Mechanistic Insights into Nonylphenol Stress on BMP2 and BMP4 Gene Expression in Red Crucian Carp (Carassius auratus Red var.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Sampling
2.2. NP Treatments of Embryos
2.3. NP Exposure and Inhibitor Intraperitoneal Injection of Adult Fish
2.4. RNA Extraction
2.5. Cloning the cDNA Sequence of BMP2 and BMP4
2.6. Sequence Identification of BMP2 and BMP4
2.7. Gene Expression Analysis by RT-qPCR
2.8. Statistical Analysis
3. Results
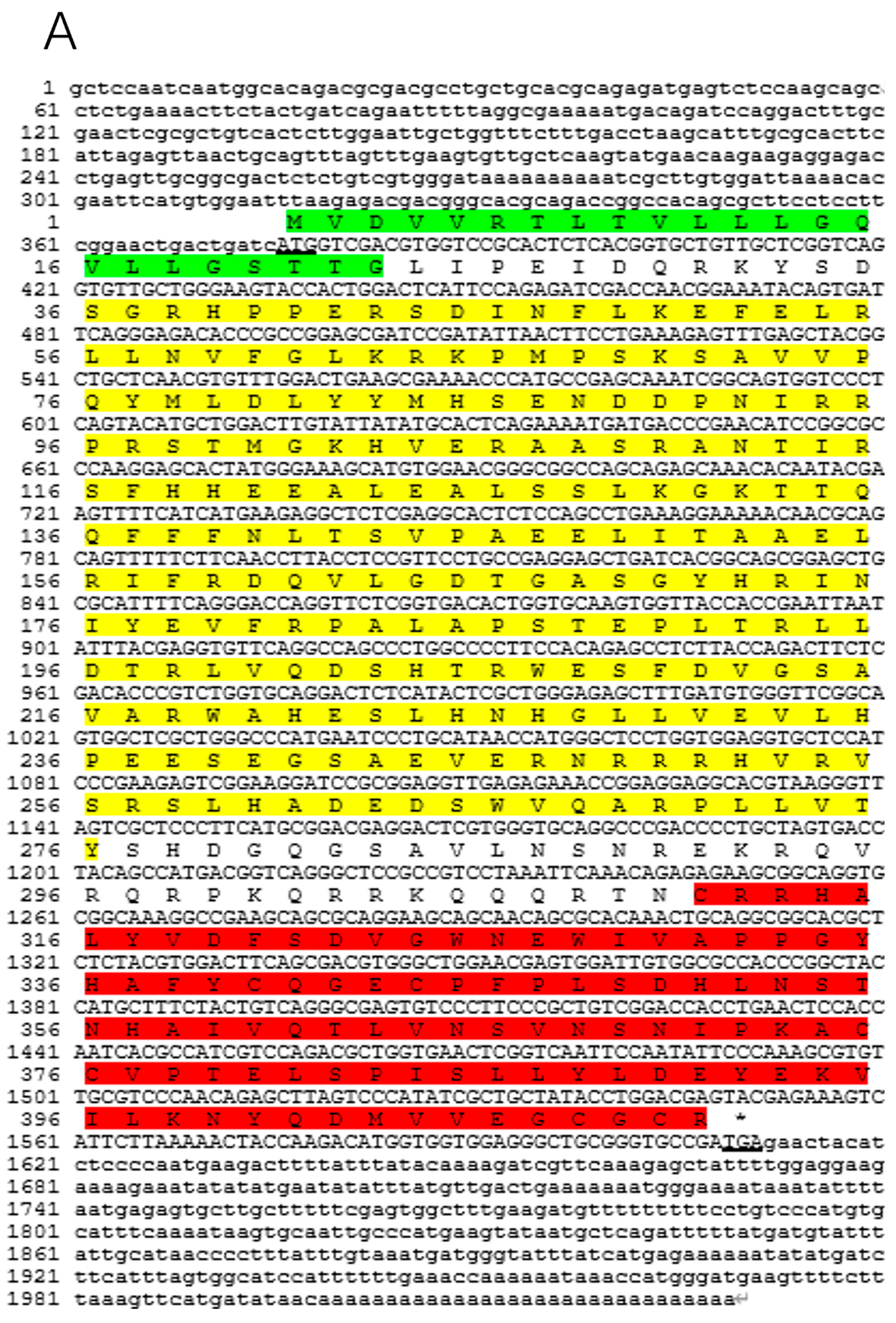
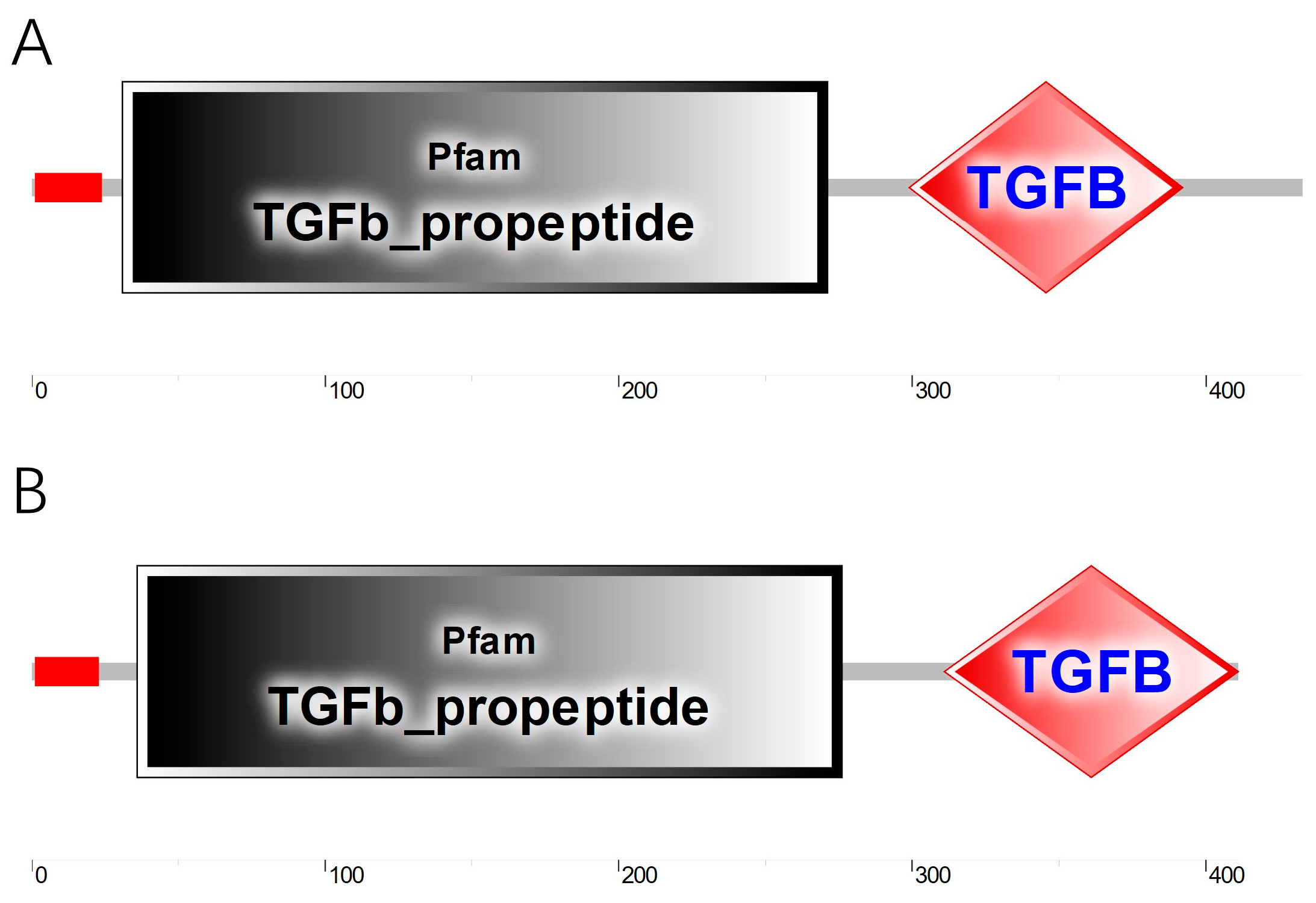
3.1. Cloning and Sequence Characteristics of BMP2 and BMP4 cDNA
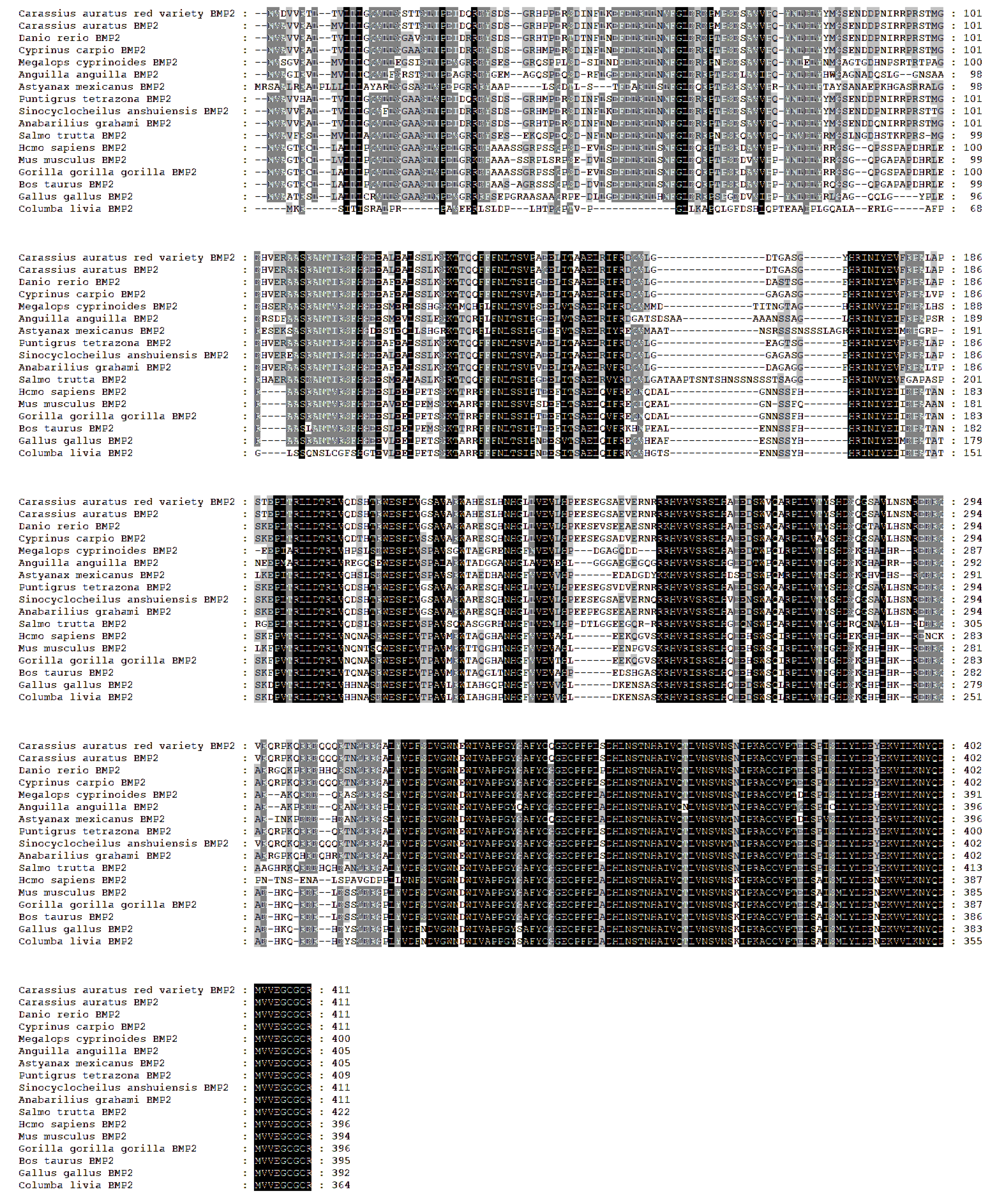
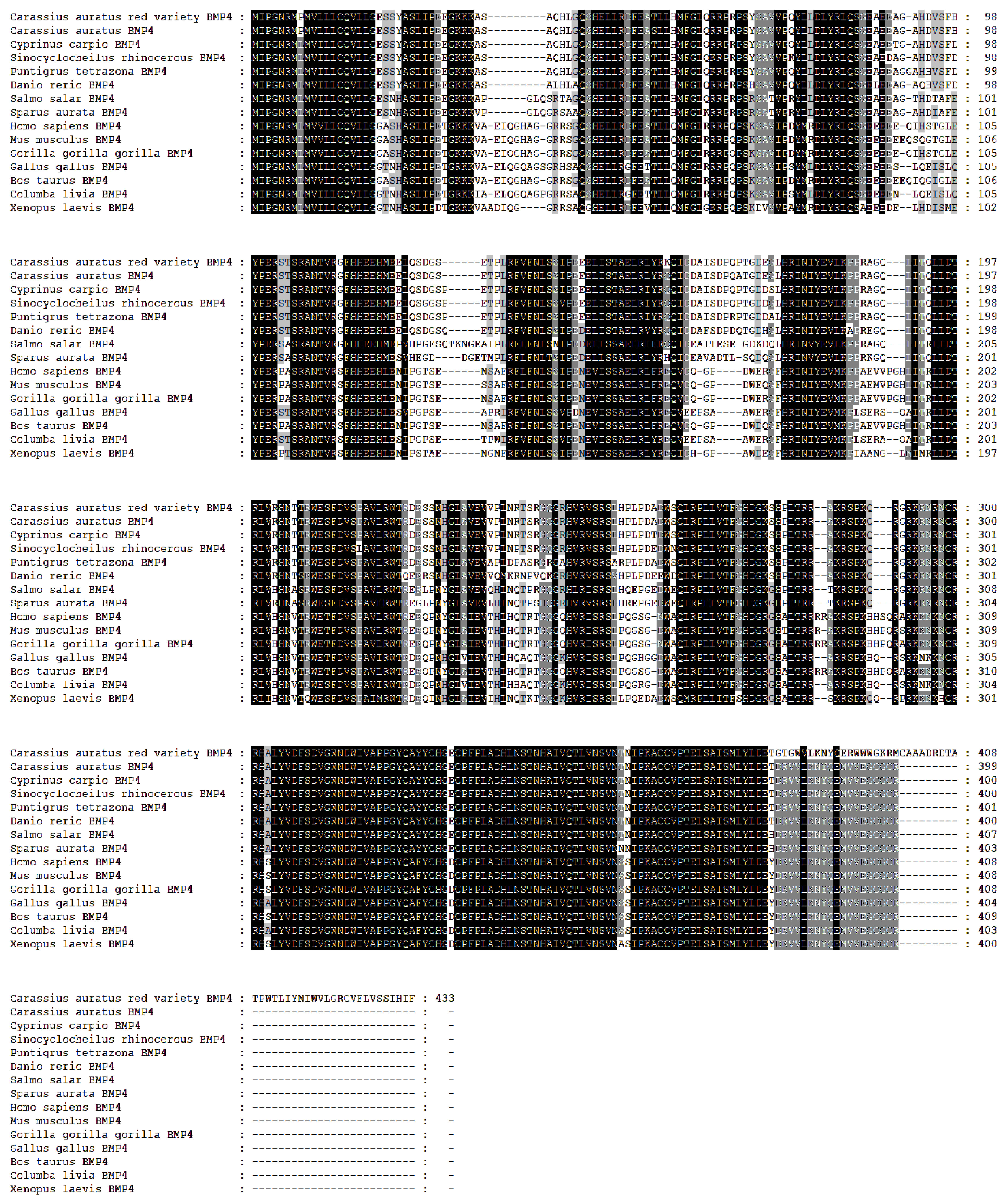
3.2. Homology and Phylogenetic Analysis
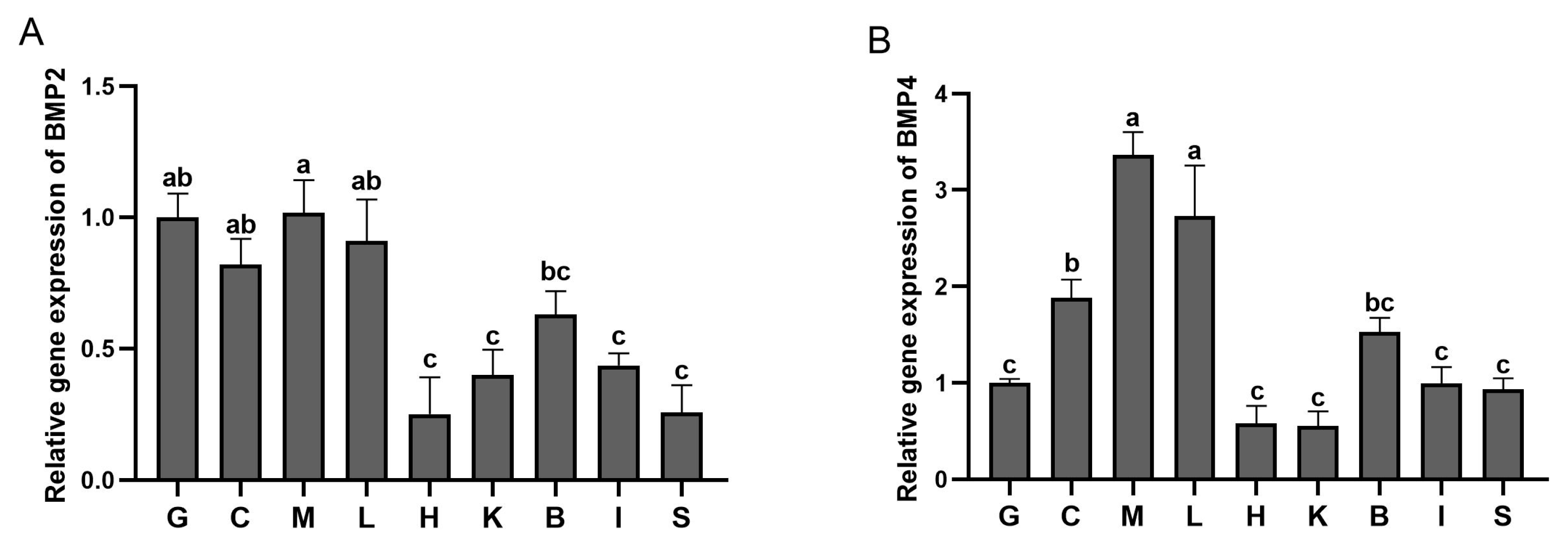
3.3. Expression Profiles of BMP2 and BMP4 in the Adult Fish
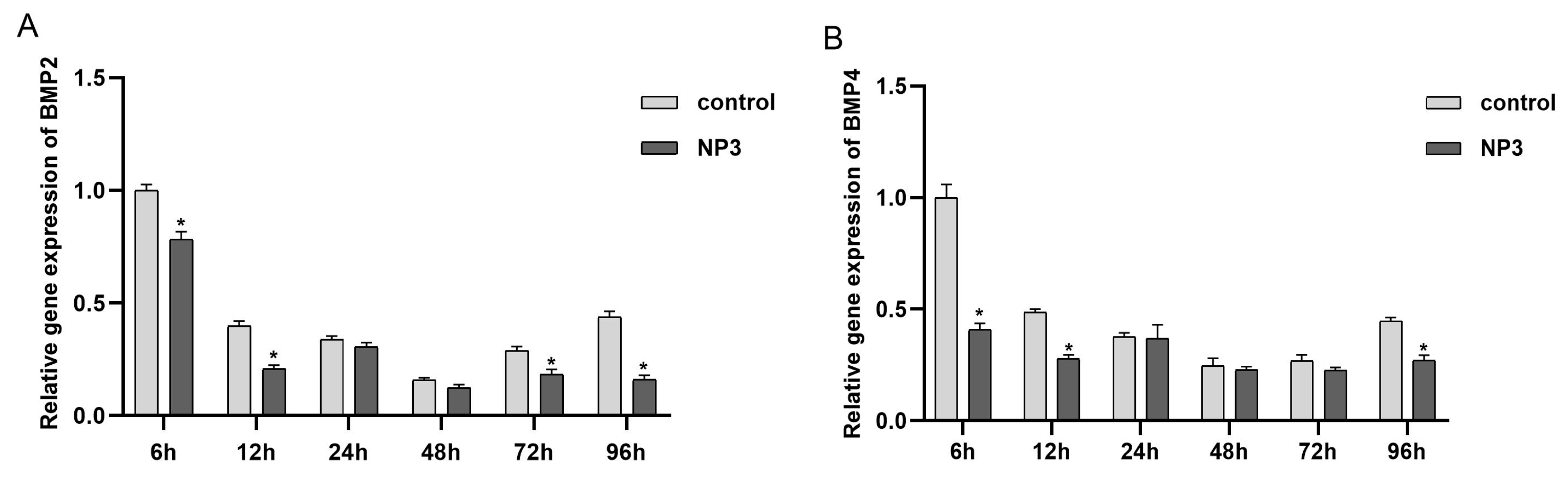
3.4. BMP2 and BMP4 Expression in Different Developmental Stages after NP Treatment
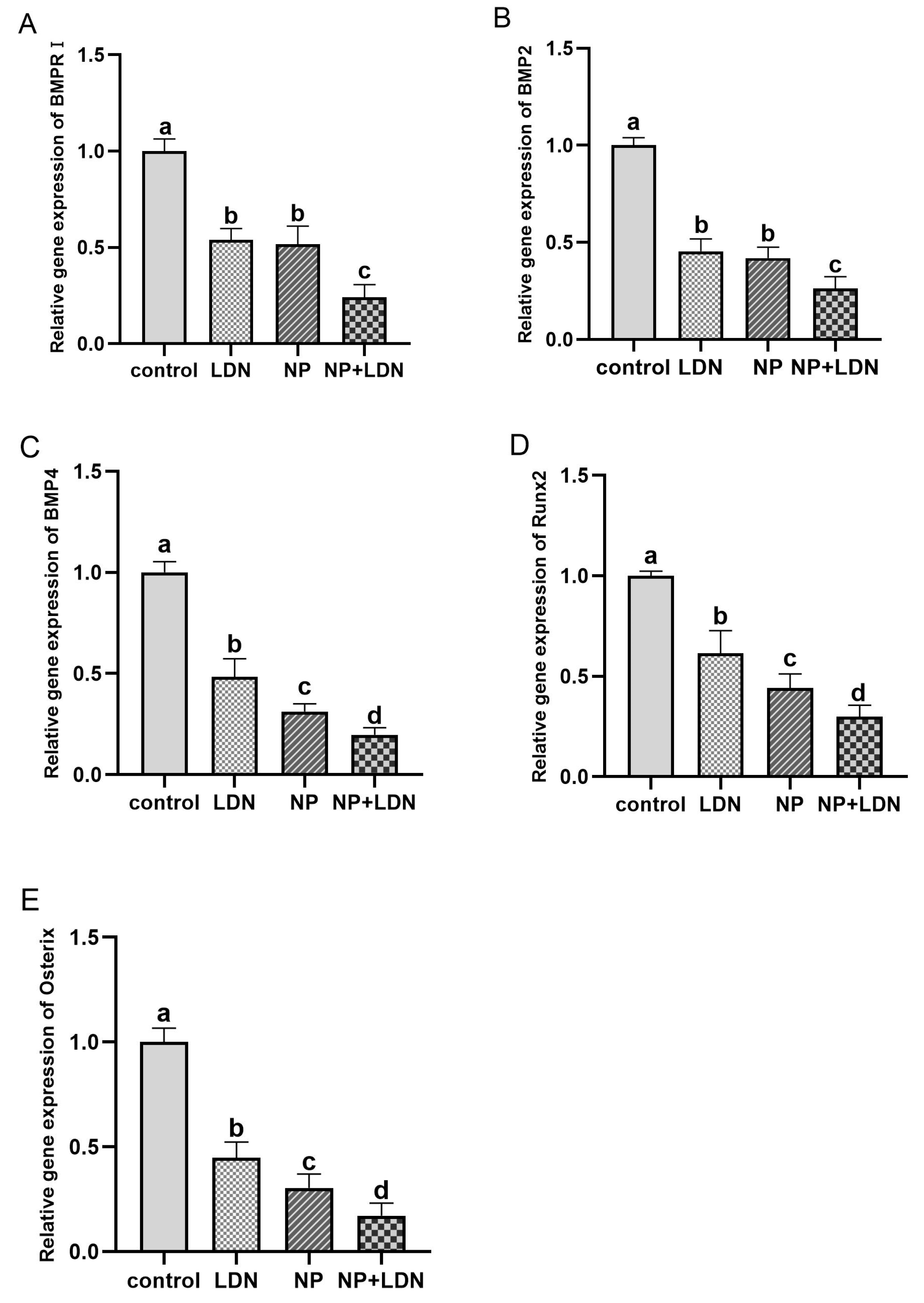
3.5. Impact of NP and LDN193189 on BMP-Smad Pathway Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishie, T.; Komaru, A.; Shiroguchi, S.; Yamaizumi, T.; Ono, Y.; Motomochi, A.; Tooyama, I.; Fujioka, Y.; Sakai, N.; Higaki, S.; et al. Nonylphenol reduced the number of haploids in in vitro spermatogenesis of the endangered cyprinid Gnathopogon caerulescens. Toxicol. Vitro. 2023, 89, 105565. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.E.; Lauth, M.J.; Hunter, C.S.; Krasny, B.R.; Hardy, K.M. Effect of 4-nonylphenol on the immune response of the pacific oyster Crassostrea gigas following bacterial infection with Vibrio campbellii. Fish. Shellfish. Immunol. 2016, 58, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Y.; Zhan, P.; Wang, Y. Effects of nonylphenol on brain gene expression profiles in F1 generation rats. Biomed. Environ. Sci. 2008, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Pan, K.; Xu, J.; Long, X.; Lin, F.; Nie, Y.; Yang, Y.; Yu, J. Effects and mechanism of perinatal nonylphenol exposure on cardiac function and myocardial mitochondria in neonatal rats. Ecotoxicol. Environ. Saf. 2023, 258, 114977. [Google Scholar] [CrossRef] [PubMed]
