Characterization of Ovarian Lipid Composition in the Largemouth Bronze Gudgeon (Coreius guichenoti) at Different Development Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish Treatment
2.2. Experiment Materials and Methods
2.2.1. H&E Staining Tests
2.2.2. Sample Preparation
2.2.3. Lipidomics
2.2.4. KEGG Annotation and Enrichment Analysis
2.3. Statistical Analysis
3. Results
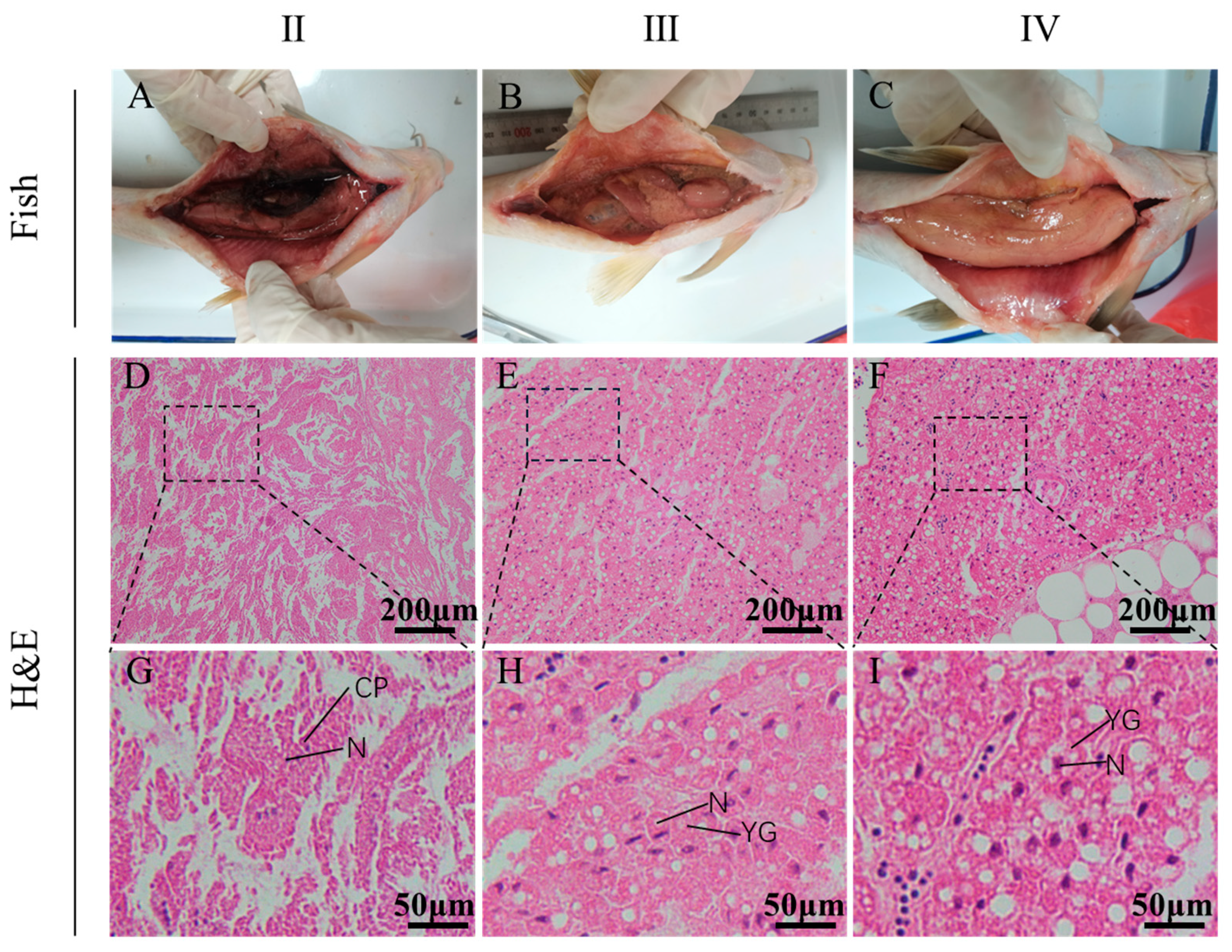
3.1. The Characteristic of Ovarian Morphology and Histology from Stage II to Stage IV
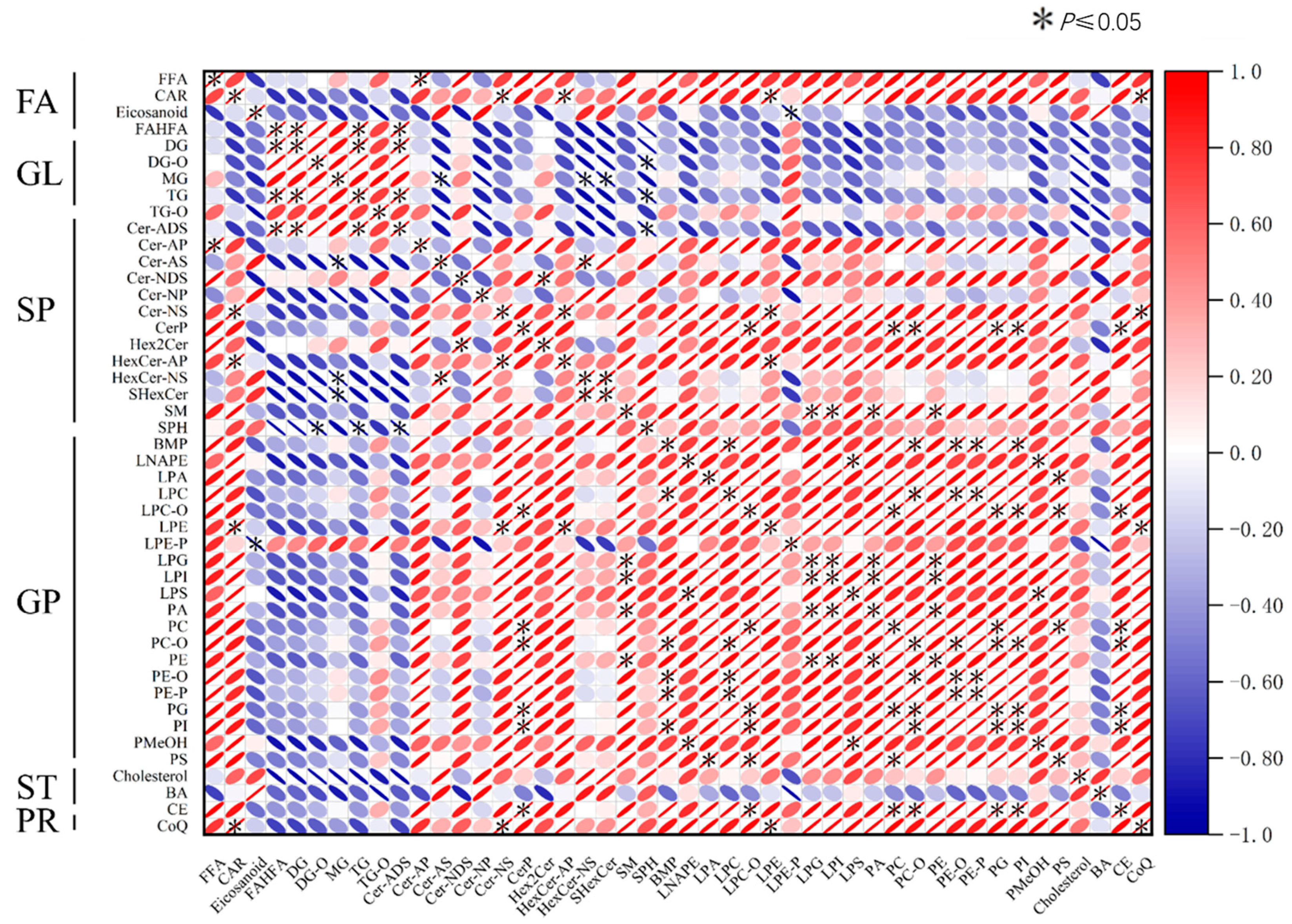
3.2. The Profile of Ovarian Lipid Composition and Correlation Analysis
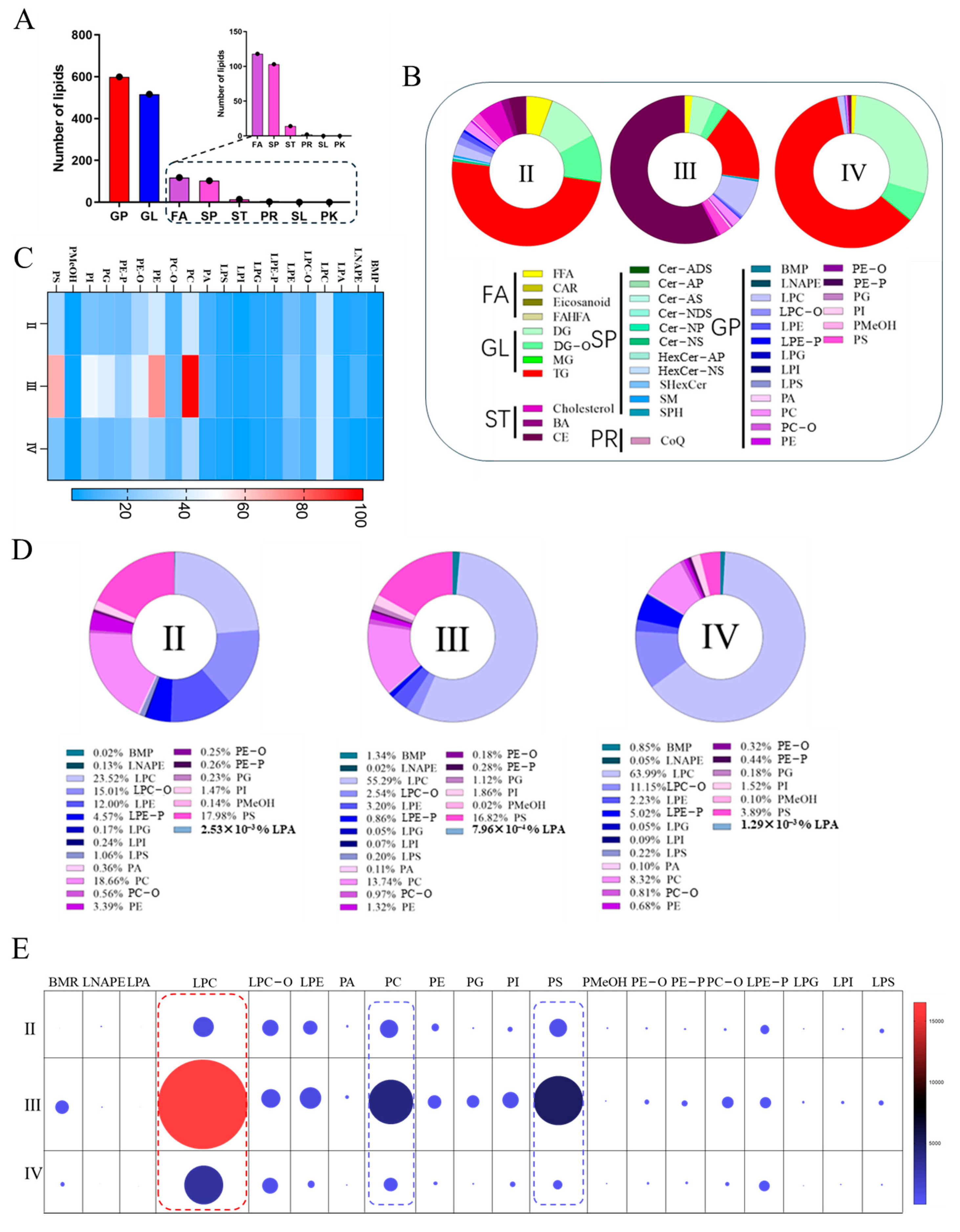
3.3. The Category and Content Analysis of Ovarian Lipid at Different Stages
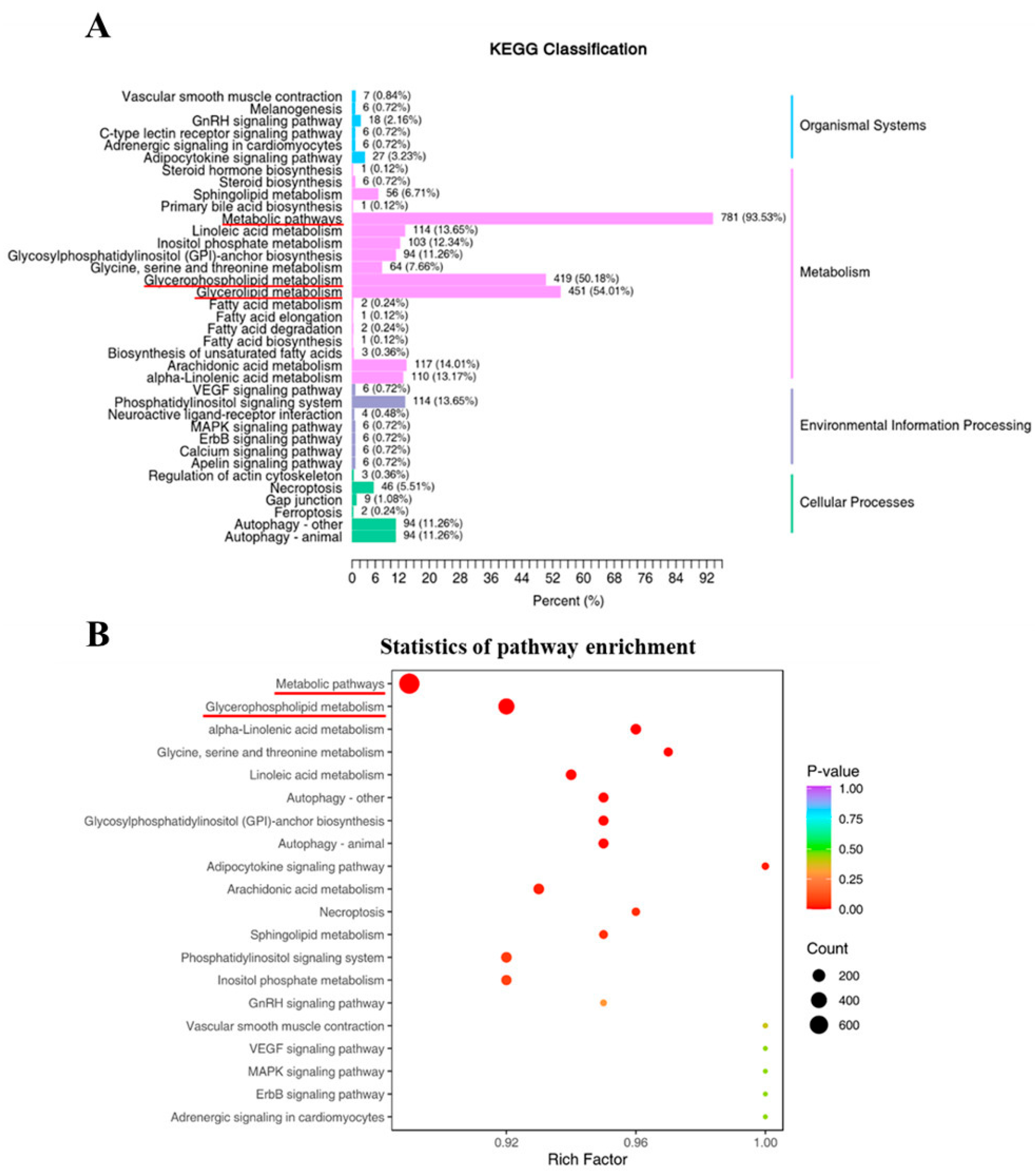
3.4. Lipid-Metabolism-Related Pathways and Their Differential Metabolic Lipids
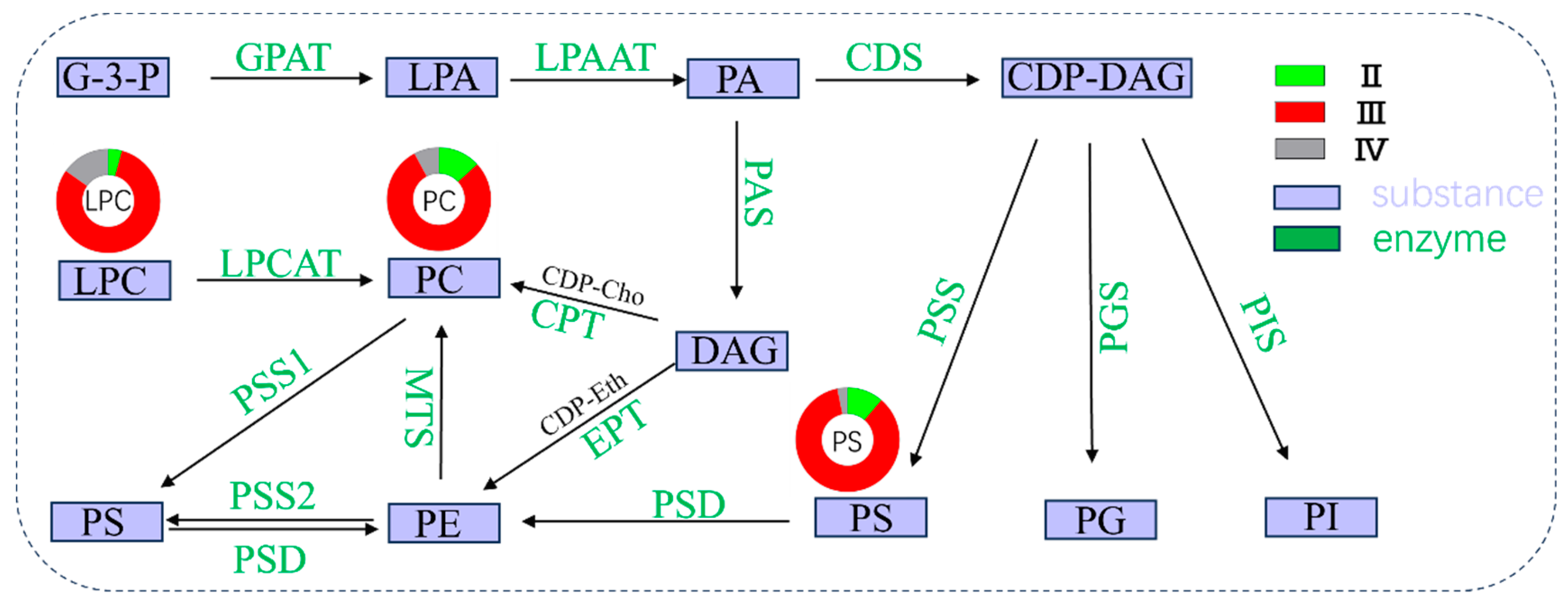
3.5. Key Phospholipid-Metabolism-Related Pathways during Ovarian Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, D.; Li, P.; Zhang, Y.; Peng, Z. Comparative study of the complete mitochondrial genomes of the bronze gudgeon (Coreius heterodon) and largemouth bronze gudgeon (Coreius guichenoti). Mitochondr. DNA 2013, 24, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Yu, X.; Chang, J.; Tong, J. Polymorphic microsatellites in largemouth bronze gudgeon (Coreius guichenoti) developed from repeat-enriched libraries and cross-species amplifications. Mol. Ecol. Notes 2007, 7, 1104–1107. [Google Scholar] [CrossRef]
