Harnessing Hue: Advances and Applications of Fish Skin Pigmentation Genetics in Aquaculture
Abstract
:1. Introduction
2. Cellular and Molecular Basis of Pigmentation
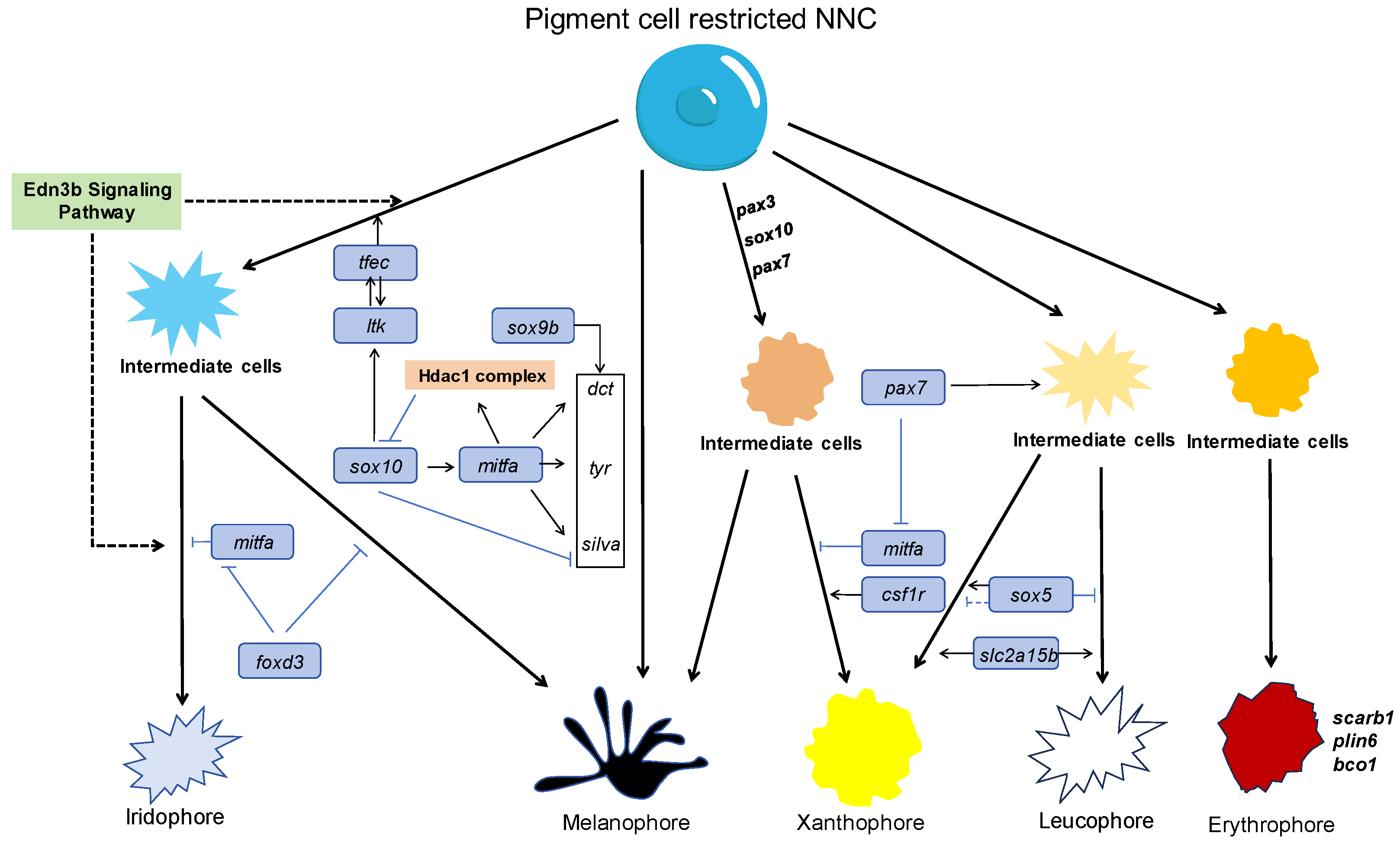
2.1. Pigment Cells and Their Development in Fish
2.2. Accumulation of Pigments and Body Color Differences
3. Factors Influencing Pigmentation
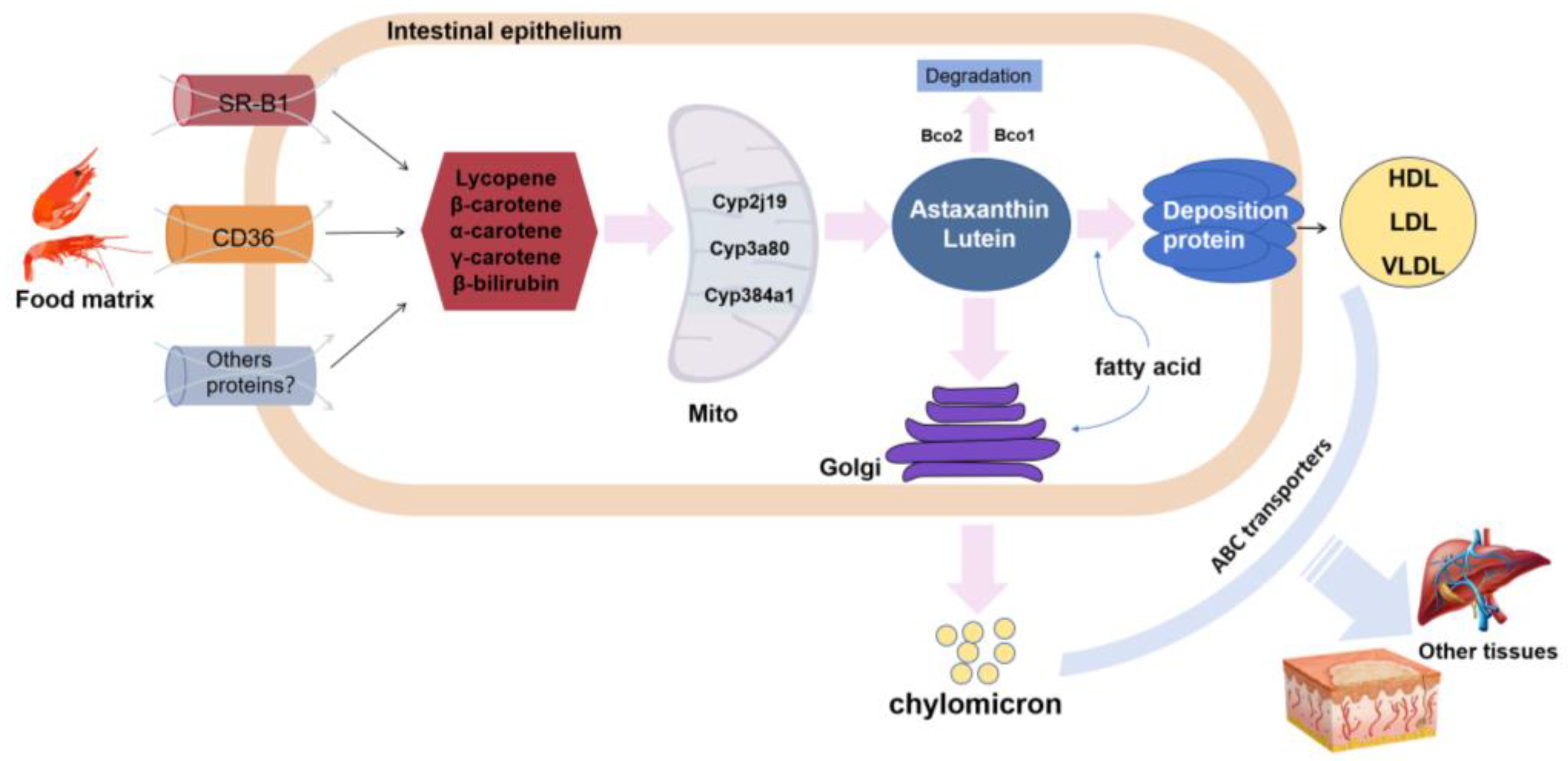
3.1. Nutritional Influences
3.2. Environmental Factors
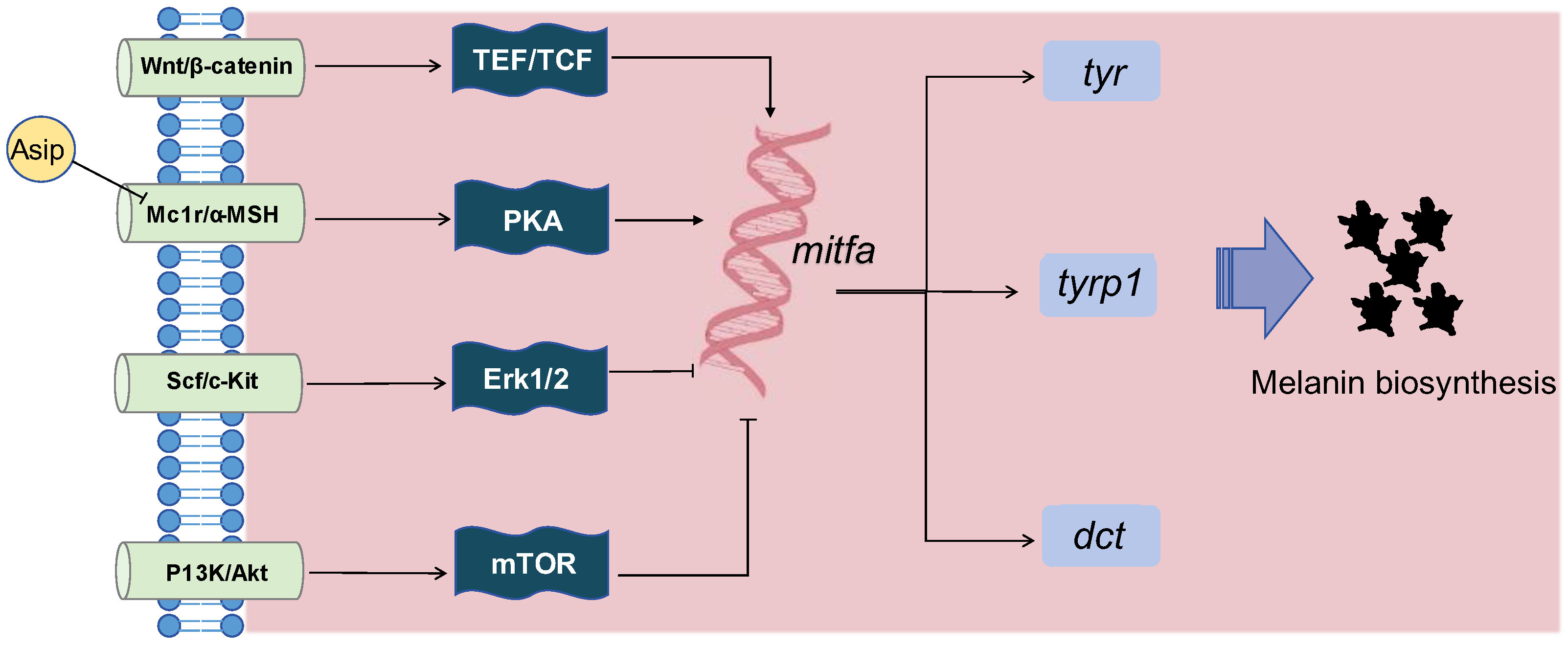
3.3. Hormonal Influences
4. Advances in Breeding Technologies for Color Traits
4.1. Gene Editing
4.2. Selective Breeding and Genome-Wide Association Studies (GWAS)
4.3. Hybrid Breeding
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hubbard, J.K.; Uy, J.A.C.; Hauber, M.E.; Hoekstra, H.E.; Safran, R.J. Vertebrate pigmentation: From underlying genes to adaptive function. Trends Genet. 2010, 26, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.M.; Kelley, J.L.; Morrell, L.J. Colour change and assortment in the western rainbowfish. Anim. Behav. 2010, 79, 1025–1030. [Google Scholar] [CrossRef]
- Üstündağ, Ü.V.; Çalıskan-Ak, E.; Ateş, P.S.; Ünal, İ.; Eğilmezer, G.; Yiğitbaşı, T.; Ata Alturfan, A.; Emekli-Alturfan, E. White LED light exposure inhibits the development and xanthophore pigmentation of zebrafish embryo. Sci. Rep. 2019, 9, 10810. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.C.; Nunes, M.S.; Montaña, C.G.; Farias, I.P.; Lovejoy, N.R. Systematics, biogeography, and evolution of the neotropical peacock basses Cichla (Perciformes: Cichlidae). Mol. Phylogenetics Evol. 2007, 44, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Icoglu Aksakal, F.; Ciltas, A. The impact of ultraviolet B (UV-B) radiation in combination with different temperatures in the early life stage of zebrafish (Danio rerio). Photochem. Photobiol. Sci. 2018, 17, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, P.; Llanos-Rivera, A.; Castro, L.; Fernandez, C. UV radiation effects on the embryos of anchoveta (Engraulis ringens) and common sardine (Strangomera bentincki) off central Chile. Mar. Freshw. Res. 2015, 67, 195–209. [Google Scholar] [CrossRef]
- Mueller, K.P.; Neuhauss, S.C. Sunscreen for fish: Co-option of UV light protection for camouflage. PLoS ONE 2014, 9, e87372. [Google Scholar] [CrossRef] [PubMed]
- Paripatananont, T.; Tangtrongpairoj, J.; Sailasuta, A.; Chansue, N. Effect of astaxanthin on the pigmentation of goldfish Carassius auratus. J. World Aquac. Soc. 1999, 30, 454–460. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhang, R.; Liu, L.; Zhu, H. Generation of golden goldfish Carassius auratus via tyrosinase gene targeting by CRISPR/Cas9. Aquaculture 2024, 583, 740594. [Google Scholar] [CrossRef]
- Eslamloo, K.; Akhavan, S.R.; Eslamifar, A.; Henry, M.A. Effects of background colour on growth performance, skin pigmentation, physiological condition and innate immune responses of goldfish, Carassius auratus. Aquac. Res. 2015, 46, 202–215. [Google Scholar] [CrossRef]
- Fang, W.; Huang, J.; Li, S.; Lu, J. Identification of pigment genes (melanin, carotenoid and pteridine) associated with skin color variant in red tilapia using transcriptome analysis. Aquaculture 2022, 547, 737429. [Google Scholar] [CrossRef]
- Hao, R.; Zhu, X.; Tian, C.; Zhu, C.; Li, G. Analysis of body color formation of leopard coral grouper Plectropomus leopardus. Front. Mar. Sci. 2022, 9, 964774. [Google Scholar] [CrossRef]
- Zhao, N.; Jiang, K.; Ge, X.; Huang, J.; Wu, C.; Chen, S.X. Neurotransmitter norepinephrine regulates chromatosomes aggregation and the formation of blotches in coral trout Plectropomus leopardus. Fish Physiol. Biochem. 2024, 50, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.G., IV; Baker, P.A.; Ibarra-García-Padilla, R.; Moore, J.A.; Rivas, L.J.; Tallman, J.J.; Singleton, E.W.; Westheimer, J.L.; Corteguera, J.A.; Uribe, R.A. An atlas of neural crest lineages along the posterior developing zebrafish at single-cell resolution. eLife 2021, 10, e60005. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, R.; Li, Y.; Lin, X.M.; Zhao, K.C.; Liu, Q.; Wang, S.W.; Yang, X.Q.; Shi, X.Y.; Ma, Y.T.; et al. Spatiotemporal mapping of gene expression landscapes and developmental trajectories during zebrafish embryogenesis. Dev. Cell 2022, 57, 1284–1298. [Google Scholar] [CrossRef]
- Pajanoja, C.; Hsin, J.; Olinger, B.; Schiffmacher, A.; Yazejian, R.; Abrams, S.; Dapkunas, A.; Zainul, Z.; Doyle, A.D.; Martin, D. Maintenance of pluripotency-like signature in the entire ectoderm leads to neural crest stem cell potential. Nat. Commun. 2023, 14, 5941. [Google Scholar] [CrossRef]
- Soldatov, R.; Kaucka, M.; Kastriti, M.E.; Petersen, J.; Chontorotzea, T.; Englmaier, L.; Akkuratova, N.; Yang, Y.; Häring, M.; Dyachuk, V. Spatiotemporal structure of cell fate decisions in murine neural crest. Science 2019, 364, eaas9536. [Google Scholar] [CrossRef] [PubMed]
- Lencer, E.; Prekeris, R.; Artinger, K.B. Single-cell RNA analysis identifies pre-migratory neural crest cells expressing markers of differentiated derivatives. eLife 2021, 10, e66078. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Carneiro, M. Pterin-based pigmentation in animals. Biol. Lett. 2021, 17, 20210221. [Google Scholar] [CrossRef]
- Luo, M.K.; Lu, G.Q.; Yin, H.R.; Wang, L.M.; Atuganile, M.; Dong, Z.J. Fish pigmentation and coloration: Molecular mechanisms and aquaculture perspectives. Rev. Aquac. 2021, 13, 2395–2412. [Google Scholar] [CrossRef]
