Growth, Metabolic, Hepatic and Redox Parameters, Digestive Enzymes and Innate Immunity in Mugil liza Fed a Citral-Supplemented Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Maintenance and Water Quality
2.2. Citral
2.3. Diet and Treatments
2.4. Growth Parameters
2.5. Sample Collection
2.6. Digestive Enzymes
2.7. Metabolic Parameters
2.8. Prooxidant and Antioxidant Analyses
2.9. Innate Immune System Analyses
2.10. Hepatic Enzymes
2.11. Liver Histology
2.12. Statistical Analysis
3. Results
3.1. Growth Parameters
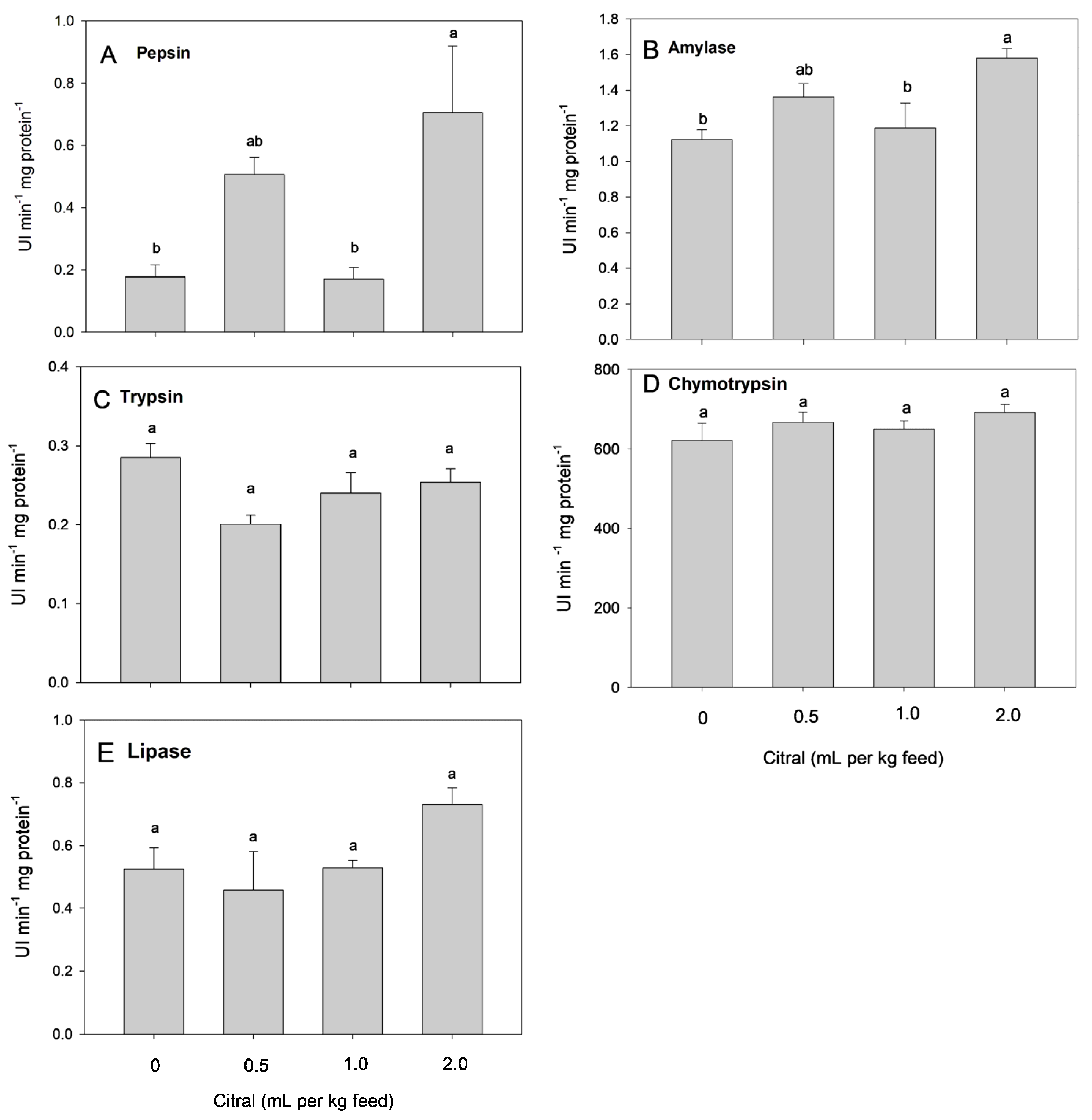
3.2. Digestive Enzymes
3.3. Metabolic Parameters
3.4. Prooxidant and Antioxidant Analyses
3.5. Innate Immune System Analyses
3.6. Plasma Levels of Alanine Aminotransferase and Aspartate Aminotransferase
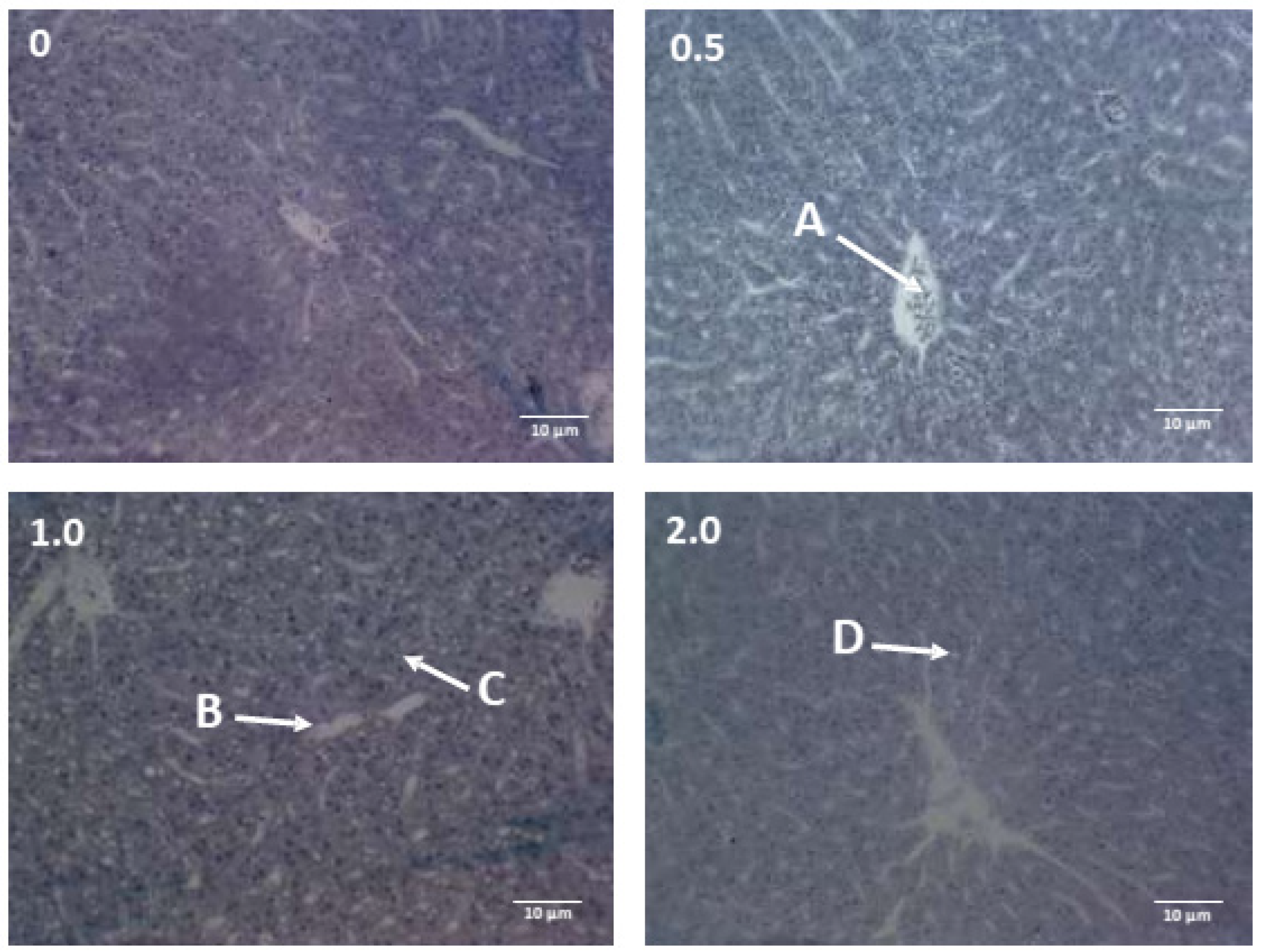
3.7. Hepatic Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, M.; Schrama, D.; Farinha, A.P.; Cerqueira, M.; Raposo de Magalhaes, C.; Carrilho, R.; Rodrigues, P. Fish pathology research and diagnosis in aquaculture of farmed fish; a proteomics perspective. Animals 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Saikia, S.K. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Guo, H.; Dixon, B. Understanding acute stress-mediated immunity in teleost fish. Fish Shellfish Immunol. 2021, 2, 100010. [Google Scholar] [CrossRef] [PubMed]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller, J.D. Stress and immune system in fish. In Biology and Physiology of Freshwater Neotropical Fish; Baldisserotto, B., Urbinati, E.C., Cyrino, J.E.P., Eds.; Academic Press: Cambridge, UK, 2020; pp. 93–114. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical properties and therapeutic potential of citral, a monoterpene isolated from lemongrass. Med. Chem. 2021, 17, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.M.; Souza, R.C.; Melo, J.F.B.; Costa, M.M.; Souza, S.A.; Souza, A.M.; Copatti, C.E. Cymbopogon flexuosus essential oil as an additive improves growth, biochemical and physiological responses and survival against Aeromonas hydrophila infection in Nile tilapia. An. Acad. Bras. Cienc. 2020, 92, e20190140. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, C.C.; Hernández, D.R.; Santinón, J.J.; Heinzmann, B.M.; Cunha, M.A.; Schmidt, D.; Baldisserotto, B. Essential oil of Aloysia triphylla as feed additive promotes growth of silver catfish (Rhamdia quelen). Aquac. Nutr. 2016, 22, 933–940. [Google Scholar] [CrossRef]
