Abstract
Polyvalent passive vaccines can act fast in resisting various bacteria with good efficacy, and they have application value in aquaculture. This study prepared live and inactivated Vibrio fluvialis mouse antisera (L-VF-antiserum and I-VF-antiserum), and administered them to goldfish (Carassius auratus) infected with V. fluvialis and Aeromonas hydrophila. The passive protective rates of live and inactivated mouse antisera against V. fluvialis were 60% (p < 0.05) and 40% (p < 0.05), and their passive cross-protective rates against A. hydrophila were 42.86% (p < 0.05) and 35.71% (p < 0.05), respectively. Furthermore, the two mouse antisera could recognize the bacteria in vitro; the content of bacteria in the C. auratus kidney decreased (p < 0.05), the phagocytic activity of C. auratus leukocytes was enhanced (p < 0.05), and the expression of inflammatory genes and activity of antioxidant factors decreased (p < 0.05). Moreover, the kidney, spleen, and intestinal tissue structures were intact, and the apoptosis and DNA damage were decreased (p < 0.05) among the kidney cells. The live V. fluvialis antiserum contained more antibodies against the outer membrane proteins of V. fluvialis than the inactivated mouse antiserum. The immunoprotective abilities of the live V. fluvialis antiserum were higher than those of the inactivated antiserum, and the antiserum of live V. fluvialis immunity demonstrated potential application value as a polyvalent passive immune vaccine in fish.
Key Contribution:
This study identified the multivalent passive immunoprotective effects of antisera obtained via live or inactivated bacterial immunity in fish, and found that the antiserum of live V. fluvialis immunity has potential application value as a polyvalent passive vaccine in fish.
1. Introduction
Aquaculture provides people with rich protein nutrition and is a pillar of the agricultural economy. With the expansion of intensive aquaculture, the prevalence of pathogenic bacteria has increased, causing large economic losses in fishery production [1]. Important pathogenic bacteria in aquaculture include Aeromonas hydrophila, Vibrio fluvialis, Vibrio alginolyticus, Edwardsiella ictaluri, and Vibrio parahaemolyticus [2,3]. V. fluvialis is extensively found in rivers and estuary water, and can infect commonly farmed fish such as crucian carp, grass carp, and Paralichthys olivaceus [4,5]. The main symptoms of infection in fish are inflammation of the body, the shedding of scales, congestion, and the erosion of fins, leading to sepsis and red skin disease. V. fluvialis is also the main pathogenic bacterial cause of diarrhea due to the consumption of unclean seafood [6]. Therefore, V. fluvialis is a zoonotic pathogen.
Antibiotics are used to prevent and control bacterial diseases in aquaculture [7]. However, the abuse of antibiotics inevitably leads to increased bacterial resistance and environmental pollution, especially drug residues, which affect the quality of aquatic products and pose a potential threat to human health [8,9]. Prevention and control methods involving Chinese herbal medicines and probiotics have some applications in aquaculture [10,11]. However, their effects are not sufficiently timely for explosive pathogen infections. Vaccines have received widespread attention due to their characteristics of no residue, low drug resistance, and minimal toxic side effects. Fish vaccines include attenuated, inactivated, and protein subunit vaccines [12]. There are few commercialized vaccines in aquaculture; most vaccines are in the laboratory research stage [13]. Research reports on V. fluvialis vaccines are especially limited.
Passive immune vaccines are immune serum or immunoglobulin antibodies that enable the body to immediately acquire specific immune abilities, and they show fast onset and good therapeutic effects [14,15]. For example, immunoglobulins, antitoxins, and antiviral serum are directly injected with antibodies to provide immune protection in clinical practice. They are generally used for emergency prevention in special situations, such as in anti-snake venom serum and anti-rabies serum [16]. In one study, the parents (male and female) of tilapia were immunized with Tilapia lake virus (TiLV), and the offspring tilapia passively received TiLV antibodies, making the tilapia seedlings resistant to TiLV [17]. We passively administered red crucian carp with egg yolk antibodies (IgY) against the outer membrane proteins (PF1380 and ExbB) of Pseudomonas fluorescens, and we observed that the two IgY antibodies could resist infections from P. fluorescens and A. hydrophila, making them candidates for multivalent passive vaccines [18]. Moreover, the development of multivalent passive vaccines that can resist multiple pathogens has great potential due to the wide variety of pathogenic bacteria in aquaculture [19]. However, there is relatively little research on passive vaccines in aquaculture.
The present study examined multivalent passive vaccines. Mice were immunized with live or inactivated V. fluvialis to prepare the live V. fluvialis mouse antiserum (L-VF-antiserum) and inactivated V. fluvialis mouse antiserum (I-VF-antiserum), and red crucian carp (Carassius auratus) were passively administered the antisera and infected with V. fluvialis or A. hydrophila. The immune abilities of live and inactivated V. fluvialis antisera were compared according to the immune protective rate, the count of bacteria in the viscera of fish, the phagocytic activity, antioxidant reactions, inflammatory reactions, the pathology of the visceral tissue, cell function analysis, and antibody chip array analysis (Figure S1). This study lays the foundation for the development of multivalent passive vaccines in aquaculture.
2. Materials and Methods
2.1. Bacterial Strains and Animals
V. fluvialis, A. hydrophila, and Staphylococcus aureus were preserved in the molecular biology laboratory of Fuyang Normal University (Fuyang, China). Leghorn laying hens (20 weeks old) were purchased from Chongqing Tengxin Biotechnology Co., Ltd. (Xi’an, China), and Carassius auratus (20 ± 1.0 g) was obtained from Fuyang Economic Fish Farming Co., Ltd. (Fuyang, China).
2.2. Preparation of the Mouse Antisera
V. fluvialis cells were collected after overnight cultivation, suspended in a 1% formaldehyde solution, inactivated in an 80 °C water bath for 90 min, and suspended in normal saline (0.9% NaCl). The mice were immunized with 100 μL of live or inactivated V. fluvialis (5 × 106 CFU) via intraperitoneal injection. Immunization was performed thrice at 14-day intervals. After the third immunization, the mice were anesthetized, and blood was collected from the eyeballs. The mouse blood was coagulated at 4 °C overnight. After centrifugation at 3000 r/min, the live V. fluvialis mouse antiserum (L-VF-antiserum) and inactivated V. fluvialis mouse antiserum (I-VF-antiserum) were obtained and stored at −80 °C [20].
2.3. Passive and Passive Cross-Protective Rates of the Mouse Antisera
The C. auratus were divided into two sets (V. fluvialis versus A. hydrophila) of three groups, with 15 fish in each group: a live V. fluvialis antiserum group, inactivated V. fluvialis antiserum group, and control group (blank antiserum). Each experimental group was intraperitoneally administered 20 μL of antiserum. After 2 h, the fish were intraperitoneally challenged with V. fluvialis (1 × 109 CFU) or A. hydrophila (4.0 × 108 CFU), respectively. The fish mortality rate was observed for 14 days. The protection rate (RPS) was calculated according to the following formula: RPS (%) = (1 − [% experimental group morality/% control morality]) × 100. The significant differences (p < 0.05) between the experimental groups and the control group were analyzed using SPSS 19.0 software [18].
2.4. Interaction between Mouse Antisera and Bacteria
The interaction between the mouse antisera and bacteria was measured as previously described [18]. Briefly, the bacteria were placed in an ELISA plate. Skim milk (5%) was added to the plate’s wells, and the plate was sealed and incubated at 4 °C overnight. Gradient-diluted antisera were added to the wells. After washing them thrice with a 0.01 M PBS solution, a rat anti-carp serum antibody (1:500) was added to the wells and incubated at 37 °C for 1 h. Next, after washing, a goat anti-rat secondary antibody (1:3000) was added to the wells, and the plate was incubated at 37 °C for 10 min with a color solution (50 μL of TMB and 50 μL of H2O2). A termination liquid (2 M of H2SO4) was used to terminate the reaction, and the plate was read immediately on an ELISA reader at OD450.
2.5. Kidney Bacterial Content
The C. auratus were administered live or inactivated mouse antisera and challenged with V. fluvialis or A. hydrophila, and the control received blank mouse antiserum. After 2 days, kidney tissues were obtained to prepare the homogenates with 400 μL of a 0.01 M PBS solution. The homogenates of the kidney tissue solution were coated with an LB medium at 30 °C overnight, and the bacterial colonies were counted.
