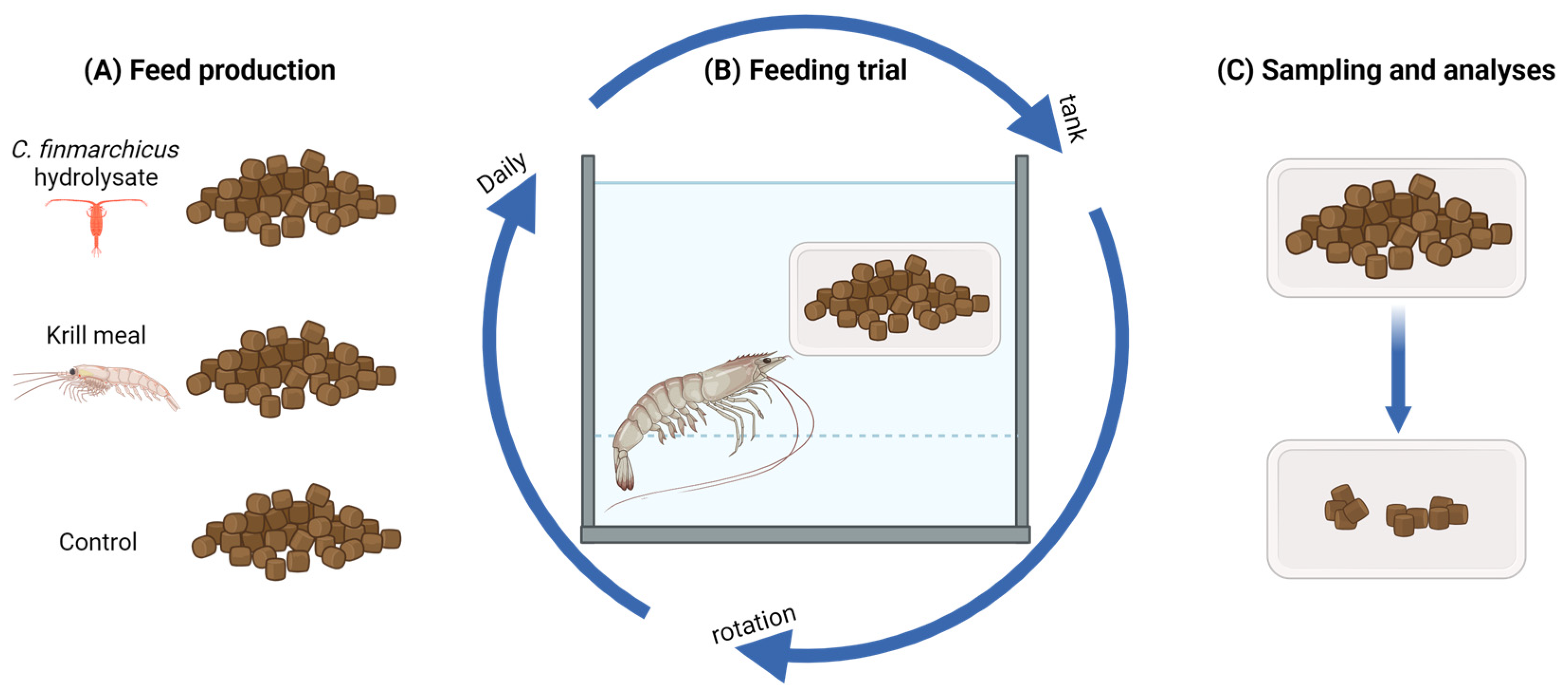
2.1. Diet Formulation and Production
2.2. Ethical Statement
2.3. Feeding Trial
2.4. Statistics
3. Results and Discussion
3.1. Feed Intake in Low/High FM Groups
3.2. Feed Intake and CH/KM Inclusion Rates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Syama, D.J.; Ponniah, A.G.; Khan, H.I.; Babu, E.P.M.; Ambasankar, K.; Vasagam, K.P.K. Shrimps—A nutritional perspective. Curr. Sci. 2013, 104, 1487–1491. [Google Scholar]
- FAO. Towards Blue Transformation. In The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2022. [Google Scholar]
- Sookying, D.; Davis, D.A.; da Silva, F.S.D. A review of the development and application of soybean-based diets for Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 441–448. [Google Scholar] [CrossRef]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the Future of Aquaculture Nutrition: Realigning Perspectives to Reflect Contemporary Issues Related to Judicious Use of Marine Resources in Aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Yuan, Y.; Chehade, S.B.; Jensen, K.E.; Barry, R.J.; Fowler, L.A.; Makowsky, R.; Powell, M.L.; Lawrence, A.L.; Watts, S.A. Feed Intake as an Estimation of Attractability in Pacific White Shrimp Litopenaeus Vannamei. Aquaculture 2021, 532, 736041. [Google Scholar] [CrossRef] [PubMed]
- Iber, B.T.; Kasan, N.A. Recent advances in Shrimp aquaculture wastewater management. Heliyon 2021, 7, e08283. [Google Scholar] [CrossRef] [PubMed]
- Eap, D.; Correa, S.; Ngo-Vu, H.; Derby, C.D. Chemosensory Basis of Feeding Behavior in Pacific White Shrimp, Litopenaeus vannamei. Biol. Bull. 2020, 239, 115–131. [Google Scholar] [CrossRef]
- Smith, D.M.; Tabrett, S.J.; Barclay, M.C.; Irvin, S.J. The efficacy of ingredients included in shrimp feeds to stimulate intake. Aquac. Nutr. 2005, 11, 263–272. [Google Scholar] [CrossRef]
- Jannathulla, R.; Sravanthi, O.; Khan, H.I.; Moomeen, H.S.; Gomathi, A.; Dayal, J.S. Chemoattractants: Their essentiality and efficacy in shrimp aquaculture. Indian J. Fish. 2021, 68, 151–159. [Google Scholar] [CrossRef]
- Nunes, A.J.; Sá, M.V.; Andriola-Neto, F.F.; Lemos, D. Behavioral response to selected feed attractants and stimulants in Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2006, 260, 244–254. [Google Scholar] [CrossRef]
- Grey, M.; Forster, I.; Dominy, W.; Ako, H.; Giesen, A.F. Validation of a Feeding Stimulant Bioassay Using Fish Hydrolysates for the Pacific White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2009, 40, 547–555. [Google Scholar] [CrossRef]
- Alves, D.R.S.; de Oliveira, S.R.; Luczinski, T.G.; Paulo, I.G.P.; Boscolo, W.R.; Bittencourt, F.; Signor, A. Palatability of Protein Hydrolysates from Industrial Byproducts for Nile Tilapia Juveniles. Animals 2019, 9, 311. [Google Scholar] [CrossRef]