- Suna, P.A.; Cengiz, O.; Ceyhan, A.; Atay, E.; Ertekin, T.; Nisari, M.; Yay, A. The protective role of curcumin against toxic effect of nonylphenol on bone development. Hum. Exp. Toxicol. 2021, 40, S63–S76. [Google Scholar] [CrossRef] [PubMed]
- Agas, D.; Sabbieti, M.G.; Marchetti, L. Endocrine disruptors and bone metabolism. Arch. Toxicol. 2013, 87, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, J.; Kamei, S.; Sakayama, K.; Yamamoto, H.; Masuno, H. 4-tert-octylphenol regulates the differentiation of C3H10T1/2 cells into osteoblast and adipocyte lineages. Toxicol. Sci. 2008, 102, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Ethen, N.J.; Williams, B.O. Wnt signaling in bone development and homeostasis. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, U.; Samanta, A.; Biswas, S.; Ghosh, S.; Das, S.; Banerjee, S.; Maitra, S. Chronic exposure to nonylphenol induces oxidative stress and liver damage in male zebrafish (Danio rerio): Mechanistic insight into cellular energy sensors, lipid accumulation and immune modulation. Chem. Biol. Interact. 2022, 351, 109762. [Google Scholar] [CrossRef]
- Desai, J.K.; Trangadia, B.J.; Patel, U.D.; Patel, H.B.; Kalaria, V.A.; Kathiriya, J.B. Neurotoxicity of 4-nonylphenol in adult zebrafish: Evaluation of behaviour, oxidative stress parameters and histopathology of brain. Environ. Pollut. 2023, 334, 122206. [Google Scholar] [CrossRef]
