Protective Effects of Roselle Aqueous Extracts against UV-Induced Damage in Zebrafish Fins
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Roselle Aqueous Extract (RAE) and UV Absorbance Assay
2.2. In Vitro Antioxidant Assay
2.3. Experimental Animals and Images
2.4. Survival Rate Analysis, UV Radiation, RAE Treatment, and Fin Morphology Recording
2.5. Real-Time PCR
2.6. Statistical Analysis
3. Results
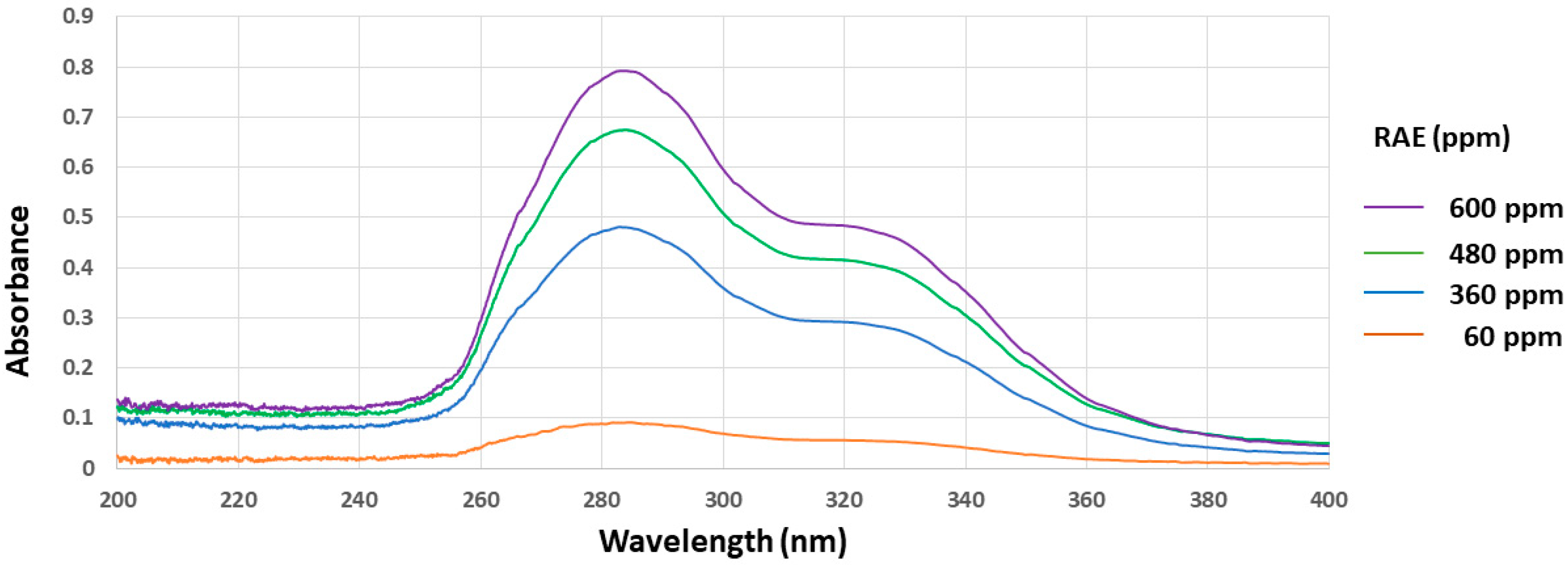
3.1. In Vitro Free-Radical Scavenging Activities and UV Absorbance of Roselle Aqueous Extract (RAE)
3.2. Survival Rate Analysis of RAE
3.3. Effects of RAE on Fin Repair
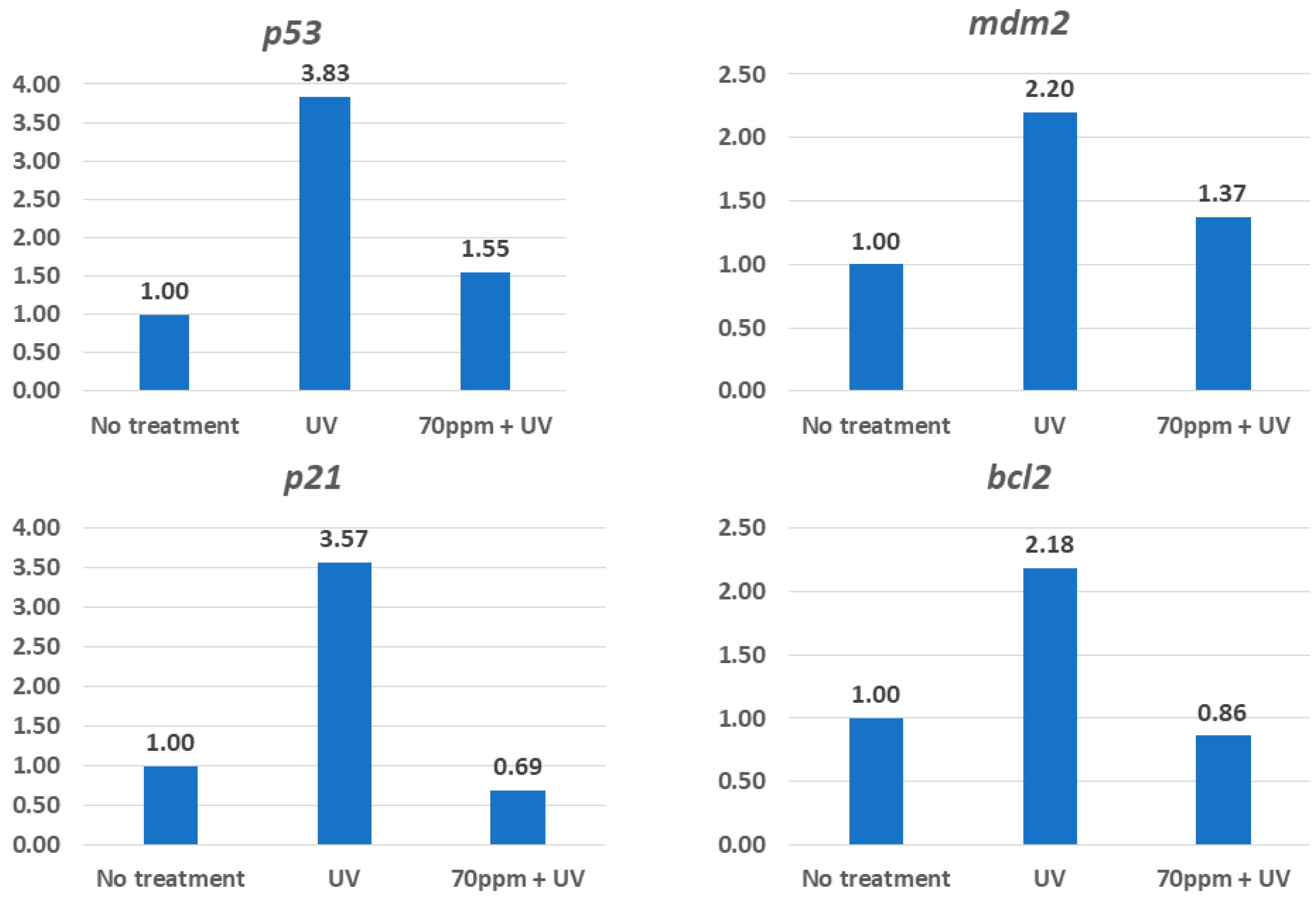
3.4. Possible Mechanisms That Underlie Protection from UV by RAE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.H.; Ho, C.T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial effects of natural bioactive compounds from Hibiscus sabdariffa L. on obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tan, L.; Zou, Y.; Tan, X.; Huang, J.; Chen, W.; Tang, Q. The effects of ultraviolet A/B treatments on anthocyanin accumulation and gene expression in dark-purple tea cultivar ‘Ziyan’ (Camellia sinensis). Molecules 2020, 25, 354. [Google Scholar] [CrossRef]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Fakeye, T. Toxicity and immunomodulatory activity of fractions of Hibiscus sabdariffa Linn (family Malvaceae) in animal models. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Seujange, Y.; Leelahavanichkul, A.; Yisarakun, W.; Khawsuk, W.; Meepool, A.; Phamonleatmongkol, P.; Saechau, W.; Onlamul, W.; Tantiwarattanatikul, P.; Oonsook, W.; et al. Hibiscus sabdariffa linnaeus aqueous extracts attenuate the progression of renal injury in 5/6 nephrectomy rats. Ren. Fail. 2013, 35, 118–125. [Google Scholar] [CrossRef]