- Yue, G.H.; Wang, L. Current status of genome sequencing and its applications in aquaculture. Aquaculture 2017, 468, 337–347. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, R.; Tian, C.; Zhang, J.; Zhu, C.; Li, G. Integrative transcriptomics and metabolomics analysis of body color formation in the leopard coral grouper (Plectropomus leopardus). Front. Mar. Sci. 2021, 8, 726102. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Li, Y.; Zhao, L.; Liu, Z. Analysis of yellow mutant rainbow trout transcriptomes at different developmental stages reveals dynamic regulation of skin pigmentation genes. Sci. Rep. 2022, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-T.; Wen, B.; Ji, Y.; Wang, Q.; Zhang, H.-R.; Zhang, Y.; Gao, J.-Z.; Chen, Z.-Z. Comparative metabolomics analysis of pigmentary and structural coloration in discus fish (Symphysodon haraldi). J. Proteom. 2021, 233, 104085. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, L.; Yan, M.; Jiang, Z.; Xue, Y.; Xu, P. Integrative transcriptomics and metabolomics analysis of body color formation in the common carp. Aquaculture 2024, 579, 740143. [Google Scholar]
- Wen, X.; Yang, M.; Zhou, K.; Huang, J.; Fan, X.; Zhang, W.; Luo, J. Transcriptomic and proteomic analyses reveal the common and unique pathway (s) underlying different skin colors of leopard coral grouper (Plectropomus leopardus). J. Proteom. 2022, 266, 104671. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mori, K.; Saitoh, K.; Oshima, K.; Mekuchi, M.; Sugaya, T.; Shigenobu, Y.; Ojima, N.; Muta, S.; Fujiwara, A.; et al. Evolutionary changes of multiple visual pigment genes in the complete genome of Pacific bluefin tuna. Proc. Natl. Acad. Sci. USA 2013, 110, 11061–11066. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Hisano, Y.; Ikawa, Y.; Kawahara, A. Multiple genome modifications by the CRISPR/Cas9 system in zebrafish. Genes Cells 2014, 19, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Dunham, R.A.; Elaswad, A. Catfish Biology and Farming. Annu. Rev. Anim. Biosci. 2018, 6, 305–325. [Google Scholar] [CrossRef]
- Balamurugan, J.; Ajith Kumar, T.T.; Kathiresan, K.; Meenakumari, B. Determination of growth, colour and other traits in F1 hybrid of Amphiprion percula (male) × A. ocellaris (female). Aquac. Res. 2017, 48, 2989–3003. [Google Scholar] [CrossRef]
- Song, H.; Dong, T.; Wang, W.; Yan, X.; Jiang, B.; Xu, S.; Hu, H. Whole-genome resequencing of Russian sturgeon (Acipenser gueldenstaedtii) reveals selection signatures associated with caviar color. Aquaculture 2024, 582, 740545. [Google Scholar] [CrossRef]
- Inaba, M.; Yamanaka, H.; Kondo, S. Pigment Pattern Formation by Contact-Dependent Depolarization. Science 2012, 335, 677. [Google Scholar] [CrossRef] [PubMed]
- Goda, M.; Fujiyoshi, Y.; Sugimoto, M.; Fujii, R. Novel dichromatic chromatophores in the integument of the mandarin fish Synchiropus splendidus. Biol. Bull. 2013, 224, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Goda, M.; Ohata, M.; Ikoma, H.; Fujiyoshi, Y.; Sugimoto, M.; Fujii, R. Integumental reddish-violet coloration owing to novel dichromatic chromatophores in the teleost fish, Pseudochromis diadema. Pigment Cell Melanoma Res. 2011, 24, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, H.E. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity 2006, 97, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M. Morphological color changes in fish: Regulation of pigment cell density and morphology. Microsc. Res. Tech. 2002, 58, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Lewis, V.M.; Foster, T.N.; Toomey, M.B.; Corbo, J.C.; Parichy, D.M. Development and genetics of red coloration in the zebrafish relative Danio albolineatus. eLife 2021, 10, e70253. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, E.; Taylor, J.F.; Migaud, H. Morphological skin colour changes in teleosts. Fish Fish. 2010, 11, 159–193. [Google Scholar] [CrossRef]
- Kimler, V.A.; Taylor, J.D. Morphological studies on the mechanisms of pigmentary organelle transport in fish xanthophores and melanophores. Microsc. Res. Tech. 2002, 58, 470–480. [Google Scholar] [CrossRef]
- Pham, M.A.; Byun, H.G.; Kim, K.D.; Lee, S.M. Effects of dietary carotenoid source and level on growth, skin pigmentation, antioxidant activity and chemical composition of juvenile olive flounder Paralichthys olivaceus. Aquaculture 2014, 431, 65–72. [Google Scholar] [CrossRef]
- Djurdjevič, I.; Kreft, M.E.; Sušnik Bajec, S. Comparison of pigment cell ultrastructure and organisation in the dermis of marble trout and brown trout, and first description of erythrophore ultrastructure in salmonids. J. Anat. 2015, 227, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Ryozo, F.; Noriko, O. Control of chromatophore movements in teleost fishes. Zool. Sci. 1986, 3, 13–47. [Google Scholar]
- Lythgoe, J.; Shand, J. The structural basis for iridescent colour changes in dermal and corneal iridophores in fish. J. Exp. Biol. 1989, 141, 313–325. [Google Scholar] [CrossRef]
- Lynn Lamoreux, M.; Kelsh, R.N.; Wakamatsu, Y.; Ozato, K. Pigment pattern formation in the medaka embryo. Pigment Cell Res. 2005, 18, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Menter, D.G.; Obika, M.; Tchen, T.; Taylor, J.D. Leucophores and iridophores of Fundulus heteroclitus: Biophysical and ultrastructural properties. J. Morphol. 1979, 160, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Begbie, J.; McGonnell, I. Significance of the cranial neural crest. Dev. Dyn. 2004, 229, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, E.J.; Kunttas, E.; Piacentino, M.L.; Howard, A.G.A.; Bronner, M.E.; Uribe, R.A. Migration and diversification of the vagal neural crest. Dev. Biol. 2018, 444, S98–S109. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Bohnsack, B.L. Neural crest derivatives in ocular development: Discerning the eye of the storm. Birth Defects Res. Part C-Embryo Today-Rev. 2015, 105, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.E.; Bronner-Fraser, M. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development 1991, 112, 913–920. [Google Scholar] [CrossRef]