- Horie, Y.; Kanazawa, N.; Takahashi, C.; Tatarazako, N.; Iguchi, T. Gonadal soma-derived factor expression is a potential biomarker for predicting the effects of endocrine-disrupting chemicals on gonadal differentiation in Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2022, 41, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Chaube, R.; Gautam, G.J.; Joy, K.P. Teratogenic effects of 4-nonylphenol on early embryonic and larval development of the catfish Heteropneustes fossilis. Arch. Environ. Contam. Toxicol. 2013, 64, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, J.L.; Shan, Z.J.; Bu, Y.Q.; Tian, F. Toxic effects of 4-nonylphenol on embryo/larva of zebrafish (Danio rerio). J. Ecol. Rural. Environ. 2017, 33, 737–742. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Sun, Y.D.; Wang, Z.J.; Hu, X.J.; Kui, X. Preliminary study on toxicity of nonylphenol to embryo development of goldfish (Carassius auratus). Prog. Mod. Biomed. 2016, 16, 3040–3043. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’Keefe, R.J.; et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Lyons, K.M. Multiple functions of BMPs in chondrogenesis. J. Cell Biochem. 2004, 93, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Cao, X. BMP signaling in skeletal development. Biochem. Biophys. Res. Commun. 2005, 328, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Min, K.H.; Choi, K.H.; Hwang, Y.C.; Jeong, I.K.; Ahn, K.J.; Chung, H.Y.; Chang, J.S. Bisphenol A reduces differentiation and stimulates apoptosis of osteoclasts and osteoblasts. Life Sci. 2013, 93, 367–372. [Google Scholar] [CrossRef]
- Ju, L.; Tang, K.; Guo, X.R.; Yang, Y.; Zhu, G.Z.; Lou, Y. Effects of embryonic exposure to polychlorinated biphenyls on zebrafish skeletal development. Mol. Med. Rep. 2012, 5, 1227–1231. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Tsuji, K.; Cox, K.; Harfe, B.D.; Rosen, V.; Tabin, C.J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Huang, Q.K.; Feng, L.L.; Guo, Y.F.; Liang, J.; Lan, G.Q. Sequence and expression differences of BMP2 and FGFR3 genes in Guangxi bama mini pig and landrace pig. J. South. Agric. 2021, 52, 1709–1718. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Liu, X.; Ge, B. