Effects of Different Stocking Densities on the Growth, Antioxidant Status, and Intestinal Bacterial Communities of Carp in the Rice–Fish Co-Culture System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Sample Collection
2.3. Growth Parameters Analysis
2.4. Biochemical and Antioxidant Status Analysis
2.5. Microbial Sequencing
2.6. Statistical Analysis
3. Results
3.1. Change in Growth Performance
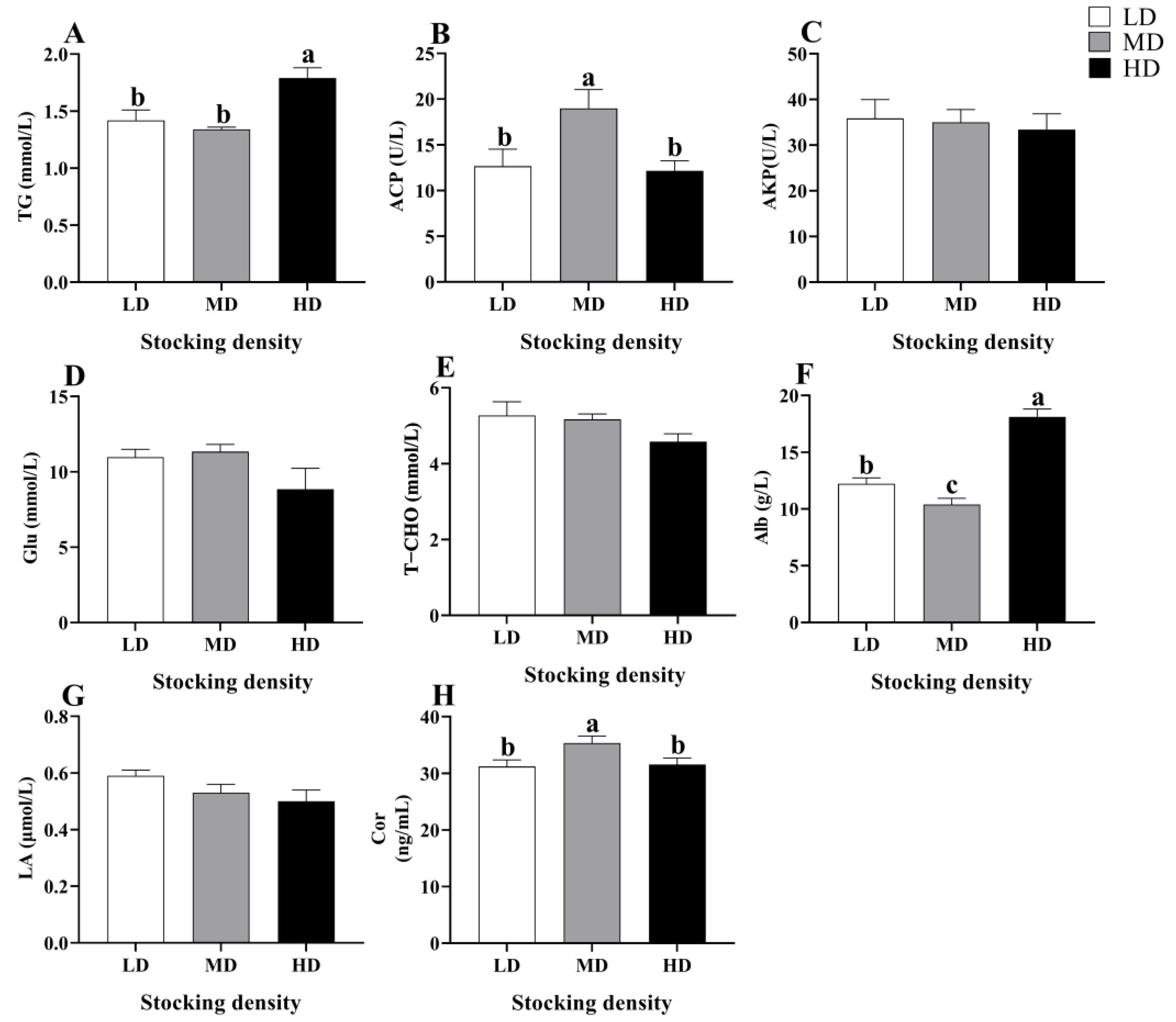
3.2. Change in Biochemical Indices in Serum
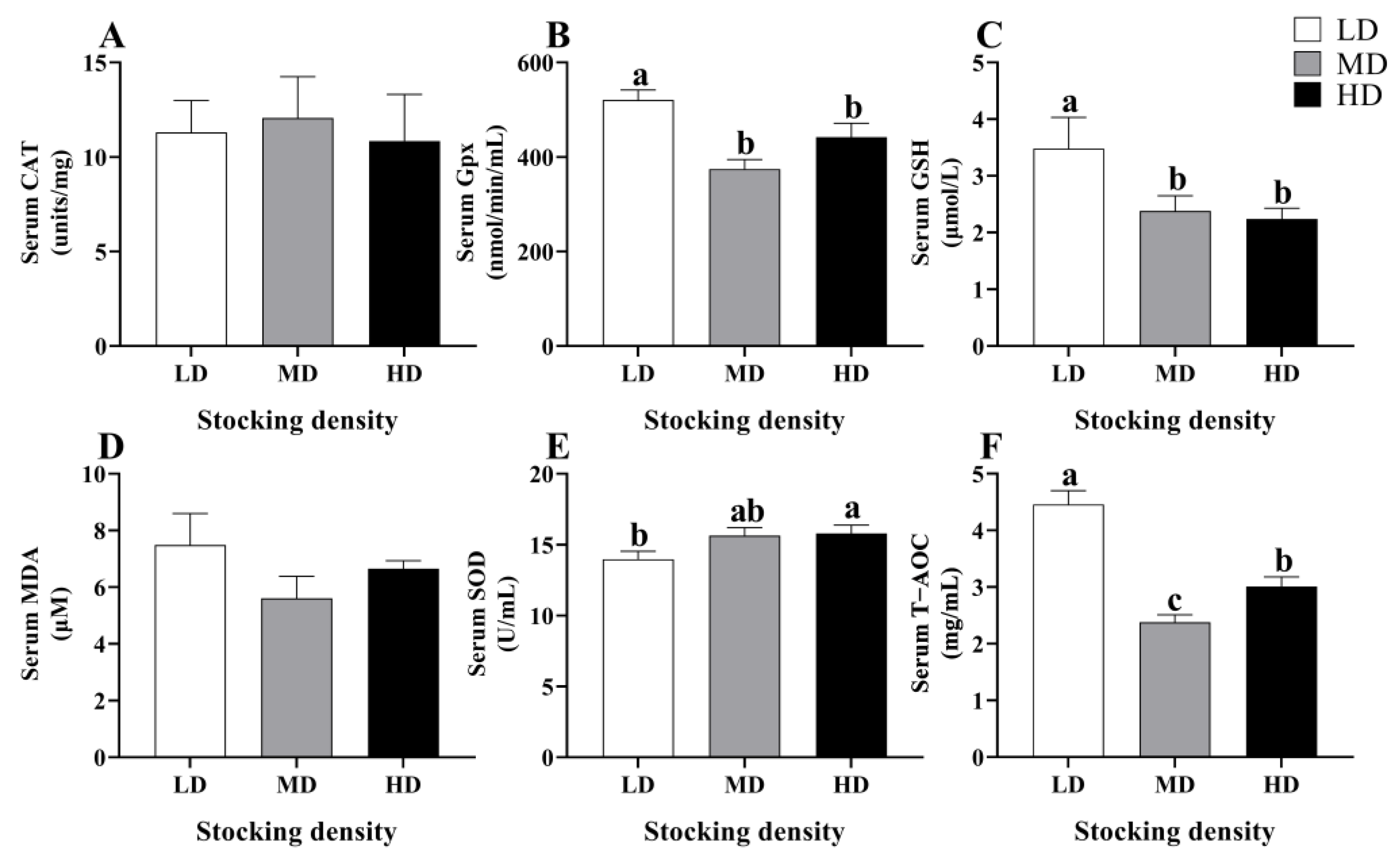
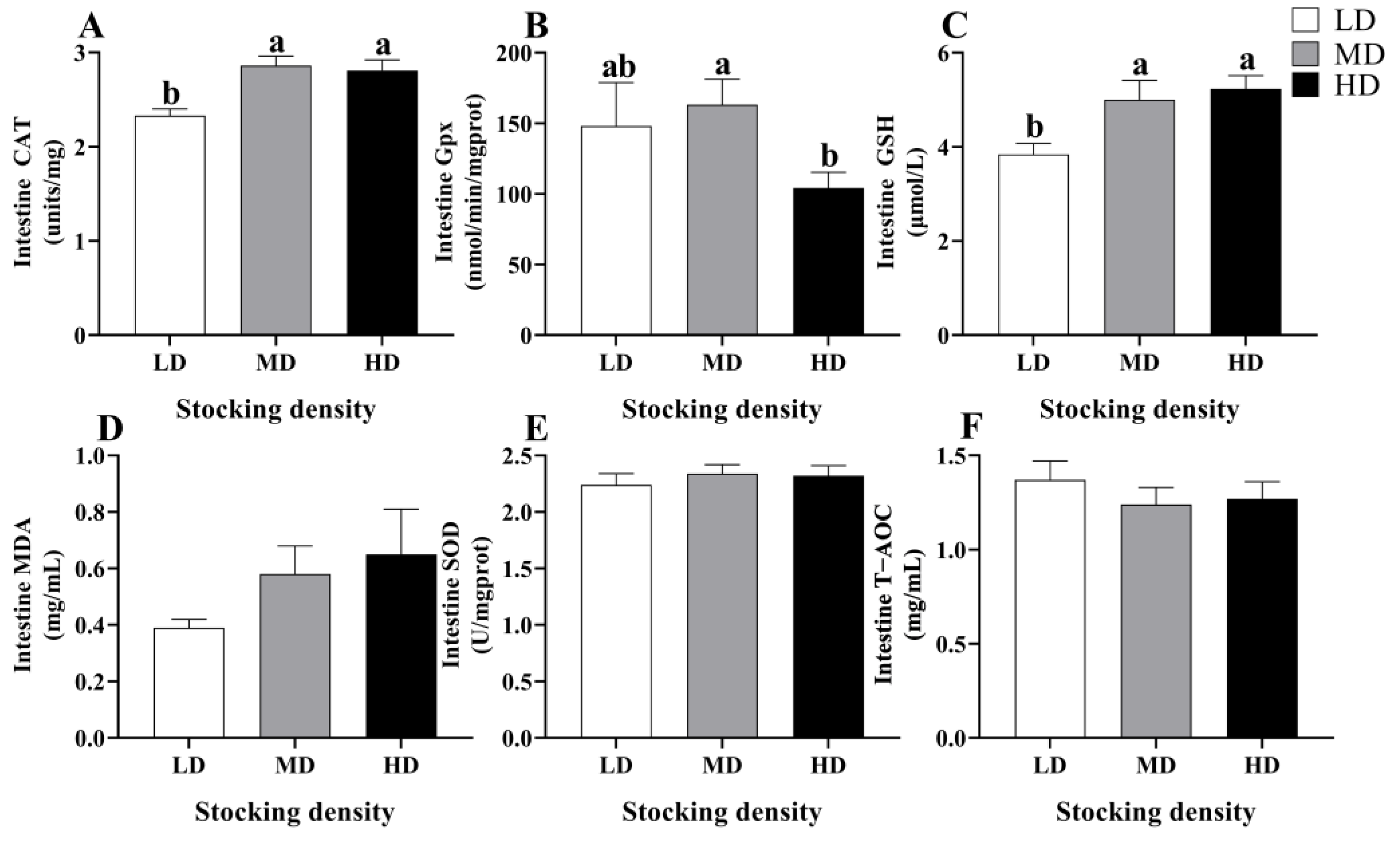
3.3. Change in Antioxidant Status in Serum and Intestine
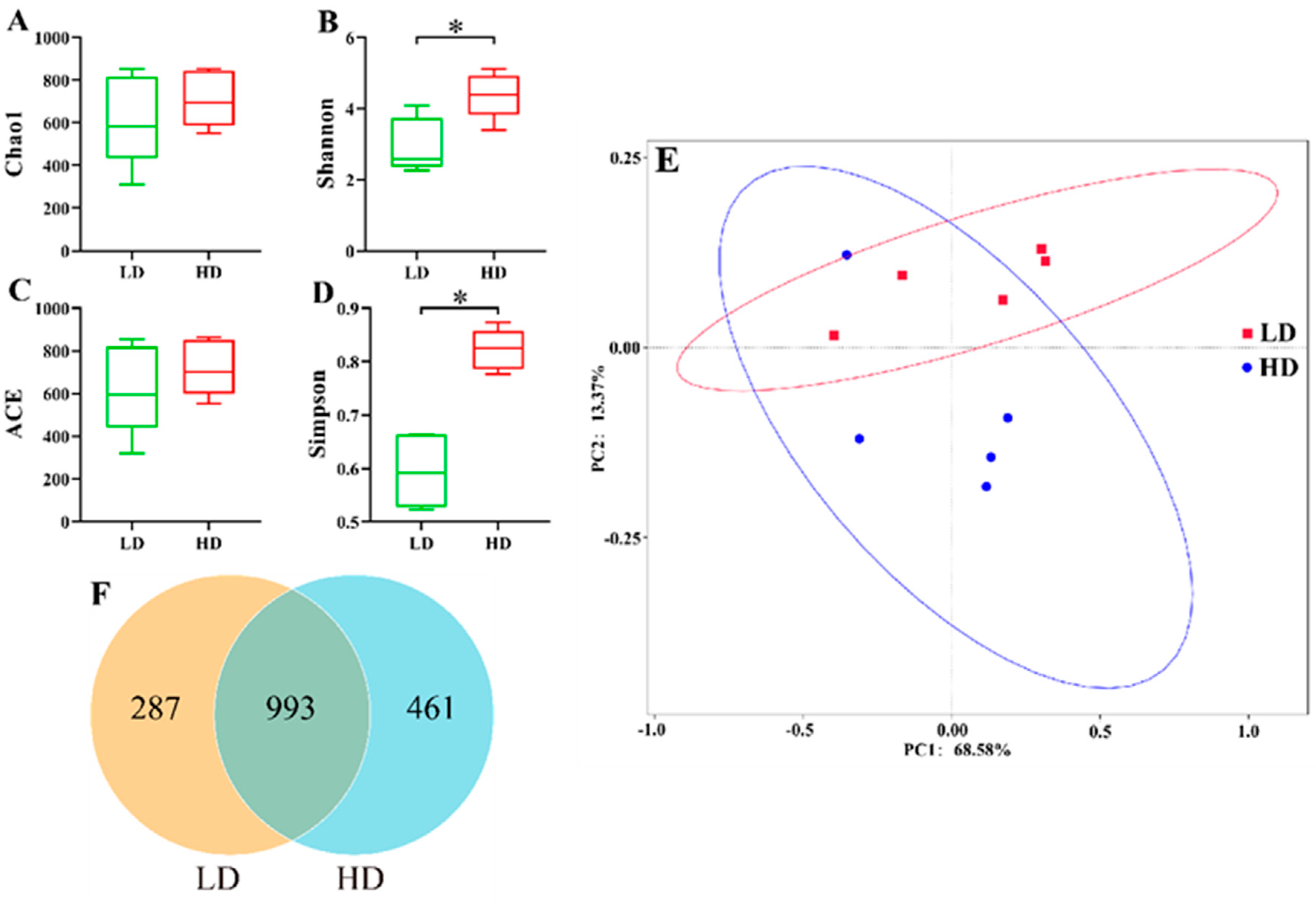
3.4. Alpha and Beta Diversities of Bacteria in Intestine
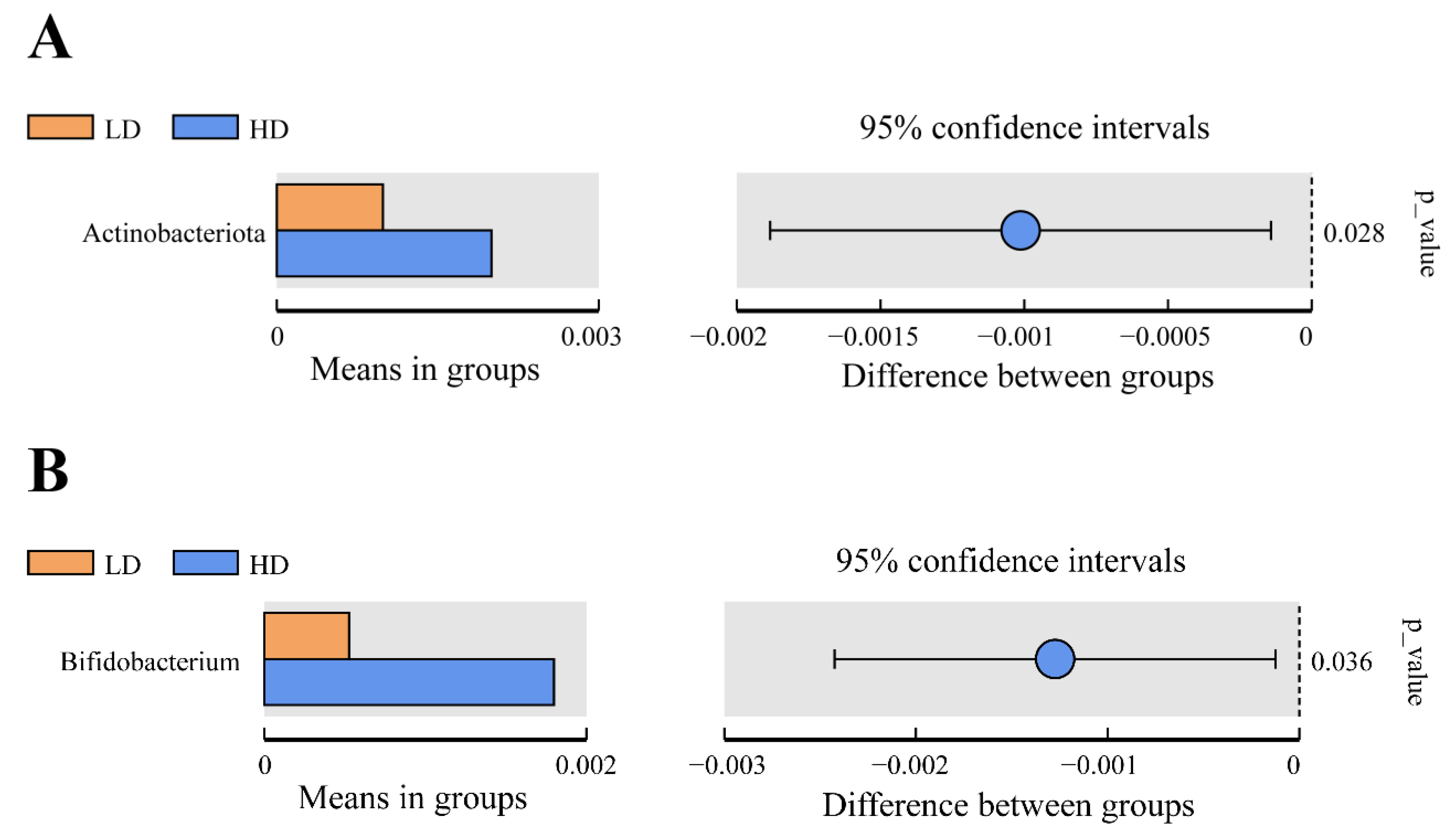
3.5. Bacterial Composition in Intestine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, P.C.M.; Silva, A.E.M.; Silva, D.A.; Silva, S.M.B.C.; Brito, L.O.; Gálvez, A.O. Effect of Stocking Density of Crassostrea sp. in a Multitrophic Biofloc System with Litopenaeus vannamei in Nursery. Aquaculture 2021, 530, 735913. [Google Scholar] [CrossRef]
- Diao, W.; Jia, R.; Hou, Y.; Dong, Y.; Li, B.; Zhu, J. Effects of Stocking Density on the Growth Performance, Physiological Parameters, Antioxidant Status and Lipid Metabolism of Pelteobagrus fulvidraco in the Integrated Rice-Fish Farming System. Animals 2023, 13, 1721. [Google Scholar] [CrossRef] [PubMed]
- Nga, B.; Lürling, M.; Peeters, E.; Roijackers, R.; Scheffer, M.; Nghia, T. Chemical and Physical Effects of Crowding on Growth and Survival of Penaeus monodon Fabricius Post-Larvae. Aquaculture 2005, 246, 455–465. [Google Scholar] [CrossRef]
- Yu, X.; Hao, X.; Dang, Z.; Yang, L. Report on the Development of China’s Integrated Rice-Fish Farming (2023). China Fish. 2023, 19–26. [Google Scholar]
