Abstract
Slimy sculpin (Uranidea cognata) inhabit coldwater streams in southeastern Minnesota, USA, many of which were subjected to probable 2000-year flood events in August 2007. Floods scoured streambeds, created new stream channels, and greatly reduced benthic invertebrate communities that serve as the primary food resource for sculpin. Diets and Fulton condition of sculpin in Gilmore Creek (with moderate flooding) and Garvin Brook (with very severe flooding) had been examined just prior to flooding and were re-examined 2 weeks after flooding to assess possible diet and condition changes. Diets, body condition, and reproductive fitness of sculpin were examined 7 months post-flood in these same two streams, plus nearby Trout Run (which also experienced very severe flooding). Sculpin condition declined slightly post-flood in Garvin Brook but improved in Gilmore Creek. Prior to spring spawning, the condition of Garvin Brook sculpin had improved, but Gilmore Creek fish condition had worsened. Sculpin diets were more diverse before and after flooding in Gilmore Creek than in Garvin Brook, although the diets of fish from both streams were dominated (>55%) by midge (Diptera: Chironomidae) larvae. Diets remained largely unchanged before versus after flooding in the more severely flooded Garvin Brook, but they changed in Gilmore Creek, becoming more midge-dominated. Prey number per sculpin stomach declined post-flood in Gilmore Creek but not in Garvin Brook, although the dry mass of prey/fish wet mass declined post-flood in both streams. Pre-spawn sculpin displayed no patterns in reproductive fitness (gonadosomatic index, hepatosomatic index, oocyte number) among the three streams that may have been related to flooding severity the previous summer. Sculpin diets and condition were not altered as expected by flooding, and food resource recovery apparently was rapid enough to prevent longer-term impacts on sculpin condition and reproductive fitness in the streams examined.
Key Contribution:
Slimy sculpin did not display consistent patterns in diets, condition, or reproductive fitness in response to differing flood severity in several small streams.
1. Introduction
River or stream flooding (i.e., out-of-banks flow) is a common phenomenon worldwide, brought on naturally by heavy rainfall or extensive snowmelt [1] or artificially by managed releases from reservoirs [2]. Flooding can occur in a wide range of systems, from tropical rivers [3] to desert streams [4], from high mountain streams [5] to lowland coastal rivers [3,6,7], and from high-gradient low-order headwaters [5,8,9] to high-order wide-floodplain systems [10,11]. Some rivers and streams are prone to frequent, recurring flooding [5,8], whereas others rarely experience floods. Floods also exhibit extreme variability in both magnitude (total discharge and/or elevation change relative to baseflow) and duration (time spent out-of-banks) [1,12].
Floods can dramatically alter the environments of both rivers [13] and their riparia [14], impacting both physical and biological components of aquatic habitats and their adjoining terrestrial corridors [3,8,9,15]. These effects can be either negative or positive, depending on flood variables, location within the stream or river system [11,16], and the physical or biological stream/riparian characteristic being examined [12,17,18]. From a human perspective, small floods may have neutral to positive effects on many of the ecosystem services (the benefits humans obtain from the natural functioning of the system) provided by a river or stream, but extreme floods cause losses in virtually every ecosystem service [19].
Extreme (also termed catastrophic or historic) floods can have truly devastating impacts on river and stream environments [1,5,9,16]. These once-in-100-year to once-in-2000-year events wreak havoc on instream and riparian physical features [3,9] and may have long-lasting, negative effects on aquatic biota [1,5,9]. However, some biota may be resistant and/or resilient to even these drastic floods, demonstrating amazing abilities to survive scouring flows and returning to pre-flood abundances only weeks or months after floodwaters subside [5,8,9].
Although many aquatic organisms can recover quickly from extreme floods, others may not. For example, species that are poor recolonizers, those that inhabit vulnerable habitats, or those that are in more sensitive life stages at the time of flooding are likely to be severely impacted by extreme floods [8]. Such species may include benthic fishes like sculpins (Cottidae) and longnose dace (Rhinichthys cataractae), which are considered poor recolonizers [8,20] and may be vulnerable to crushing by bedload movement of their cobble/boulder habitats during catastrophic flooding [8,21]. Young-of-year or juvenile life stages of these and other species may also be at greater risk than larger or adult fish during flood events due to both small size and poor swimming ability [22].
Sculpin are common fish of marine and freshwater systems, especially in circumpolar regions of the Northern Hemisphere [23,24]. Many species inhabit cold and coolwater rivers and streams, where they can numerically dominate fish communities [20,25]. The slimy sculpin (Uranidea cognatus), with the widest geographical distribution of any sculpin species in North America [26,27], has been suggested as an excellent species for environmental effects monitoring due to its small size, limited mobility, early maturity, fast growth rate, high reproductive output, and high abundance [20,28,29].
A once-in-2000-year flood impacted many coldwater streams and rivers across southeastern Minnesota in August 2007 [9,30], scouring stream habitats and reducing aquatic macroinvertebrate densities by up to 95% [9]. Post-flood surveys on several regional streams indicated that salmonids (brook trout, Salvelinus fontinalis, and brown trout, Salmo trutta) and slimy sculpin had survived the floods, although numbers were greatly reduced (N. Mundahl, unpublished data). However, there was concern that reduced aquatic prey resources might result in poorer fish condition, which ultimately could impact subsequent spawning success. Consequently, the current study was undertaken to assess the impact of flooding on the autumn diets and body condition of slimy sculpin in two area streams that differed in severity of flooding and where, fortuitously, sculpin had been collected 1 to 8 weeks prior to flooding for examination of fish condition and diets. It was hypothesized that sculpin would exhibit poorer condition and reduced diets (reduced prey and reduced prey biomass) post- versus pre-flood in both streams, with reductions greater in the stream with more severe flooding. In addition, sculpin in these two streams, plus one additional stream that also had experienced catastrophic flooding, were examined the following spring (7 months post-flood) just prior to spawning to assess diets, fish condition, and reproductive fitness. It was expected that sculpin in the two streams that had the worst flooding the previous summer would display poorer diets and condition and reduced reproductive fitness relative to sculpin in the stream that had less severe flooding.