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: A pilot study. Clin. Orthop. Relat. Res. 2010, 468, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Barberá, J.P.; Toresson, H.; Da Rocha, S.; Krauss, S. Cloning and expression of three members of the zebrafish BMP family: BMP2A, BMP2B and BMP4. Gene 1997, 198, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Shi, Z.Y.; Chen, X.W.; Cheng, Q.Q. Skeleton development of Japanese flounder (Paralichthy solivaceus) and expression analysis of related genes (SOX9, BMP4 and BMP2) during metamorphosis. Mar. Fish. 2009, 31, 337–346. [Google Scholar] [CrossRef]
- Ma, L.X.; Dong, Z.J.; Su, S.Y.; Zhang, J.Q.; Liu, W.; Li, L.L.; Yuan, X.H. Cloning of fragments of bone morphogenetic protein gene (BMP2B) of Cyprinus carpio var. Jian and its expression analysis. Jiangsu J. Agr. Sci. 2013, 29, 370–378. [Google Scholar] [CrossRef]
- Chen, J.; Lü, Y.P.; Dai, Q.M.; Zhang, L.; Xu, C.B.; Lu, J. Molecular characterization of a BMP2A homologue in barbel steed (Hemibarbus labeo) and its involvement in intermuscular bone development. Acta Hydrobiol. Sin. 2021, 45, 8–13. [Google Scholar] [CrossRef]
- Cao, X.Y.; Ma, C.X.; Xia, X.; Zhang, R.Q.; Chen, X.W.; Zhao, J.L. Cloning and expression analysis of jaw remodeling developmental gene BMP4 in Siniperca chuatsi. Genom. Appl. Biol. 2020, 39, 5075–5083. [Google Scholar] [CrossRef]
- Huang, L.; Deng, X.; Yang, X.; Tang, Z.; Fan, S.; Zhou, Z.; Tao, M.; Liu, S. Cloning, distribution, and effects of growth regulation of MC3R and MC4R in red crucian carp (Carassius auratus red var.). Front. Endocrinol. 2024, 14, 1310000. [Google Scholar] [CrossRef]
- Tian, Y.S.; Sun, Y.D.; Ou, M.; Liu, Y.F.; Cui, X.J.; Zhou, D.G.; Che, W.A.; Chen, K.C. Preliminary studies on the mechanism of nonylphenol-induced malformation of Carassius auratus red var. J. Fish. China 2020, 44, 1619–1636. [Google Scholar] [CrossRef]