- Liu, J.; Zhao, Z.; Luo, L.; Wang, S.; Zhang, R.; Guo, K.; Bai, Q.; Li, H.; Li, M. Effects of Different Crab Stocking Density on Production Performance and Environmental Factors under Integrated Cultivation of Rice Crab in the Cold Areas. Fresh Water Fish. 2022, 52, 89–97. [Google Scholar]
- Guan, W.; Liu, K.; Shi, W.; Xuan, F.; Wang, W. Scientific Paradigm of Integrated Farming of Rice and Fish. Acta Ecol. Sin. 2020, 40, 5451–5464. [Google Scholar]
- Mabaya, G.; Unami, K.; Yoshioka, H.; Takeuchi, J.; Fujihara, M. Robust Optimal Diversion of Agricultural Drainage Water from Tea Plantations to Paddy Fields During Rice Growing Seasons and Non-Rice Growing Seasons. Paddy Water Environ. 2016, 14, 247–258. [Google Scholar] [CrossRef]
- Nakasone, H. Runoff Water Quality Characteristics in a Small Agriculture Watershed. Paddy Water Environ. 2003, 1, 183–188. [Google Scholar] [CrossRef]
- Huang, Z. In Vitro Bacteriostatic Test of Traditional Chinese Medicine against Aeromonas hydrophila of Cyprinus carpio Origin. North. Chin. Fish. 2023, 42, 437–440. [Google Scholar]
- Dong, Z.; Luo, M. Main Methods, Genetic Analysis and Prospect of Common Carp (Cyprinus carpio) Breeding in China. J. Fish. China 2023, 47, 39–55. [Google Scholar]
- Jui, R.A.; Haque, M.M.; Rahmatullah, S. Growth Performance of Silver Carp Hypophthalmichthys molitrix in Cage Stocked at Different Densities. J. Bangladesh Agric. Univ. 2018, 16, 322–327. [Google Scholar] [CrossRef]
- Stanivuk, J.; Nagy, L.B.; Gyalog, G.; Ardó, L.; Vitál, Z.; Plavša, N.; Krstović, S.; Fazekas, G.L.; Horváth, Á.; Ljubobratović, U. The Rank of Intensification Factors Strength in Intensive Pond Production of Common Carp (Cyprinus carpio L.). Aquaculture 2024, 583, 740584. [Google Scholar] [CrossRef]