- Zeppenfeld, C.; Saccol, E.; Pes, T.; Salbego, J.; Koakoski, G.; dos Santos, A.; Heinzmann, B.; da Cunha, M.; Barcellos, L.; Pavanato, M.; et al. Aloysia triphylla essential oil as food additive for Rhamdia quelen–Stress and antioxidant parameters. Aquac. Nutr. 2017, 23, 1362–1367. [Google Scholar] [CrossRef]
- Copatti, C.E.; Felix e Silva, A.; Lorenzo, V.P.; Costa, M.M.; Melo, J.F.B. Addition of essential oil from lemongrass to the tambaqui (Colossoma macropomum) diet: Effects on growth, intestinal enzymes, haematological and metabolic variables, and antimicrobial challenge. Aquac. Res. 2022, 53, 5656–5666. [Google Scholar] [CrossRef]
- Mori, N.C.; Michelotti, B.T.; Pês, T.d.S.; Bressan, C.A.; Sutili, F.; Kreutz, L.C.; Garlet, Q.; Baldisserotto, B.; Pavanato, M.A.; Cerqueira, V.R.; et al. Citral as a dietary additive for Centropomus undecimalis juveniles: Redox, immune innate profiles, liver enzymes and histopathology. Aquaculture 2019, 501, 14–21. [Google Scholar] [CrossRef]
- Michelotti, B.T.; Mori, N.C.; Magnotti, C.C.F.; Heinzmann, B.M.; Almeida, A.P.G.; Cerqueira, V.R.; Baldisserotto, B. Citral as food additive for common snook—Zootechnical parameters and digestive enzymes. Cien. Rural 2020, 50, e20190577. [Google Scholar] [CrossRef]
- Lemos, V.M.; Cabral, H.; Pasquaud, S.; Vieira, J.P. Occurrence and abundance of young mullet Mugil liza (Teleostei: Mugilidae) in the surf zone along the southern coast of Brazil. Sci. Mar. 2021, 85, 245–255. [Google Scholar] [CrossRef]
- Zeppenfeld, C.C.; Toni, C.; Becker, A.G.; dos Santos Miron, D.; Parodi, T.V.; Heinzmann, B.M.; Barcellos, L.J.G.; Koakoski, G.; Da Rosa, J.G.S.; Loro, V.L. Physiological and biochemical responses of silver catfish, Rhamdia quelen, after transport in water with essential oil of Aloysia triphylla (L‘Herit) Britton. Aquaculture 2014, 418, 101–107. [Google Scholar] [CrossRef]
- Michelotti, B.T.; Passini, G.; Carvalho, C.; Salbego, J.; Mori, N.C.; Vieira, R.; Baldisserotto, B.; Cerqueira, V.R. Growth and metabolic parameters of common snook juveniles raised in freshwater with different water hardness. Aquaculture 2018, 482, 31–35. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Urea, E.; Sanz, A. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 1999, 170, 267–283. [Google Scholar] [CrossRef]
- Hummel, B.C.W. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can. J. Biochem. Physiol. 1959, 37, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Gawlicka, A.; Parent, B.; Horn, M.H.; Ross, N.; Opstad, I.; Torrissen, O.J. Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): Indication of readiness for first feeding. Aquaculture 2000, 184, 303–314. [Google Scholar] [CrossRef]