- Stemple, D.L.; Anderson, D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 1992, 71, 973–985. [Google Scholar] [CrossRef]
- Hari, L.; Miescher, I.; Shakhova, O.; Suter, U.; Chin, L.; Taketo, M.; Richardson, W.D.; Kessaris, N.; Sommer, L. Temporal control of neural crest lineage generation by Wnt/β-catenin signaling. Development 2012, 139, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M. Cell line segregation during peripheral nervous system ontogeny. Science 1986, 231, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Baroffio, A.; Dupin, E.; Le Douarin, N.M. Clone-forming ability and differentiation potential of migratory neural crest cells. Proc. Natl. Acad. Sci. USA 1988, 85, 5325–5329. [Google Scholar] [CrossRef] [PubMed]
- Baroffio, A.; Dupin, E.; Douarin, N.M.L. Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development 1991, 112, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Weston, J. Regulation of neural crest cell migration and differentiation. In Cell Interactions and Development: Molecular Mechanisms; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1983; pp. 153–184. [Google Scholar]
- Weston, J.A. 6 sequential segregation and fate of developmentally restricted intermediate cell populations in the neural crest lineage. Curr. Top. Dev. Biol. 1991, 25, 133–153. [Google Scholar] [PubMed]
- Krispin, S.; Nitzan, E.; Kassem, Y.; Kalcheim, C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development 2010, 137, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Ruhrberg, C.; Schwarz, Q. In the beginning: Generating neural crest cell diversity. Cell Adhes. Migr. 2010, 4, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Reedy, M.V.; Faraco, C.D.; Erickson, C.A. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev. Biol. 1998, 200, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, E.; Krispin, S.; Pfaltzgraff, E.R.; Klar, A.; Labosky, P.A.; Kalcheim, C. A dynamic code of dorsal neural tube genes regulates the segregation between neurogenic and melanogenic neural crest cells. Development 2013, 140, 2269–2279. [Google Scholar] [CrossRef]
- Quigley, I.K.; Parichy, D.M. Pigment pattern formation in zebrafish: A model for developmental genetics and the evolution of form. Microsc. Res. Tech. 2002, 58, 442–455. [Google Scholar] [CrossRef]
- Lister, J.A. Development of pigment cells in the zebrafish embryo. Microsc. Res. Tech. 2002, 58, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Nüsslein-Volhard, C. Zebrafish stripes as a model for vertebrate colour pattern formation. Curr. Biol. 2015, 25, R81–R92. [Google Scholar] [CrossRef] [PubMed]
- Kenny, C.; Dilshat, R.; Seberg, H.E.; Van Otterloo, E.; Bonde, G.; Helverson, A.; Franke, C.M.; Steingrímsson, E.; Cornell, R.A. TFAP2 paralogs facilitate chromatin access for MITF at pigmentation and cell proliferation genes. PLoS Genet. 2022, 18, e1010207. [Google Scholar] [CrossRef] [PubMed]
- Nord, H.; Dennhag, N.; Muck, J.; von Hofsten, J. Pax7 is required for establishment of the xanthophore lineage in zebrafish embryos. Mol. Biol. Cell 2016, 27, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Petratou, K.; Subkhankulova, T.; Lister, J.A.; Rocco, A.; Schwetlick, H.; Kelsh, R.N. A systems biology approach uncovers the core gene regulatory network governing iridophore fate choice from the neural crest. PLoS Genet. 2018, 14, e1007402. [Google Scholar] [CrossRef] [PubMed]
- Elworthy, S.; Lister, J.A.; Carney, T.J.; Raible, D.W.; Kelsh, R.N. Transcriptional regulation of mitfa accounts for the sox10 requirement in zebrafish melanophore development. Development 2003, 130, 2809–2818. [Google Scholar] [CrossRef]
- Opdecamp, K.; Nakayama, A.; Nguyen, M.-T.T.; Hodgkinson, C.A.; Pavan, W.J.; Arnheiter, H. Melanocyte development in vivo and in neural crest cell cultures: Crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development 1997, 124, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, E.R.; Rocco, A.; Vibert, L.; Nikaido, M.; Kelsh, R.N. An iterative genetic and dynamical modelling approach identifies novel features of the gene regulatory network underlying melanocyte development. PLoS Genet. 2011, 7, e1002265. [Google Scholar] [CrossRef] [PubMed]
- Vibert, L.; Aquino, G.; Gehring, I.; Subkankulova, T.; Schilling, T.F.; Rocco, A.; Kelsh, R.N. An ongoing role for Wnt signaling in differentiating melanocytes in vivo. Pigment Cell Melanoma Res. 2017, 30, 219–232. [Google Scholar] [CrossRef]
- Dorsky, R.I.; Moon, R.T.; Raible, D.W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 1998, 396, 370–373. [Google Scholar] [CrossRef]
- Petratou, K.; Spencer, S.A.; Kelsh, R.N.; Lister, J.A. The MITF paralog tfec is required in neural crest development for fate specification of the iridophore lineage from a multipotent pigment cell progenitor. PLoS ONE 2021, 16, e0244794. [Google Scholar] [CrossRef]
- Ben-Neriah, Y.; Bauskin, A.R. Leukocytes express a novel gene encoding a putative transmembrane protein-kinase devoid of an extracellular domain. Nature 1988, 333, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.A.; Cooper, C.; Nguyen, K.; Modrell, M.; Grant, K.; Raible, D.W. Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Dev. Biol. 2006, 290, 92–104. [Google Scholar] [CrossRef]