2.6. White Blood Cell Phagocytosis Analysis
The C. auratus were administered mouse antisera and challenged with pathogenic bacteria, and leukocytes were then collected from the caudal vein in anticoagulant tubes. A total of 0.2 mL of leukocytes and 1% inactivated S. aureus (6 × 108 CFU) were mixed and incubated in a water bath for 60 min at 25 °C. The blood solution was placed on a glass slide and stained with a Giemsa staining kit (Sangon Biotech Co., Ltd., Shanghai, China). The phagocytic cells were counted under a microscope and calculated as phagocytic percentage (PP%) = number of cells participating in phagocytosis in 100 phagocytic cells/100 × 100%; phagocytic index (PI%) = number of bacteria in phagocytic cells/number of cells participating in phagocytosis × 100% [18].
2.7. Organizational Pathological Analysis
Tissue pathology slices were prepared as previously described [21]. Briefly, the kidneys, spleens, and intestines were obtained after the C. auratus were administered mouse antisera, challenged with V. fluvialis or A. hydrophila, and then soaked in Davidson’s fixative and 10% formaldehyde solution for 24 h. Then, the tissues were subjected to gradient ethanol dehydration treatment and xylene transparency treatment and embedded in paraffin at 60 °C and cut into 5 μm slices with a microtome (Leica, Wetzlar, Germany). The slices were placed on a glass slide to dry at 37 °C overnight, and hematoxylin and eosin (H&E) staining was performed. After xylene transparency, the slices were sealed in neutral balsam and photographed using a microscope (Leica, Wetzlar, Germany).
2.8. Kidney Immunofluorescence Analysis
The kidney tissue slices were placed in xylene for dewaxing and rehydrated in ethanol with a decreasing gradient concentration. After being treated with an antigen repair solution, 50 μL of BSA sealing solution (5%) was added to the slices at room temperature for 1.5 h to seal the tissue. Then, a monoclonal antibody for p53 or γH2A.X (1:1000) was added, and the slices were stored at 4 °C overnight. After washing thrice, a secondary antibody solution (1:3000) was added at 37 °C for 1 h. DAPI solution was used for tissue nucleus staining, and two drops of anti-fluorescence quenching agent were added to the tissue. Finally, the slices were photographed with a fluorescence microscope (Leica, Wetzlar, Germany) [21].
2.9. Antioxidant Factor Activity Analysis
Serum was obtained from the caudal vein after the C. auratus were administered mouse antisera and challenged with bacteria. The activity of the antioxidant factors superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) were assessed according to the instructions of the detection kit (Sangon Biotechnology Co., Ltd., Shanghai, China).
2.10. mRNA Expression of Inflammatory Factors
The kidneys and spleens were obtained on Day 2 after the C. auratus were administered mouse antisera and challenged with V. fluvialis or A. hydrophila, and the tissues were also ground with liquid nitrogen. The tissues’ RNA was extracted and reverse transcribed to cDNA according to the instructions of the reagent kit (Takara, Beijing, China). qRT-PCR was performed according to the manufacturer’s instructions with a SYBR® Green Premix kit (Takara, Beijing, China) and synthetic primers (Table 1). The significance of differences was assessed with SPSS 19.0 software [21].

Table 1.
Primers used for the qRT-PCR.
2.11. Protein Chip Array to Assess the Outer Membrane Protein Antibodies in the Mouse Antisera
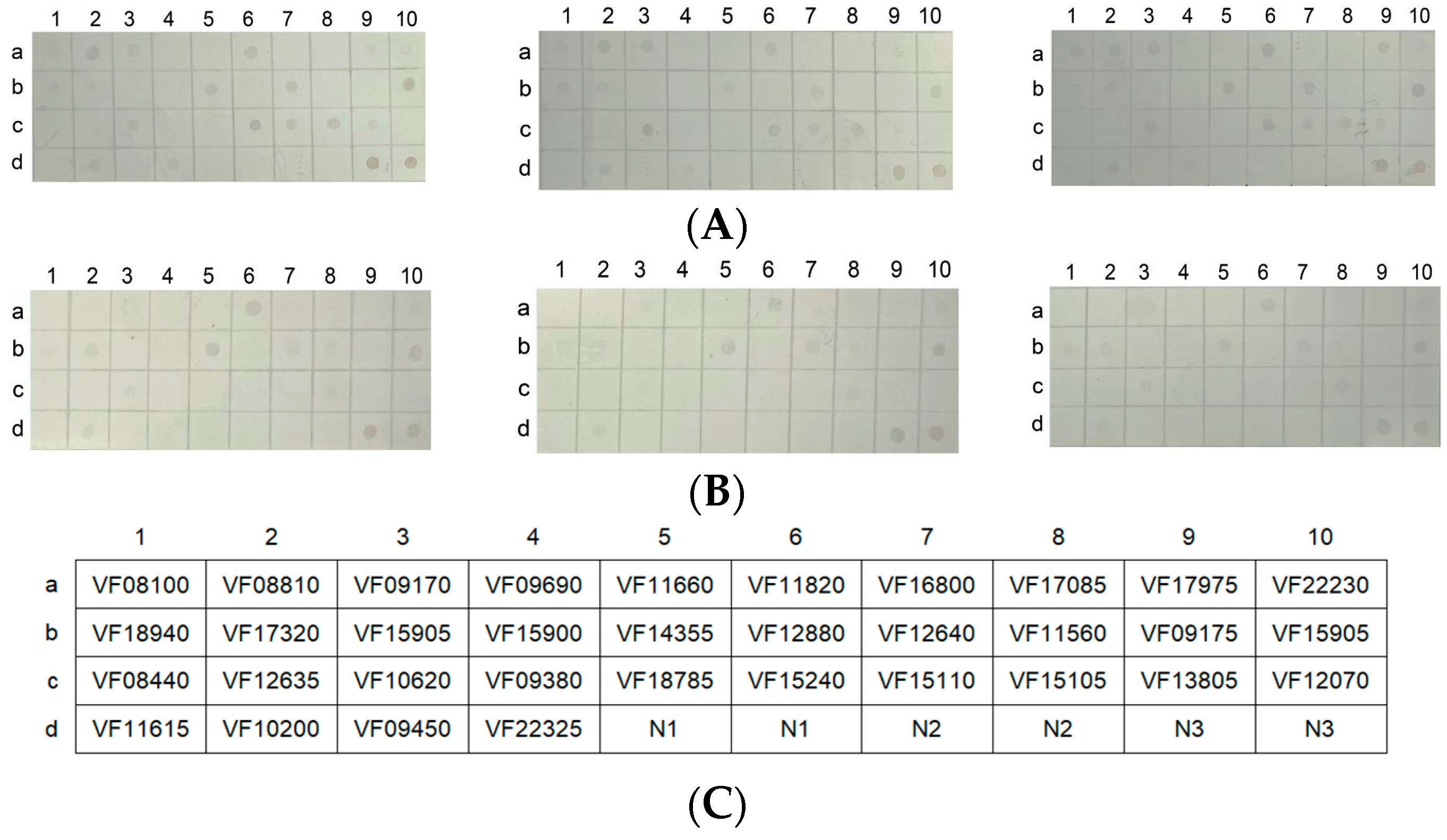
The nitrocellulose (NC) membrane was arrayed into uniformly sized squares of 0.7 cm × 0.7 cm. The protein array consisted of a 4 × 10 matrix with 40 squares, including 34 outer membrane proteins (Table S1) and 6 squares as controls (Figure 8C). A total of 34 purified outer membrane proteins (OMPs) of V. fluvialis were adjusted to a concentration of 0.25 μg/μL, and 2 μL of the solution was added to the squares of the NC membrane. A 5% BSA solution was used to seal the NC membrane at room temperature for 2 h. Then, the NC membrane was incubated with mouse antisera (1:200) at 37 °C for 1 h. After washing thrice, a secondary antibody (1:2000) was added to the NC membrane at 37 °C for 1 h. Finally, the NC membrane was stained with a DAB solution [18].