- Derby, C.D.; Elsayed, F.H.; Williams, S.A.; González, C.; Choe, M.; Bharadwaj, A.S.; Chamberlain, G.W. Krill meal enhances performance of feed pellets through concentration-dependent prolongation of consumption by Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2016, 458, 13–20. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Sabry-Neto, H.; Oliveira-Neto, S.; Burri, L. Feed preference and growth response of juvenile Litopenaeus vannamei to supplementation of marine chemoattractants in a fishmeal-challenged diet. J. World Aquac. Soc. 2019, 50, 1048–1063. [Google Scholar] [CrossRef]
- Kaur, K.; Kortner, T.M.; Benitez-Santana, T.; Burri, L. Effects of Antarctic Krill Products on Feed Intake, Growth Performance, Fillet Quality, and Health in Salmonids. Aquac. Nutr. 2022, 2022, 3170854. [Google Scholar] [CrossRef]
- Torrecillas, S.; Montero, D.; Carvalho, M.; Benitez-Santana, T.; Izquierdo, M. Replacement of fish meal by Antarctic krill meal in diets for European sea bass Dicentrarchus labrax: Growth performance, feed utilization and liver lipid metabolism. Aquaculture 2021, 545, 737166. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Xie, F.; Shen, H.; Gao, W.; Zhang, W.; Mai, K. Influences of replacing dietary fish meal by Antarctic krill meal on growth performance, immunity and muscle quality of white shrimp Litopenaeus vannamei. Aquac. Rep. 2022, 25, 101256. [Google Scholar] [CrossRef]
- Skjoldal, H.R. The Norwegian Sea Ecosystem; Tapir Academic Press: Trondheim, Norway, 2004. [Google Scholar]
- Broms, C.; Strand, E.; Utne, K.R.; Hjøllo, S.; Sundby, S.; Melle, W. Vitenskapelig bakgrunnsmateriale for forvaltninsplan for raudåte. In Fisken og Havet; Rapport 8; Havforskningsinstituttet: Bergen, Norway, 2016. [Google Scholar]
- Eysteinsson, S.T.; Gudjónsdóttir, M.; Jónasdóttir, S.H.; Arason, S. Review of the composition and current utilization of Calanus finmarchicus—Possibilities for human consumption. Trends Food Sci. Technol. 2018, 79, 10–18. [Google Scholar] [CrossRef]
- Pedersen, A.M.; Vang, B.; Olsen, R.L. Oil from Calanus finmarchicus—Composition and Possible Use: A Review. J. Aquat. Food Prod. Technol. 2014, 23, 633–646. [Google Scholar] [CrossRef]
- Albrektsen, S.; Kortet, R.; Skov, P.V.; Ytteborg, E.; Gitlesen, S.; Kleinegris, D.; Mydland, L.-T.; Hansen, J.Ø.; Lock, E.-J.; Mørkøre, T.; et al. Future feed resources in sustainable salmonid production: A review. Rev. Aquac. 2022, 14, 1790–1812. [Google Scholar] [CrossRef]
- Bøgwald, I.; Østbye, T.-K.K.; Pedersen, A.M.; Rønning, S.B.; Dias, J.; Eilertsen, K.-E.; Wubshet, S.G. Calanus finmarchicus hydrolysate improves growth performance in feeding trial with European sea bass juveniles and increases skeletal muscle growth in cell studies. Sci. Rep. 2023, 13, 12295. [Google Scholar] [CrossRef]
- ISO 16634-1; Food products—Determination of the total nitrogen content by combustion according to the Dumas principle and calculation of the crude protein content. Part 1: Oilseeds and animal feeding stuffs. ISO: Geneva, Switzerland, 2008.