- Ponpandy, N.; Kumar, V.A.; Subodh, G.; Annamalai, J.; Mallikarjun, H.C. Effects of Different Stocking Densities on Haematological, Non-Specific Immune, and Antioxidant Defence Parameters of Striped Catfish (Pangasianodon hypophthalmus) Fingerlings Reared in Finger Millet-Based Biofloc System. Aquac. Int. 2022, 30, 3229–3245. [Google Scholar]
- Ray, G.W. Effects of Replacing Fishmeal with Alternative Plant Proteins Surce Including Spc, Wgm and Ddgs on Growth, Serum Biochemical Indices, and Antioxidative Functions, and Microbiota for Juvenile Shrimp Litopenaeus vannamei. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2020. [Google Scholar]
- Wang, J. Hypoxia Tolerance and Physiological Alterations in Turbot (Scophthalmus maximus). Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar]
- Hu, S.; Lin, Y.; Shi, X.; Chi, C. Effects of Dietary Supplementation of Silphium Perfoliatum on Growth Performance, Antioxidant Capacity and Lipid Metabolism of Juvenile Megalobrama amblycephal. J. Fish. China 2024, 48, 323–336. [Google Scholar]
- Ren, Y.; Luo, J.; Wang, R.; Zhou, Z. Effects of Different Zinc Sources on the Intestinal Morphology, Digestive Enzymes, Antioxidant Capacity, and Mineral Element Deposition of Pearl Gentian Grouper. Guangdong Feed J. 2023, 32, 31–34. [Google Scholar]
- Yong, S.; Yi, H.; Ziqin, W.; Jiancheng, Z.; Junzhi, Z.; Huan, Z.; Guihong, F.; Lei, Z. The Protective Effect of Taurine on Oxidized Fish-Oil-Induced Liver Oxidative Stress and Intestinal Barrier-Function Impairment in Juvenile Ictalurus punctatus. Antioxidants 2021, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Uparse: Highly Accurate Otu Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of Rrna Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Muscle: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Minchin, P.R. An Evaluation of the Relative Robustness of Techniques for Ecological Ordination. Vegetatio 2004, 69, 89–107. [Google Scholar] [CrossRef]