3. Results
3.1. Passive and Passive Cross-Protective Rates of the Mouse Antisera in C. auratus
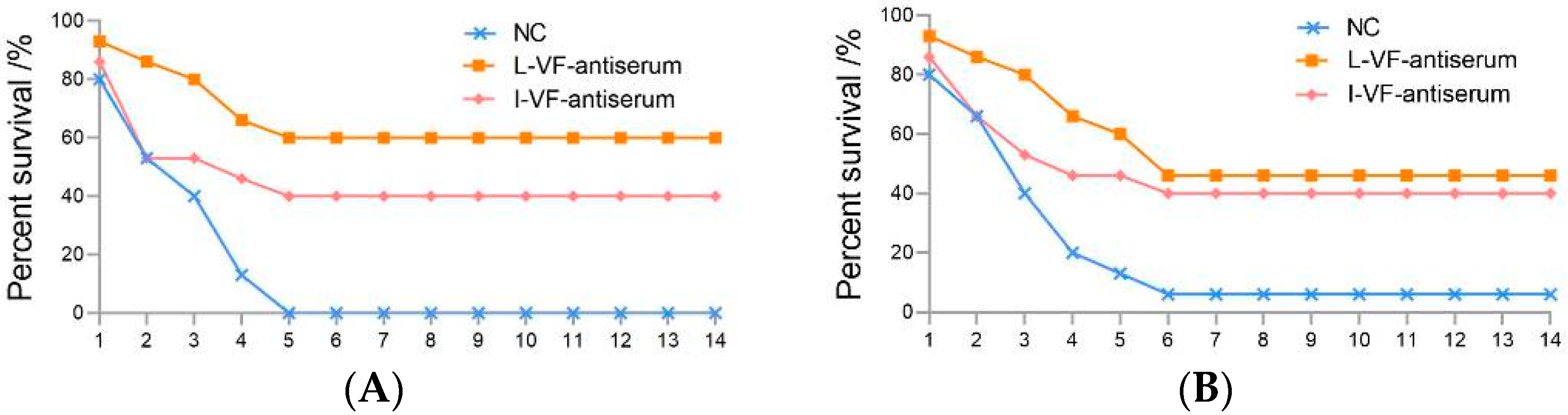
The C. auratus were administered mouse antisera and challenged with V. fluvialis or A. hydrophila in order to study the difference in the protective rates of the mouse antisera against live and inactivated V. fluvialis. After being challenged with bacteria, the C. auratus exhibited a bleeding epidermis and swollen belly, and many of them died within 6 days. The mortality gradually stabilized from Day 7 (Figure 1), and the passive protective rates of the live and inactivated mouse antisera against V. fluvialis were 60% (p < 0.05) and 40% (p < 0.05), respectively. In addition, the passive cross-protective rates of those against A. hydrophila were 42.86% (p < 0.05) and 35.71% (p < 0.05), respectively (Table 2). Therefore, the live and inactivated V. fluvialis mouse antisera provided protection, and the passive and passive cross-protective rates of the live V. fluvialis mouse antiserum were higher than those of inactivated mouse antiserum.
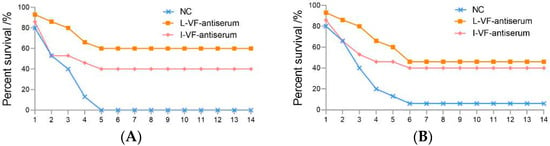
Figure 1.
The survival rates for C. auratus following pathogenic bacterium challenge. (A,B) represent attacks by V. fluvialis and A. hydrophila, respectively.

Table 2.
The passive and passive cross-protective rates of the mouse antisera.
3.2. Immune Binding Capacity between the Mouse Antisera and Bacteria In Vitro
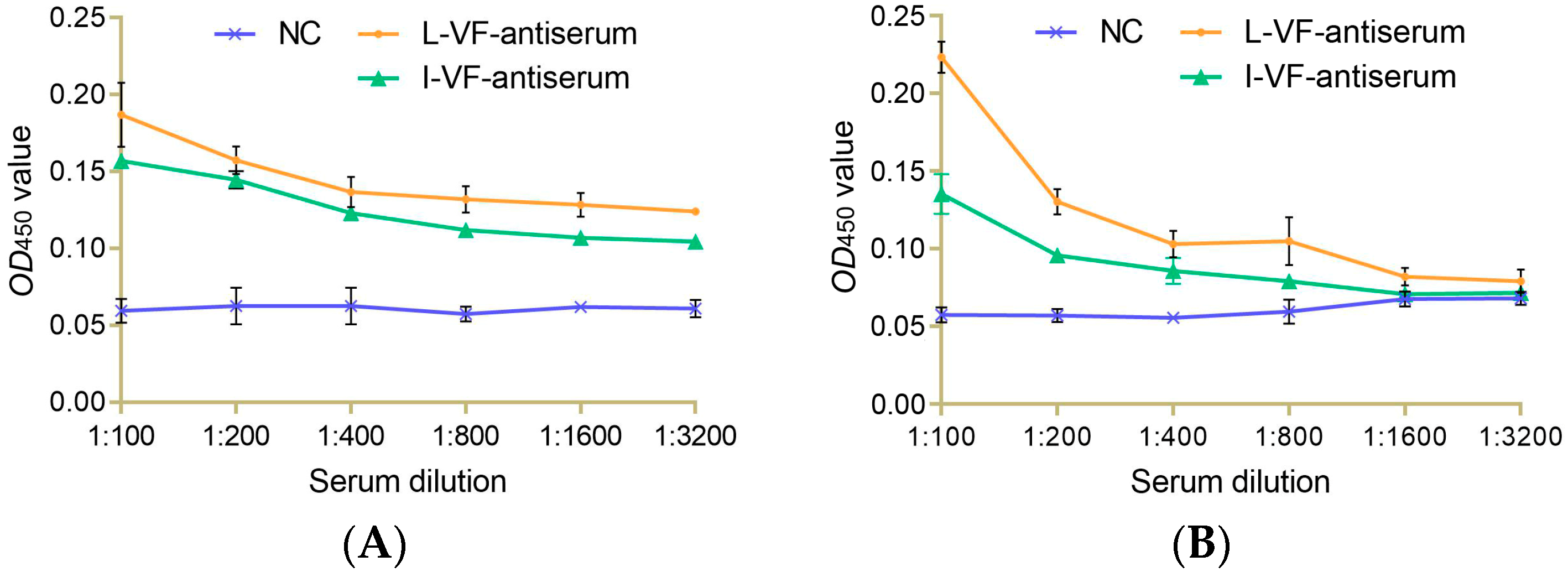
Different dilutions of the live and inactivated V. fluvialis mouse antisera were incubated with bacteria in vitro. The results showed that the binding capacity between the antisera and bacteria gradually weakened as the dilution of the antisera increased. The two antisera had a binding capacity with V. fluvialis at a titer of 1:1600 and 1:400 for A. hydrophila. The binding capacity of the live mouse antiserum was higher than that of the inactivated mouse antiserum (Figure 2).
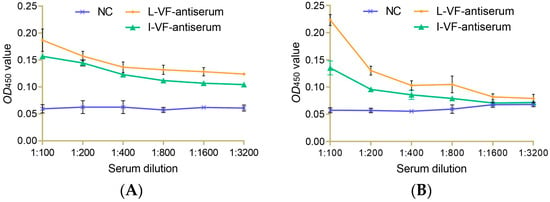
Figure 2.
The immune binding capacity between the mouse antisera and bacteria. (A,B) V. fluvialis and A. hydrophila, respectively.