- Zhang, C.; Fujiwara, M.; Pawluk, M.; Liu, H.; Cao, W.; Gao, X. Changes in taxonomic and functional diversity of fish communities after catastrophic habitat alteration caused by construction of Three Gorges Dam. Ecol. Evol. 2020, 10, 5829–5839. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Z.; Cai, L.; Qiao, Y.; Chen, X.; Chang, J. Effects of upstream and downstream dam operation on the spawning habitat suitability of Coreius guichenoti in the middle reach of the Jinsha River. Ecol. Eng. 2018, 120, 198–208. [Google Scholar] [CrossRef]
- Xiong, M.; Yan, S.; Shao, K.; Li, W.; Zhu, B.; Xu, N. Development of twenty-nine polymorphic microsatellite loci from largemouth bronze gudgeon (Coreius guichenoti). J. Genet. 2014, 93, 100–103. [Google Scholar] [CrossRef]
- Li, X.; Yan, Q.; Ringø, E.; Wu, X.; He, Y.; Yang, D. The influence of weight and gender on intestinal bacterial community of wild largemouth bronze gudgeon (Coreius guichenoti, 1874). BMC Microbiol. 2016, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Long, M.; Ji, C.; Shen, Z.; Gatesoupe, F.J.; Zhang, X.; Zhang, Q.; Zhang, L.; Zhao, Y.; Liu, X.; et al. Alterations of the gut microbiome of largemouth bronze gudgeon (Coreius guichenoti) suffering from furunculosis. Sci. Rep. 2016, 28, 30606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhao, Y.; Yang, J.; Li, Y.; Chen, P.; Huantao, Q. Feeding frequency affects liver health in largemouth bronze gudgeon Coreius guichenoti: Implications for lipid metabolism, oxidative stress, and inflammation response. Aquac. Rep. 2024, 35, 101941. [Google Scholar] [CrossRef]
