Coexisting in the Surf Zone: Age and Feeding Habits of the Spotted Seabass (Dicentrarchus punctatus) and European Seabass (Dicentrarchus labrax) on the Gulf of Cádiz Beaches (Southwest Iberian Peninsula)
Abstract
:1. Introduction
2. Materials and Methods
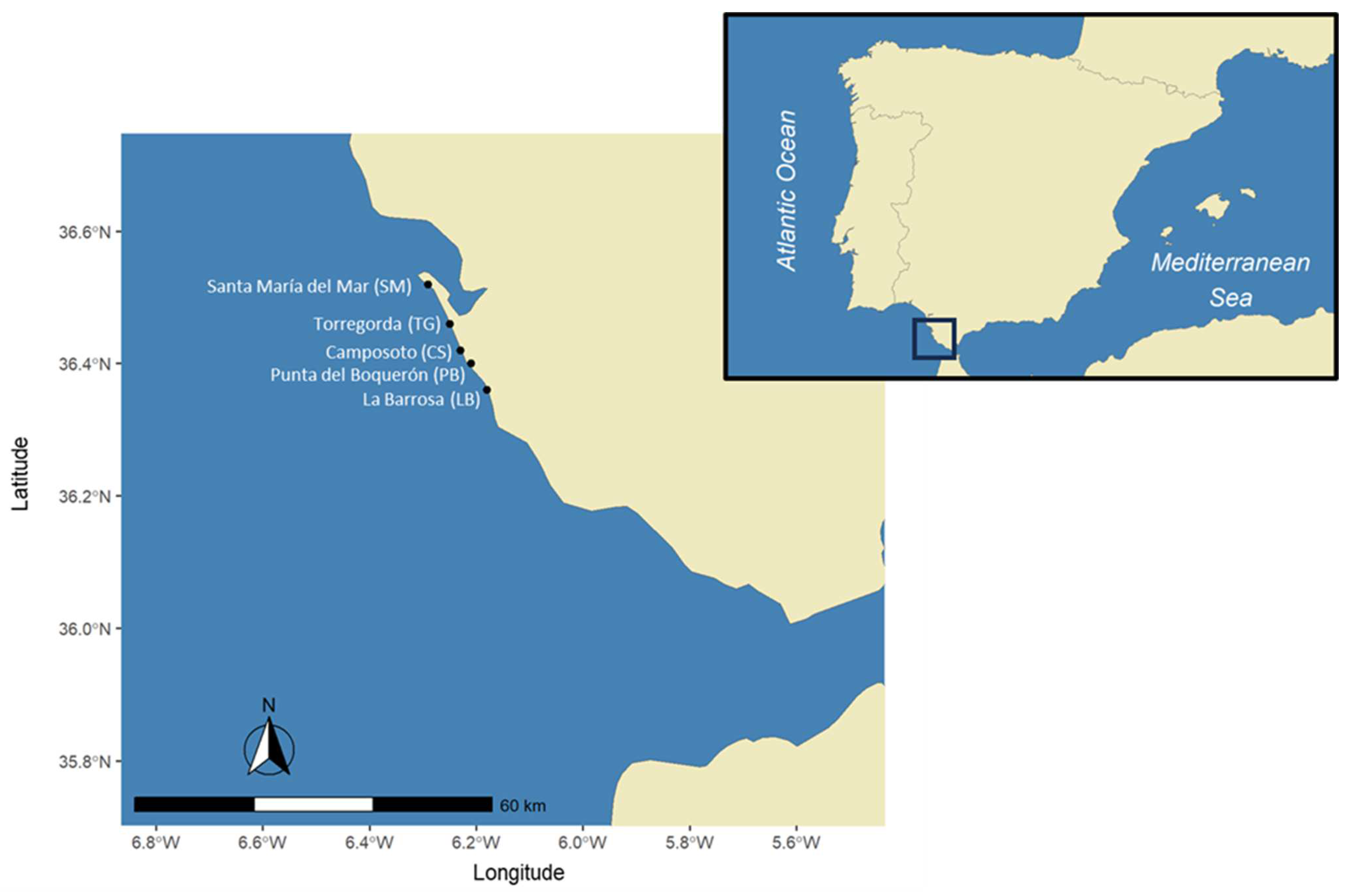
2.1. Sampling Methods
2.2. Fish Sampling
2.3. Data Analysis
3. Results
3.1. Spotted Seabass, Dicentrarchus punctatus
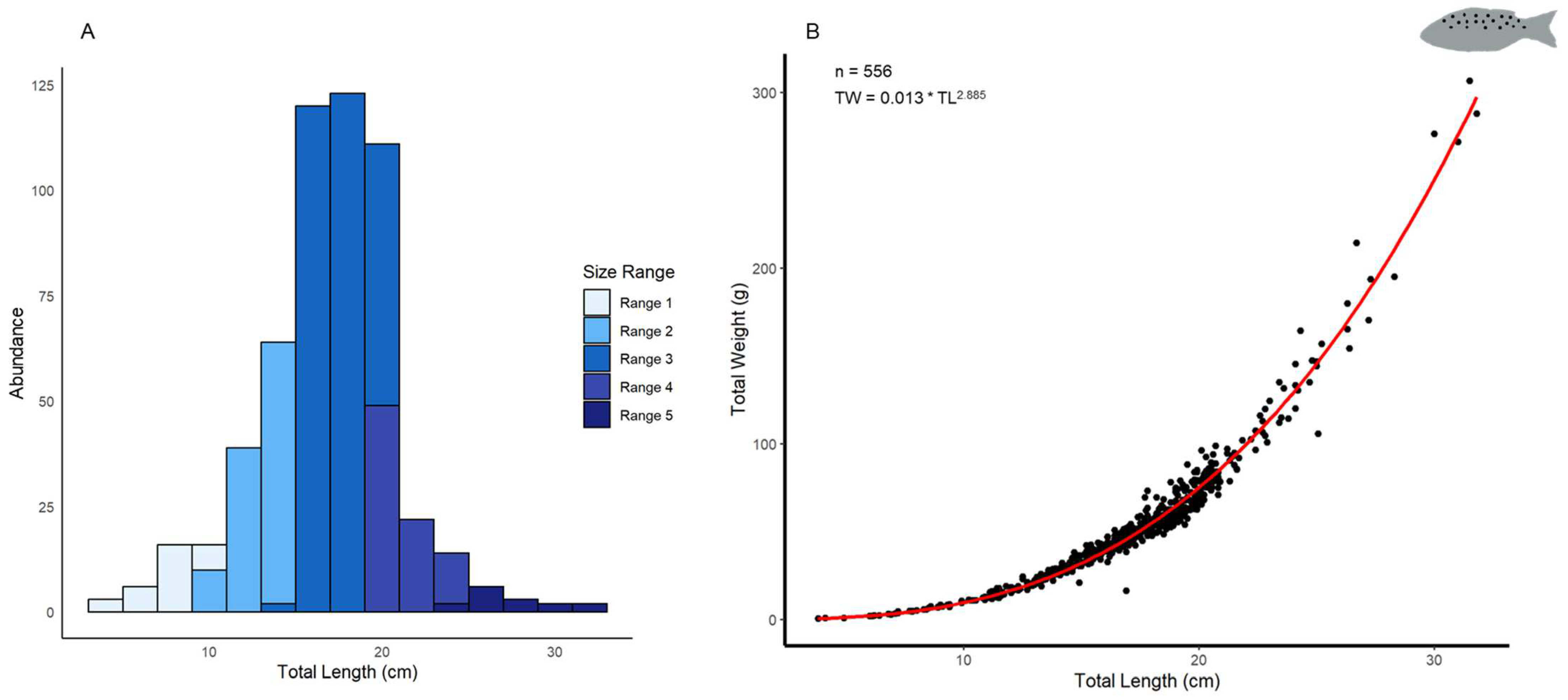
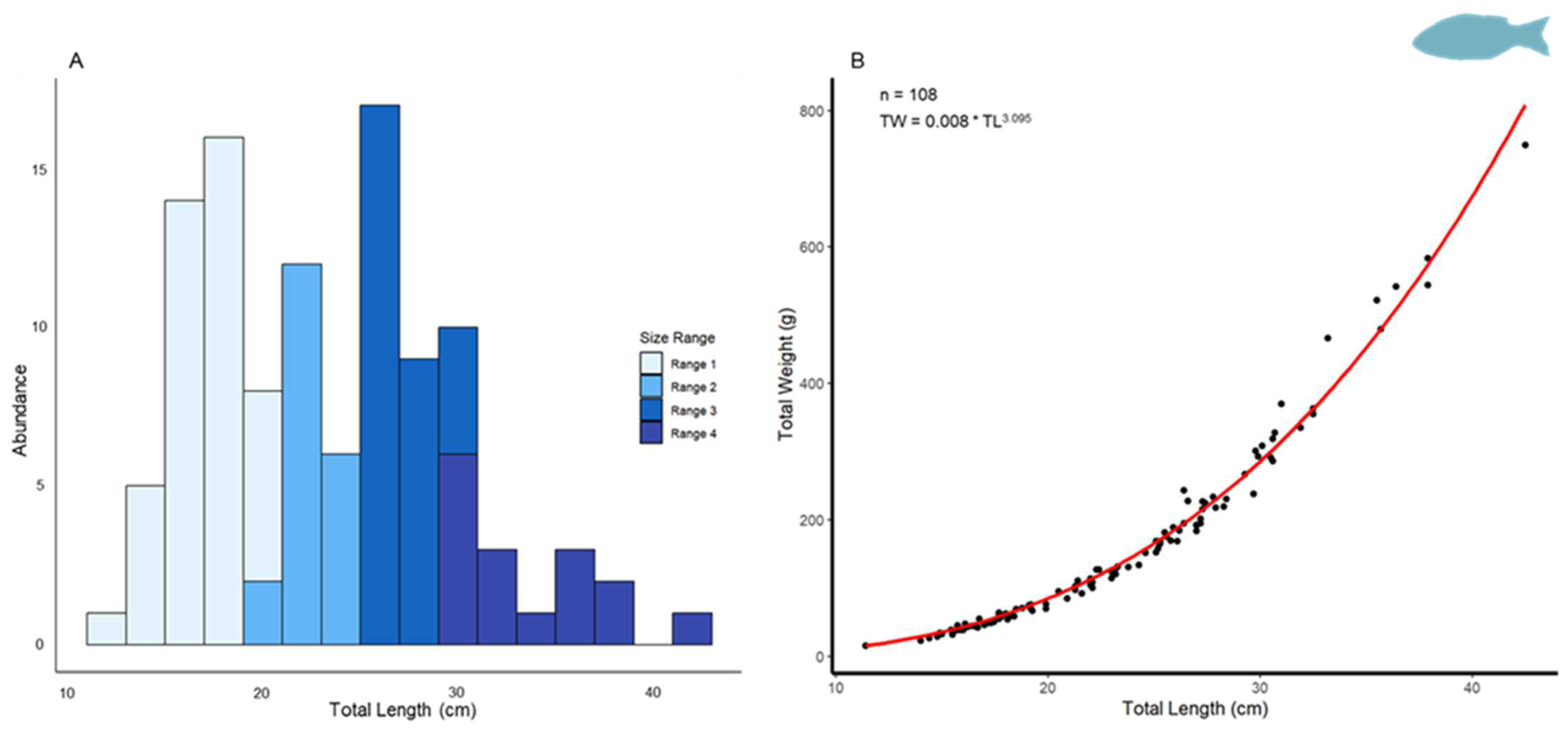
3.1.1. Biometric Analysis
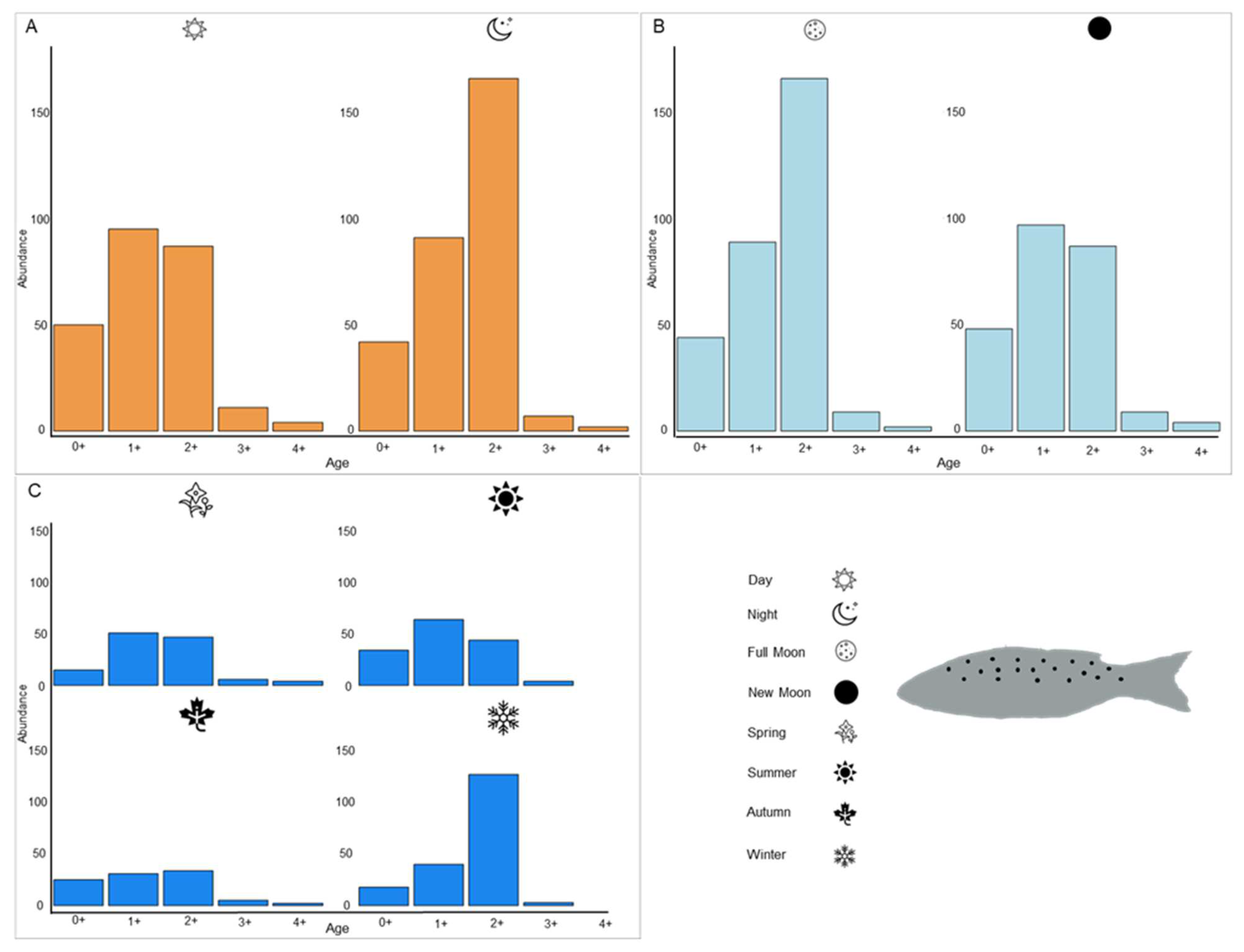