- Bernfeld, P.; Colowick, S.P. Methods in Enzymology; Academic Press: New York, NY, USA, 1995; p. 149. [Google Scholar]
- Park, J.T.; Johnson, M.J. A submicrodetemination of glucose. J. Biol. Chem. 1949, 181, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farra, L.; Randall, R.J. Protein measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Harrower, J.R.; Brown, C.H. Blood lactic acid–a micromethod adapted to field collection of microliter samples. J. Appl. Physiol. 1972, 32, 709–711. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Södergren, E.; Nourooz-Zadeh, J.; Berglund, L.; Vessby, B. Re-evaluation of the ferrous oxidation in xylenol orange assay for the measurement of plasma lipid hydroperoxides. J. Biochem. Biophys. Methods 1998, 37, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A.; Chance, B. Mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Ellman, J. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.B.; Sharp, G.J.E.; Secombes, C.J.; Robertsen, B. Effect of a yeast-cell-wall glucan on the bactericidal activity of rainbow trout macrophages. Fish Shellfish Immun. 1993, 3, 267–277. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Garcia, A.F.S.; Garcia, A.M.; Vollrath, S.R.; Schneck, F.; Silva, C.F.M.; Marchetti, I.J.; Vieira, J.P. Spatial diet overlap and food resource in two congeneric mullet species revealed by stable isotopes and stomach content analyses. Community Ecol. 2018, 19, 116–124. [Google Scholar] [CrossRef]
- Mitsch, P.; Zitterl-Eglseer, K.; Köhler, B.; Gabler, C.; Losa, R.; Zimpernik, I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 2004, 83, 669–675. [Google Scholar] [CrossRef]
- Sutili, F.J.; Kreutz, L.C.; Flores, F.C.; da Silva, C.D.B.; Kirsten, K.S.; Voloski, A.P.D.S.; Frandoloso, R.; Pinheiro, C.G.; Heinzmann, B.M.; Baldisserotto, B. Effect of dietary supplementation with citral-loaded nanostructured systems on innate immune responses and gut microbiota of silver catfish (Rhamdia quelen). J. Funct. Foods 2019, 60, 103454. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Xu, L.; Zhang, K.; Mei, Y.; Chen, J.; Wang, M.; Guan, Y.; Pang, H.; Wang, Y. The effect of dietary lactic acid bacteria on intestinal microbiota and immune responses of crucian carp (Carassius auratus) under water temperature decrease. Front. Microbiol. 2022, 13, 847167. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef]