- Ni, M.; Wen, H.; Li, J.; Chi, M.; Bu, Y.; Ren, Y.; Zhang, M.; Song, Z.; Ding, H. The Physiological Performance and Immune Responses of Juvenile Amur Sturgeon (Acipenser schrenckii) to Stocking Density and Hypoxia Stress. Fish Shellfish Immunol. 2014, 36, 325–335. [Google Scholar] [CrossRef] [PubMed]
- De las Heras, V.; Martos-Sitcha, J.A.; Yúfera, M.; Mancera, J.M.; Martínez-Rodríguez, G. Influence of Stocking Density on Growth, Metabolism and Stress of Thick-Lipped Grey Mullet (Chelon labrosus) Juveniles. Aquaculture 2015, 448, 29–37. [Google Scholar] [CrossRef]
- Gunjan, K.; Kumar, D.B.; Puthiyottil, M.; Tasso, T.; Suman, K.; Kumar, S.U.; Kanti, D.A.; Yusuf, A. Impact of Stocking Density on Growth, Feed Utilization and Survival of Cage Reared Minor Carp, Labeo bata (Hamilton, 1822) in Maithon Reservoir, India. Aquaculture 2021, 532, 736078. [Google Scholar]
- Ezhilmathi, S.; Ahilan, B.; Uma, A.; Felix, N.; Cheryl, A.; Somu Sunder Lingam, R. Effect of Stocking Density on Growth Performance, Digestive Enzyme Activity, Body Composition and Gene Expression of Asian Seabass Reared in Recirculating Aquaculture System. Aquac. Res. 1963, 53, 1963–1972. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Yang, M.; Gan, H.; Lu, X.; Ruan, Z.; Lv, M. Effects of Stocking Density and Antimicrobial Peptides on Growth in Yellow Catfish Pelteobagrus Fulvidraco Cultured in Net Cages. Chin. J. Fish. 2020, 33, 66–71. [Google Scholar]
- CristianAlin, B.; Mihaela, R.C.; Marian, B.; Lenuta, D.; LiviuDan, M.; Stefan, B.R.; Gabriela, D.; Elena, T. Comparative Study of Flesh Quality, Blood Profile, Antioxidant Status, and Intestinal Microbiota of European Catfish (Silurus glanis) Cultivated in a Recirculating Aquaculture System (Ras) and Earthen Pond System. Life 2023, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Xie, C.; Tang, R.; Qin, X.; Wang, D.; Li, D. Effect of Stocking Density on Growth, Physiological Responses, and Body Composition of Juvenile Blunt Snout Bream, Megalobrama amblycephala. J. World Aquac. Soc. 2016, 47, 358–368. [Google Scholar] [CrossRef]