- Firestone, D. AOCS official Method Ba 3-38. In Offical methods and recommended practices of the AOCS, 6th ed.; AOCS Press: Urbana, IL, USA, 2009. [Google Scholar]
- ISO 5984; Animal feeding stuffs – Determination of crude ash. ISO: Geneva, Switzerland, 2002.
- ISO 6496; Animal feeding stuffs – Determination of moisture and other volatile matter content. ISO: Geneva, Switzerland, 1999.
- Wyban, J.; Walsh, W.A.; Godin, D.M. Temperature effects on growth, feeding rate and feed conversion of the Pacific white shrimp (Penaeus vannamei). Aquaculture 1995, 138, 267–279. [Google Scholar] [CrossRef]
- Holland, K.N.; Borski, R.J. A palatability bioassay for determining ingestive stimuli in the marine shrimp Penaeus wannamei. Aquaculture 1993, 109, 153–164. [Google Scholar] [CrossRef]
- Lee, P.G.; Meyers, S.P. Chemoattraction and feeding stimulation in crustaceans. Aquac. Nutr. 1996, 2, 157–164. [Google Scholar] [CrossRef]
- Suresh, A.V.; Kumaraguru Vasagam, K.P.; Nates, S. Attractability and palatability of protein ingredients of aquatic and terrestrial animal origin, and their practical value for blue shrimp, Litopenaeus stylirostris fed diets formulated with high levels of poultry byproduct meal. Aquaculture 2011, 319, 132–140. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Dalen, L.L.; Leonardi, G.; Burri, L. Developing sustainable, cost-effective and high-performance shrimp feed formulations containing low fish meal levels. Aquac. Rep. 2022, 27, 101422. [Google Scholar] [CrossRef]
- Sabry-Neto, H.; Lemos, D.; Raggi, T.; Nunes, A.J.P. Effects of soy protein ratio, lipid content and minimum level of krill meal in plant-based diets over the growth and digestibility of the white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2017, 23, 293–303. [Google Scholar] [CrossRef]
- El-Sayed, A.M. Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev. Aquac. 2014, 6, 241–255. [Google Scholar] [CrossRef]
- Shi, M.; Yao, X.; Qu, K.; Liu, Y.; Tan, B.; Xie, S. Effects of taurine supplementation in low fishmeal diet on growth, immunity and intestinal health of Litopenaeus vannamei. Aquac. Rep. 2023, 32, 101713. [Google Scholar] [CrossRef]
- To, V.-A.; Liou, C.-H. Taurine supplementation enhances the replacement level of fishmeal by soybean concentrate in diets of juvenile Pacific white shrimp (Litopenaeus vannamei Boone, 1931). Aquac. Res. 2021, 52, 3771–3784. [Google Scholar] [CrossRef]
- To, V.-A.; Liou, C.-H.; Yang, S.-D. Can dietary with a taurine supplement improve lipid utilization, growth performance, haemolymph parameters and immune responses of white shrimp (Litopenaeus vannamei)? Aquac. Res. 2021, 52, 6612–6625. [Google Scholar] [CrossRef]