- Carvalho e Martins, M.D.C.; Martins Oliveira, A.S.S.S.; da Silva, L.A.A.; Primo, M.G.S.; Lira, V.B.D.C. Biological indicators of oxidative stress [malondialdehyde, catalase, glutathione peroxidase, and superoxide dismutase] and their application in nutrition. In Biomarkers in Nutrition; Biomarkers in Disease: Methods, Discoveries and Applications; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–25. [Google Scholar] [CrossRef]
- Vélez-Alavez, M.; De Anda-Montañez, J.A.; Galván-Magaña, F.; Zenteno-Savín, T. Comparative study of enzymatic antioxidants in muscle of elasmobranch and teleost fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 61–65. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Surai, A. Antioxidant defence systems in health and diseases. In Silymarin Puzzle; Surai, P.F., Surai, A., Eds.; Wageningen Academic: Leiden, The Netherlands, 2023; pp. 85–102. [Google Scholar]
- Averill-Bates, D.A. The antioxidant glutathione. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 109–141. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; Mahmoud, H.K.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquac. Nutr. 2017, 24, 1006–1014. [Google Scholar] [CrossRef]
- Ming, J.H.; Ye, J.Y.; Zhang, Y.X.; Xu, P.; Xie, J. Effects of dietary reduced glutathione on growth performance, non-specific immunity, antioxidant capacity and expression levels of IGF-I and HSP70 mRNA of grass carp (Ctenopharyngodon idella). Aquaculture 2015, 438, 39–46. [Google Scholar] [CrossRef]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharm. 2015, 289, 361–370. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyamoto, M.; Murakami, A.; Ohigashi, H.; Osawa, T.; Uchida, K. A phase II detoxification enzyme inducer from lemongrass: Identification of citral and involvement of electrophilic reaction in the enzyme induction. Biochem. Biophys. Res. Commun. 2003, 302, 593–600. [Google Scholar] [CrossRef]
- Dallas, J.W.; Warne, R.W. Captivity and animal microbiomes: Potential roles of microbiota for influencing animal conservation. Microb. Ecol. 2023, 85, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mei, J.; Xie, J. The effects of lemon balm (Melissa officinalis L.) essential oil on the stress response, anti-oxidative ability, and kidney metabolism of sea bass during live transport. Animals 2022, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, T.; Varshney, C.; Khan, A.; Singh, B.; Jainer, S.; Tiwari, A.K.; Nagarjan, K.; Singh, A.P. Pharmacological potential of lemongrass oil: A systematic review and meta analysis. Biochem. Cell. Arch. 2024, 24, 587–593. [Google Scholar] [CrossRef]