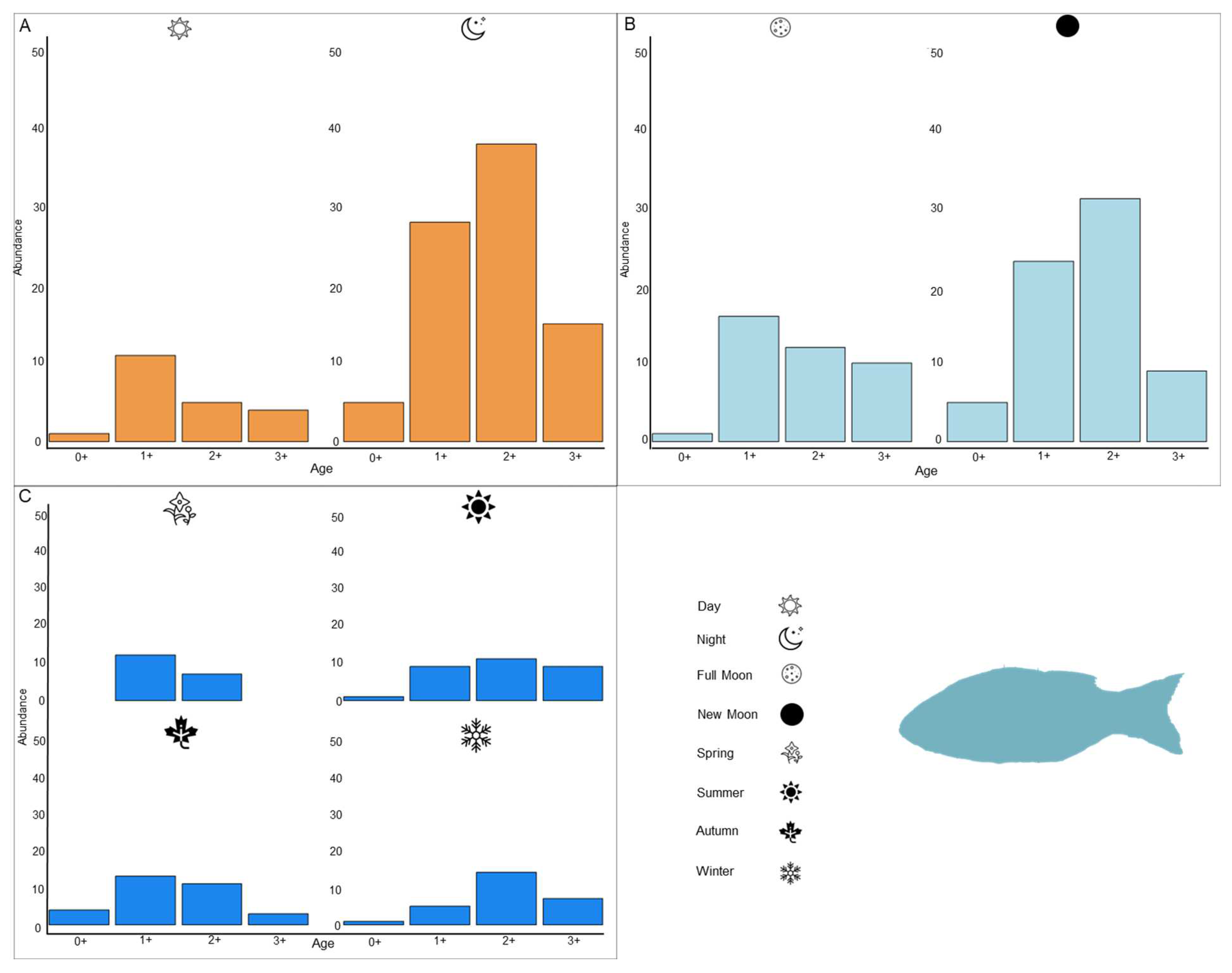
3.1.2. Age Determination
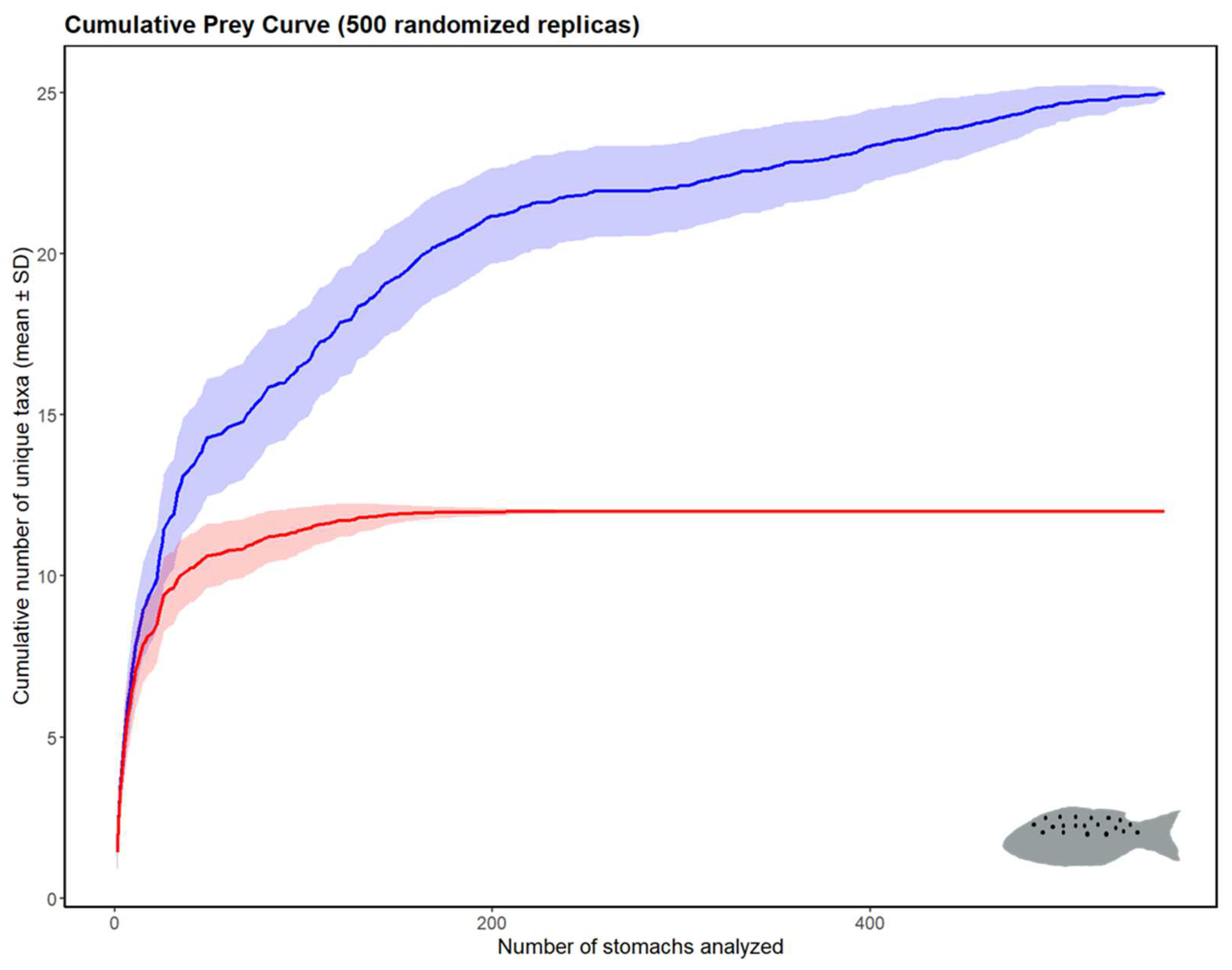
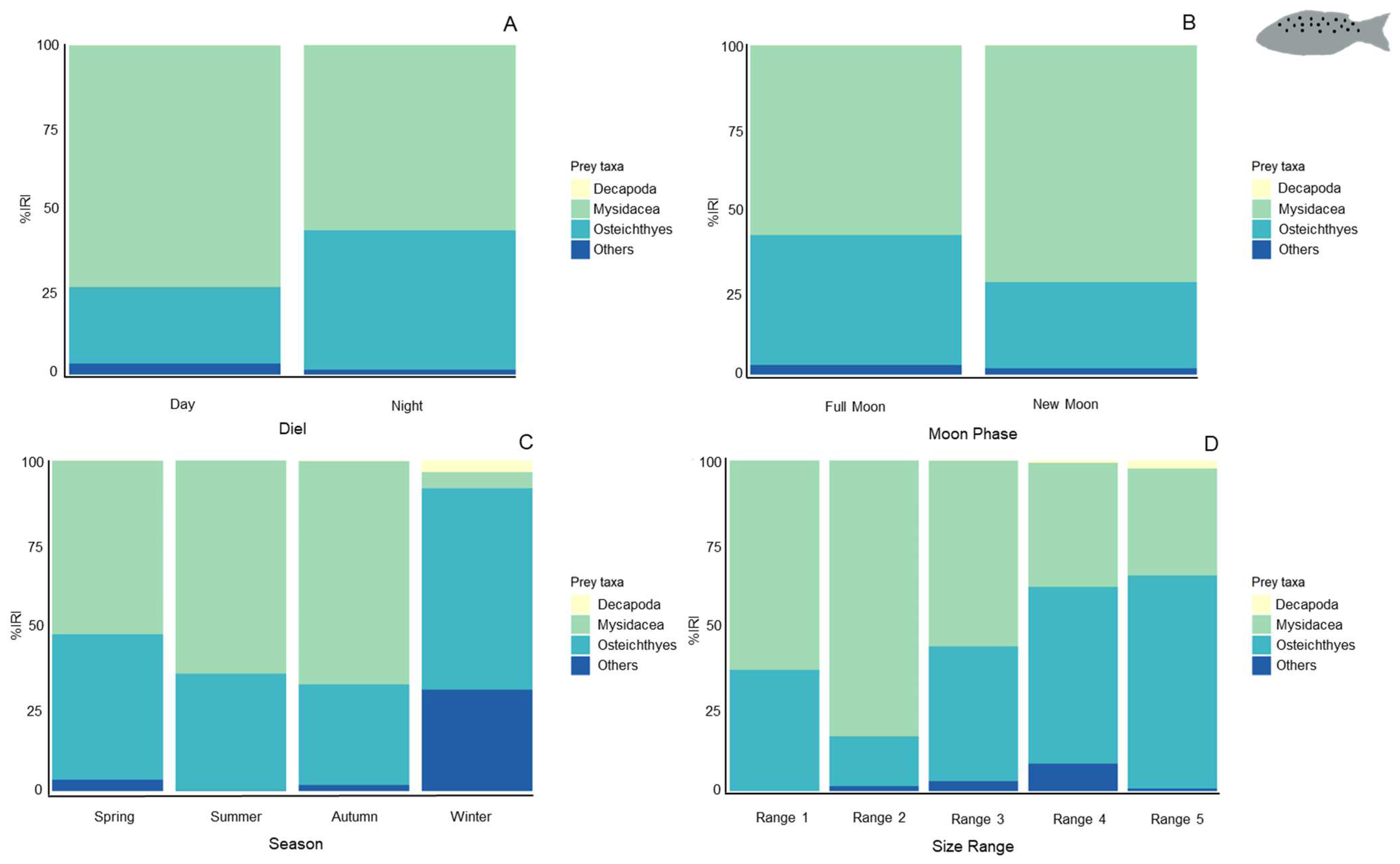
3.1.3. Feeding Habits
3.2. European Seabass, Dicentrarchus labrax
3.2.1. Biometric Analysis
3.2.2. Age Determination
3.2.3. Feeding Habits
3.3. Feeding Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehanna, S. Fisheries regulations based on yield per recruit analysis for the spotted seabass Dicentrarchus punctatus (Moronidae) at Bardawil lagoon, Mediterranean coast of Sinai, Egypt. Egypt. J. Aquat. Biol. Fish. 2006, 10, 129–145. [Google Scholar] [CrossRef]