- Guo, H.Y.; Dong, X.Y.; Zhang, X.M.; Zhang, P.D.; Li, W.T. Survival, Growth and Physiological Responses of Juvenile Japanese Flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846) Exposed to Different Dissolved Oxygen Concentrations and Stocking Densities. J. Appl. Ichthyol. 2017, 33, 731–739. [Google Scholar] [CrossRef]
- Tolussi, C.E.; Hilsdorf, A.W.S.; Caneppele, D.; Moreira, R.G. The Effects of Stocking Density in Physiological Parameters and Growth of the Endangered Teleost Species Piabanha, Brycon Insignis (Steindachner, 1877). Aquaculture 2010, 310, 221–228. [Google Scholar] [CrossRef]
- Marchi, A.; Bonaldo, A.; Di Biase, A.; Cerri, R.; Scicchitano, D.; Nanetti, E.; Candela, M.; Picone, G.; Capozzi, F.; Dondi, F.; et al. Towards a Free Wild-Caught Fishmeal, Fish Oil and Soy Protein in European Sea Bass Diet Using by-Products from Fishery and Aquaculture. Aquaculture 2023, 573, 739571. [Google Scholar] [CrossRef]
- Shi, X. The Stress-Influences of Rearing Densities on Juvenile Amur Sturgeon, Acipenser schrenckii. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2006. [Google Scholar]
- Wang, X.; Fang, X.; Peng, S.; Wang, Q.; Shi, Z. Impact of Abrupt Salinity Changes on Activitiy of Metabolic Enzymes, Antioxidant Enzymes and Cortisol Content in Serum and Liver of Lateolabrax maculatus. Mar. Fish. 2021, 43, 340–349. [Google Scholar] [CrossRef]
- Ran, F.; Jin, W.; Huang, S.; Liu, C.; Li, Z.; Li, C. Research Progress on the Effects of Salinity Change on Fish. Northwest A F Univ. (Nat. Sci. Ed.) 2020, 48, 10–18. [Google Scholar]