- Wang, Z.; Aweya, J.J.; Yao, D.; Zheng, Z.; Wang, C.; Zhao, Y.; Li, S.; Zhang, Y. Taurine metabolism is modulated in Vibrio-infected Penaeus vannamei to shape shrimp antibacterial response and survival. Microbiome 2022, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.-R.; Liu, Y.-J.; Tian, L.-X.; Gan, L.; Yang, H.-J.; Liang, G.-Y.; He, J.-Y. The effect of dietary taurine supplementation on growth performance, feed utilization and taurine contents in tissues of juvenile white shrimp (Litopenaeus vannamei, Boone, 1931) fed with low-fishmeal diets. Aquac. Res. 2013, 44, 1317–1325. [Google Scholar] [CrossRef]
- Hansen, B.H.; Degnes, K.; Øverjordet, I.B.; Altin, D.; Størseth, T.R. Metabolic fingerprinting of arctic copepods Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus. Polar Biol. 2013, 36, 1577–1586. [Google Scholar] [CrossRef]
- Papatryphon, E.; Soares, J.H. Optimizing the levels of feeding stimulants for use in high-fish meal and plant feedstuff-based diets for striped bass, Morone saxatilis. Aquaculture 2001, 202, 279–288. [Google Scholar] [CrossRef]
- Kasper, C.S.; White, M.R.; Brown, P.B. Betaine can replace choline in diets for juvenile Nile Tilapia, Oreochromis niloticus. Aquaculture 2002, 205, 119–126. [Google Scholar] [CrossRef]
- Mohseni, M.; Saltanat, N.L.; Rastravan, M.E.; Golalipour, Y. Effects of betaine supplementation in plant-protein-based diets on growth performance, haemato-immunological parameters, antioxidant status and digestive enzyme activities of juvenile Caspian trout (Salmo trutta, Kessler, 1877). Aquac. Nutr. 2021, 27, 2132–2141. [Google Scholar] [CrossRef]


| Component | CH 1 (WW) | KM 2 (WW) | CH 3 (DW) | KM 3 (DW) |
|---|---|---|---|---|
| Dry matter, % | 53.3 | 91.6 | 100.0 | 100.0 |
| Crude protein, % | 33.6 | 55.0 | 63.0 | 60.0 |
| Crude fat, % | 0.6 | 25.2 | 1.1 | 27.5 |
| Ash, % | 10.9 | 8.6 | 20.5 | 9.3 |
| Phosphorus, % | 0.8 | 1.2 | 1.5 | 1.3 |
| Gross energy, kJ/g | 9.8 | 24.7 | 18.4 | 27.0 |
| Arginine, % | 2.23 | 3.39 | 4.18 | 3.65 |
| Histidine, % | 0.46 | 1.24 | 0.86 | 1.43 |
| Isoleucine, % | 1.21 | 2.87 | 2.27 | 2.98 |
| Leucine, % | 2.07 | 4.42 | 3.88 | 4.61 |
| Lysine, % | 2.26 | 4.03 | 4.24 | 4.21 |
| Threonine, % | 1.23 | 2.43 | 2.31 | 2.60 |
| Valine, % | 1.58 | 2.91 | 2.96 | 3.09 |
| Methionine, % | 0.62 | 2.00 | 1.16 | 1.68 |
| Cysteine, % | 0.33 | 0.43 | 0.62 | 0.45 |
| Phenylalanine, % | 1.08 | 2.93 | 2.03 | 3.17 |
| Tyrosine, % | 1.19 | 3.25 | 2.23 | 3.55 |
| Aspartic acid, % | 2.56 | 5.73 | 4.80 | 6.26 |
| Glutamic acid, % | 3.85 | 7.05 | 7.22 | 7.70 |
| Alanine, % | 2.12 | 2.99 | 3.98 | 3.26 |
| Glycine, % | 2.29 | 2.58 | 4.30 | 2.82 |
| Proline, % | 1.13 | 2.18 | 2.12 | 2.38 |
| Serine, % | 1.11 | 2.22 | 2.08 | 2.42 |
| Taurine, % | 0.56 | 0.14 | 1.05 | 0.15 |
| Ingredients (%) | Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | Diet 7 | Diet 8 | Diet 9 | Diet 10 | Diet 11 | Diet 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fish meal a | 10 | 10 | 10 | 10 | 10 | 10 | 20 | 20 | 20 | 20 | 20 | 20 |
| Calanus hydrolysate b | 0 | 2 | 4 | 6 | 0 | 0 | 0 | 2 | 4 | 6 | 0 | 0 |
| Krill meal c | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 2 | 3 |