- Fritsch, M.; Morizur, Y.; Lambert, E.; Bonhomme, F.; Guinand, B. Assessment of sea bass (Dicentrarchus labrax, L.) stock delimitation in the Bay of Biscay and the English Channel based on mark-recapture and genetic data. Fish. Res. 2007, 83, 123–132. [Google Scholar] [CrossRef]
- Freyhof, J.; Kottelat, M. Dicentrarchus labrax . IUCN Red List Threat. Species 2008, 2008, e.T135606A4159287. [Google Scholar] [CrossRef]
- Trunov, I.A.; Kukuev, E.I.; Sukhorukova, V.S. A finding in the Pregolya River (Kaliningrad oblast) of the black-spotted bass Dicentrarchus punctatus (Moronidae). J. Ichthyol. 2006, 46, 345–346. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. 2005. Available online: http://www.fishbase.org (accessed on 14 February 2024).
- Carpenter, K.E.; Smith-Vaniz, W.F.; de Bruyne, G.; de Morais, L. Dicentrarchus punctatus . IUCN Red List Threat Species 2015, 2015, e.T198671A21913001. [Google Scholar] [CrossRef]
- FAO. FishStat. Global Capture Production QUANTITY (1950–2021). 2023. Available online: https://fao.org/fishery/statistics-query/en/capture/capture_quantity (accessed on 21 December 2023).