3.3. Bacterial Count in the Kidneys of the C. auratus
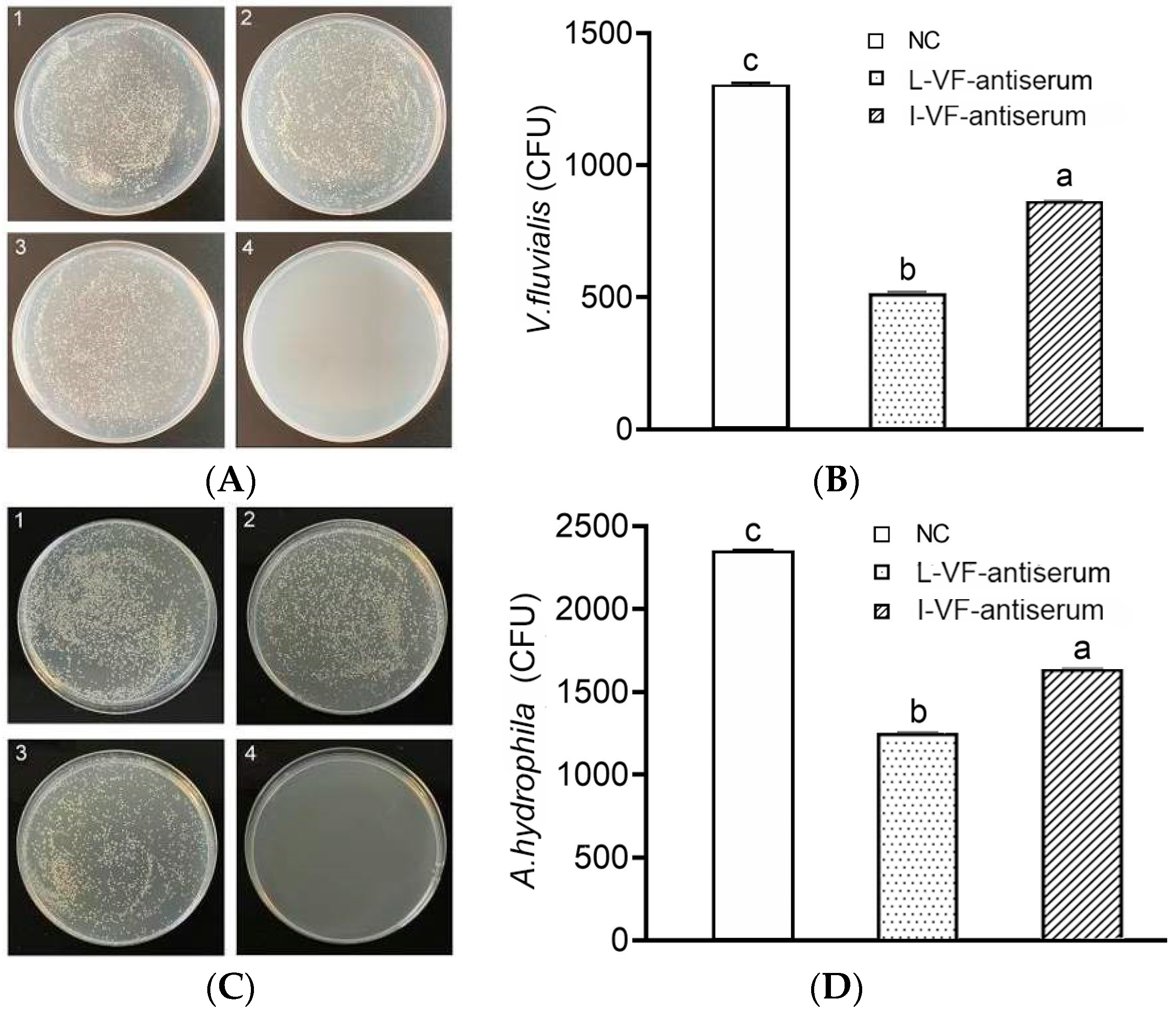
After the administration of mouse antisera and challenge with bacteria, C. auratus kidney homogenate was coated in LB medium. Compared with that in the control group, the count of V. fluvialis or A. hydrophila decreased (p < 0.05) in the groups with the live or inactivated V. fluvialis mouse antisera, and the bacterial count with live mouse antiserum was lower than that with inactivated mouse antiserum (p < 0.05) (Figure 3). Thus, the live and inactivated mouse antisera both demonstrated the ability to clear bacteria in C. auratus, and live mouse antiserum was better than inactivated mouse antiserum.
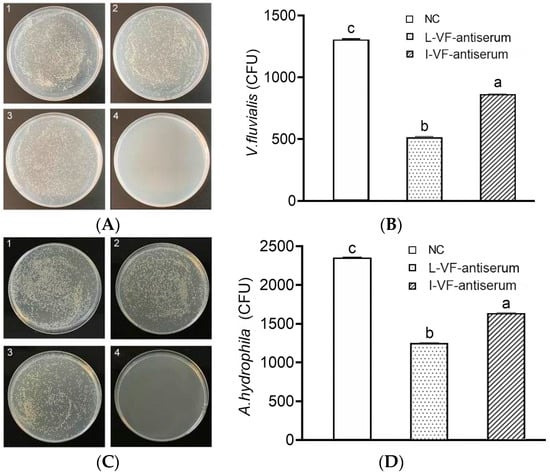
Figure 3.
Bacterial count in C. auratus’ kidneys. (A,B) The bacterial colony and the bacterial count statistics for V. fluvialis, respectively. (C,D) The bacterial colony and the bacterial count statistics for A. hydrophila, respectively. Labels 1–3 represent the blank mouse antiserum as the control, the live mouse antiserum, and the inactivated mouse antiserum, respectively. Label 4 represents a kidney without challenge from bacteria. Labels a–c indicate statistically different groups (p < 0.05).
3.4. The Phagocytic Activity of C. auratus Leukocytes
In the groups of live and inactivated mouse antisera, the phagocytic percentage (PP%) and phagocytic index (PI%) were increased (p < 0.05) in the C. auratus leukocytes after the C. auratus were administered mouse antisera and challenged with V. fluvialis or A. hydrophila. The phagocytic percentage (PP%) of the live mouse antiserum was higher than that of the inactivated mouse antiserum (Table 3).

Table 3.
Phagocytic activity of C. auratus leukocytes.
3.5. Pathological Observation of the Visceral Tissue Morphology in C. auratus
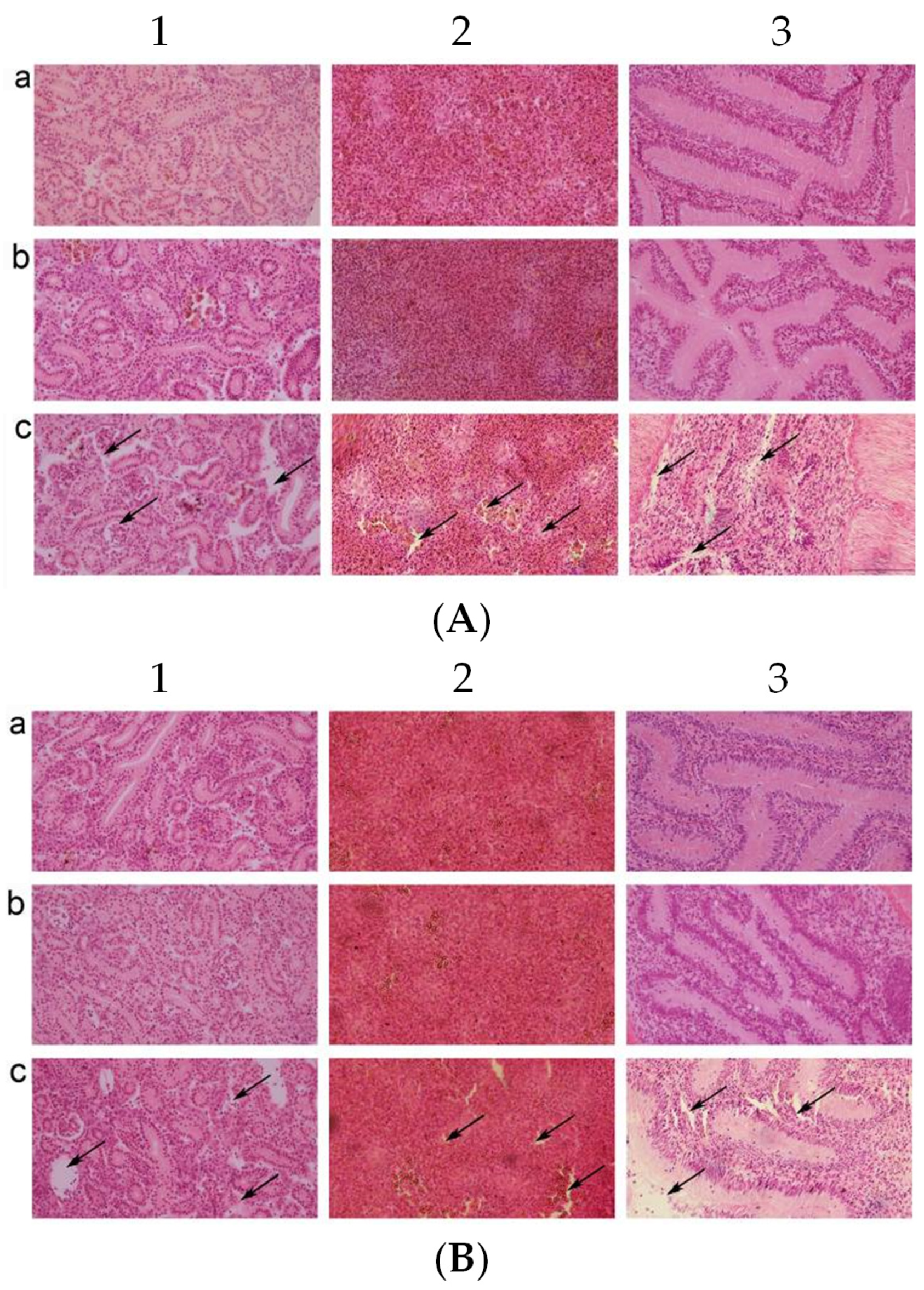
The kidneys, spleens, and intestines were taken for pathological section and observation after the C. auratus were administered live or inactivated mouse antiserum and challenged with V. fluvialis or A. hydrophila. In the control group, the kidney tissue structures were loose and incomplete with atrophy, degeneration of glomeruli and renal tubules, and cell apoptosis (Figure 4(A1c,B1c)). In addition, the spleen tissue was incomplete with a decrease in nuclear density and a heightened occurrence of cell apoptosis (Figure 4(A2c,B2c)), and the lamina propria of the intestinal mucosa was shrunk and presented with an incomplete structure and cell apoptosis (Figure 4(A3c,B3c)). The kidneys, spleens, and intestines were complete and tight in the live and inactivated mouse antiserum groups (Figure 4). Thus, the live and inactivated mouse antisera could protect the integrity of the organizational structure for bacterial infection in C. auratus.