- Ignatius, M.S.; Moose, H.E.; El-Hodiri, H.M.; Henion, P.D. colgate/hdac1 Repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev. Biol. 2008, 313, 568–583. [Google Scholar] [CrossRef]
- Curran, K.; Raible, D.W.; Lister, J.A. Foxd3 controls melanophore specification in the zebrafish neural crest by regulation of Mitf. Dev. Biol. 2009, 332, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.; Lister, J.A.; Kunkel, G.R.; Prendergast, A.; Parichy, D.M.; Raible, D.W. Interplay between Foxd3 and Mitf regulates cell fate plasticity in the zebrafish neural crest. Dev. Biol. 2010, 344, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.; Frohnhöfer, H.G.; Walderich, B.; Maischein, H.M.; Weiler, C.; Irion, U.; Nüsslein-Volhard, C. Endothelin signalling in iridophore development and stripe pattern formation of zebrafish. Biol. Open 2014, 3, 503–509. [Google Scholar] [CrossRef]
- Parichy, D.M.; Mellgren, E.M.; Rawls, J.F.; Lopes, S.S.; Kelsh, R.N.; Johnson, S.L. Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio. Dev. Biol. 2000, 227, 294–306. [Google Scholar] [CrossRef]
- Hozumi, S.; Shirai, M.; Wang, J.X.; Aoki, S.; Kikuchi, Y. The N-terminal domain of gastrulation brain homeobox 2 (Gbx2) is required for iridophore specification in zebrafish. Biochem. Biophys. Res. Commun. 2018, 502, 104–109. [Google Scholar] [CrossRef]
- Jang, H.S.; Chen, Y.; Ge, J.; Wilkening, A.N.; Hou, Y.; Lee, H.J.; Choi, Y.R.; Lowdon, R.F.; Xing, X.; Li, D. Epigenetic dynamics shaping melanophore and iridophore cell fate in zebrafish. Genome Biol. 2021, 22, 282. [Google Scholar] [CrossRef]
- D’Agati, G.; Beltre, R.; Sessa, A.; Burger, A.; Zhou, Y.; Mosimann, C.; White, R.M. A defect in the mitochondrial protein Mpv17 underlies the transparent casper zebrafish. Dev. Biol. 2017, 430, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Minchin, J.E.; Hughes, S.M. Sequential actions of Pax3 and Pax7 drive xanthophore development in zebrafish neural crest. Dev. Biol. 2008, 317, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Miyadai, M.; Takada, H.; Shiraishi, A.; Kimura, T.; Watakabe, I.; Kobayashi, H.; Nagao, Y.; Naruse, K.; Higashijima, S.-i.; Shimizu, T. A gene regulatory network combining Pax3/7, Sox10 and Mitf generates diverse pigment cell types in medaka and zebrafish. Development 2023, 150, dev202114. [Google Scholar] [CrossRef]
- Parichy, D.M.; Ransom, D.G.; Paw, B.; Zon, L.I.; Johnson, S.L. An orthologue of the kit-related gene is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development 2000, 127, 3031–3044. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Takada, H.; Miyadai, M.; Adachi, T.; Seki, R.; Kamei, Y.; Hara, I.; Taniguchi, Y.; Naruse, K.; Hibi, M.; et al. Distinct interactions of Sox5 and Sox10 in fate specification of pigment cells in medaka and zebrafish. PLoS Genet. 2018, 14, e1007260. [Google Scholar] [CrossRef] [PubMed]
- Bajec, S.S.; Djurdjevic, I.; Andújar, C.L.; Kreft, M.E. Genetic and correlative light and electron microscopy evidence for the unique differentiation pathway of erythrophores in brown trout skin. Sci. Rep. 2022, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.G.; Shi, H.J.; Han, T.; Jiang, D.N.; Lu, B.Y.; Shi, G.; Zhu, C.H.; Li, G.L. Pigment Identification and Gene Expression Analysis during Erythrophore Development in Spotted Scat (Scatophagus argus) Larvae. Int. J. Mol. Sci. 2023, 24, 15356. [Google Scholar] [CrossRef] [PubMed]
- Wakamátsu, Y.; Pristyazhnyuk, S.; Kinoshita, M.; Tanaka, M.; Ozato, K. The see-through medaka: A fish model that is transparent throughout life. Proc. Natl. Acad. Sci. USA 2001, 98, 10046–10050. [Google Scholar] [CrossRef]
- Kimura, T.; Nagao, Y.; Hashimoto, H.; Yamamoto-Shiraishi, Y.; Yamamoto, S.; Yabe, T.; Takada, S.; Kinoshita, M.; Kuroiwa, A.; Naruse, K. Leucophores are similar to xanthophores in their specification and differentiation processes in medaka. Proc. Natl. Acad. Sci. USA 2014, 111, 7343–7348. [Google Scholar] [CrossRef]
- Nagao, Y.; Suzuki, T.; Shimizu, A.; Kimura, T.; Seki, R.; Adachi, T.; Inoue, C.; Omae, Y.; Kamei, Y.; Hara, I.; et al. Sox5 Functions as a Fate Switch in Medaka Pigment Cell Development. PLoS Genet. 2014, 10, e1004246. [Google Scholar] [CrossRef]
- Hearing, V.J.; Tsukamoto, K. Enzymatic control of pigmentation in mammals. FASEB J. 1991, 5, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. Natural Melanogenesis Inhibitors Acting Through the Down-Regulation of Tyrosinase Activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Bauer, G.L.; Praetorius, C.; Bergsteinsdóttir, K.; Hallsson, J.H.; Gísladóttir, B.K.; Schepsky, A.; Swing, D.A.; O’Sullivan, T.N.; Arnheiter, H.; Bismuth, K.; et al. The Role of MITF Phosphorylation Sites During Coat Color and Eye Development in Mice Analyzed by Bacterial Artificial Chromosome Transgene Rescue. Genetics 2009, 183, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.; Kelsh, R.N.; Scholpp, S. The Role of Wnt/β-Catenin Signalling in Neural Crest Development in Zebrafish. Front. Cell Dev. Biol. 2021, 9, 782445. [Google Scholar] [CrossRef] [PubMed]