- Lu, X.H.; Gu, Y.; Song, Y. Toxicity and tissue accumulation of nonylphenol in Carassius auratus red variety, Grass Carp and Sliver Carp. J. Hyg. Res. 2012, 41, 785–789. [Google Scholar] [CrossRef]
- Rajaram, S.; Patel, S.; Uggini, G.K.; Desai, I.; Balakrishnan, S. BMP signaling regulates the skeletal and connective tissue differentiation during caudal fin regeneration in sailfin molly (Poecilia latipinna). Dev. Growth Differ. 2017, 59, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Schlange, T.; Andrée, B.; Arnold, H.H.; Brand, T. BMP2 is required for early heart development during a distinct time period. Mech. Dev. 2000, 91, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xiao, Q.; Yip, H.K. Regulation of retinal progenitor cell differentiation by bone morphogenetic protein 4 is mediated by the smad/id cascade. Invest. Ophthalmol. Vis. Sci. 2010, 51, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Takahashi, N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral. Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef]
- Miyazono, K.; Maeda, S.; Imamura, T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor. Rev. 2005, 16, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P.; Archer, S.J.; Qian, S.W.; Roberts, A.B.; Sporn, M.B.; Weatherbee, J.A.; Tsang, M.L.; Lucas, R.; Zhang, B.L.; Wenker, J.; et al. Transforming growth factor beta 1: Three-dimensional structure in solution and comparison with the X-ray structure of transforming growth factor beta 2. Biochemistry 1996, 35, 8517–8534. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, C.; Kong, S.; Zhang, J.; Li, X.; Xu, P. Genome wide identification, phylogeny, and expression of bone morphogenetic protein genes in tetraploidized common carp (Cyprinus carpio). Gene 2017, 627, 157–163. [Google Scholar] [CrossRef]
- Shin, D.; Shin, C.H.; Tucker, J.; Ober, E.A.; Rentzsch, F.; Poss, K.D.; Hammerschmidt, M.; Mullins, M.C.; Stainier, D.Y. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development 2007, 134, 2041–2050. [Google Scholar] [CrossRef]