2. Study Sites and Flooding
Gilmore Creek, Garvin Brook, and Trout Run are first- to second-order coldwater trout streams in southeastern Minnesota, USA (Figure 1). They lie in adjacent but separate watersheds, each draining to the Mississippi River. All three streams contain similar fish communities comprised of native brook trout, slimy sculpin, and non-native brown trout.
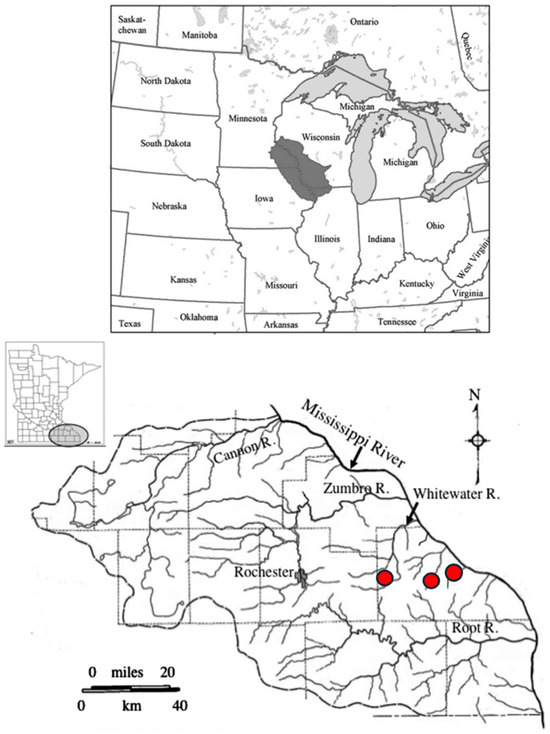
Figure 1.
Maps indicating location of the study area within the upper midwestern USA (upper: Driftless Area Ecoregion highlighted in gray) and locations of the three study sites (lower: sites indicated by red dots) within the stream network of southeastern Minnesota (inset at middle left highlights southeastern Minnesota). Sites (left to right) were located on Trout Run, Garvin Brook, and Gilmore Creek.
During a 48 h period in August 2007, the southeastern Minnesota region experienced a historic precipitation event that produced 30 to 43 cm of rainfall across a wide area. This rain event produced variable but substantial flooding on many regional streams and rivers, resulting in loss of human life and widespread damage to homes, businesses, roadways, bridges, farmlands, and parks. Numerous hillslope rockslides delivered materials to valley floors, and floodwaters cut new stream channels and filled old channels with rocks and debris. Whitewater State Park (containing Trout Run) and Farmers Community Park (Garvin Brook) were closed to the public for many months to repair damaged and destroyed bridges and other infrastructure. Both Garvin Brook and Trout Run exhibited severe channel scour, new channel formation, and large instream and riparian deposits of cobbles and boulders. In contrast, flooding in Gilmore Creek was less severe, with much less channel scour, no new channels, and no major damage to bridges or roadways. Gilmore Creek flows returned within banks only a few hours after cresting > 3 m above normal water level [31]. None of these streams have gauging stations to monitor stream elevations or discharge.
3. Methods and Materials
3.1. Field Work
One to eight weeks prior to and two weeks after flooding, slimy sculpin were collected from Gilmore Creek and Garvin Brook with a backpack electrofisher. After assessing that sculpin populations were adequate to support collections, a small, representative sample of fish from each stream was anesthetized and euthanized in a solution of tricaine methanesulfonate (MS-222; 300–400 ppm), fixed in 7% formalin, and preserved in 70% ethanol. These fish were used to assess general fish condition and the composition of their diets. Seven months post-flood (during early March 2008), just prior to the spring spawning season of slimy sculpin in the region, additional sculpin were collected from Gilmore Creek and Garvin Brook and also from nearby Trout Run (after first assessing for adequate abundance). Fish were again euthanized and preserved as described previously. As with earlier collections, these fish were used to assess sculpin condition and diets, as well as to determine their reproductive fitness.
3.2. Laboratory Work
In the laboratory, sculpin were processed to gather data on size, body condition, diet, and reproductive condition. Individual fish were first towel dried, then weighed (nearest 0.01 g wet mass) and measured (mm total length (TL)). Masses and TLs were used to calculate a Fulton condition factor (K, where K = (mass/TL3) × 100,000) for each fish [32,33]. Fish were dissected, and the entire stomach was removed, cut open, and the contents washed into a watch glass. Prey items were identified (primarily to family or order) [34,35] and counted with the aid of a dissecting microscope (8 to 50× magnification). For each fish, all prey items were placed into a pre-weighed aluminum pan, dried at room temperature under a constant-flow fume hood, then weighed (nearest 0.0001 g) to determine the total dry mass of all prey consumed. This mass was divided by the fish’s total wet mass to calculate a standardized prey mass (mg dry mass of prey/fish total wet mass).
Fish collected during spring (March) were examined further to assess reproductive fitness immediately prior to spawning. During dissections, the entire liver was removed and weighed (nearest 0.0001 g wet mass), and a hepatosomatic index (HSI, where HSI = (mg liver/mg total fish mass) × 100) was calculated as an assessment of fish health and reproductive status [33]. In addition, both gonads (either ovaries or testes) were removed and weighed (nearest 0.0001 g wet mass), and a gonadosomatic index (GSI, where GSI = (mg gonads/mg total fish mass) × 100) was calculated to assess reproductive condition [36]. After weighing ovaries, the total oocytes in one ovary were counted by placing the ovary in a watch glass, splitting open the ovary membrane, and counting oocytes with the aid of a dissecting microscope. Average oocyte mass was calculated by dividing the combined weights of both ovaries by the estimated total oocyte count (count in one ovary × 2).
3.3. Data Analyses
Sculpin size data were compared between/among streams in two ways. First, log10-transformed lengths and weights from all three sampling periods were combined and compared among streams (single-factor ANOVA, Tukey’s HSD test) to examine possible sculpin size differences among streams [37]. Second, length–frequency distributions (10-mm size classes) were compared between/among streams for each sampling period using contingency table Chi-square tests to determine whether sculpin size distributions differed within each time period [37].