- Dorsky, R.I.; Raible, D.W.; Moon, R.T. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000, 14, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, H.; Cong, X.; Huo, D.; Cong, L.; Wu, G. α-Msh-Pe38kdel kills melanoma cells Via modulating Erk1/2/Mitf/Tyr signaling in an Mc1r-dependent manner. OncoTargets Ther. 2020, 13, 12457–12469. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, C.; Martínez-Vicente, I.; Maresca, V. The α-melanocyte-stimulating hormone/melanocortin-1 receptor interaction: A driver of pleiotropic effects beyond pigmentation. Pigment Cell Melanoma Res. 2021, 34, 748–761. [Google Scholar] [CrossRef]
- Wang, T.-Z.; Wu, Q.; Zhang, N.; Wang, D.-J.; Xu, Z.; Luo, W.; Du, Z.-J. Advances in research on melanin synthesis and signaling pathway in fish. China Biotechnol. 2020, 40, 84–93. [Google Scholar]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol Inhibits α-MSH-Induced Melanogenesis through the Acceleration of ERK and PI3K/Akt-Mediated MITF Degradation. BioMed Res. Int. 2014, 2014, 842569. [Google Scholar] [CrossRef]
- Wu, L.-C.; Lin, Y.-Y.; Yang, S.-Y.; Weng, Y.-T.; Tsai, Y.-T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signaling pathways. J. Biomed. Sci. 2011, 18, 74. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.-H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef] [PubMed]
- Phung, B.; Sun, J.; Schepsky, A.; Steingrimsson, E.; Rönnstrand, L. C-KIT signaling depends on microphthalmia-associated transcription factor for effects on cell proliferation. PLoS ONE 2011, 6, e24064. [Google Scholar] [CrossRef] [PubMed]
- Del Ama, L.F.; Jones, M.; Walker, P.; Chapman, A.; Braun, J.A.; Mohr, J.; Hurlstone, A.F. Reprofiling using a zebrafish melanoma model reveals drugs cooperating with targeted therapeutics. Oncotarget 2016, 7, 40348. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, I.; McDonaldo, T.; Hesslinger, C.; Pelletier, I.; Boyle, P. Development of the pteridine pathway in the zebrafish, Danio rerio. J. Biol. Chem. 2000, 275, 18926–18932. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.A. Larval but not adult xanthophore pigmentation in zebrafish requires GTP cyclohydrolase 2 (gch2) function. Pigment Cell Melanoma Res. 2019, 32, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Liang, P.; Wu, S.; Li, Y.; Qiao, L.; Hu, H.; Xiang, Z.; Lu, C.; Dai, F. Disruption of PTPS gene causing pale body color and lethal phenotype in the silkworm, Bombyx mori. Int. J. Mol. Sci. 2018, 19, 1024. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Vachali, P.; Gorusupudi, A.; Nelson, K.; Bernstein, P.S. All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch. Biochem. Biophys. 2017, 634, 21–28. [Google Scholar] [CrossRef]
- Sakudoh, T.; Kuwazaki, S.; Iizuka, T.; Narukawa, J.; Yamamoto, K.; Uchino, K.; Sezutsu, H.; Banno, Y.; Tsuchida, K. CD36 homolog divergence is responsible for the selectivity of carotenoid species migration to the silk gland of the silkworm Bombyx mori. J. Lipid Res. 2013, 54, 482–495. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Wang, G.; Huang, S. De novo assembly transcriptome analysis reveals the genes associated with body color formation in the freshwater ornamental shrimps Neocaridina denticulate sinensis. Gene 2022, 806, 145929. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Canene-Adams, K.; Erdman, J.W., Jr. Absorption, transport, distribution in tissues and bioavailability. In Carotenoids: Volume 5: Nutrition and Health; Springer: Basel, Switzerland, 2009; pp. 115–148. [Google Scholar]
- Keen, J.N.; Caceres, I.; Eliopoulos, E.E.; Zagalsky, P.F.; Findlay, J.B. Complete sequence and model for the C1 subunit of the carotenoprotein crustacyanin, and model for the dimer, β-crustacyanin, formed from the C1 and A2 subunits with astaxanthin. Eur. J. Biochem. 1991, 202, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zagalsky, P.; Haxo, F.; Hertzberg, S.; Liaaen-Jensen, S. Studies on a blue carotenoprotein, linckiacyanin, isolated from the starfish Linckia laevigata (Echinodermata: Asteroidea). Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 93, 339–353. [Google Scholar] [CrossRef]
- dela Seña, C.; Narayanasamy, S.; Riedl, K.M.; Curley, R.W.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant human β-carotene 15, 15′-oxygenase (BCO1). J. Biol. Chem. 2013, 288, 37094–37103. [Google Scholar] [CrossRef] [PubMed]
- dela Seña, C.; Sun, J.; Narayanasamy, S.; Riedl, K.M.; Yuan, Y.; Curley, R.W.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant chicken β-carotene 9′, 10′-oxygenase (BCO2). J. Biol. Chem. 2016, 291, 14609–14619. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Tian, H.; Winograd, N.; Benkovic, S.J. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science 2020, 368, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Uribe, R.A.; Yieh, L.; Nuckels, R.; Gross, J.M. Zebrafish mutations in gart and paics identify crucial roles for de novo purine synthesis in vertebrate pigmentation and ocular development. Development 2009, 136, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takehana, Y.; Naruse, K. pnp4a Is the Causal Gene of the Medaka Iridophore Mutant guanineless. G3 Genes Genomes Genet. 2017, 7, 1357–1363. [Google Scholar] [CrossRef]
- Goda, M.; Miyagi, A.; Kitamoto, T.; Kondo, M.; Hashimoto, H. Uric acid is a major chemical constituent for the whitish coloration in the medaka leucophores. Pigment Cell Melanoma Res. 2023, 36, 416–422. [Google Scholar] [CrossRef]
- Estevez, A.; Kanazawa, A. Effect of (n-3) PUFA and vitamin A Artemia enrichment on pigmentation success of turbot, Scophthalmus maximus. Aquac. Nutr. 1995, 1, 159–168. [Google Scholar] [CrossRef]
- Heath, P.L.; Moore, C.G. Rearing Dover sole larvae on Tisbe and Artemia diets. Aquac. Int. 1997, 5, 29–39. [Google Scholar] [CrossRef]