- Rafael, M.S.; Laizé, V.; Cancela, M.L. Identification of Sparus aurata bone morphogenetic protein 2: Molecular cloning, gene expression and in silico analysis of protein conserved features in vertebrates. Bone 2006, 39, 1373–1381. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sugizaki, T.; Tsukamoto, Y.; Senoh, E.; Goto, T.; Ishihara, Y. Effects of alkylphenols on bone metabolism in vivo and in vitro. Toxicol. Lett. 2008, 181, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sabbieti, M.G.; Agas, D.; Palermo, F.; Mosconi, G.; Santoni, G.; Amantini, C.; Farfariello, V.; Marchetti, L. 4-nonylphenol triggers apoptosis and affects 17-β-estradiol receptors in calvarial osteoblasts. Toxicology 2011, 290, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, A.; Nie, C.; Gao, Z.X.; Wan, S.M. Functional differentiation of BMP2A and BMP2B genes in zebrafish. Gene Expr. Patterns 2022, 46, 119288. [Google Scholar] [CrossRef]
- Hussein, A.M.; Sina, M. p-Nonylphenol impairment of osteogenic differentiation of mesenchymal stem cells was found to be due to oxidative stress and down-regulation of RUNX2 and BMP. Endocr. Metab. Immune Disord. Drug Targets. 2020, 20, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhou, Z.L.; Gu, J.H. Effect of nonylphenol on the gonadal differentiation and development in gobbid fish (Odontobutis potamophila). Fish. Sci. 2009, 28, 15–19. [Google Scholar] [CrossRef]
- Fan, X.; Wu, L.; Hou, T.; He, J.; Wang, C.; Liu, Y.; Wang, Z. Maternal bisphenol A exposure impaired endochondral ossification in craniofacial cartilage of rare minnow (Gobiocypris rarus) offspring. Ecotoxicol. Environ. Saf. 2018, 163, 514–520. [Google Scholar] [CrossRef]
- Pu, S.Y.; Hamid, N.; Ren, Y.W.; Pei, D.S. Effects of phthalate acid esters on zebrafish larvae: Development and skeletal morphogenesis. Chemosphere 2020, 246, 125808. [Google Scholar] [CrossRef]