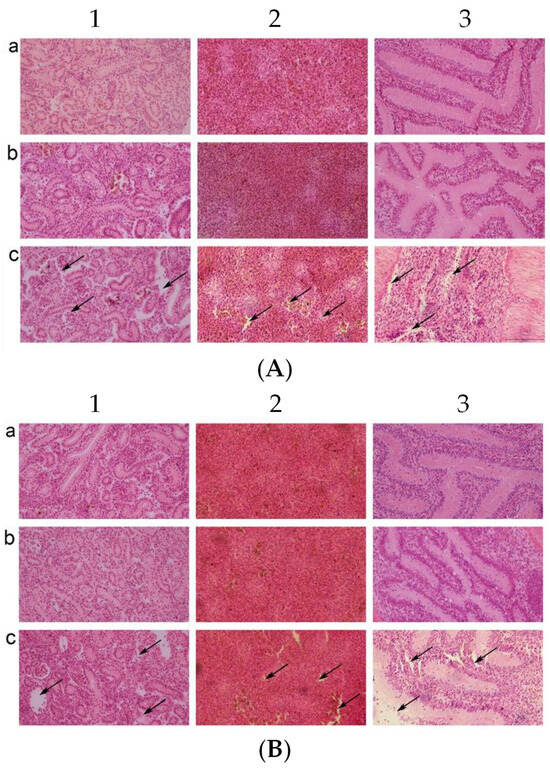
Figure 4.
Pathological sections of the C. auratus visceral tissues. (A,B) The C. auratus that were challenged with V. fluvialis and A. hydrophila, respectively. Labels a–c represent fish administered live mouse antiserum, inactivated mouse antiserum, and blank mouse antiserum as a control, respectively. Numbers 1, 2, and 3 represent kidney, spleen and intestine, respectively. In the control group, the kidney (A1c,B1c), spleen (A2c,B2c), and intestine (A3c,B3c) exhibited structural incompleteness and cell apoptosis. The visceral tissues were intact without cell apoptosis in the live and inactivated mouse antiserum groups.
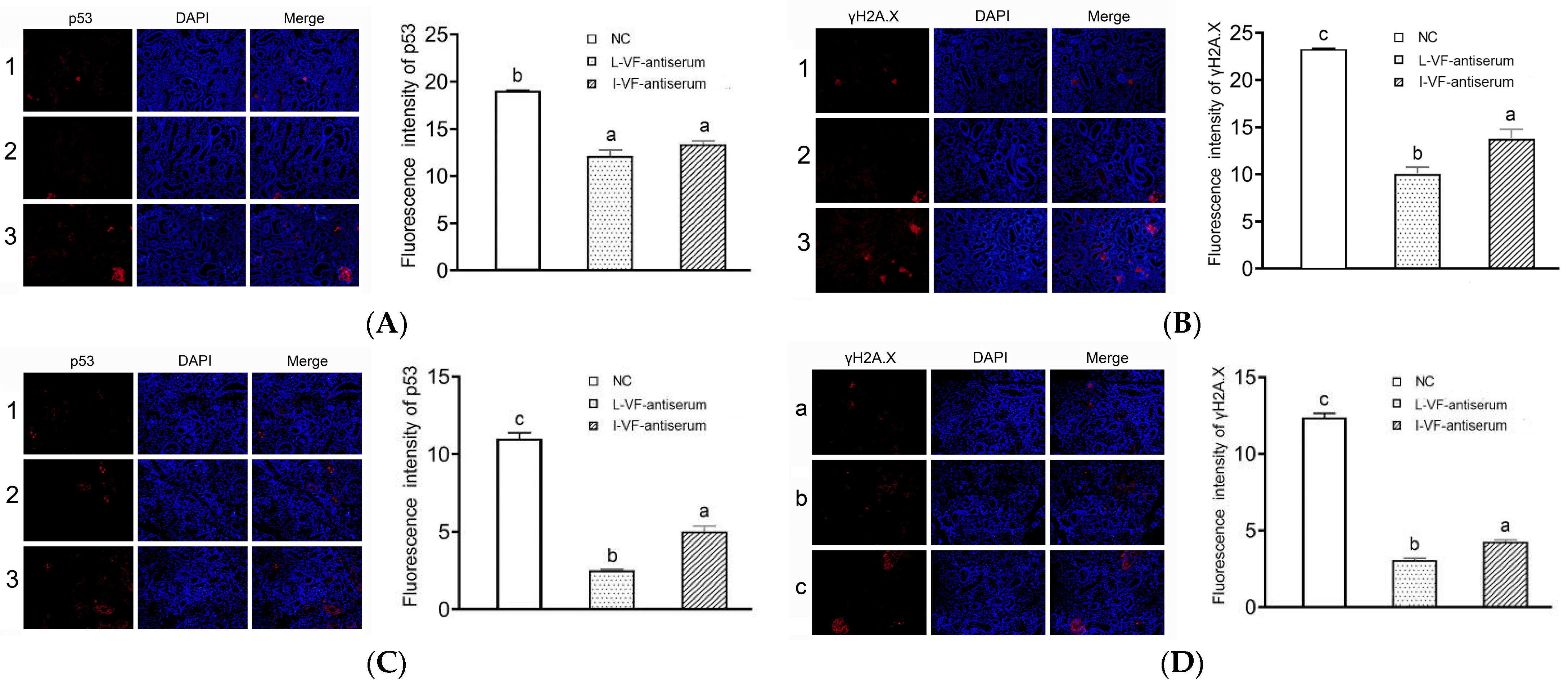
3.6. Immunofluorescence of p53 and γH2A.X in the C. auratus’ Kidneys
The apoptosis and DNA damage of the C. auratus kidney cells were assessed according to the immunofluorescence of p53 and γH2A.X proteins, respectively. After the C. auratus were administered mouse antisera and challenged with V. fluvialis or A. hydrophila, the expression of p53 and γH2A.X was decreased (p < 0.05) compared to the control group. Moreover, the expression of p53 and γH2A.X with the live mouse antiserum was lower than that with the inactivated mouse antiserum (p < 0.05) (Figure 5). Thus, the passive administration of live and inactivated mouse antisera could reduce apoptosis and DNA damage in C. auratus kidney cells, and the immune effects of the live mouse antiserum were better than those of the inactivated mouse antiserum.
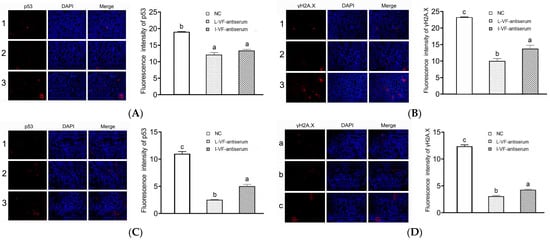
Figure 5.
Immunofluorescence of p53 and γH2A.X in the kidney cells. (A,B) Challenges with V. fluvialis. (C,D) Challenges with A. hydrophila. Labels 1–3 represent the C. auratus administered the live mouse antiserum, inactivated mouse antiserum, and blank mouse antiserum (control), respectively. Labels a–c indicate statistically different groups (p < 0.05). The immunofluorescence of p53 and γH2A.X decreased (p < 0.05) compared to the control, and the immunofluorescence with the live mouse antiserum was lower than that with the inactivated mouse antiserum.