- Papadaki, C.; Soulis, K.; Muñoz-Mas, R.; Martinez-Capel, F.; Zogaris, S.; Ntoanidis, L.; Dimitriou, E. Potential impacts of climate change on flow regime and fish habitat in mountain rivers of the south-western Balkans. Sci. Total. Environ. 2016, 540, 18–28. [Google Scholar] [CrossRef]
- Li, T.; Chen, Q.; Zhang, Q.; Feng, T.; Zhang, J.; Lin, Y.; Yang, P.; He, S.; Zhang, H. Transcriptomic Analysis on the Effects of Altered Water Temperature Regime on the Fish Ovarian Development of Coreius guichenoti under the Impact of River Damming. Biology 2022, 11, 1829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qiao, Y.; Schineider, M.; Chang, J.; Mutzner, R.; Fluixá-Sanmartín, J.; Yang, Z.; Fu, R.; Chen, X.; Cai, L.; et al. Using a hierarchical model framework to assess climate change and hydropower operation impacts on the habitat of an imperiled fish in the Jinsha River, China. Sci. Total. Environ. 2019, 646, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Sullivan, C.V.; Govoni, J.J. Oogenesis and spawn information in the invasive lionfish, Pterois miles and Pterois volitans. Sci. Mar. 2011, 75, 147–154. [Google Scholar] [CrossRef]
- Abascal, F.J.; Medina, A. Ultrastructure of oogenesis in the bluefin tuna, Thunnus thynnus. J. Morphol. 2005, 264, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, M.; Marammazi, J.G.; Kochanian, P.; Savari, A.; Yavari, V.; Haghi, M. Effects of protein and lipid concentrations in broodstock diets on growth, spawning performance and egg quality of yellowfin sea bream (Acanthopagrus latus). Aquaculture 2009, 295, 99–105. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Li, G.L.; Zhu, C.H.; Deng, S.P. Effects of fish oil on ovarian development in spotted scat (Scatophagus argus). Anim. Reprod. Sci. 2013, 141, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.F.; Tan, X.Y.; Pan, Y.X.; Zhang, L.H.; Chen, Q.L. Fatty Acid β-Oxidation Is Essential in Leptin-Mediated Oocytes Maturation of Yellow Catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2018, 19, 1457. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Y.; Shi, Z.; Li, W.; Zhou, H.; Lv, W. Lipid content and fatty acid composition in wild-caught silver pomfret (Pampus argenteus) broodstocks: Effects on gonad development. Aquaculture 2010, 310, 192–199. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, N.; Todo, T.; Sullivan, C.V.; Schilling, J.; Reading, B.J.; Matsubara, T.; Ryu, Y.W.; Mizuta, H.; Luo, W.; Nishimiya, O.; et al. Ovarian yolk formation in fishes: Molecular mechanisms underlying formation of lipid droplets and vitellogenin-derived yolk proteins. Gen. Comp. Endocrinol. 2015, 221, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Zhang, Y.Y.; Huang, X.; Wang, H.P.; Dong, X.; Zhu, B.; Qin, L. Analysis of Lipid Molecule Profiling and Conversion Pathway in Mandarin Fish (Siniperca chuatsi) during Fermentation via Untargeted Lipidomics. J. Agric. Food Chem. 2023, 71, 8673–8684. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Li, J.; Sun, H.; Mao, W.; Lu, Z.; Zhao, Q.; Han, C.; Gong, X.; Jiang, B.; Chua, G.H.; et al. Quantitative Lipidomics and Spatial MS-Imaging Uncovered Neurological and Systemic Lipid Metabolic Pathways Underlying Troglomorphic Adaptations in Cave-Dwelling Fish. Mol. Biol. Evol. 2022, 39, msac050. [Google Scholar] [CrossRef] [PubMed]