| Primer Names | Sequence (5′ to 3′) | Application |
|---|---|---|
| BMP2-F | GCTGTTGCTCGGTCAGGTGT | Partial sequence obtaining |
| BMP2-R | CAGCCCTCCACCACCATGT | Partial sequence obtaining |
| BMP4-F | GAAGGGAAGAAGAAAGCGTCG | Partial sequence obtaining |
| BMP4-R | GACCTCTTTGCTTCGGGCTG | Partial sequence obtaining |
| BMP2-5′GSP | GTGGAGCACCTCAACCAGAAGCCCG | 5′-RACE PCR |
| BMP2-3′GSP | AGCATGGCCCCTTCCAAAGAGCCTC | 3′-RACE PCR |
| BMP4-5′GSP | CTCCCGTGGGTTGGGGATCTGAGAC | 5′-RACE PCR |
| BMP4-3′GSP | CGAGTCGAGCGAACACCGTGAGAGG | 3′-RACE PCR |
| BMP2-5′NGSP | AGCCCGTGGTTGTGCTGGGATTCGC | Nested 5′-RACE PCR |
| BMP2-3′NGSP | TGTTCAGGCCAGCATGGCCCCTTCC | Nested 3′-RACE PCR |
| BMP4-5′NGSP | TGGGGATCTGAGACTGCATCATCTATCT | Nested 5′-RACE PCR |
| BMP4-3′NGSP | CACCGTGAGAGGATTCCATCATGAAGAG | Nested 3′-RACE PCR |
| BMP2-QF | GCGATCCGATATTAACTTCCTG | qRT-PCR |
| BMP2-QR | GCTTTCCCATAGTGCTCCTTG | qRT-PCR |
| BMP4-QF | TGAACTGCTGCGGGACTTTG | qRT-PCR |
| BMP4-QR | GACTCGTGGACCTCTCGGGAT | qRT-PCR |
| Runx2-QF | CACAGAGCCATAAAGGTCACGG | qRT-PCR |
| Runx2-QR | GGAGTTGGGGTTGCTAAGCG | qRT-PCR |
| Osterix-QF | CAAACCCGTCCCATCTTCTG | qRT-PCR |
| Osterix-QR | GCACCAAGCCTCTCCAACTC | qRT-PCR |
| BMPRI-QF | TGGCGTACTCTGCAGCCTGT | qRT-PCR |
| BMPRI-QR | TGGGATGTCCACTTCATTTGTG | qRT-PCR |
| β-actin-QF | TCCCTTGCTCCTTCCACCA | qRT-PCR |
| β-actin-QR | GGAAGGGCCAGACTCATCGTA | qRT-PCR |
| Protein Name | GenBank Access. No. |
|---|---|
| Carassius auratus red variety BMP2 | PP411940 |
| Carassius auratus BMP2 | BAN17326.1 |
| Danio rerio BMP2 | AAI14257.1 |
| Cyprinus carpio BMP2 | XP_042602743.1 |
| Megalops cyprinoides BMP2 | XP_036398004.1 |
| Anguilla Anguilla BMP2 | XP_035277091.1 |
| Astyanax mexicanus BMP2 | KAG9268738.1 |
| Puntigrus tetrazona BMP2 | XP_043074619.1 |
| Sinocyclocheilus anshuiensis BMP2 | XP_016337425.1 |
| Anabarilius grahami BMP2 | ROL52452.1 |
| Salmo trutta BMP2 | XP_029587557.1 |
| Homo sapiens BMP2 | ACV32596.1 |