- Cabral, H.; Duque, J.; Costa, M.J. Discards of the beach seine fishery in the central coast of Portugal. Fish. Res. 2003, 63, 63–71. [Google Scholar] [CrossRef]
- Jennings, S.; Pawson, M.G. The origin and recruitment of bass, Dicentrarchus labrax, larvae to nursery areas. J. Mar. Biol. Assoc. U. K. 1992, 72, 199–212. [Google Scholar] [CrossRef]
- Dufour, V.; Cantou, M.; Lecomte, F. Identification of sea bass (Dicentrarchus labrax) nursery areas in the north-western Mediterranean Sea. J. Mar. Biol. Assoc. U. K. 2009, 89, 1367–1374. [Google Scholar] [CrossRef]
- McLachlan, A.; Brown, A.C. The Ecology of Sandy Shores, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2006; 373p. [Google Scholar]
- Suda, Y.; Inoue, T.; Uchida, H. Fish communities in the surf zone of a protected sandy beach at Doigahama, Yamaguchi prefecture, Japan. Estuar. Coast. Shelf Sci. 2002, 55, 81–96. [Google Scholar] [CrossRef]
- Nash, R.D.; Santos, R.S.; Hawkins, S.J. Diel fluctuations of a sandy beach fish assemblage at Porto Pim, Faial Island. Azores. Arquipélago. Life Mar. Sci. 1994, 12, 75–86. [Google Scholar]
- Taal, I.; Saks, L.; Rohtla, M.; Jürgens, K.; Svirgsden, R.; Kesler, M.; Verliin, A.; Hubel, K.; Albert, A.; Eschbaum, R. Diel changes in the fish assemblage in a coastal surf-zone area in the eastern Baltic Sea. Boreal Environ. Res. 2017, 22, 83–96. [Google Scholar]
- Du Preez, H.H.; McLachlan, A.; Marais, J.F.K.; Cockcroft, A.C. Bioenergetics of fishes in a high-energy surf-zone. Mar. Biol. 1990, 106, 1–12. [Google Scholar] [CrossRef]
- Esposito, V.; Andaloro, F.; Bianca, D.; Natalotto, A.; Romeo, T.; Scotti, G.; Castriota, L. Diet and prey selectivity of the red mullet, Mullus barbatus (Pisces: Mullidae), from the southern Tyrrhenian Sea: The role of the surf zone as a feeding ground. Mar. Biol. Res. 2014, 10, 167–178. [Google Scholar] [CrossRef]
- Watt-Pringle, P.; Strydom, N.A. Habitat use by larval fishes in a temperate South African surf zone. Estuar. Coast. Shelf Sci. 2003, 58, 765–774. [Google Scholar] [CrossRef]
- Costa, L.L.; Landmann, J.G.; Gaelzer, L.R.; Zalmon, I.R. Does human pressure affect the community structure of surf zone fish in sandy beaches? Cont. Shelf Res. 2017, 132, 1–10. [Google Scholar] [CrossRef]
- Biagi, F.; Gambaccini, S.; Zazzetta, M. Settlement and recruitment in fishes: The role of coastal areas. Ital. J. Zool. 1998, 65, 269–274. [Google Scholar] [CrossRef]
- Omar, S.A.; Ibrahim, G.D.; Ahmed, M.S.; AbdElnabi, H.E. Length-weight relationship, condition factor and growth parameters of spotted sea bass Dicentrarchus punctatus in Bardawil lagoon North Sinai Egypt. Sinai J. Appl. Sci. 2022, 11, 909–920. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Mohammed-AbdAllah, E. Assessing fishery status and sustainable exploitation of spotted seabass (Dicentrarchus punctatus) in Bardawil Lagoon, Eastern Mediterranean, Egypt. Egypt. J. Aquat. Res. 2023, 49, 353–359. [Google Scholar] [CrossRef]
- Ahmed, A.I. Biology of food and feeding of juvenile Dicentrarchus punctatus (Bloch, 1792) along Port-Said coast on the Mediterranean Sea, Egypt. Egypt. J. Aquat. Res. 2009, 35, 165–176. [Google Scholar]
- Attia, S.; El Aiatt, A.; Hassanen, G.D.; Ahmad, M.S.; Elnabi, H.E.A. Reproductive Biology of the Spotted Sea bass, Dicentrarchus punctatus, in Bardawil Lagoon, North Sinai, Egypt. Egypt. J. Aquat. Biol. Fish. 2023, 27, 459–475. [Google Scholar] [CrossRef]
- Baldó, F.; Drake, P. A multivariate approach to the feeding habits of small fishes in the Guadalquivir Estuary. J. Fish Biol. 2002, 61, 21–32. [Google Scholar] [CrossRef]
- Mehanna, S.F.; El-Aiatt, A.A.; Ameran, M.; Salem, M. Population dynamics and fisheries regulations for the European seabass Dicentrarchus labrax (Moronidae) at Bardawil lagoon, Egypt. In Proceedings of the 3rd International Conference on Fisheries and Aquaculture, Cairo, Egypt, 29 November–1 December 2010; Volume 29. [Google Scholar]
- Shalloof, K.A.S.; El-Aiatt, A.; Mohammed, S.M. Biological aspects of the European sea bass (Dicentrarchus labrax L., 1758) from Bardawil lagoon, North Sinai, Egypt. Egypt. J. Zool. 2019, 72, 11–21. [Google Scholar] [CrossRef]
- Jennings, S.; Lancaster, J.E.; Ryland, J.S.; Shackley, S.E. The age structure and growth dynamics of young-of-the-year bass, Dicentrarchus labrax, populations. J. Mar. Biol. Assoc. U. K. 1991, 71, 799–810. [Google Scholar] [CrossRef]
- Abdel-Hakim, N.F.; Mehanna, S.F.; Eisa, I.A.; Hussein, M.S.; Al-Azab, D.A.; Ahmed, A.S. Length weight relationship, condition factor and stomach contents of the European sea bass, Dicentrarchus labrax at Bardawil Lagoon, North Sinai, Egypt. In Proceeding of the 3rd Global Fisheries and Aquaculture Research Conference, Cairo, Egypt, 29 November–1 December 2010; pp. 1–14. [Google Scholar]
- Laffaille, P.; Lefeuvre, J.C.; Schricke, M.T.; Feunteun, E. Feeding ecology of o-group sea bass, Dicentrarchus labrax, in salt marshes of Mont Saint Michel Bay (France). Estuaries 2001, 24, 116–125. [Google Scholar] [CrossRef]
- Cabral, H.; Costa, M.J. Abundance, feeding ecology and growth of 0-group sea bass, Dicentrarchus labrax, within the nursery areas of the Tagus estuary. J. Mar. Biol. Assoc. U. K. 2001, 81, 679–682. [Google Scholar] [CrossRef]
- Kara, M.H. Cycle sexuel et fécondité du Loup Dicentrarchus labrax (Poisson Moronidé) du golfe d’Annaba. Cah. De Biol. Mar. 1997, 38, 161–168. [Google Scholar]
- Arias, A.M.; Drake, P. Estados Juveniles de la Ictiofauna en los Caños de las Salinas de la Bahía de Cádiz; CSIC-Instituto de Ciencias Marinas de Andalucía (ICMAN): Cádiz, Spain, 1990. [Google Scholar]
- Lloris, D. Ictiofauna Marina: Manual de Identificación de los Peces Marinos de la Península Ibérica y Balears; 954 especies; Omega: Moscow, Russia, 2015. [Google Scholar]
- Waldron, M.E.; Kerstan, M. Age validation in horse mackerel (Trachurus trachurus) otoliths. ICES J. Mar. Sci. 2001, 58, 806–813. [Google Scholar] [CrossRef]
- Hayward, P.J.; Ryland, J.S. (Eds.) . Handbook of the Marine Fauna of North-West Europe; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; Bulletin of the Fisheries Research Board of Canada: Ottawa, Canada, 1975. [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Gibson, R.N.; Ezzi, I.A. Feeding relationships of a demersal fish assemblage on the west coast of Scotland. J. Fish Biol. 1987, 31, 55–69. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Longman: London, UK, 1989. [Google Scholar]
- Ferry, L.A. Sample size and data analysis: Are we characterizing and comparing diet properly? In Proceedings of the International Congress on the Biology of Fshes, San Francisco, CA, USA, 14–18 July 1996; pp. 71–80. [Google Scholar]
- Scenna, L.B.; García De La Rosa, S.B.; Díaz de Astarloa, J.M. Trophic ecology of the Patagonian skate, Bathyraja macloviana, on the Argentine continental shelf. ICES J. Mar. Sci. 2006, 63, 867–874. [Google Scholar] [CrossRef]
- Matić-Skoko, S.; Tutman, P.; Bojanić Varezić, D.; Skaramuca, D.; Đikić, D.; Lisičić, D.; Skaramuca, B. Food preferences of the Mediterranean moray eel, Muraena helena (Pisces: Muraenidae), in the southern Adriatic Sea. Mar. Biol. Res. 2014, 10, 807–815. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Anderson, M.J.; ter Braak, C.J.F. Permutation tests for multifactorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, T.W.; Wang, Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. dplyr: A Grammar of Data Manipulation; R package Version 1.1.2; Posit: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Grolemund, G.; Wickham, H. Dates and times made easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package; R Package Version 2.6-4. 2022. Available online: https://CRAN.R560project.org/package=vegan (accessed on 10 February 2024).