- Santos, E.L.R.; Rezende, F.P.; Moron, S.E. Stress-Related Physiological and Histological Responses of Tambaqui (Colossoma macropomum) to Transportation in Water with Tea Tree and Clove Essential Oil Anesthetics. Aquaculture 2020, 523, 735164. [Google Scholar] [CrossRef]
- Lin, L.; Sun, X.; Xing, K.; Guo, Y. Effects of Stocking Density on Grouwth and Blood Biochemical Parameters in Plectropomus ieopardus. Fish. Sci. 2017, 1, 1003–1111. [Google Scholar]
- Zhu, M.; Kong, F.; Zhao, Q. Exercise Regulates Lactic Acid Metabolism. Chin. J. Tissue Eng. Res. 2023, 27, 322–328. [Google Scholar]
- Refaey, M.M.; Li, D.; Tian, X.; Onxayvieng, K.; Tang, R. Physiological Responses of Channel Catfish (Ictalurus punctatus) Reared at Different Stocking Densities in a Recirculating Aquaculture System. Aquaculture 2022, 557, 738329. [Google Scholar] [CrossRef]
- Lv, B.; Ye, Y.; Luo, H.; Ma, J.; Wang, Z.; Wu, D.; Tang, F.; Pu, Q. Effects of Dietary Sargassum Powder on Growth Performance, Tissue Enzyme Activity and Serum Biochemical Indices of Yellow Catfish (Pelteobagrus fulvidraco). Feed Ind. 2022, 43, 52–59. [Google Scholar]
- Zhang, Q.; Hou, J.; Yang, J.; Liao, J.; Lu, J.; He, X. Captive Density on Growth Performance and Health Status of Largemouth Bass (Micropterus salmoides). Acta Hydrobiol. Sin. 2022, 46, 671–678. [Google Scholar]
- Wojtczyk-Miaskowska, A.; Schlichtholz, B. DNA Damage and Oxidative Stress in Long-Lived Aquatic Organisms. DNA Repair 2018, 69, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, J.S.; Herrejón, G.A.P.; Becerra, H.A. Fish Responses to Alternative Feeding Ingredients under Abiotic Chronic Stress. Animals 2024, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Zhang, Y.; Liang, X.; Tang, S. The Influence of Stocking Density on the Growth, Blood Biochemistry, and Antioxidant Capacity Indicators of Chinese Perch (Siniperca chuatsi). Acta Hydrobiol. Sin. 2023, 47, 473–478. [Google Scholar]