- Hamre, K.; Moren, M.; Solbakken, J.; Opstad, I.; Pittman, K. The impact of nutrition on metamorphosis in Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 2005, 250, 555–565. [Google Scholar] [CrossRef]
- Darias, M.J.; Andree, K.B.; Boglino, A.; Rotllant, J.; Cerdá-Reverter, J.M.; Estévez, A.; Gisbert, E. Morphological and Molecular Characterization of Dietary-Induced Pseudo-Albinism during Post-Embryonic Development of Solea senegalensis (Kaup, 1858). PLoS ONE 2013, 8, e68844. [Google Scholar] [CrossRef]
- Maoka, T.; Sato, W.; Nagai, H.; Takahashi, T. Carotenoids of red, brown, and black specimens of plectropomus leopardus, the coral trout (Suziara in Japanese). J. Oleo Sci. 2017, 66, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.H.; Chien, Y.H. Effects of dietary supplementation of alga Haematococcus pluvialis (Flotow), synthetic astaxanthin and β-carotene on survival, growth, and pigment distribution of red devil, Cichlasoma citrinellum (Günther). Aquac. Res. 2009, 40, 871–879. [Google Scholar] [CrossRef]
- Tejera, N.; Cejas, J.R.; Rodríguez, C.; Bjerkeng, B.; Jerez, S.; Bolaños, A.; Lorenzo, A. Pigmentation, carotenoids, lipid peroxides and lipid composition of skin of red porgy (Pagrus pagrus) fed diets supplemented with different astaxanthin sources. Aquaculture 2007, 270, 218–230. [Google Scholar] [CrossRef]
- Kalinowski, C.T.; Robaina, L.E.; Fernández-Palacios, H.; Schuchardt, D.; Izquierdo, M.S. Effect of different carotenoid sources and their dietary levels on red porgy (Pagrus pagrus) growth and skin colour. Aquaculture 2005, 244, 223–231. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Nelson, R.J. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 2017, 7, e1017. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.F.; Britt, L.L.; Cook, M.A.; Wade, T.H.; Berejikian, B.A.; Goetz, F.W. Effect of light intensity and feed density on feeding behaviour, growth and survival of larval sablefish Anoplopoma fimbria. Aquac. Res. 2017, 48, 4438–4448. [Google Scholar] [CrossRef]
- Ali, B.; Mishra, A. Effects of monochromatic lights on the melanophores arrangement in the spotted snakehead fish Channa punctata (Bloch, 1793). J. Fish Biol. 2023, 102, 1415–1424. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, H.W.; Hu, P.F.; Liu, W.L.; Shen, X.F.; Cui, X.; Wu, Y.M.; Yuan, Z.; Zhang, L.; Zhang, Y.X.; et al. Growth and survival of Takifugu rubripes larvae cultured under different light conditions. Fish Physiol. Biochem. 2019, 45, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Kasagi, S.; Miura, M.; Okazaki, T.; Mizusawa, K.; Takahashi, A. Effects of tank color brightness on the body color, somatic growth, and endocrine systems of rainbow trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2020, 298, 113581. [Google Scholar] [CrossRef] [PubMed]
- Song, F.B.; Shi, L.P.; Yao, F.C.; Gu, Y.; Zheng, D.; Zhang, W.W.; Liang, Y.S.; Zhang, K.X.; Yang, M.; Wang, L.; et al. The Effect of Background Color on Skin Color Variation of Juvenile Plectropomus leopardus. Animals 2022, 12, 3349. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Kasagi, S.; Takahashi, A.; Mizusawa, K. Effects of background color and feeding status on the expression of genes associated with body color regulation in the goldfish Carassius auratus. Gen. Comp. Endocrinol. 2021, 312, 113860. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Mattioli, C.C.; Silva, W.S.; Takata, R.; Leme, F.O.P.; Oliveira, A.L.; Luz, R.K. The effect of environmental colour on the growth, metabolism, physiology and skin pigmentation of the carnivorous freshwater catfish Lophiosilurus alexandri. J. Fish Biol. 2017, 90, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Rema, P. Effect of microalgal biomass concentration and temperature on ornamental goldfish (Carassius auratus) skin pigmentation. Aquac. Nutr. 2005, 11, 19–23. [Google Scholar] [CrossRef]
- No, H.K.; Storebakken, T. Pigmentation of rainbow trout with astaxanthin and canthaxanthin in freshwater and saltwater. Aquaculture 1992, 101, 123–134. [Google Scholar] [CrossRef]
- Tveit, G.M.; Anders, N.; Bondo, M.S.; Mathiassen, J.R.; Breen, M. Atlantic mackerel (Scomber scombrus) change skin colour in response to crowding stress. J. Fish Biol. 2022, 100, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Bertolesi, G.E.; Zhang, J.Z.; McFarlane, S. Plasticity for colour adaptation in vertebrates explained by the evolution of the genes pomc, pmch and pmchl. Pigment Cell Melanoma Res. 2019, 32, 510–527. [Google Scholar] [CrossRef]
- Baker, B.I.; Bird, D.J.; Buckingham, J.C. Effects of chronic administration of melanin-concentrating hormone on corticotrophin, melanotrophin, and pigmentation in the trout. Gen. Comp. Endocrinol. 1986, 63, 62–69. [Google Scholar] [CrossRef]
- Benedet, S.; Björnsson, B.T.; Taranger, G.L.; Andersson, E. Cloning of somatolactin alpha, beta forms and the somatolactin receptor in Atlantic salmon: Seasonal expression profile in pituitary and ovary of maturing female broodstock. Reprod. Biol. Endocrinol. 2008, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Kakisawa, S.; Kaneko, T.; Hasegawa, S.; Hirano, T. Effects of feeding, fasting, background adaptation, acute stress, and exhaustive exercise on the plasma somatolactin concentrations in rainbow trout. Gen. Comp. Endocrinol. 1995, 98, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cánepa, M.M.; Zhu, Y.; Fossati, M.; Stiller, J.W.; Vissio, P.G. Cloning, phylogenetic analysis and expression of somatolactin and its receptor in Cichlasoma dimerus: Their role in long-term background color acclimation. Gen. Comp. Endocrinol. 2012, 176, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, S.; Sugimoto, M.; Mitani, H.; Shima, A. Somatolactin selectively regulates proliferation and morphogenesis of neural-crest derived pigment cells in medaka. Proc. Natl. Acad. Sci. USA 2004, 101, 10661–10666. [Google Scholar] [CrossRef] [PubMed]