Diets of slimy sculpin were compared before and after flooding using several different variables. First, after NMDS ordination and k-means clustering (JMP Pro 17.0 statistical software) failed to detect differences in the diets of individual sculpin among time periods for either Garvin Brook or Gilmore Creek (85% and 77% of the diets of individual sculpin could not be statistically separated, respectively), the diets of all fish from a specific stream and date were combined to form a composite diet sample. Then, a percent similarity index [38] was calculated separately for each of three pairwise comparisons of these composite diet samples (before flooding versus after flooding, before flooding versus spring, after flooding versus spring) for each stream. Percent similarity index values range from 0.0 (no similarity) to 1.0 (complete similarity) [38]. Diets were not compared between streams because sculpin diets typically vary among streams within this region under normal conditions [39]. Diets were also compared among dates and streams using repeated measures two-factor analysis of variance (ANOVA) with replication for number of prey taxa per stomach, numbers of prey per fish stomach, and standardized prey weights.
The general body condition of slimy sculpin was examined relative to flooding in two ways. Length–weight relationships (linear least-squares regression on log-transformed data) of sculpin for each stream–time period combination were compared with analysis of covariance (ANCOVA) [40] in three instances: (1) before versus after flooding in Garvin Brook, (2) before versus after flooding in Gilmore Creek, and (3) among all three streams prior to spring spawning. Fulton condition of fish in Gilmore Creek and Garvin Brook were assessed separately across the three sampling periods with single-factor ANOVAs and Tukey’s HSD tests to determine whether condition changed relative to flooding.
During spring, a series of single-factor ANOVAs and Tukey’s HSD tests were used to compare fish lengths, Fulton condition, and reproductive fitness (GSIs, HSIs, oocyte numbers, and oocyte wet mass) among fish from the three different streams. Separate comparisons were made for male and female fish. Prior to analyses, data were log10-transformed to meet the normality assumptions of ANOVA.
4. Results
4.1. Sculpin Size and Body Condition
Slimy sculpin collected from three coldwater trout streams in Minnesota ranged in size from 25 to 101 mm TL and 0.1 to 18.0 g wet mass (Table 1). Neither sculpin length nor mass differed significantly among the three streams. Size distributions of sculpin (Figure 2) did not differ between/among streams after flooding (contingency table Chi-square = 11.9, df = 8, p = 0.156) or just prior to spawning the following spring (contingency table Chi-square = 17.4, df = 14, p = 0.238). However, size distributions of sculpin examined before flooding differed significantly (contingency table Chi-square = 19.2, df = 7, p = 0.008), with more small young-of-year sculpin and fewer adult sculpin in Gilmore Creek compared to Garvin Brook.

Table 1.
Size ranges of slimy sculpin collected from three streams in southeastern Minnesota before and after catastrophic flooding in 2007 and 2008. Single-factor ANOVA test statistics comparing log-transformed length and mass among streams are included.
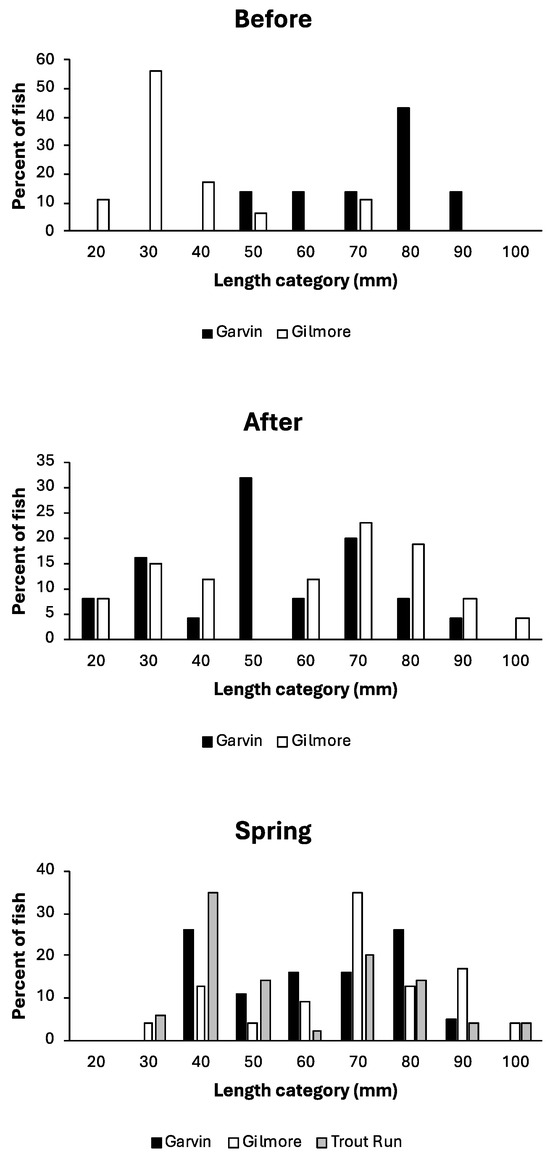
Figure 2.
Length–frequency distributions of slimy sculpin in southeastern Minnesota streams before and after August 2007 flooding and prior to 2008 spring spawning.
All sculpin length–weight relationships for each stream–time period combination were highly significant (p < 0.001), with correlation coefficients (r2) all >0.95. When these relationships were compared before versus after the flood with ANCOVA, sculpin in Gilmore Creek after the flood had significantly (p = 0.004) greater weights at a given length than they had before the flood, whereas sculpin in Garvin Brook were slightly, but not significantly (p = 0.071), heavier before than after the flood. ANCOVA of length–weight relationships prior to spring spawning found that weights at length differed significantly among the three streams for both female (p < 0.001) and male (p = 0.002) sculpin, with those in Garvin Brook being the heaviest and those in Gilmore Creek being the lightest.
There were significant differences in sculpin Fulton condition among time periods within both Gilmore Creek (ANOVA F2,63 = 11.67, p < 0.0001) and Garvin Brook (ANOVA F2,54 = 9.29, p = 0.0004), but the pattern of change differed between streams (Figure 3). In Gilmore Creek, sculpin were in significantly better condition immediately after flooding compared with before flooding or prior to spring spawning (based on Tukey’s HSD). In contrast, sculpin in Garvin Brook were in significantly better condition before flooding and prior to spawning than they were immediately after flooding (based on Tukey’s HSD). The condition of sculpin in Trout Run prior to spring spawning was intermediate between those of fish from the other two streams (Figure 3).