- Dyková, I.; Žák, J.; Blažek, R.; Reichard, M.; Součková, K.; Slabý, O. Histology of major organ systems of Nothobranchius fishes: Short-lived model species. J. Vertebr. Biol. 2022, 71, 21074. [Google Scholar] [CrossRef]

| Ingredients | (g kg−1) | Proximate Average Composition | (g kg−1) |
|---|---|---|---|
| Fish meal | 700 | Protein | 537.3 |
| Fresh squid | 120 | Dry matter | 943.2 |
| Fish oil | 24 | Mineral matter | 207.3 |
| Soy lecithin | 10 | Ether extract | 91.9 |
| Starch | 140 | Acid detergent fiber | 20.4 |
| Minerals and vitamins (premix) * | 5 | Neutral detergent fiber | 14.3 |
| Vitamin C | 1 |
| Citral (mL per kg Feed) | ||||
|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 2.0 | |
| Weight gain | 7.35 ± 0.10 b | 8.93 ± 0.90 ab | 9.26 ± 0.69 ab | 10.74 ± 0.76 a |
| Specific growth rate | 2.00 ± 0.07 a | 2.45 ± 0.26 a | 2.29 ± 0.16 a | 2.56 ± 0.16 a |
| Condition factor | 2.43 ± 0.06 a | 2.76 ± 0.05 a | 2.49 ± 0.06 a | 2.85 ± 0.04 a |
| Feed intake | 12.75 ± 0.87 a | 14.35 ± 0.43 a | 14.20 ± 0.40 a | 17.39 ± 0.50 a |
| Feed conversion rate | 1.74 ± 0.02 a | 1.64 ± 0.15 a | 1.55 ± 0.11 a | 1.64 ± 0.12 a |
| Protein retention efficiency | 50.3 ± 0.7 b | 68.8 ± 6.9 b | 70.6 ± 5.2 b | 100.3 ± 7.1 a |
| Citral (mL per kg Feed) | ||||
|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 2.0 | |
| HG | 312.4 ± 15.02 a | 197.31 ± 9.27 b | 272.11 ± 32.24 ab | 327.82 ± 26.16 a |
| MG | 187.83 ± 11.02 a | 212.13 ± 5.53 a | 203.1 ± 23.94 a | 240.49 ± 20.29 a |
| HL | 1.38 ± 0.19 a | 1.84 ± 0.05 ab | 1.70 ± 0.08 ab | 2.24 ± 0.11 b |
| ML | 3.48 ± 0.35 a | 1.79 ± 0.24 b | 1.95 ± 0.27 b | 2.33 ± 0.17 ab |
| Citral (mL per kg Feed) | ||||
|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 2.0 | |
| Liver | ||||
| LOOH | 4.60 ± 0.02 a | 2.19 ± 0.18 b | 4.61 ± 0.30 a | 1.98 ± 0.13 b |
| SOD | 2.02 ± 0.02 a | 2.49 ± 0.04 b | 2.6 ± 0.11 b | 2.31 ± 0.09 ab |
| CAT | 3.18 ± 0.06 a | 2.67 ± 0.39 a | 3.52 ± 0.19 a | 2.78 ± 0.008 a |
| GPx | 1.96 ± 0.22 c | 9.79 ± 0.59 b | 10.06 ± 0.34 b | 16.22 ± 0.65 a |
| GST | 113.8 ± 2.64 b | 95.42 ± 4.20 b | 163.56 ± 5.83 a | 173.42 ± 12.79 a |
| NPSH | 4.15 ±0.10 a | 3.73 ± 0.20 a | 3.90 ± 0.08 a | 3.94 ± 0.04 a |
| Gills | ||||
| LOOH | 7.64 ± 0.29 a | 5.84 ± 0.36 a | 3.68 ± 0.35 b | 6.76 ± 0.68 a |
| SOD | 1.24 ± 0.11 bc | 1.02 ± 0.11 c | 1.98 ± 0.11 a | 2.32 ± 0.08 a |