- Marqueño, A.; Blanco, M.; Maceda-Veiga, A.; Porte, C. Skeletal Muscle Lipidomics as a New Tool to Determine Altered Lipid Homeostasis in Fish Exposed to Urban and Industrial Wastewaters. Environ. Sci. Technol. 2019, 53, 8416–8425. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hogstrand, C.; Chen, G.; Lv, W.; Song, Y.; Xu, Y.; Luo, Z. Zn Induces Lipophagy via the Deacetylation of Beclin1 and Alleviates Cu-Induced Lipotoxicity at Their Environmentally Relevant Concentrations. Environ. Sci. Technol. 2021, 55, 4943–4953. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, L.; Yuan, X.; Huang, Y.; Johnson, D. Aerobic swimming performance of juvenile largemouth bronze gudgeon (Coreius guichenoti) in the Yangtze River. J. Exp. Zool. A Ecol. Genet. Physiol. 2012, 317, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Selman, K.; Wallace, R.A.; Sarka, A.; Qi, X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J. Morphol. 1993, 218, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Chen, M.J. The Effect of Steroid Hormones on Ovarian Follicle Development. Vitam. Horm. 2018, 107, 155–175. [Google Scholar]
- Walters, K.A.; Allan, C.M.; Handelsman, D.J. Androgen actions and the ovary. Biol. Reprod. 