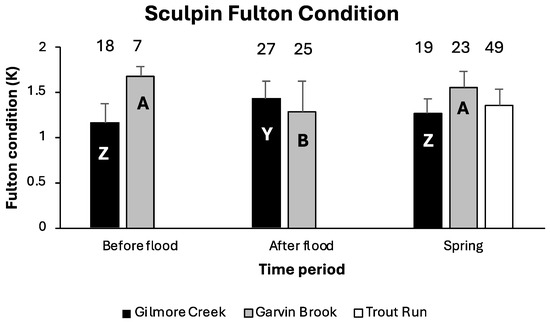
Figure 3.
Mean (+one standard deviation) Fulton condition factors of slimy sculpin in southeastern Minnesota streams before and after August 2007 flooding and prior to 2008 spring spawning. Numbers above bars are sample sizes. Within a given stream, bars with different letters are significantly different from one another (ANOVA and Tukey’s HSD).
4.2. Sculpin Diets
In total, 4069 individual prey organisms representing 19 different taxa were consumed by slimy sculpin examined from the three streams (Table 2). Midge larvae (Diptera: Chironomidae) dominated diets in all three streams (Figure 4), comprising 55 to 99% of individuals consumed in the different streams. Overall, diets were most diverse in Gilmore Creek and least diverse in Garvin Brook (Figure 4, Table 3).

Table 2.
Prey taxa included in the diets of slimy sculpins from three streams in southeastern Minnesota, 2007–2008. Unid. = unidentified.
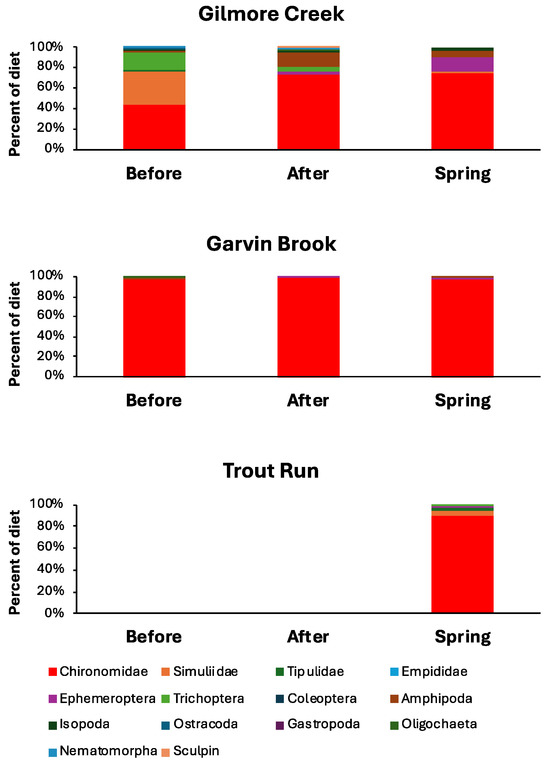
Figure 4.
Relative abundances of various prey items in the diets of slimy sculpin in three southeastern Minnesota streams before and after August 2007 flooding and prior to the 2008 spring spawning season.

Table 3.
Diet variables based on invertebrate prey organisms removed from stomachs of slimy sculpin collected from three streams in southeastern Minnesota before and after August 2007 flooding and prior to 2008 spring spawning. Taxa/fish values are means with standard deviations in parentheses. Shannon diversity values are based on the combined diets of all fish examined.
Before and immediately after the flood, sculpin diets in Gilmore Creek contained 13 total taxa, whereas the diets of Garvin Brook sculpin contained only eight total taxa. Seven taxa in Gilmore Creek but only one taxon in Garvin Brook were consumed by sculpin both before and after flooding. Sculpin stomachs generally contained one to three different invertebrate prey taxa, regardless of the time period, with low proportions of fish with empty stomachs observed only post-flood (Table 3). Taxa per stomach differed significantly both between streams (F1,113 = 17.51, p < 0.001) and among time periods (F2,113 = 9.91, p < 0.001), with a pattern of change similar in both streams (time period X stream interaction: F2,113 = 0.94, p = 0.394). Taxa per sculpin stomach were significantly higher in Gilmore Creek than in Garvin Brook, with the number of taxa declining significantly (based on Tukey’s HSD test) post-flood in both streams before recovering by spring in Gilmore but not Garvin (Table 3).
Diets of Garvin Brook sculpin before and after flooding were very similar (percent similarity index = 0.968), whereas those of Gilmore Creek sculpin were less similar (percent similarity index = 0.532) before versus after flooding. Additional diet percent similarity comparisons between time periods suggest that the diets of Garvin Brook sculpin remained very consistent throughout the study (before versus spring = 0.982, after versus spring = 0.972), whereas diets of Gilmore Creek sculpin were more variable (before versus spring = 0.505, after versus spring = 0.847).
The total number and dry mass of prey contained in sculpin stomachs both displayed significant variation (Figure 5). Prey numbers varied both between streams (two-factor ANOVA: between streams F1,113 = 33.54, p < 0.0001) and among sampling dates (among dates F2,113 = 4.66, p = 0.011), although the pattern of change varied between streams (stream × date interaction: F2,113 = 11.94, p < 0.0001). The number of prey in stomachs decreased immediately after flooding for Gilmore Creek sculpin before increasing again in spring, but prey numbers increased after flooding for Garvin Brook sculpin before decreasing again in spring (Figure 5a). Prey numbers consumed by Garvin Brook sculpin post-flood were significantly higher (p < 0.01, Tukey’s HSD test) than those for all other date–stream combinations. Dry masses of prey in stomachs were significantly higher in Gilmore Creek than in Garvin Brook (two-factor ANOVA: between streams F1,94 = 4.69, p = 0.033), and sculpin in both streams displayed significant declines in prey dry mass post-flood, which carried through into spring (among dates F2,94 = 12.69, p < 0.0001) (Figure 5b). The pattern of change in prey dry mass was consistent between streams (stream × date interaction: F2,94 = 0.40, p = 0.672).