- Hoseini, S.M.; Yousefi, M.; Mirghaed, A.T.; Paray, B.A.; Hoseinifar, S.H.; Van Doan, H. Effects of Rearing Density and Dietary Tryptophan Supplementation on Intestinal Immune and Antioxidant Responses in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2020, 528, 735537. [Google Scholar] [CrossRef]
- Choi, C.Y.; Choi, J.Y.; Choi, Y.J.; Kim, B.-S.; Kim, J.-W. Effects of Green Wavelength Light on Antioxidant and Non-Specific Immune Responses of the Olive Flounder Paralichthys olivaceus Maintained at Different Stocking Densities. Aquac. Eng. 2019, 84, 23–28. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Sun, P.; Tang, B. Physiological Response of Larimichthys Crocea under Differentstocking Densities and Screening of Stress Sensitive Biomarkers. Mar. Fish. 2021, 43, 327–339. [Google Scholar] [CrossRef]
- Zhang, Y. Effect of Air Exposure and Transportation Stress on Plasma Biochemical Profiles and Hsp70 Mrna Expression of American Shad (Alosa sapidissima) Broodstock. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Ma, C.; Chen, C.X.; Jia, L.; He, X.X.; Zhang, B. Comparison of the Intestinal Microbiota Composition and Function in Healthy and Diseased Yunlong Grouper. AMB Express 2019, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tao, H.; Liao, X.; Hu, S.; Zhao, J.; He, Z.; Han, X.; Chen, Z.; Sun, C. Effects of Stocking Density on Bacterial Community Characterization of Biofloc and Intestine of Fenneropenaeus merguiensis. J. Hunan Agric. Univ. (Nat. Sci.) 2020, 46, 608–615. [Google Scholar]
- Nie, Z.; Xu, G.; Shao, N.; Wang, B.; Gao, J.; Xu, P.; He, J. Comparison of Gut Microbiota in Carps from Fish Monoculture Ponds and the Rice-Fish Co-Culture System in Hani Terraces. Acta Microbiol. Sin. 2022, 62, 1473–1484. [Google Scholar]