- Bertolesi, G.E.; McFarlane, S. Melanin-concentrating hormone like and somatolactin. A teleost-specific hypothalamic-hypophyseal axis system linking physiological and morphological pigmentation. Pigment Cell Melanoma Res. 2021, 34, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Goto, M. Prolactin signaling in erythrophores and xanthophores of teleost fish. Pigment Cell Res. 2000, 13, 35–40. [Google Scholar] [CrossRef]
- Oshima, N.; Makino, M.; Iwamuro, S.; Bern, H.A. Pigment dispersion by prolactin in cultured xanthophores and erythrophores of some fish species. J. Exp. Zool. 1996, 275, 45–52. [Google Scholar] [CrossRef]
- McMenamin, S.K.; Bain, E.J.; McCann, A.E.; Patterson, L.B.; Eom, D.S.; Waller, Z.P.; Hamill, J.C.; Kuhlman, J.A.; Eisen, J.S.; Parichy, D.M. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science 2014, 345, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.M.; Mishra, A.K.; Aman, A.J.; Lewis, V.M.; Toomey, M.B.; Packer, J.S.; Qiu, X.J.; McFaline-Figueroa, J.L.; Corbo, J.C.; Trapnell, C.; et al. Thyroid hormone regulates distinct paths to maturation in pigment cell lineages. eLife 2019, 8, e45181. [Google Scholar] [CrossRef]
- Volkov, L.I.; Kim-Han, J.S.; Saunders, L.M.; Poria, D.; Hughes, A.E.; Kefalov, V.J.; Parichy, D.M.; Corbo, J.C. Thyroid hormone receptors mediate two distinct mechanisms of long-wavelength vision. Proc. Natl. Acad. Sci. USA 2020, 117, 15262–15269. [Google Scholar] [CrossRef]
- Lu, J.; Fang, W.; Huang, J.; Li, S. The application of genome editing technology in fish. Mar. Life Sci. Technol. 2021, 3, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Gutási, A.; Hammer, S.E.; El-Matbouli, M.; Saleh, M. Recent Applications of Gene Editing in Fish Species and Aquatic Medicine. Animals 2023, 13, 1250. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.A.; Østbye, T.K.K.; Kettunen, A.H.; Coates, A.; Barrett, L.T.; Robledo, D.; Dempster, T. A guide to assess the use of gene editing in aquaculture. Rev. Aquac. 2024, 16, 775–784. [Google Scholar] [CrossRef]
- Xu, X.; Chen, H.; Mandal, B.K.; Si, Z.; Wang, J.; Wang, C. Duplicated Tyr disruption using CRISPR/Cas9 reveals melanophore formation in Oujiang color common carp (Cyprinus carpio var. color). Reprod. Breed. 2022, 2, 37–45. [Google Scholar] [CrossRef]
- Mandal, B.K.; Chen, H.; Si, Z.; Hou, X.; Yang, H.; Xu, X.; Wang, J.; Wang, C. Shrunk and scattered black spots turn out due to MC1R knockout in a white-black Oujiang color common carp (Cyprinus carpio var. color). Aquaculture 2020, 518, 734822. [Google Scholar] [CrossRef]
- Jao, L.-E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, R.B.; Leininger, S.; Kleppe, L.; Skaftnesmo, K.O.; Wargelius, A. Targeted mutagenesis in Atlantic salmon (Salmo salar L.) using the CRISPR/Cas9 system induces complete knockout individuals in the F0 generation. PLoS ONE 2014, 9, e108622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qi, Y.; Liang, Q.; Song, J.; Liu, J.; Li, W.; Shu, Y.; Tao, M.; Zhang, C.; Qin, Q. Targeted disruption of tyrosinase causes melanin reduction in Carassius auratus cuvieri and its hybrid progeny. Sci. China Life Sci. 2019, 62, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Du, J.; Si, Z.; Yang, H.; Xu, X.; Wang, C. ASIP disruption via CRISPR/Cas9 system induces black patches dispersion in Oujiang color common carp. Aquaculture 2019, 498, 230–235. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Kocher, T.D.; Li, M.; Wang, D. CRISPR knockouts of pmela and pmelb engineered a golden tilapia by regulating relative pigment cell abundance. J. Hered. 2022, 113, 398–413. [Google Scholar] [CrossRef]
- Wang, C.; Kocher, T.D.; Lu, B.; Xu, J.; Wang, D. Knockout of hermansky-pudlak syndrome 4 (hps4) leads to silver-white tilapia lacking melanosomes. Aquaculture 2022, 559, 738420. [Google Scholar] [CrossRef]
- Lu, B.; Wang, C.; Liang, G.; Xu, M.; Kocher, T.D.; Sun, L.; Wang, D. Generation of ornamental Nile tilapia with distinct gray and black body color pattern by csf1ra mutation. Aquac. Rep. 2022, 23, 101077. [Google Scholar] [CrossRef]
- Kratochwil, C.F.; Liang, Y.; Gerwin, J.; Woltering, J.M.; Urban, S.; Henning, F.; Machado-Schiaffino, G.; Hulsey, C.D.; Meyer, A. Agouti-related peptide 2 facilitates convergent evolution of stripe patterns across cichlid fish radiations. Science 2018, 362, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, W.K. Selective breeding in aquaculture. Food Rev. Int. 1990, 6, 359–372. [Google Scholar] [CrossRef]
- Risch, N.; Merikangas, K. The future of genetic studies of complex human diseases. Science 1996, 273, 1516–1517. [Google Scholar] [CrossRef] [PubMed]
- Doan, Q.K.; Vandeputte, M.; Chatain, B.; Haffray, P.; Vergnet, A.; Breuil, G.; Allal, F. Genetic variation of resistance to Viral Nervous Necrosis and genetic correlations with production traits in wild populations of the European sea bass (Dicentrarchus labrax). Aquaculture 2017, 478, 1–8. [Google Scholar] [CrossRef]
- Liu, G.J.; Han, Z.F.; Jiang, D.; Li, W.B.; Zhang, W.J.; Ye, K.; Gu, L.L.; Dong, L.S.; Fang, M.; Wang, Z.Y. Genome-wide association study identifies loci for traits related to swim bladder in yellow drum (Nibea albiflora). Aquaculture 2020, 526, 735327. [Google Scholar] [CrossRef]