2008, 78, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.A.; Selman, K. Major protein changes during vitellogenesis and maturation of Fundulus oocytes. Dev. Biol. 1985, 110, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Stifani, S.; Le Menn, F.; Rodriguez, J.N.; Schneider, W.J. Regulation of oogenesis: The piscine receptor for vitellogenin. Biochim. Biophys. Acta 1990, 1045, 271–279. [Google Scholar] [CrossRef]
- Mizuta, H.; Luo, W.; Ito, Y.; Mushirobira, Y.; Todo, T.; Hara, A.; Reading, B.J.; Sullivan, C.V.; Hiramatsu, N. Ovarian expression and localization of a vitellogenin receptor with eight ligand binding repeats in the cutthroat trout (Oncorhynchus clarki). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 166, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Menn, F.L.; Cerdà, J.; Babin, P.J. Ultrastructural Aspects of the Ontogeny and Differentiation of Ray-Finned Fish Ovarian Follicles; Springer: Dordrecht, The Netherlands, 2007; pp. 1–37. [Google Scholar]
- Johnson, R.B. Lipid Deposition in Oocytes of Teleost Fish During Secondary Oocyte Growth. Rev. Fish. Sci. 2009, 17, 78–89. [Google Scholar] [CrossRef]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and β-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, H.; García-Ulloa, M.; Hernández-Llamas, A.; Villarreal, H. Effect of dietary protein level on spawning and egg quality of redclaw crayfish Cherax quadricarinatus. Aquaculture 2006, 257, 412–419. [Google Scholar] [CrossRef]
- Li, Q.Q.; Xiang, Q.Q.; Lian, L.H.; Chen, Z.Y.; Luo, X.; Ding, C.Z.; Chen, L.Q. Metabolic profiling of nanosilver toxicity in the gills of common carp. Ecotoxicol. Environ. Saf. 2021, 222, 112548. [Google Scholar] [CrossRef] [PubMed]