| CAT | 0.41 ± 0.13 a | 0.47 ± 0.01 a | 0.53 ± 0.004 a | 0.64 ± 0.09 a |
| GPx | 4.71 ± 0.19 b | 4.91 ± 0.44 b | 4.50 ± 0.09 b | 9.79 ± 0.20 a |
| GST | 7.23 ± 0.82 b | 7.71 ± 0.06 b | 14.10 ± 0.48 a | 14.20 ± 0.04 a |
| NPSH | 7.79 ± 0.09 b | 7.33 ± 0.17 b | 7.22 ± 0.13 b | 12.07 ± 0.45 a |
| Brain | ||||
| LOOH | 7.50 ± 0.04 a | 3.44 ± 0.24 b | 3.22 ± 0.001 b | 3.52 ± 0.09 b |
| SOD | 1.27 ± 0.06 bc | 2.59 ± 0.08 a | 0.67 ± 0.02 c | 1.69 ± 0.06 b |
| CAT | 0.04 ± 0.02 a | 0.03 ± 0.00 a | 0.03 ± 0.001 a | 0.05 ± 0.001 a |
| GPx | 16.01 ± 0.69 c | 36.73 ± 1.19 a | 30.59 ± 0.89 b | 27.99 ± 0.14 b |
| GST | 129.16 ± 5.45 c | 140.98 ± 5.98 bc | 150.39 ± 4.03 ab | 213.35 ± 1.20 a |
| NPSH | 11.26 ± 0.07 b | 11.32 ± 0.12 b | 8.60 ± 1.53 b | 15.42 ± 0.30 a |
| Citral (mL per kg Feed) | AST (U mL−1) | ALT (U mL−1) |
|---|---|---|
| 0.0 | 55.15 ± 13.28 | 7.89 ± 0.47 |
| 0.5 | 68.72 ± 4.84 | 11.28 ± 1.64 |
| 1.0 | 47.04 ± 4.60 | 7.61 ± 1.65 |
| 2.0 | 58.65 ± 11.40 | 10.39 ±1.53 |
| Citral (mL per kg Feed) | ||||
|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 2.0 | |
| Area of the hepatocytes (µm2) | 67.33 ± 3.51 | 65.63 ± 3.51 | 70.69 ± 1.49 | 63.34 ± 0.05 |
| Diameter of sinusoidal capillaries (µm) | 4.28 ± 0.15 | 4.74 ± 0.04 | 3.90 ± 0.55 | 5.03 ± 0.04 |
| Diameter of lobular central vein (µm) | 1469 ± 421.03 | 2079 ± 449.3 | 2362 ± 218.68 | 2005 ± 458.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, N.C.; Michelotti, B.T.; Magnotti, C.C.F.; Bressan, C.A.; Bianchin, L.B.; Sutili, F.J.; Almeida, A.P.G.; Kreutz, L.C.; Pavanato, M.A.; Cerqueira, V.R.; et al. Growth, Metabolic, Hepatic and Redox Parameters, Digestive Enzymes and Innate Immunity in Mugil liza Fed a Citral-Supplemented Diet. Fishes 2024, 9, 240. https://doi.org/10.3390/fishes9060240
Mori NC, Michelotti BT, Magnotti CCF, Bressan CA, Bianchin LB, Sutili FJ, Almeida APG, Kreutz LC, Pavanato MA, Cerqueira VR, et al. Growth, Metabolic, Hepatic and Redox Parameters, Digestive Enzymes and Innate Immunity in Mugil liza Fed a Citral-Supplemented Diet. Fishes. 2024; 9(6):240. https://doi.org/10.3390/fishes9060240
Chicago/Turabian StyleMori, Natacha C., Bruna T. Michelotti, Caio C. F. Magnotti, Caroline A. Bressan, Letícia B. Bianchin, Fernando J. Sutili, Ana Paula G. Almeida, Luiz C. Kreutz, Maria A. Pavanato, Vinicius R. Cerqueira, and et al. 2024. "Growth, Metabolic, Hepatic and Redox Parameters, Digestive Enzymes and Innate Immunity in Mugil liza Fed a Citral-Supplemented Diet" Fishes 9, no. 6: 240. https://doi.org/10.3390/fishes9060240