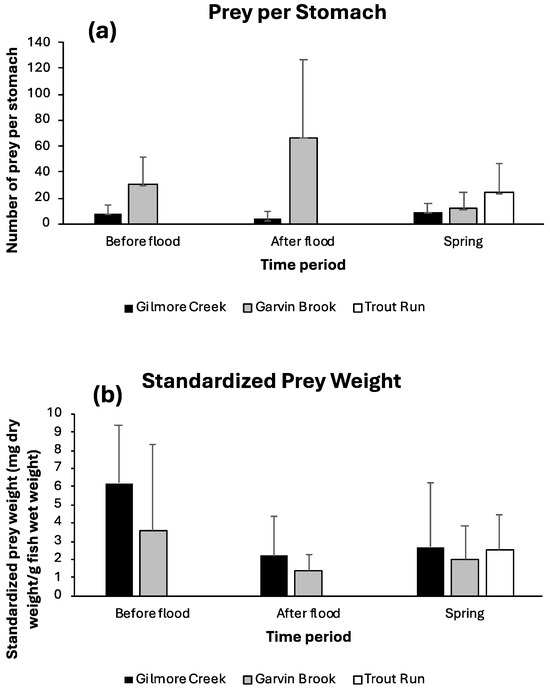
Figure 5.
Mean (+one standard deviation) prey abundance per stomach (a) and standardized dry prey mass per fish (b) for slimy sculpin in southeastern Minnesota streams before and after August 2007 flooding and prior to 2008 spring spawning.
4.3. Sculpin Size, Condition, and Reproductive Variables during Spring Pre-Pawn
The sizes of reproductive-age slimy sculpin did not differ among the three streams examined just prior to spring spawning (Table 4). There were significant differences for Fulton condition among the creeks for each sex, with lower mean values for Gilmore Creek. HSIs, GSIs, and oocyte numbers did not differ significantly among the streams. However, mean oocyte wet masses differed among all three streams, being the highest in Trout Run and the lowest in Gilmore Creek (Table 4).

Table 4.
Size, condition, and reproductive variables of female and male slimy sculpin from three streams in southeastern Minnesota, March 2008. Values are means, with standard deviations in parentheses. ANOVA test statistics (F and p values) are included for each variable–sex combination. Within a row, values not sharing the same letter are significantly different (single-factor ANOVA, Tukey’s HSD). F = female, M = male, n = sample size, K = Fulton condition, GSI = gonadosomatic index, HSI = hepatosomatic index.
5. Discussion
Flooding during August 2007 had severe effects on streams, rivers, and the surrounding landscape throughout southeastern Minnesota and west–central Wisconsin. Damage to human structures was extensive (272 million USD in losses) across a broad geographic area, and flooding resulted in 10 human fatalities. Streams and rivers crested many meters above flood stage [31], flowing with enough power to destroy bridges and roadways, lift houses off their foundations, sweep cars off roads, and obliterate agricultural crops [41]. Rock and mud landslides blocked some streams, forcing the creation of new channels. In the aftermath of such devastation, concern eventually shifted from anthropogenic losses to the status of fish that had inhabited these streams prior to flooding [30].
The impacts of extreme flooding on lotic fish communities can be highly variable and complicated to understand or explain [1]. Although juvenile or small-bodied fishes may be highly susceptible to heavy losses during extreme floods in some systems [1], in other systems, this same size group of fish may be unaffected or actually increase in abundance post-flood [16]. Habitat complexity may play a crucial role in reducing flood impacts on fish [8]. In addition, many fish species display remarkable resistance and resilience to even extreme flooding [2,5,8,16], depending on both regular seasonal and periodic catastrophic floods to clean spawning substrates, deepen channels and pools, reduce non-native fish abundance, and reset food webs [1,5,8,16,42]. Long-term exposure of many fish species to the variable abiotic environments of lotic systems has allowed them to evolve and/or adapt unique sets of characteristics (e.g., morphological, physiological, behavioral) that allow them to better cope with flood conditions [1].
Despite the catastrophic flooding, surveys after the flooding in southeastern Minnesota indicated that many native brook trout and introduced brown trout had survived [30]. Most juvenile trout (5–8 cm total length) that had hatched out 5 months prior to the floods were lost, but all sizes of older fish remained in the streams, although in reduced numbers [30]. The resilience of those survivors was impressive, as they spawned 2 to 3 months after flooding and produced a record hatch of young trout during spring 2008 [30]. The same positive, post-flood response to late summer flooding has been observed previously for brown trout, attributed to improved fall spawning conditions (larger and higher-quality gravel beds) and enhanced environments for incubating trout eggs and, later, emerging fry (reduced fine sediments within spawning gravels) [5].
The presence of slimy sculpin in all streams after catastrophic flooding was encouraging, especially since small benthic species like sculpins are considered highly vulnerable to such destructive events [8,21,22]. Powerful flooding in Garvin Brook and Trout Run produced severe channel scouring and displacement of boulders > 40 cm in diameter, simultaneously destroying sculpin habitat and food resources. The presence of sculpin in Gilmore Creek and Garvin Brook only 2 weeks post-flood suggests that either fish were able to find refuges [5,8] that enabled them to survive mass bedload rearrangement, or they were able to quickly recolonize stream reaches from nearby, less-impacted systems [43,44]. Although slimy sculpin are often considered sedentary and unlikely to exhibit more than small-scale movements [29], there is increasing evidence that they are capable of both rapid and long-range movements, especially when recolonizing defaunated stream reaches [43,44,45]. An apparent lack of less-impacted reaches in Garvin Brook (severe flooding affected the entire stream, extending from headwater springs downstream to the confluence with the Mississippi River) suggests that survival in refuges was most likely, especially since even young-of-year sculpin were large enough by August to significantly reduce their vulnerability to elevated current velocities [5,22]. Slimy sculpin also have the ability to squeeze into extremely tight spaces by compressing their skull widths by up to 20% [46], which would permit them to retreat into very small openings beneath large boulders or into cracks between layers of sedimentary limestone bedrock that are common in regional streams [47], allowing them to wait out catastrophic flows in protected spaces. A study conducted in Gilmore Creek reported that slimy sculpin were more abundant a short time after the flood than they were prior to flooding [31].