- Wu, Y.D.; Zhou, Z.X.; Pan, Y.; Zhao, J.; Bai, H.Q.; Chen, B.H.; Zhang, X.Y.; Pu, F.; Chen, J.; Xu, P. GWAS identified candidate variants and genes associated with acute heat tolerance of large yellow croaker. Aquaculture 2021, 540, 736696. [Google Scholar] [CrossRef]
- Wen, X.; Tang, H.; Zhou, M.; Yang, M.; Huang, J.; Liu, J.; Zhou, K.; Fan, X.; Zhang, W.; Luo, J. Genome-wide association study of red skin color in leopard coral grouper (Plectropomus leopardus) based on genome resequencing. Aquaculture 2023, 563, 739014. [Google Scholar] [CrossRef]
- Valette, T.; Leitwein, M.; Lascaux, J.M.; Desmarais, E.; Berrebi, P.; Guinand, B. Redundancy analysis, genome-wide association studies and the pigmentation of brown trout (Salmo trutta L.). J. Fish Biol. 2023, 102, 96–118. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.F.; Wang, X.M.; Li, J.T.; Liu, G.M.; Kuang, Y.Y.; Xu, J.; Zheng, X.H.; Ren, L.F.; Wang, G.L.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Z.; Liu, J.C.; Wang, Z.R.; Zhang, L.J.; Yang, M.; Huang, J.; Wen, X.; Luo, J. Genome-wide association study (GWAS) analysis of black color trait in the leopard coral grouper (Plectropomus leopardus) using whole genome resequencing. Comp. Biochem. Physiol. D-Genom. Proteom. 2023, 48, 101138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Li, B.J.; Yu, M.H.; Chang, S.H.; Li, S.Q.; Xu, J.; Feng, J.X.; Zhang, Q.; Zhang, H.Y.; Xu, P. Genome-wide association study and gene editing reveals the causal gene responsible for abnormal red skin color in Yellow River carp. Aquaculture 2022, 560, 738530. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.P.; Li, Y.J.; Yu, J.C.; Liao, H.; Wang, S.Y.; Lv, J.; Liang, J.; Huang, X.T.; Bao, Z.M. A Genome-Wide Association Study Identifies the Genomic Region Associated with Shell Color in Yesso Scallop, Patinopecten yessoensis. Mar. Biotechnol. 2017, 19, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.; Yang, Y.; Li, B.J.; Huang, W.H.; Wang, X.; Liu, X.C.; Meng, Z.N.; Xia, J.H. First Genome-wide Association Analysis for Growth Traits in the Largest Coral Reef-Dwelling Bony Fishes, the Giant Grouper (Epinephelus lanceolatus). Mar. Biotechnol. 2019, 21, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Han, K.H.; Wu, Y.D.; Bai, H.Q.; Ke, Q.Z.; Pu, F.; Wang, Y.L.; Xu, P. Genome-Wide Association Study of Growth and Body-Shape-Related Traits in Large Yellow Croaker (Larimichthys crocea) Using ddRAD Sequencing. Mar. Biotechnol. 2019, 21, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Lavanchy, G.; Schwander, T. Hybridogenesis. Curr. Biol. 2019, 29, R9–R11. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, S.; Okamoto, M.; Kondo, S. Blending of animal colour patterns by hybridization. Nat. Commun. 2010, 1, 66. [Google Scholar] [CrossRef]
- Miyazawa, S. Pattern blending enriches the diversity of animal colorations. Sci. Adv. 2020, 6, eabb9107. [Google Scholar] [CrossRef]
- Zhou, K.X.; Zhang, K.X.; Fan, X.; Zhang, W.W.; Liang, Y.S.; Wen, X.; Luo, J. The skin-color is associated with its physiological state: A case study on a colorful variety, hybrid grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus). Aquaculture 2022, 549, 737719. [Google Scholar] [CrossRef]
- Mohamad, S.N.; Noordin, W.N.M.; Ismail, N.F.; Hamzah, A. Red hybrid tilapia (Oreochromis spp.) broodstock development programme in Malaysia: Status, challenges and prospects for future development. Asian Fish. Sci 2021, 34, 73–81. [Google Scholar] [CrossRef]
- Nwachi, O.F.; Irabor, A.E.; Umehai, M.C.; Omonigho, T.; Sanubi, J.O. Pattern of color inheritance in African catfish (Clarias gariepinus): An expression of a Mendelian law. Fish Physiol. Biochem. 2023. [Google Scholar] [CrossRef]
- Subkhankulova, T.; Camargo Sosa, K.; Uroshlev, L.A.; Nikaido, M.; Shriever, N.; Kasianov, A.S.; Yang, X.; Rodrigues, F.S.; Carney, T.J.; Bavister, G. Zebrafish pigment cells develop directly from persistent highly multipotent progenitors. Nat. Commun. 2023, 14, 1258. [Google Scholar] [CrossRef] [PubMed]
- Dawes, J.H.; Kelsh, R.N. Cell fate decisions in the neural crest, from pigment cell to neural development. Int. J. Mol. Sci. 2021, 22, 13531. [Google Scholar] [CrossRef] [PubMed]
- Irion, U.; Nüsslein-Volhard, C. The identification of genes involved in the evolution of color patterns in fish. Curr. Opin. Genet. Dev. 2019, 57, 31–38. [Google Scholar] [CrossRef]
- Lewis, V.M.; Saunders, L.M.; Larson, T.A.; Bain, E.J.; Sturiale, S.L.; Gur, D.; Chowdhury, S.; Flynn, J.D.; Allen, M.C.; Deheyn, D.D. Fate plasticity and reprogramming in genetically distinct populations of Danio leucophores. Proc. Natl. Acad. Sci. USA 2019, 116, 11806–11811. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yin, M.; Ye, Z.; Hu, J.; Bao, Z. Harnessing Hue: Advances and Applications of Fish Skin Pigmentation Genetics in Aquaculture. Fishes 2024, 9, 220. https://doi.org/10.3390/fishes9060220
Liu J, Yin M, Ye Z, Hu J, Bao Z. Harnessing Hue: Advances and Applications of Fish Skin Pigmentation Genetics in Aquaculture. Fishes. 2024; 9(6):220. https://doi.org/10.3390/fishes9060220
Chicago/Turabian StyleLiu, Jialong, Miaomiao Yin, Zhi Ye, Jingjie Hu, and Zhenmin Bao. 2024. "Harnessing Hue: Advances and Applications of Fish Skin Pigmentation Genetics in Aquaculture" Fishes 9, no. 6: 220. https://doi.org/10.3390/fishes9060220
APA StyleLiu, J., Yin, M., Ye, Z., Hu, J., & Bao, Z. (2024). Harnessing Hue: Advances and Applications of Fish Skin Pigmentation Genetics in Aquaculture. Fishes, 9(6), 220. https://doi.org/10.3390/fishes9060220