- Tan, C.K.; Natrah, I.; Suyub, I.B.; Edward, M.J.; Kaman, N.; Samsudin, A.A. Comparative Study of Gut Microbiota in Wild and Captive Malaysian Mahseer (Tor tambroides). Microbiologyopen 2019, 8, e00734. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, P.; Balaraman, D.; Thirunavukkarasu, R.; George, E.G.J.; Subaramaniyan, K.; Manikkam, S.; Sadayappan, B. Probiotics in Aquaculture. Drug Invent. Today 2013, 5, 55–59. [Google Scholar] [CrossRef]
- Wen, J.; Sun, X. Research Progress on Gut Microbiota Regulation in Aquatic Animals. Feed Ind. 2009, 68–70. [Google Scholar] [CrossRef]

| Groups | Initial Weight (g) | Final Weight (g) | WG (%) | SGR (%/d) |
|---|---|---|---|---|
| LD | 105.55 ± 0.33 | 178.3 ± 10.19 a | 1.15 ± 0.096 a | 0.84 ± 0.092 a |
| MD | 105.72 ± 0.18 | 155.41 ± 8.15 ab | 0.78 ± 0.054 b | 0.62 ± 0.086 ab |
| HD | 105.43 ± 0.24 | 136.36 ± 5.09 b | 0.49 ± 0.047 c | 0.41 ± 0.063 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, W.; Jia, R.; Hou, Y.; Gong, J.; Zhang, L.; Li, B.; Zhu, J. Effects of Different Stocking Densities on the Growth, Antioxidant Status, and Intestinal Bacterial Communities of Carp in the Rice–Fish Co-Culture System. Fishes 2024, 9, 244. https://doi.org/10.3390/fishes9070244
Diao W, Jia R, Hou Y, Gong J, Zhang L, Li B, Zhu J. Effects of Different Stocking Densities on the Growth, Antioxidant Status, and Intestinal Bacterial Communities of Carp in the Rice–Fish Co-Culture System. Fishes. 2024; 9(7):244. https://doi.org/10.3390/fishes9070244
Chicago/Turabian StyleDiao, Weixu, Rui Jia, Yiran Hou, Jianyou Gong, Liqiang Zhang, Bing Li, and Jian Zhu. 2024. "Effects of Different Stocking Densities on the Growth, Antioxidant Status, and Intestinal Bacterial Communities of Carp in the Rice–Fish Co-Culture System" Fishes 9, no. 7: 244. https://doi.org/10.3390/fishes9070244