- Abdul Hamid, Z.; Lin, W.H.; Abdalla, B.J.; Bee Yuen, O.; Latif, E.S.; Mohamed, J.; Rajab, N.F.; Paik Wah, C.; Wak Harto, M.K.; Budin, S.B. The role of Hibiscus sabdariffa L. (Roselle) in maintenance of ex vivo murine bone marrow-derived hematopoietic stem cells. Sci. World J. 2014, 2014, 258192. [Google Scholar] [CrossRef]
- Murillo Pulgarín, J.A.; García Bermejo, L.F.; Carrasquero Durán, A. A fast and simple FIA-chemiluminescence method for the evaluation of Roselle flowers as scavenger of the free radicals generated by UV irradiated antibiotics. J. Pharm. Biomed. Anal. 2019, 164, 630–635. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, C.Y.; Chang, C.F.; Chen, P.C.; Lee, Y.T.; Chern, C.Y.; Tsai, J.N. Pro-angiogenic effects of chalcone derivatives in zebrafish embryos in vivo. Molecules 2015, 20, 12512–12524. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chou, C.Y.; Chang, Y.C.; Wang, H.W.; Wen, C.C.; Chen, Y.H. Protective role of comfrey leave extracts on UV-induced zebrafish fin damage. J. Toxicol. Pathol. 2014, 27, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, Y.J.; Chou, C.Y.; Wen, C.C.; Cheng, C.C. UV-protective activities of pineapple leaf extract in zebrafish embryos. Res. Chem. Intermed. 2019, 45, 65–75. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wen, C.C.; Yang, Z.S.; Cheng, C.C.; Tsai, J.N.; Ku, C.C.; Wu, H.J.; Chen, Y.H. Development of a whole-organism model to screen new compounds for sun protection. Mar. Biotechnol. 2009, 11, 419–429. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Mullendlers, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Yang, D.; Ding, X.Y.; Xu, H.X.; Guo, Y.X.; Zhang, Q.F. Chemical profile of roselle extract and its inhibitory activities on three digestive enzymes in vitro and in vivo. Int. J. Biol. Macromol. 2023, 253, 126902. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Vega, J.A.; Arteaga-Badillo, D.A.; Sánchez-Gutiérrez, M.; Morales-González, J.A.; Vargas-Mendoza, N.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Delgado-Olivares, L.