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H. Forcats: Tools for Working with Categorical Variables (Factors); Posit: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H. Modelr: Modelling Functions that Work with the Pipe. R Package Version 0.1.11. 2023. Available online: https://CRAN.R-project.org/package=modelr (accessed on 10 February 2024).
- Auguie, B. gridExtra: Miscellaneous Functions for “Grid” Graphics_. R Package Version 2.3. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 10 February 2024).
- Wickham, H.; Hester, J.; Chang, W.; Bryan, J. devtools: Tools to Make Developing R Packages Easier_. R Package Version 2.4.5. 2022. Available online: https://CRAN.R-project.org/package=devtools (accessed on 10 February 2024).
- Hassan, I.; El-Feky, A. Age and Growth of Spotted Sea Bass Dicentrarchus punctatus (Bloch, 1792) in the Bitter Lakes, Suez Canal, Egypt. J. Anim. Poult. Fish Prod. 2022, 11, 1–7. [Google Scholar] [CrossRef]
- Rogdakis, Y.; Ramfos, A.; Koukou, K.; Dimitriou, E.; Katselis, G. Feeding habits and trophic level of sea bass (Dicentrarchus labrax) in the Messolonghi-Etoliko lagoons complex (Western Greece). J. Biol. Res. 2010, 13, 13–26. [Google Scholar]
- Gonçalves, J.M.S.; Bentes, L.; Lino, P.G.; Ribeiro, J.; Canario, A.V.M.; Erzini, K. Weight–length relationships for selected fish species of the small-scale demersal fisheries of the south and south-west coast of Portugal. Fish. Res. 1997, 30, 253–256. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Moutopoulos, D.K. A review of length–weight relationships of fishes from Greek Marine Waters. ICLARM Quart 2001, 24, 23–39. [Google Scholar]
- Koutrakis, E.T.; Tsikliras, A.C. Length–weight relationships of fishes from three northern Aegean estuarine systems (Greece). J. Appl. Ichthyol. 2003, 19, 258–260. [Google Scholar] [CrossRef]
- Dulčić, J.; Glamuzina, B. Length–weight relationships for selected fish species from three eastern Adriatic estuarine systems (Croatia). J. Appl. Ichthyol. 2006, 22, 254–256. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Marcos, C. Ecology and Distribution of Dicentrarchus labrax (Linneaus, 1758); Vázquez, F.J.S., Muñoz-Cueto, J.A., Eds.; Biology of European Sea Bass; CRC Press: Boca Raton, FL, USA, 2014; pp. 3–33. [Google Scholar]
- Gibson, R.N.; Pihl, L.; Burrows, M.T.; Modin, J.; Wennhage, H.; Nickell, L.A. Diel movements of juvenile plaice Pleuronectes platessa in relation to predators, competitors, food availability and abiotic factors on a microtidal nursery ground. Mar. Ecol. Prog. Ser. 1998, 165, 145–159. [Google Scholar] [CrossRef]
- Layman, C.A. Fish assemblage structure of the shallow ocean surf zone on the eastern shore of Virginia Barrier Islands. Estuar. Coast. Shelf Sci. 2000, 51, 201–213. [Google Scholar] [CrossRef]
- Lewin, W.C.; Okun, N.; Mehner, T. Determinants of the distribution of juvenile fish in the littoral area of a shallow lake. Freshw. Biol. 2004, 49, 410–424. [Google Scholar] [CrossRef]
- Contente, R.F.; Marion, C.; Silva, J.V.; Soeth, M.; Condini, M.V.L.; Lopes Almeida, L.; Spach, S.L.; Hostim-Silva, M. Surf-zone fish assemblage structure and its diel variability in an ocean beach of Espírito Santo (central Brazilian coast). Lat. Am. J. Aquat. Res. 2023, 51, 133–144. [Google Scholar] [CrossRef]
- Gaelzer, L.R.; Machado, G.R.; Baptista, O.R.; Zalmon, I.R. Surf zone ichthyofauna diel variation in Arraial do Cabo, southeastern Brazil. J. Coast. Res. 2006, 39, 1114–1117. [Google Scholar]
- Ley, J.A.; Halliday, I.A. Diel variation in mangrove fish abundances and trophic guilds of northeastern Australian estuaries with a proposed trophodynamic model. Bull. Mar. Sci. 2007, 80, 681–720. [Google Scholar]
- Rodríguez-García, C.; Abad-Rodríguez, I.; López-Báez, J.; Cabrera-Castro, R. A life in the surf zone: Age and feeding habits of the pompano (Trachinotus ovatus, Linnaeus, 1758) on the beaches of the Gulf of Cádiz (south-west Iberian Peninsula). J. Fish Biol. 2024, 104, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.; França, S.; Cabral, H.N. Diel and semi-lunar patterns in the use of an intertidal mudflat by juveniles of Senegal sole, Solea senegalensis. Estuar. Coast. Shelf Sci. 2006, 69, 246–254. [Google Scholar] [CrossRef]
- Moreira, F.; Assis, C.A.; Almeida, P.R.; Costa, J.L.; Costa, M.J. Trophic relationships in the community of the upper Tagus estuary (Portugal): A preliminary approach. Estuar. Coast. Shelf Sci. 1992, 34, 617–623. [Google Scholar] [CrossRef]
- Pasquaud, S.; Pillet, M.; David, V.; Sautour, B.; Elie, P. Determination of fish trophic levels in an estuarine system. Estuar. Coast. Shelf Sci. 2010, 86, 237–246. [Google Scholar] [CrossRef]
- Cardoso, J.F.M.F.; Freitas, V.; Quilez, I.; Jouta, J.; Witte, J.I.; Van Der Veer, H.W. The European sea bass Dicentrarchus labrax in the Dutch Wadden Sea: From visitor to resident species. J. Mar. Biol. Assoc. U. K. 2015, 95, 839–850. [Google Scholar] [CrossRef]
- DeLancey, L.B. The summer zooplankton of the surf zone at Folly Beach, South Carolina. J. Coast. Res. 1987, 3, 211–217. [Google Scholar]