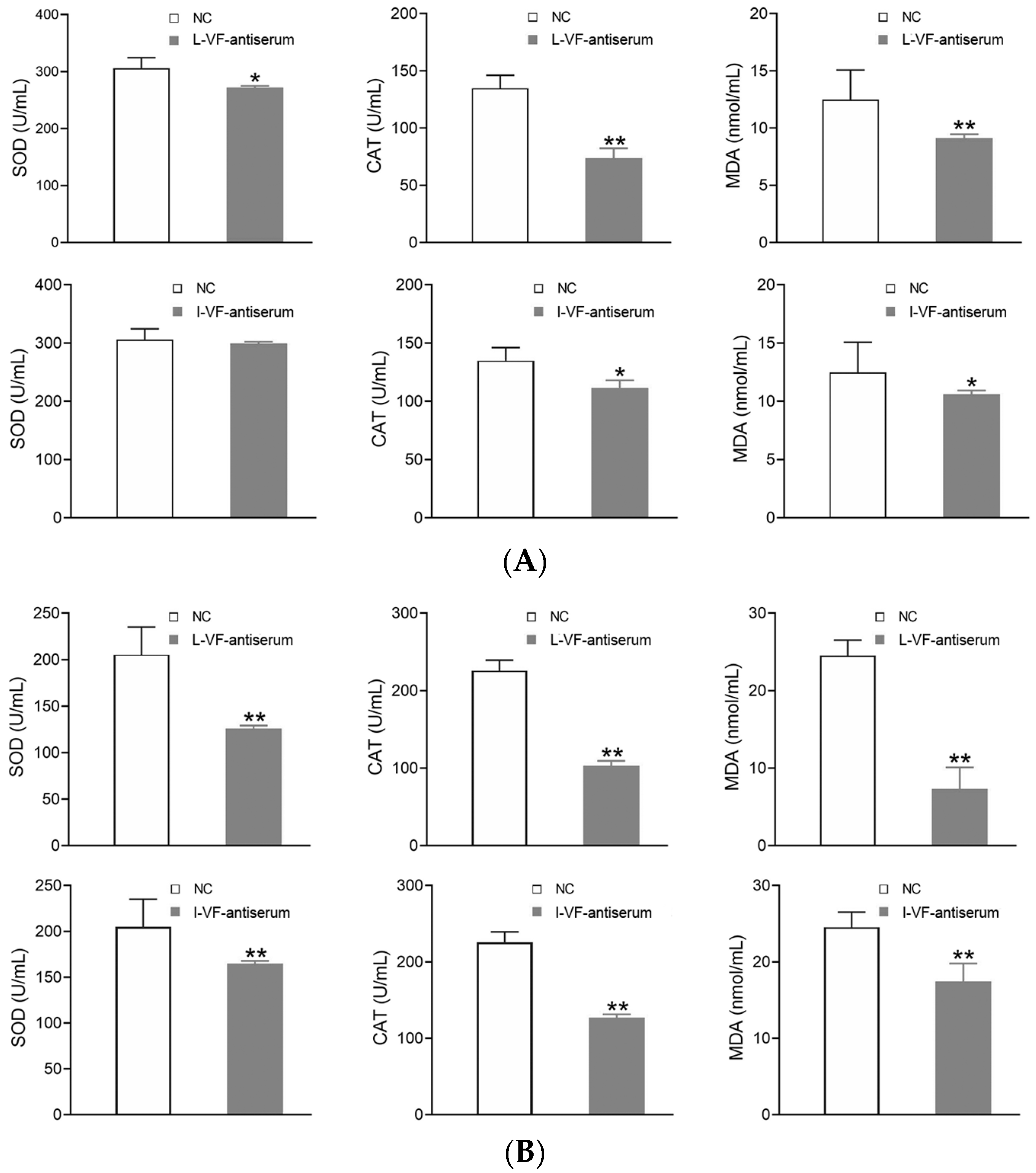
3.7. Detection of the Antioxidant Factors’ Activity in the C. auratus Serum
The C. auratus serum was obtained on Day 2 after the fish were administered mouse antisera and bacteria were introduced. In the treatment groups, most of the antioxidant factors’ activity (i.e., SOD, CAT, and MDA) decreased (p < 0.05) after challenge with V. fluvialis or A. hydrophila when compared with the control group (Figure 6). Thus, the live and inactivated mouse antisera promoted antioxidant activity in C. auratus.
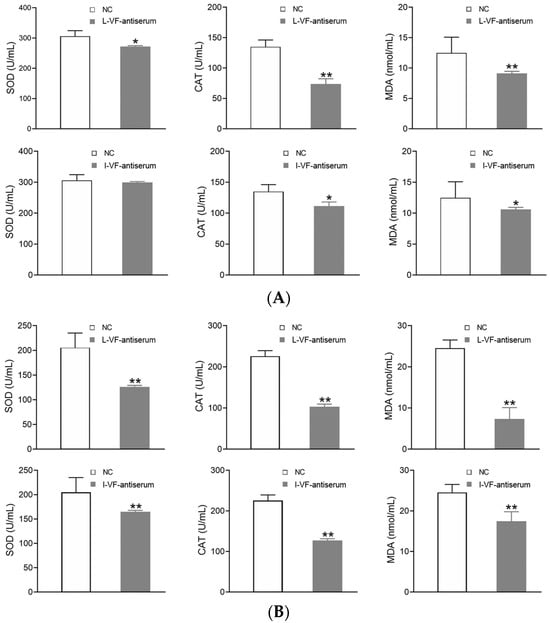
Figure 6.
The activity of the antioxidant factors in the C. auratus serum. (A,B) represent challenges with V. fluvialis and A. hydrophila, respectively. * p < 0.05 and ** p < 0.01 (compared with the control). The activity of most antioxidant factors (i.e., SOD, CAT, and MDA) decreased (p < 0.05).