Based on examination of slimy sculpin body condition and diets before and after catastrophic flooding, it was apparent that differing severities of flooding did not impact these variables exactly as expected. Based on a percent similarity index, pre- and post-flood diets did not differ in Garvin Brook, where flooding was most severe (likely due to the heavy reliance on midge larvae during all time periods examined), but they differed more in Gilmore Creek, where flooding was less intense. However, sculpin in both streams consumed lower prey biomass after the flood, even though fish in Garvin Brook increased the number of prey consumed. The condition of sculpin declined post-flood in Garvin Brook as expected, but sculpin condition improved after flooding in Gilmore Creek. In the spring, prior to spawning, sculpin condition had improved in Garvin Brook but declined in Gilmore Creek. Just prior to spawning, sculpin from the two most severely flood-impacted streams were in better condition than those in the less-impacted stream, but no measure of reproductive fitness other than oocyte mass differed among streams.
Reduced prey biomass in sculpin stomachs after flooding suggests that prey availability was limited [9]. The dominance of sculpin diets by Chironomidae (midge) larvae was not surprising, as these organisms were the most abundant aquatic invertebrates after the flood and comprised a large proportion of the invertebrate assemblage for many months thereafter [9]. Sculpin are opportunistic feeders, taking advantage of whatever prey are available and abundant [39], so their ability to find and consume prey after a flood should be adequate. Differing pre- versus post-flood diets in Gilmore Creek, but not in Garvin Brook, and the increased numbers of prey in Garvin Brook sculpin stomachs relative to the pre-flood survey both appear counterintuitive. The reduced condition of sculpin post-flood in Garvin Brook suggests that fish may have experienced a 1–2-week period of food deprivation during and after flooding, followed by compensatory increases in feeding rate and prey consumption [48]. However, the small size of midge larvae (especially if they were recently hatched, post-flood colonizers) still resulted in reductions in ingested biomass post-flood relative to pre-flood feeding. The reduced body condition of sculpin has been reported previously when fish consumed smaller, lower-energy prey [49].
As expected, the body condition of sculpin in flood-damaged Garvin Brook declined after the flood, likely attributable to a combination of reduced prey resources and increased stress post-flood [9,32,33]. In contrast, sculpin in Gilmore Creek exhibited improved condition post-flood, despite reductions in numbers and dry mass of prey in stomachs. Condition is a responsive indicator of exposure of sculpin to a variety of stressors [29,50], so significant reductions in condition post-flood in Garvin Brook are suggestive of stressful environmental conditions in the few weeks following floods. Environmental conditions apparently were better in less-impacted Gilmore Creek since fish displayed improved condition after flooding. This result is difficult to explain from a diet perspective since Gilmore Creek sculpin contained fewer prey items in their stomachs and reduced dry-weight prey biomass after flooding compared with before. Perhaps the more diverse diet of Gilmore Creek sculpin, containing organisms considerably larger than midge larvae (especially caddisfly larvae, a variety of crustaceans, and small fish), may have provided nutrients sufficient to maintain and even improve overall sculpin body condition post-flood [49].
Prior to spring spawning, temperate zone fishes, in general, and sculpin, in particular, direct energy resources to their reproductive organs to maximize their reproductive fitness [51,52]. Prey resources often are abundant and diverse during this time period [9], although territorial behaviors among fish may result in reduced food intake in those individuals relegated to poorer habitats [51,53]. As cold-adapted species capable of feeding effectively at winter temperatures [52], sculpin should lose little of their endogenous energy reserves and maintain condition through the winter months. However, reduced food (energy) intake during winter and early spring due to reduced prey resources post-flooding [9], while reproductive organs were enlarging in preparation for spawning, could have resulted in the reduced condition factors observed for sculpin in Gilmore Creek (both males and females). It appears contradictory that the stream that experienced the least flooding (Gilmore Creek) would have sculpin with poorer body condition prior to spawning due to inadequate diet, whereas sculpin in a more severely flooded system (Garvin Brook) would exhibit significantly better body condition. Additional factors (e.g., intraspecific competition, predation threat) may have been acting on sculpin in Gilmore Creek to affect their spring body condition [31].
The lack of any significant differences in GSI or HSI indices or in oocyte numbers of sculpin among the three streams examined indicates that there were no observable effects of differing flood magnitude on sculpin reproduction 7 months post-flood. Although differing availabilities of prey in the various systems may have resulted in altered food intake and possible declines in overall fish condition, this was not translated into reduced reproductive fitness. Even under adverse environmental conditions, reproductive tradeoffs in fish are common [52]. Many fish species, apparently including sculpin, can allocate significant energy reserves toward maximizing their reproductive output, even if that allocation may compromise the post-spawning survival of some fish [52]. Such allocations toward reproduction have been reported in other fish species deprived of food prior to spawning [52].
6. Conclusions
Slimy sculpin condition (but not diets) changed immediately post-flood in the more severely flooded stream, but post-flood prey recovery was rapid enough to prevent any extended impacts on sculpin condition or reproductive fitness. Pre-spawn slimy sculpin displayed no obvious negative impacts to reproductive fitness (GSI, HSI, oocyte number) among three streams 7 months after flooding that may have been related to flooding severity the previous summer. Taken together, these observations suggest that slimy sculpin are extremely resilient to even catastrophic flooding, able to survive and reproduce after exposure to destructive flows that rearranged stream habitats, reduced prey availability, and caused numerous human fatalities and extensive loss of infrastructure.
Funding
Partial funding for this project was provided by the Minnesota Department of Natural Resources, Division of Parks and Trails.
Institutional Review Board Statement
Fish collections were carried out under special permits (Numbers 14055, 14728) from the Minnesota Department of Natural Resources, Division of Fish and Wildlife, Section of Fisheries, and were conducted with the approval of the Winona State University Animal Care and Use Committee (1310073-2). This research complied with all ethical standards.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the author upon reasonable request.