- Cortes, V.A.; Busso, D.; Maiz, A.; Arteaga, A.; Nervi, F.; Rigotti, A. Physiological and pathological implications of cholesterol. Front. Biosci. 2014, 19, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R.; Bendiksen, E.Å.; Campbell, P.J.; Bell, J.G. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Kitagawa, T.; Matsue, Y.; Hashidume, M.; Wada, S. Lipid class and fatty acid composition of phospholipids from the gonads of skipjack tuna. Fish. Sci. 2004, 70, 903–909. [Google Scholar] [CrossRef]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.Y.; Wu, X.G.; Wille, M.; Cheng, Y.X.; Sorgeloos, P. Effect of dietary soybean lecithin on reproductive performance of Chinese mitten crab Eriocheir sinensis (H. Milne-Edwards) Broodstock. Aquacult. Int. 2008, 17, 45–56. [Google Scholar] [CrossRef]
- Tomohiro, S.; Kawaguti, A.; Kawabe, Y.; Kitada, S.; Kuge, O. Purification and characterization of human phosphatidylserine synthases 1 and 2. Biochem. J. 2009, 418, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.; Tocher, D.R.; Roy, W.J.; Robertson, D.A. Egg quality determinants in cod (Gadus morhua L.): Egg performance and lipids in eggs from farmed and wild broodstock. Aquac. Res. 2005, 36, 1488–1499. [Google Scholar] [CrossRef]
- Cowey, C.B.; Bell, J.G.; Knox, D.; Fraser, A.; Youngson, A. Lipids and antioxidant systems in developing eggs of salmon (Salmo salar). Lipids 1985, 20, 567–572. [Google Scholar] [CrossRef]