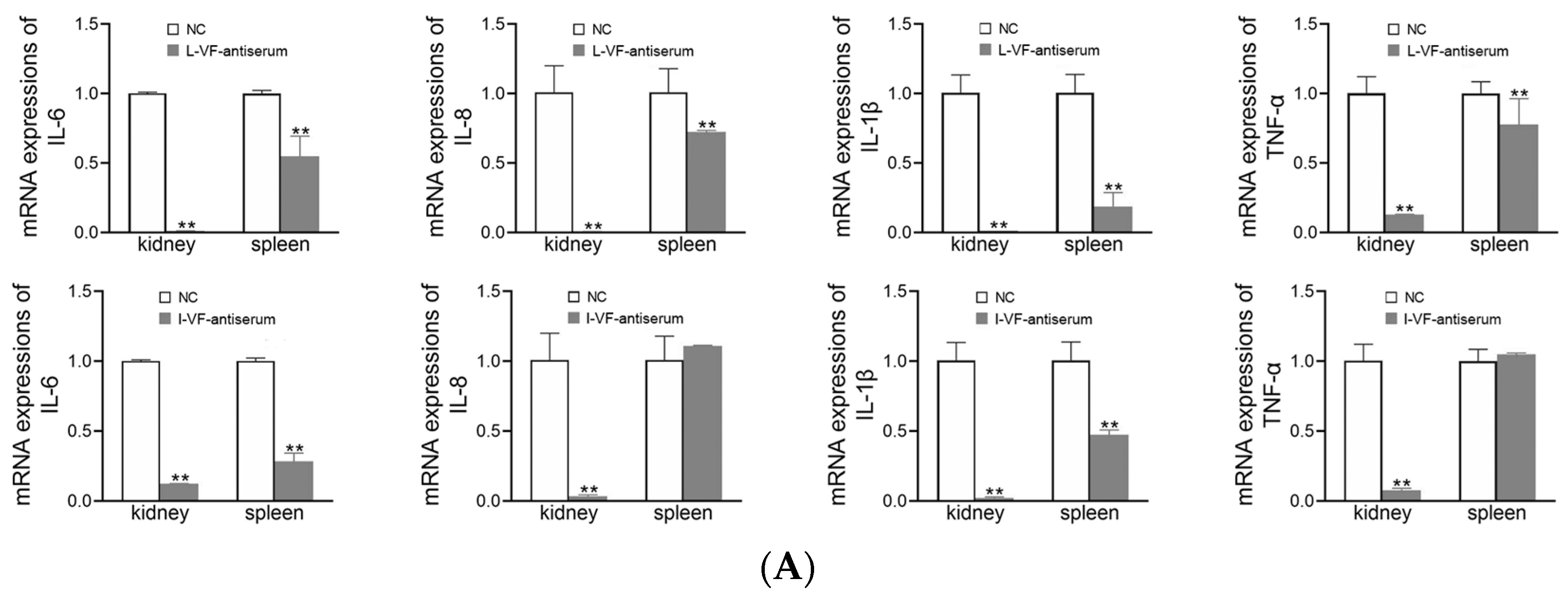
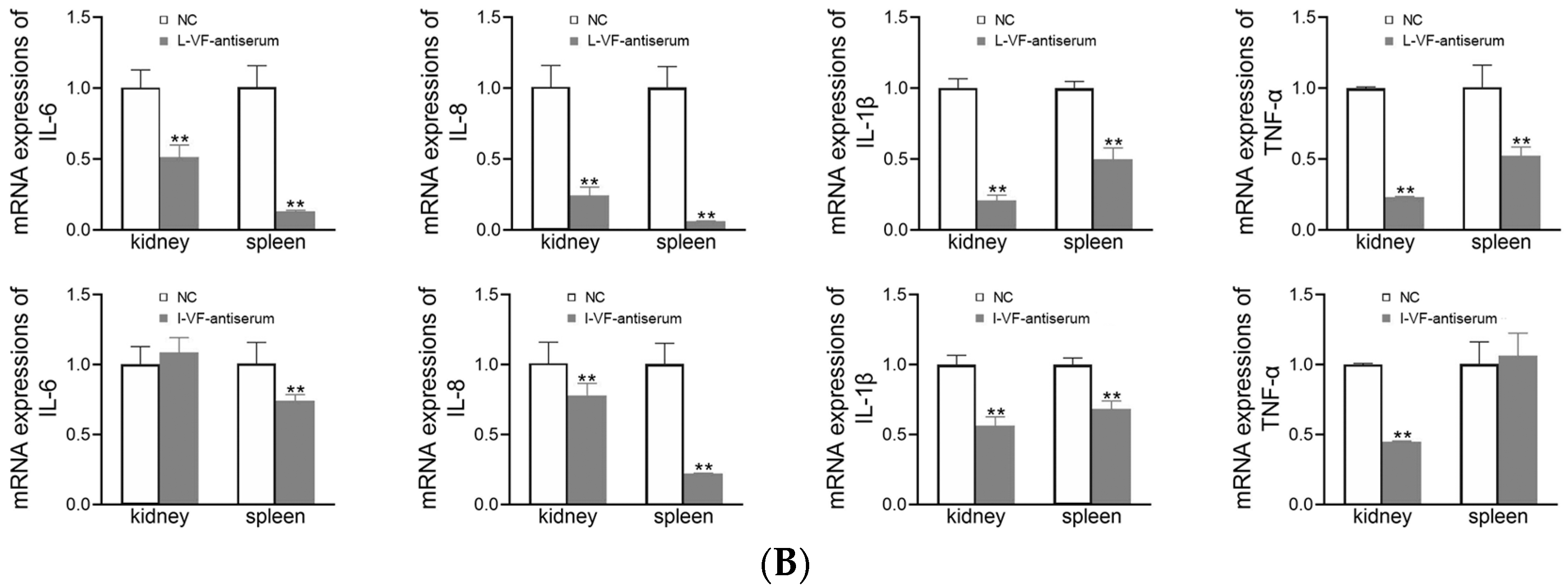
3.8. Inflammatory Gene Expression in C. auratus
The kidneys and spleens of C. auratus were obtained on Day 2 after the fish were administered mouse antisera and challenged with bacteria. In the treatment groups, the mRNA expression of inflammatory genes (i.e., IL-6, IL-8, TNF-α, and IL-1β) was decreased (p < 0.05) compared with the control group (Figure 7). Thus, the live and inactivated mouse antisera promoted anti-inflammatory activity in C. auratus.
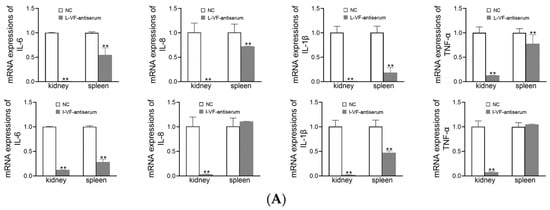
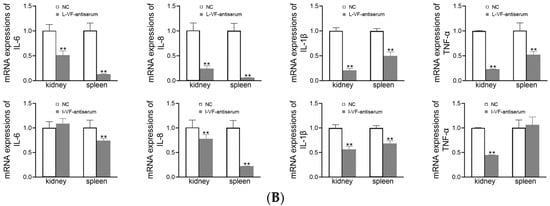
Figure 7.
mRNA expression of the inflammatory genes in the kidney and spleen of C. auratus. (A,B) Challenges with V. fluvialis and A. hydrophila, respectively. ** p < 0.01 (compared with the control). The mRNA expression of the inflammatory genes generally decreased (p < 0.05).
3.9. Differences in the Antibody Responses of the Outer Membrane Proteins Induced by Live and Inactivated V. fluvialis
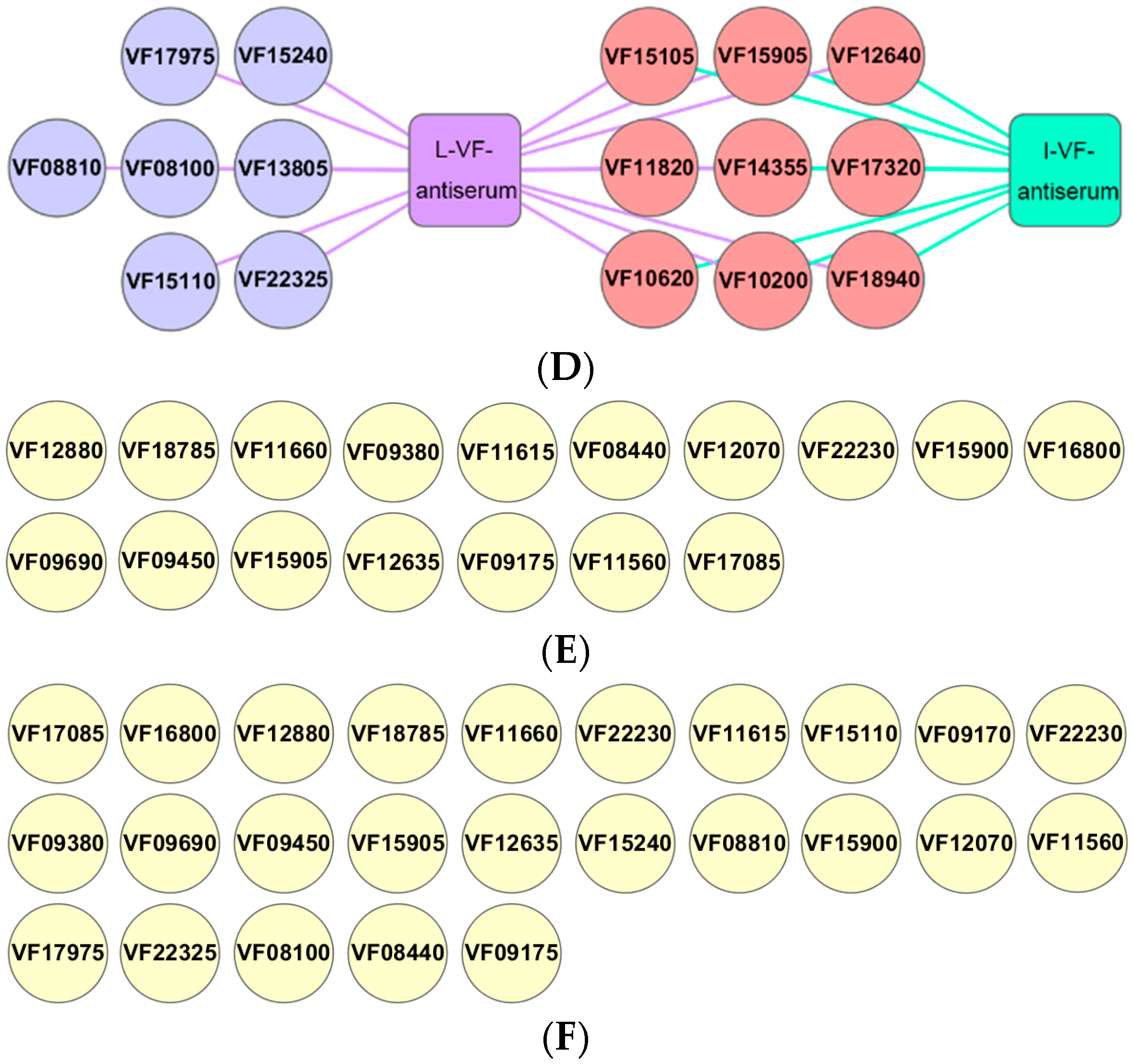
Bacterial outer membrane proteins have good immunogenicity and are targeted proteins for vaccine research [18]. The live and inactivated mouse antisera were interacted with a chip array that included 34 outer membrane proteins of V. fluvialis via western blotting in order to study the antibody responses for the outer membrane proteins induced by live and inactivated V. fluvialis. The results showed that 16 outer membrane protein antibodies were present with the live mouse antiserum and 9 with the inactivated mouse antiserum (Figure 8D). Furthermore, the live V. fluvialis immunity that activated the production of antibodies against the outer membrane proteins included that of the inactivated V. fluvialis immunity (Figure 8D). Therefore, live V. fluvialis immunity could induce more of an antibody response against outer membrane proteins than the inactivated V. fluvialis in mice.