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Organic acids from roselle (Hibiscus sabdariffa L.)-A brief review of its pharmacological effects. Biomedicines 2020, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, S.; Karasiewicz, M.; Kolodziejski, W. Convenient UV-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J. Food Drug Anal. 2017, 25, 717–722. [Google Scholar] [CrossRef]
- Wen, J.; Kang, L.; Liu, H.; Xiao, Y.; Zhang, X.; Chen, Y. A validated UV-HPLC method for determination of chlorogenic acid in Lepidogrammitis drymoglossoides (Baker) Ching, Polypodiaceae. Pharmacogn. Res. 2012, 4, 148–153. [Google Scholar]
- Buchweitz, M.; Kroon, P.A.; Rich, G.T.; Wilde, P.J. Quercetin solubilisation in bile salts: A comparison with sodium dodecyl sulphate. Food Chem. 2016, 211, 356–364. [Google Scholar] [CrossRef]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R.; Khan, Z.; Banerjee, K. Anthocyanin profiling using UV-Vis spectroscopy and liquid chromatography mass spectrometry. J. AOAC Int. 2020, 103, 23–39. [Google Scholar] [CrossRef]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and stability of a formulation containing antioxidants-enriched castanea sativa shells extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]
- Ryan, E.M.; Duryee, M.J.; Hollins, A.; Dover, S.K.; Pirruccello, S.; Sayles, H.; Real, K.D.; Hunter, C.D.; Thiele, G.M.; Mikuls, T.R. Antioxidant properties of citric acid interfere with the uricase-based measurement of circulating uric acid. J. Pharm. Biomed. Anal. 2019, 164, 460–466. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Rybałtowska-Kawałko, P.; Skrzydlewska, E. Rutin as a mediator of lipid metabolism and cellular signaling pathways interactions in fibroblasts altered by UVA and UVB radiation. Oxidative Med. Cell. Longev. 2017, 2017, 4721352. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem. Toxicol. 2005, 43, 1557–1566. [Google Scholar] [CrossRef]