- Lasiak, T.A.; McLachlan, A. Opportunistic utilization of mysid shoals by surf-zone teleosts. Mar. Ecol. Prog. Ser. 1987, 37, 7. [Google Scholar] [CrossRef]
- Jarrin, J.R.M.; Shanks, A.L. Spatio-temporal dynamics of the surf-zone faunal assemblages at a southern Oregon sandy beach. Mar. Ecol. 2011, 32, 232–242. [Google Scholar] [CrossRef]
- De Los Ríos-Méndez, J.; Kanamori, G.A.; Landaeta, M.F. Lunar influences in the diet habits and selectivity of larval clingfish Gobiesox marmoratus. J. Fish Biol. 2019, 95, 833–846. [Google Scholar] [CrossRef]
- Wootton, R.J. Ecology of Teleost Fishes; Springer Science Business Media: Berlin, Germany, 1998; Volume 1. [Google Scholar]
- Beyst, B.; Buysse, D.; Dewicke, A.; Mees, J. Surf zone hyperbenthos of Belgian sandy beaches: Seasonal patterns. Estuar. Coast. Shelf Sci. 2001, 53, 877–895. [Google Scholar] [CrossRef]
- Millán, M. Reproductive characteristics and condition status of anchovy Engraulis encrasicolus L. from the Bay of Cadiz (SW Spain). Fish. Res. 1999, 41, 73–86. [Google Scholar] [CrossRef]
- Martin, M.P.; Valle, F.R. Especies de Interés Pesquero en el Litoral de Andalucía; Consejería de Agricultura y Pesca: Málaga, Spain, 2001; 442p. [Google Scholar]
- Sá, R.; Bexiga, C.; Veiga, P.; Vieira, L.; Erzini, K. Feeding ecology and trophic relationships of fish species in the lower Guadiana River Estuary and Castro Marim e Vila Real de Santo António Salt Marsh. Estuar. Coast. Shelf Sci. 2006, 70, 19–26. [Google Scholar] [CrossRef]
- Riley, W.D.; Ibbotson, A.T.; Beaumont, W.R.; Pawson, M.G.; Cook, A.C.; Davison, P.I. Predation of the juvenile stages of diadromous fish by sea bass (Dicentrarchus labrax) in the tidal reaches of an English chalk stream. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 307–312. [Google Scholar] [CrossRef]
- Gerking, S.D. Feeding Ecology of Fish; Academic Press: San Diego, CA, USA, 1994; 415p. [Google Scholar]
- Aranda, A.; Sánchez-Vázquez, F.J.; Madrid, J.A. Influence of water temperature on demand-feeding rhythms in sea bass. J. Fish Biol. 1999, 55, 1029–1039. [Google Scholar] [CrossRef]
- Lasiak, T.A. Juveniles, food and the surf zone habitat: Implications for teleost nursery areas. S. Afr. J. Zool. 1986, 21, 51–56. [Google Scholar] [CrossRef]
- Guerreiro, M.A.; Marques, S.C.; Martinho, F.; Azeiteiro, U.M.; Pardal, M.Â.; Primo, A.L. Surf zone zooplankton communities from the west coast of the Iberian Peninsula—Influence of season, substrate type and environmental factors. Reg. Stud. Mar. Sci. 2021, 48, 102050. [Google Scholar] [CrossRef]
- Bachiller, E.; Irigoien, X. Trophodynamics and diet overlap of small pelagic fish species in the Bay of Biscay. Mar. Ecol. Prog. Ser. 2015, 534, 179–198. [Google Scholar] [CrossRef]
- Macpherson, E. Régime alimentaire de Galeus melastomus Rafinesque, 1810 Etmopterus spinax (L., 1758) et Scymnorhinus licha (Bonnaterre, 1788) en Méditerranée occidentale. Vie Et Milieu/Life Environ. 1980, 30, 139–148. [Google Scholar]
- Valls, M.; Quetglas, A.; Ordines, F.; Moranta, J. Feeding ecology of demersal elasmobranchs from the shelf and slope off the Balearic Sea (western Mediterranean). Sci. Mar. 2011, 75, 633–639. [Google Scholar] [CrossRef]
- Bachiller, E.; Giménez, J.; Albo-Puigserver, M.; Pennino, M.G.; Marí-Mena, N.; Esteban, A.; Lloret-Lloret, E.; Bellido, J.M.; Coll, M. Trophic niche overlap between round sardinella (Sardinella aurita) and sympatric pelagic fish species in the Western Mediterranean. Ecol. Evol. 2021, 11, 16126–16142. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Gonçalves Neto, J.B.; García-Romero, C.; Domínguez-Bustos, Á.R.; Cabrera-Castro, R. Feeding habits of two shark species: Velvet belly, Etmopterus spinax (Linnaeus, 1758) and blackmouth catshark, Galeus melastomus (Rafinesque, 1810), present in fishing discards in the Gulf of Cádiz. Environ. Biol. Fishes 2024, 107, 159–172. [Google Scholar] [CrossRef]
- Pennino, M.G.; Coll, M.; Albo-Puigserver, M.; Fernández-Corredor, E.; Steenbeek, J.; Giráldez, A.; González, M.; Esteban, A.; Bellido, J.M. Current and future influence of environmental factors on small pelagic fish distributions in the Northwestern Mediterranean Sea. Front. Mar. Sci. 2020, 7, 622. [Google Scholar] [CrossRef]


| Age | n | % | Mean TL (cm) | Standard Deviation | TL Range (cm) | Mean Size Increment (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D. punctatus | D. labrax | D. punctatus | D. labrax | D. punctatus | D. labrax | D. punctatus | D. labrax | D. punctatus | D. labrax | D. punctatus | D. labrax | |
| 0+ | 91 | 7 | 16.46 | 6.48 | 11.12 | 14.94 | 2.94 | 1.78 | 4.1–16.9 | 11.4–16.4 | - | - |
| 1+ | 186 | 39 | 33.63 | 36.11 | 15.82 | 19.60 | 2.17 | 4.12 | 9.9–22.7 | 14.4–29.3 | 4.70 | 4.66 |
| 2+ | 252 | 43 | 45.57 | 39.81 | 19.05 | 24.73 | 1.89 | 4.97 | 13.8–25.0 | 15.5–37.9 | 3.23 | 5.13 |
| 3+ | 18 | 16 | 3.25 | 14.81 | 24.46 | 28.97 | 2.45 | 4.04 | 20.5–28.3 | 22.0–37.9 | 5.41 | 4.24 |
| 4+ | 6 | 3 | 1.08 | 2.78 | 28.87 | 37.90 | 3.48 | 3.98 | 24.1–31.8 | 35.5–42.5 | 4.41 | 8.93 |
| Dicentrarchus punctatus | Dicentrarchus labrax | |||||||
|---|---|---|---|---|---|---|---|---|
| Taxa | %F | %Cn | %Cw | %IRI | %F | %Cn | %Cw | %IRI |
| ANNELIDA | ||||||||