Acknowledgments
I thank the personnel of Farmers Community Park and Whitewater State Park for granting access to the parks while they were closed to the public after flooding, as well as the Winona State Biology students who assisted with fish collections.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hickey, J.T.; Salas, J.D. Environmental effects of extreme floods. Hydrometeorol. Impacts Manag. Extreme Floods 1995, 7, 13–17. [Google Scholar]
- Schultz, A.A.; Maughan, O.E.; Bonar, S.A.; Matter, W.J. Effects of flooding on abundance of native and nonnative fishes downstream from a small impoundment. N. Am. J. Fish. Manag. 2003, 23, 503–511. [Google Scholar] [CrossRef]
- Rayner, T.S.; Pusey, B.J.; Pearson, R.G. Seasonal flooding, instream habitat structure and fish assemblages in the Mulgrave River, north-east Queensland: Towards a new conceptual framework for understanding fish-habitat dynamics in small tropical rivers. Mar. Freshw. Res. 2008, 59, 97–116. [Google Scholar] [CrossRef]
- Eby, L.A.; Fagan, W.F.; Minckley, W.L. Variability and dynamics of a desert stream community. Ecol. Appl. 2003, 13, 1566–1579. [Google Scholar] [CrossRef]
- George, S.D.; Baldigo, B.P.; Smith, A.J.; Robinson, G.R. Effects of extreme floods on trout populations and fish communities in a Catskill Mountain river. Freshw. Biol. 2015, 60, 2511–2522. [Google Scholar] [CrossRef]
- Adams, S.M.; Greeley, M.S.; Law, J.M.; Noga, E.J.; Zelikoff, J.T. Application of multiple sublethal stress indicators to assess the health of fish in Pamlico Sound following extensive flooding. Estuaries 2003, 26, 1365–1382. [Google Scholar] [CrossRef]
- Blewett, D.A.; Stevens, P.W.; Carter, J. Ecological effects of river flooding on abundance and body condition of a large, euryhaline fish. Mar. Ecol. Prog. Ser. 2017, 563, 211–218. [Google Scholar] [CrossRef]
- Pearsons, T.N.; Li, H.W.; Lamberti, G.A. Influence of habitat complexity on resistance to flooding and resilience of stream fish assemblages. Trans. Am. Fish. Soc. 1992, 121, 427–436. [Google Scholar] [CrossRef]
- Mundahl, N.D.; Hunt, A.M. Recovery of stream invertebrates after catastrophic flooding in southeastern Minnesota, USA. J. Freshw. Ecol. 2011, 26, 445–457. [Google Scholar] [CrossRef]
- King, A.J.; Humphries, P.; Lake, P.S. Fish recruitment on floodplains: The roles of patterns of flooding and life history characteristics. Can. J. Fish. Aquat. Sci. 2003, 60, 773–786. [Google Scholar] [CrossRef]
- Pereira, L.S.; Tencatt, L.F.C.; Dias, R.M.; de Oliveira, A.G.; Agostinho, A.A. Effects of long and short flooding years on the feeding ecology of piscivorous fish in floodplain river systems. Hydrobiologia 2017, 795, 65–80. [Google Scholar] [CrossRef]
- Death, R.D. The effect of floods on aquatic invertebrate communities. In Aquatic Insects: Challenges to Populations; Lancaster, J., Briers, R.A., Eds.; Proceedings of the Royal Society’s 24th Symposium; CAB International: Oxfordshire, UK, 2008; pp. 103–121. [Google Scholar]
- Allan, J.D.; Castillo, M.M.; Cappas, K.A. Stream Ecology: Structure and Function of Running Waters, 3rd ed.; Springer Nature Switzerland: Cham, Switzerland, 2021. [Google Scholar]
- Naiman, R.J.; Décamps, H.; McClain, M.E. Riparia: Ecology, Conservation, and Management of Streamside Communities; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Yin, Y.; Nelson, J.C.; Swenson, G.V.; Langrehr, H.A.; Blackburn, T.A. Tree mortality in the upper Mississippi River and floodplain following an extreme flood in 1993. In Long Term Resource Monitoring Program 1993 Flood Observations (LTRMP 94-S011); National Biological Service, Environmental Management Technical Center: Onalaska, WI, USA, 1994. [Google Scholar]
- Carlson, A.K.; Fincel, M.J.; Longhenry, C.M.; Graeb, B.D.S. Effects of flooding on fishes and aquatic habitats in a Missouri River delta. J. Freshw. Ecol. 2016, 31, 271–288. [Google Scholar] [CrossRef]
- Harrell, H.L. Response of the Devil’s River (Texas) fish community to flooding. Copeia 1978, 1978, 60–68. [Google Scholar] [CrossRef]
- Piniewski, M.; Prudhomme, C.; Acreman, M.C.; Tylec, L.; Oglęcki, P.; Okruszko, T. Responses of fish and invertebrates to floods and droughts in Europe. Ecohydrology 2017, 10, e1793. [Google Scholar] [CrossRef]
- Talbot, J.J.; Bennett, E.M.; Cassell, K.; Hanes, D.M.; Minor, E.C.; Paerl, H.; Raymond, P.A.; Vargas, R.; Vidon, P.G.; Wollheim, W.; et al. The impact of flooding on aquatic ecosystem services. Biogeochemistry 2018, 141, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.A.; Cunjak, R.A. Influence of water temperature and streambed stability on the abundance and distribution of slimy sculpin (Cottus cognatus). Environ. Biol. Fishes 2006, 80, 9–22. [Google Scholar] [CrossRef]
- Erman, D.C.; Andrews, E.D.; Yoder-Williams, M. Effects of winter floods on fishes in the Sierra Nevada. Can. J. Fish. Aquat. Sci. 1988, 45, 2195–2200. [Google Scholar] [CrossRef]
- Harvey, B.C. Susceptibility of young-of-the-year fishes to downstream displacement by flooding. Trans. Am. Fish. Soc. 1987, 116, 851–855. [Google Scholar] [CrossRef]
- Berra, T.M. Freshwater Fish Distribution; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Baldigo, B.P.; Lawrence, G.B. Composition of fish communities in relation to stream acidification and habitat in the Neversink River, New York. Trans. Am. Fish. Soc. 2000, 129, 60–76. [Google Scholar] [CrossRef]