- Lo, C.W.; Huang, H.P.; Lin, H.M.; Chien, C.T.; Wang, C.J. Effect of Hibiscus anthocyanins-rich extract induces apoptosis of proliferating smooth muscle cell via activation of P38 MAPK and p53 pathway. Mol. Nutr. Food Res. 2007, 51, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Fratantonio, D.; Ferrari, D.; Saija, A.; Cimino, F.; Speciale, A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol. Med. Rep. 2016, 14, 1397–1403. [Google Scholar] [CrossRef]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef]



| Experimental Group | n (Number of Embryos) | Mean Time of Malformation | SE of Mean Time |
|---|---|---|---|
| UV only | 96 | 3.36 | 0.103 |
| UV + 36 ppm | 48 | 3.56 | 0.110 |
| UV + 48 ppm | 48 | 4.44 | 0.110 |
| UV + 60 ppm | 48 | 3.08 | 0.128 |
| Experimental Group | Relative Probability | p-Value |
|---|---|---|
| UV + 36 ppm | 0.963 | 0.834 |
| UV + 48 ppm | 0.496 | <0.001 |
| UV + 60 ppm | 1.360 | 0.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-T.; Huang, C.-Y.; Su, W.-L.; Truong, T.M.; Wen, C.-C.; Wang, B.-C.; Chen, Y.-H. Protective Effects of Roselle Aqueous Extracts against UV-Induced Damage in Zebrafish Fins. Fishes 2024, 9, 199. https://doi.org/10.3390/fishes9060199
Lee I-T, Huang C-Y, Su W-L, Truong TM, Wen C-C, Wang B-C, Chen Y-H. Protective Effects of Roselle Aqueous Extracts against UV-Induced Damage in Zebrafish Fins. Fishes. 2024; 9(6):199. https://doi.org/10.3390/fishes9060199
Chicago/Turabian StyleLee, I-Ting, Ching-Yuan Huang, Wei-Lin Su, Tran M. Truong, Chi-Chung Wen, Bo-Chang Wang, and Yau-Hung Chen. 2024. "Protective Effects of Roselle Aqueous Extracts against UV-Induced Damage in Zebrafish Fins" Fishes 9, no. 6: 199. https://doi.org/10.3390/fishes9060199
APA StyleLee, I.-T., Huang, C.-Y., Su, W.-L., Truong, T. M., Wen, C.-C., Wang, B.-C., & Chen, Y.-H. (2024). Protective Effects of Roselle Aqueous Extracts against UV-Induced Damage in Zebrafish Fins. Fishes, 9(6), 199. https://doi.org/10.3390/fishes9060199