| Mus musculus BMP2 | AAI00345.1 |
| Gorilla gorilla gorilla BMP2 | XP_004061840.1 |
| Bos taurus BMP2 | AAI42130.1 |
| Gallus gallus BMP2 | NP_001385099.1 |
| Columba livia BMP2 | XP_021150021.1 |
| Carassius auratus red variety BMP4 | PP411941 |
| Carassius auratus BMP4 | XP_026085110.1 |
| Cyprinus carpio BMP4 | XP_042630442.1 |
| Sinocyclocheilu rhinocerous BMP4 | XP_016424235.1 |
| Puntigrus tetrazona BMP4 | XP_043118272.1 |
| Danio rerio BMP4 | NP_571417.1 |
| Salmo salar BMP4 | XP_014066471.1 |
| Sparus aurata BMP4 | XP_030298905.1 |
| Homo sapiens BMP4 | NP_001334843.1 |
| Mus musculus BMP4 | AAH13459.1 |
| Gorilla gorilla gorilla BMP4 | XP_030857865.1 |
| Gallus gallus BMP4 | NP_990568.4 |
| Bos taurus BMP4 | NP_001039342.1 |
| Columba livia BMP4 | XP_005510342.1 |
| Xenopus laevis BMP4 | NP_001081501.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Cui, X.; Chen, S.; Xu, J.; Li, Y.; Zhang, Q.; Sun, Y. Mechanistic Insights into Nonylphenol Stress on BMP2 and BMP4 Gene Expression in Red Crucian Carp (Carassius auratus Red var.). Fishes 2024, 9, 159. https://doi.org/10.3390/fishes9050159
Li D, Cui X, Chen S, Xu J, Li Y, Zhang Q, Sun Y. Mechanistic Insights into Nonylphenol Stress on BMP2 and BMP4 Gene Expression in Red Crucian Carp (Carassius auratus Red var.). Fishes. 2024; 9(5):159. https://doi.org/10.3390/fishes9050159
Chicago/Turabian StyleLi, Die, Xiaojuan Cui, Shuailin Chen, Jia Xu, Yujing Li, Qiongyu Zhang, and Yuandong Sun. 2024. "Mechanistic Insights into Nonylphenol Stress on BMP2 and BMP4 Gene Expression in Red Crucian Carp (Carassius auratus Red var.)" Fishes 9, no. 5: 159. https://doi.org/10.3390/fishes9050159
APA StyleLi, D., Cui, X., Chen, S., Xu, J., Li, Y., Zhang, Q., & Sun, Y. (2024). Mechanistic Insights into Nonylphenol Stress on BMP2 and BMP4 Gene Expression in Red Crucian Carp (Carassius auratus Red var.). Fishes, 9(5), 159. https://doi.org/10.3390/fishes9050159