- Singh, P.B.; Singh, V.; Srivastava, S.; Pandey, S. Effects of estradiol-17beta and 17alpha, 20beta-dihydroxy-4-pregnen-3-one on different phospholipids metabolism and histological changes in ovary during reproductive growth in the catfish, Heteropneustes fossilis (Bloch). J. Environ. Biol. 2007, 28, 771–778. [Google Scholar] [PubMed]
- Nutautaitė, M.; Racevičiūtė-Stupelienė, A.; Andalibizadeh, L.; Šašytė, V.; Bliznikas, S.; Pockevičius, A.; Vilienė, V. Improving broiler chickens’ health by using lecithin and lysophosphatidylcholine emulsifiers: A comparative analysis of physiological indicators. Iran. J. Vet. Res. 2021, 22, 33–39. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Hu, N.; Xiao, Y.; Lai, X.; Wang, L.; Song, Y. Characterization of Ovarian Lipid Composition in the Largemouth Bronze Gudgeon (Coreius guichenoti) at Different Development Stages. Fishes 2024, 9, 291. https://doi.org/10.3390/fishes9070291
Zhu J, Hu N, Xiao Y, Lai X, Wang L, Song Y. Characterization of Ovarian Lipid Composition in the Largemouth Bronze Gudgeon (Coreius guichenoti) at Different Development Stages. Fishes. 2024; 9(7):291. https://doi.org/10.3390/fishes9070291
Chicago/Turabian StyleZhu, Jian, Nanjun Hu, Yao Xiao, Xiaohong Lai, Lingjiao Wang, and Yufeng Song. 2024. "Characterization of Ovarian Lipid Composition in the Largemouth Bronze Gudgeon (Coreius guichenoti) at Different Development Stages" Fishes 9, no. 7: 291. https://doi.org/10.3390/fishes9070291
APA StyleZhu, J., Hu, N., Xiao, Y., Lai, X., Wang, L., & Song, Y. (2024). Characterization of Ovarian Lipid Composition in the Largemouth Bronze Gudgeon (Coreius guichenoti) at Different Development Stages. Fishes, 9(7), 291. https://doi.org/10.3390/fishes9070291