| Fish oil, anchovy d | 1.37 | 1.39 | 1.4 | 1.42 | 1.09 | 0.94 | 0.46 | 0.47 | 0.49 | 0.5 | 0.17 | 0.03 |
| Wheat gluten meal e | 11.54 | 10.77 | 10.01 | 9.24 | 9.87 | 9.04 | 10.56 | 9.81 | 9.07 | 8.32 | 8.92 | 8.1 |
| Wheat grain, standard | 6.36 | 6.22 | 6.07 | 5.93 | 6.54 | 6.64 | 16.97 | 16.72 | 16.46 | 16.21 | 17 | 17.01 |
| Soybean meal f | 25 | 25 | 25 | 25 | 25 | 25 | 16 | 16 | 16 | 16 | 16 | 16 |
| Rice bran | 15 | 15 | 15 | 15 | 15 | 15 | 14 | 14 | 14 | 14 | 14 | 14 |
| Sunflower meal | 20 | 20 | 20 | 20 | 20 | 20 | 12 | 12 | 12 | 12 | 12 | 12 |
| Lecithin soya crude | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Diatomaceous earth | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Cholesterol, feed grade | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Carboxymethylcellulose | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Mono ammonium phosphate | 2.73 | 2.68 | 2.62 | 2.57 | 2.63 | 2.59 | 2.43 | 2.38 | 2.33 | 2.27 | 2.34 | 2.29 |
| Vitamins g | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Minerals h | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| DL-Methionine i | 0.08 | 0.06 | 0.04 | 0.02 | 0.05 | 0.04 | ||||||
| L-Lysine HCL i | 0.23 | 0.17 | 0.11 | 0.05 | 0.14 | 0.1 |
| Low Fish Meal | High Fish Meal | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proximate (%) | CTRL | CH2 | CH4 | CH6 | KM2 | KM3 | CTRL | CH2 | CH4 | CH6 | KM2 | KM3 |
| Moisture | 7.6 | 7.3 | 6.7 | 6.9 | 5.8 | 5.2 | 6.3 | 6.4 | 6.8 | 7.7 | 7.6 | 7.6 |
| Protein | 41.11 | 40.4 | 41 | 40.82 | 41.13 | 41.24 | 39.87 | 40.17 | 39.59 | 39.62 | 39.3 | 39.24 |
| Fat | 6.6 | 6.9 | 6.7 | 6.7 | 6.6 | 6.8 | 6.5 | 6.7 | 6.6 | 6.5 | 6.4 | 6.5 |
| Ash | 10.3 | 10.6 | 10.7 | 10.7 | 10.6 | 10.8 | 10.9 | 11.1 | 11.2 | 10.9 | 10.9 | 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bøgwald, I.; Herrig, S.; Pedersen, A.M.; Wubshet, S.G.; Eilertsen, K.-E. Effect of Calanus finmarchicus Hydrolysate Inclusion on Diet Attractiveness for Whiteleg Shrimp (Litopenaeus vannamei). Fishes 2024, 9, 134. https://doi.org/10.3390/fishes9040134
Bøgwald I, Herrig S, Pedersen AM, Wubshet SG, Eilertsen K-E. Effect of Calanus finmarchicus Hydrolysate Inclusion on Diet Attractiveness for Whiteleg Shrimp (Litopenaeus vannamei). Fishes. 2024; 9(4):134. https://doi.org/10.3390/fishes9040134
Chicago/Turabian StyleBøgwald, Isak, Simon Herrig, Alice Marie Pedersen, Sileshi Gizachew Wubshet, and Karl-Erik Eilertsen. 2024. "Effect of Calanus finmarchicus Hydrolysate Inclusion on Diet Attractiveness for Whiteleg Shrimp (Litopenaeus vannamei)" Fishes 9, no. 4: 134. https://doi.org/10.3390/fishes9040134
APA StyleBøgwald, I., Herrig, S., Pedersen, A. M., Wubshet, S. G., & Eilertsen, K.-E. (2024). Effect of Calanus finmarchicus Hydrolysate Inclusion on Diet Attractiveness for Whiteleg Shrimp (Litopenaeus vannamei). Fishes, 9(4), 134. https://doi.org/10.3390/fishes9040134