- Wallace, R.L.; McAllister, D.E.; Rankin, M. Cottus cognatus Richardson, slimy sculpin. In Atlas of North American Freshwater Fishes; Lee, D.S., Gilbert, C.R., Hocutt, C.H., Jenkins, R.E., McAllister, D.E., Stauffer, J.R., Jr., Eds.; North Carolina Biological Survey Publication #1980-12; North Carolina Museum of Natural Sciences: Raleigh, NC, USA, 1980; p. 808. [Google Scholar]
- Page, L.M.; Burr, B.M. A Field Guide to Freshwater Fishes: North America North of Mexico; Houghton Mifflin Company: Boston, MA, USA, 1991. [Google Scholar]
- Gray, M.A.; Munkittrick, K.R. An effects-based assessment of slimy sculpin (Cottus cognatus) populations in agricultural regions of northwestern New Brunswick. Water Qual. Res. J. Can. 2005, 40, 16–27. [Google Scholar] [CrossRef]
- Gray, M.A.; Curry, R.A.; Arciszewski, T.J.; Munkittrick, K.R.; Brasfield, S.M. The biology and ecology of slimy sculpin: A recipe for effective environmental monitoring. Facets 2018, 3, 103–127. [Google Scholar] [CrossRef]
- Keillor, L. Fishing after the flood. Minn. Conserv. Vol. 2010, 73, 8–17. [Google Scholar]
- Cochran, P.A.; Stagg, T.W. Response of a fish assemblage to severe flooding in Gilmore Creek, a southeastern Minnesota trout stream. J. Freshw. Ecol. 2011, 26, 77–84. [Google Scholar] [CrossRef]
- Ney, J.J. Practical use of biological statistics. In Inland Fisheries Management in North America, 2nd ed.; Kohler, C.C., Hubert, W.A., Eds.; American Fisheries Society: Bethesda, MD, USA, 1999; pp. 167–191. [Google Scholar]
- Kaller, M.D.; Green, C.C.; Haukenes, A.H. Growth and development. In Methods for Fish Biology, 2nd ed.; Midway, S.R., Hasler, C.T., Chakrabarty, P., Eds.; American Fisheries Society: Bethesda, MD, USA, 2022; pp. 347–398. [Google Scholar]
- Hilsenhoff, W.L. Aquatic Insects of Wisconsin: Generic Keys and Notes on Biology, Ecology and Distribution; Technical Bulletin No. 89; Wisconsin Department of Natural Resources: Madison, WI, USA, 1975. [Google Scholar]
- Pennak, R.W. Fresh-Water Invertebrates of the United States, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Luckenbach, J.A.; Guzmán, J.M. Reproduction. In Methods for Fish Biology, 2nd ed.; Midway, S.R., Hasler, C.T., Chakrabarty, P., Eds.; American Fisheries Society: Bethesda, MD, USA, 2022; pp. 399–448. [Google Scholar]
- Neumann, R.M.; Allen, M.S. Size structure. In Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 375–421. [Google Scholar]
- Brower, J.E.; Zar, J.H.; von Ende, C.N. Field and Laboratory Methods for General Ecology, 4th ed.; WCB McGraw-Hill: Boston, MA, USA, 1998. [Google Scholar]
- Mundahl, N.D.; Mundahl, D.E.; Merten, E.C. Success of slimy sculpin reintroductions in Minnesota trout streams: Influence of feeding and diets. Am. Midl. Nat. 2012, 168, 162–183. [Google Scholar] [CrossRef]
- Gray, M.A.; Curry, A.R.; Munkittrick, K.R. Non-lethal sampling methods for assessing environmental impacts using a small-bodied sentinel fish species. Water Qual. Res. J. Can. 2002, 37, 195–211. [Google Scholar] [CrossRef]
- Strachura, S. Six Dead as Flooding Hits SE Minnesota. Minnesota Public Radio News, 19 August 2007. Available online: https://www.mprnews.org/story/2007/08/19/flood (accessed on 5 April 2024).
- Power, M.E.; Parker, M.S.; Dietrich, W.E. Seasonal reassembly of a river food web: Floods, droughts, and impacts of fish. Ecol. Monogr. 2008, 78, 263–282. [Google Scholar] [CrossRef]
- Clarke, A.D.; Telmer, K.H.; Shrimpton, J.M. Movement patterns of fish revealed by otolith microchemistry: A comparison of putative migratory and resident species. Environ. Biol. Fish. 2015, 98, 1583–1597. [Google Scholar] [CrossRef]
- Weinstein, S.Y.; Coombs, J.A.; Nislow, K.H.; Riley, C.; Roy, A.H.; Whiteley, A.R. Evaluating the effects of barriers on slimy sculpin movement and population connectivity using novel sibship-based and traditional genetic metrics. Trans. Am. Fish. Soc. 2019, 148, 1117–1131. [Google Scholar] [CrossRef]
- Mundahl, N.D. Recovery of slimy sculpin (Uranidea cognata) after an autumn fish kill in the headwaters of a Minnesota trout stream. Water 2024, 16, 283. [Google Scholar] [CrossRef]
- Marsden, J.E.; Tobi, H. Sculpin predation on lake trout eggs in interstices: Skull compression as a novel foraging mechanism. Copeia 2014, 2014, 654–658. [Google Scholar] [CrossRef]
- Waters, T. The Streams and Rivers of Minnesota; University of Minnesota Press: Minneapolis, MN, USA, 1977. [Google Scholar]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- McGinley, E.J.; Raesley, R.L.; Seddon, W.L. The effects of embeddedness on the seasonal feeding of mottled sculpin. Am. Midl. Nat. 2013, 170, 213–228. [Google Scholar] [CrossRef]
- Hards, A.R.; Gray, M.A.; Noël, S.C.; Cunjak, R.A. Utility of condition indices as predictors of lipid content in slimy sculpin (Cottus cognatus). Diversity 2019, 11, 71. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Collins, N.C.; Cabana, G. A comparative study of sexual selection and reproductive investment in the slimy sculpin, Cottus cognatus. Oikos 1987, 51, 156–162. [Google Scholar] [CrossRef]
- Diana, J.S. Biology and Ecology of Fishes, 2nd ed.; Cooper Publishing Group: Traverse City, MI, USA, 2004. [Google Scholar]
- Petty, J.T.; Grossman, G.D. Patch selection by mottled sculpin (Pisces: Cottidae) in a southern Appalachian stream. Freshw. Biol. 1996, 35, 261–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).