| Polychaeta | ||||||||
| Polychaeta unid. | 0.70 | 0.01 | 0.01 | <0.01 | ||||
| CHELICERATA | ||||||||
| Pycnogonida | ||||||||
| Pycnogonida unid. | 0.70 | 0.04 | 0.00 | <0.01 | ||||
| CRUSTACEA | ||||||||
| Amphipoda | ||||||||
| Amphipoda unid. | 31.47 | 1.73 | 0.29 | 0.54 | 9.64 | 6.98 | 0.08 | 2.66 |
| Cumacea | ||||||||
| Cumacea unid. | 2.10 | 4.39 | 0.02 | 0.08 | 1.20 | 1.75 | 0.001 | 0.08 |
| Decapoda | ||||||||
| Brachyura | ||||||||
| Carcinus maenas | 0.70 | 0.01 | 0.03 | <0.01 | 3.61 | 0.62 | 2.59 | 0.45 |
| Liocarcinus holsatus | 1.20 | 0.12 | 1.15 | 0.06 | ||||
| Majidae unid. | 1.20 | 0.12 | 0.02 | 0.01 | ||||
| Pachygrapsus marmortatus | 10.84 | 1.37 | 8.12 | 4.03 | ||||
| Portumnus latipes | 3.61 | 1.00 | 4.94 | 0.84 | ||||
| Portunidae unid. | 1.20 | 0.12 | 0.62 | 0.04 | ||||
| Brachyura unid. | 2.10 | 0.04 | 0.02 | <0.01 | 13.25 | 1.87 | 5.34 | 3.74 |
| Caridea | ||||||||
| Alpheus sp. | 2.10 | 0.04 | 0.13 | <0.01 | ||||
| Crangon crangon | 7.69 | 0.25 | 1.14 | 0.09 | ||||
| Palaemon elegans | 6.02 | 1.00 | 2.29 | 0.78 | ||||
| Palaemon sp. | 0.70 | 0.02 | 0.17 | <0.01 | ||||
| Caridea unid. | 5.59 | 0.13 | 0.22 | 0.02 | 3.61 | 1.12 | 0.18 | 0.18 |
| Gebiidea | ||||||||
| Upogebia sp. | 0.70 | 0.01 | 0.13 | <0.01 | ||||
| Decapoda unid. | 4.20 | 0.13 | 0.20 | 0.01 | 2.41 | 0.37 | 0.47 | 0.08 |
| Isopoda | ||||||||
| Isopoda unid. | 44.06 | 2.98 | 0.95 | 1.48 | 8.43 | 1.87 | 0.22 | 0.69 |
| Mysidacea | ||||||||
| Mysidacea unid. | 93.71 | 74.22 | 8.65 | 66.57 | 16.87 | 57.98 | 0.48 | 38.57 |
| Other Crustacea | 5.59 | 0.10 | 0.01 | 0.01 | 1.20 | 0.12 | 0.001 | 0.01 |
| INSECTA | ||||||||
| Formicidae | 4.20 | 1.80 | 0.003 | 0.06 | ||||
| Other Insecta | 1.40 | 0.02 | 0.00001 | <0.01 | 1.20 | 4.74 | 0.00004 | 0.22 |
| MOLLUSCA | ||||||||
| Bivalvia | ||||||||
| Bivalvia unid. | 1.20 | 0.25 | 0.23 | 0.02 | ||||
| Cephalopoda | ||||||||
| Cephalopoda unid. | 1.40 | 0.02 | 0.24 | <0.01 | ||||
| Gastropoda | ||||||||
| Patellidae | 1.20 | 0.75 | 1.08 | 0.09 | ||||
| OSTEICHTYES | ||||||||
| Atherinidae | ||||||||
| Atherinidae unid. | 13.25 | 2.24 | 41.32 | 22.58 | ||||
| Bleniidae | ||||||||
| Lipophrys trigloides | 1.20 | 0.25 | 4.73 | 0.23 | ||||
| Bleniidae unid. | 1.20 | 0.12 | 1.98 | 0.10 | ||||
| Clupeiformes | ||||||||
| Engraulis encrasicolus | 10.49 | 0.98 | 11.46 | 1.12 | ||||
| Sardina pilchardus | 14.69 | 3.93 | 25.54 | 3.71 | 2.41 | 4.49 | 1.58 | 0.57 |
| Clupeiformes unid. | 51.05 | 6.61 | 23.17 | 13.03 | 4.82 | 3.62 | 2.47 | 1.15 |
| Gobiidae | ||||||||
| Gobiidae unid. | 0.70 | 0.01 | 0.37 | <0.01 | 6.02 | 1.25 | 11.46 | 2.99 |
| Pomatomidae | ||||||||
| Pomatomus saltatrix | 1.40 | 0.05 | 0.24 | <0.01 | ||||
| Sparidae | ||||||||
| Sparidae unid. | 2.10 | 0.05 | 1.22 | 0.02 | ||||
| Unidentified Fish | 55.24 | 2.43 | 25.52 | 13.24 | 34.94 | 5.86 | 8.64 | 19.82 |
| ALGAE | ||||||||
| Algae unid. | 1.20 | 0.12 | 0.01 | 0.01 | ||||
| Source | df | Sum of Squares | Mean Squares | Pseudo-F | p-Value | Permutations |
|---|---|---|---|---|---|---|
| Moon Phase | 1 | 1.552 | 1.552 | 8.204 | *** | 9999 |
| Season | 3 | 3.701 | 1.234 | 6.519 | *** | |
| Time of the day | 1 | 0.112 | 0.112 | 0.591 | - | |
| Size Range | 4 | 1.533 | 0.383 | 2.026 | * | |
| Moon Phase × Season | 3 | 1.009 | 0.336 | 1.777 | - | |
| Moon Phase × Time of the day | 1 | 0.432 | 0.432 | 2.282 | - | |
| Moon Phase × Size range | 4 | 1.213 | 0.303 | 1.603 | - | |
| Season × Time of the day | 3 | 1.139 | 0.380 | 2.007 | - | |
| Season × Size range | 10 | 1.450 | 0.145 | 0.767 | - | |
| Time of the day × Size range | 4 | 0.577 | 0.144 | 0.762 | - | |
| Residual | 302 | 57.143 | ||||
| Total | 336 | 69.962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-García, C.; Toro-Podadera, A.; Sarmiento-Carbajal, J.; Cabrera-Castro, R. Coexisting in the Surf Zone: Age and Feeding Habits of the Spotted Seabass (Dicentrarchus punctatus) and European Seabass (Dicentrarchus labrax) on the Gulf of Cádiz Beaches (Southwest Iberian Peninsula). Fishes 2024, 9, 173. https://doi.org/10.3390/fishes9050173
Rodríguez-García C, Toro-Podadera A, Sarmiento-Carbajal J, Cabrera-Castro R. Coexisting in the Surf Zone: Age and Feeding Habits of the Spotted Seabass (Dicentrarchus punctatus) and European Seabass (Dicentrarchus labrax) on the Gulf of Cádiz Beaches (Southwest Iberian Peninsula). Fishes. 2024; 9(5):173. https://doi.org/10.3390/fishes9050173
Chicago/Turabian StyleRodríguez-García, Carlos, Ana Toro-Podadera, Jesica Sarmiento-Carbajal, and Remedios Cabrera-Castro. 2024. "Coexisting in the Surf Zone: Age and Feeding Habits of the Spotted Seabass (Dicentrarchus punctatus) and European Seabass (Dicentrarchus labrax) on the Gulf of Cádiz Beaches (Southwest Iberian Peninsula)" Fishes 9, no. 5: 173. https://doi.org/10.3390/fishes9050173
APA StyleRodríguez-García, C., Toro-Podadera, A., Sarmiento-Carbajal, J., & Cabrera-Castro, R. (2024). Coexisting in the Surf Zone: Age and Feeding Habits of the Spotted Seabass (Dicentrarchus punctatus) and European Seabass (Dicentrarchus labrax) on the Gulf of Cádiz Beaches (Southwest Iberian Peninsula). Fishes, 9(5), 173. https://doi.org/10.3390/fishes9050173