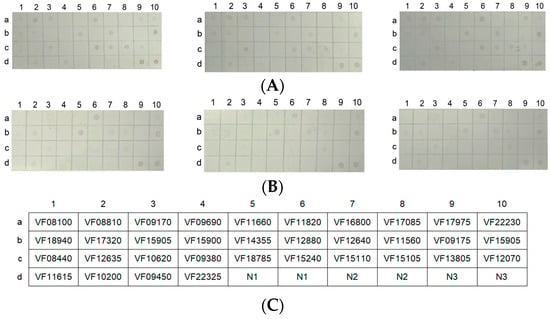
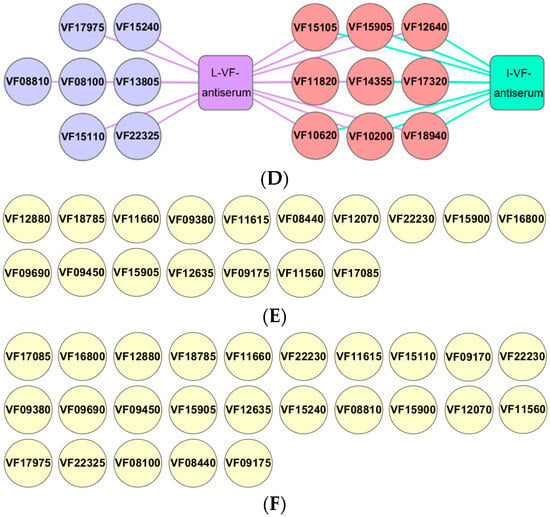
Figure 8.
The chip array of the 34 outer membrane proteins. (A,B) The chip recognized with live or inactivated mouse antisera (which were repeated three times), respectively. (C) The location of the outer membrane proteins on the microarray. (D) The antibody types of the outer membrane proteins in the live and inactivated mouse antisera. (E,F) The outer membrane proteins that did not produce antibodies with the live and inactivated mouse antisera, respectively. N1 and N2 represent the BSA solution and 50 mM Tris-HCl as negative controls, respectively. N3 represents 0.5 μg of the outer membrane protein solution as a positive control.
In addition, the entire OMPs of A. hydrophila were purified to prepare a chip array. Then, different dilutions of live or inactivated V. fluvialis antiserum were interacted with the chip array via western blotting. The results showed that the titer of the live bacterial antiserum reached 1:800, as well as also showing a titer of 1:400 for the inactivated bacterial antiserum (Supplementary Figure S2). Thus, there was an immune binding capacity between the live or inactivated bacterial antiserum and A. hydrophila OMPs, and the binding activity of the live bacterial antiserum was higher than that of inactivated bacterial antiserum.
4. Discussion
Against the wide variety of pathogenic bacteria and rapid disease development, the prevention and control of bacterial diseases pose enormous challenges in aquaculture [22,23]. Passive vaccines have timely bacterial prevention and control capabilities, which render them especially suitable for explosive pathogenic bacteria in fish [21]. However, polyvalent passive vaccines that can resist multiple bacterial infections are relatively rare in aquaculture [24]. One study passively administered mice the glycoprotein gp42 monoclonal antibody and found that the antibody had a passive protection rate of 100% (p < 0.05) against Epstein–Barr virus infection [25]. Lu et al. prepared specific IgY antibodies via immunizing laying hens with white spot syndrome virus, and found that the IgY antibody had a passive protective rate of 73.3% (p < 0.05) against white spot syndrome virus in shrimp [26]. Peng et al. prepared 18 rabbit antisera against Vibrio parahaemolyticus outer membrane proteins. After the passive administration of these antisera to zebrafish, they found that two antisera (VP1667 and VP2369) had significant immunoprotective effects against V. parahaemolyticus infection [27]. We have identified 4 protective outer membrane protein antibodies (OmpW, OmpAII, P5, and AHA2685) of A. hydrophila from 15 types of mouse antibodies, and they could provide a basis for the development of passive vaccines in aquaculture [20]. This study prepared live and inactivated mouse antisera in order to contribute to passive vaccine development. After administering the C. auratus with live or inactivated mouse antisera and challenging them with V. fluvialis or A. hydrophila, the C. auratus serum demonstrated a binding capacity with V. fluvialis or A. hydrophila. Furthermore, the passive protective rates of the live and inactivated mouse antisera against V. fluvialis were 60% (p < 0.05) and 40% (p < 0.05), respectively, whereas the passive cross-protective rates against A. hydrophila were 42.86% (p < 0.05) and 35.71% (p < 0.05), respectively. In addition, the bacterial count decreased (p < 0.05) in the C. auratus kidneys in both groups (i.e., the live and inactivated mouse antiserum groups), and the bacterial count of the live mouse antiserum was lower than that of the inactivated mouse antiserum. Moreover, the phagocytic activity of the live and inactivated mouse antisera increased (p < 0.05) in the C. auratus leukocytes, but it was higher with the live mouse antiserum than with the inactivated mouse antiserum. Most of the interactions between bacteria and antisera are considered to occur shortly after injection. Furthermore, it becomes crucial to understand how long antibody molecules remain in the body after administration, how long their effects persist and the considerations that need to be made for effective administration methods. In previous experiments, it was found that 2 or 2.5 h was the optimal time for attacking bacteria after antisera are administrated to fish [18,20,21]. In this experiment, we found that two hours was the optimal time for attacking the pathogenic bacteria in a preliminary experiment after the antisera were injected into the peritoneal cavity, as there may be some time required for the fish to adapt the antibodies to exert their immune activity. These results show that the live and inactivated mouse antisera have passive immune protective abilities and that the live mouse antiserum has a higher efficacy than the inactivated mouse antiserum.
The morphological integrity of the cells reflects the health status of animals and can be used to evaluate the immunological function of drugs [28]. Organizational pathology is measured with the preparation of pathological sections and microscopic observation, and it can be used to observe cell morphology [29]. Huang et al. improved tumor boundary recognition and clinical pathological diagnosis using 2D/3D Raman images of human tissue slices [30]. Other researchers have prepared the VP19 protein to display on the spore surface of Bacillus subtilis and orally immunized groupers. After challenge with the Singapore grouper iridovirus, histopathological sections showed that the pathological damage to the grouper kidney and liver was significantly reduced. This indicated that recombinant VP19 have a protective effect on the integrity of the organizational structure [31]. In the current study, C. auratus were administered live or inactivated mouse antisera and challenged with bacteria; following this, the structures of the kidneys, spleens, and intestines were found to be intact and tight. Thus, the live and inactivated mouse antisera used in this study could protect the integrity of organizational structures under bacterial infection in C. auratus.
Using immunofluorescence to perform p53 and γH2A.X fluorescence value analysis can indirectly indicate cell apoptosis and DNA damage [32]. The p53 and γH2A.X proteins are targeted proteins for cell function identification [33]. Researchers have shown that the expression of p53 and γH2A.X in aging cells is significantly increased (p < 0.05), indicating an increase in cell apoptosis and DNA damage [34]. Through immunofluorescence detection, Suresh et al. found that N-(4-(benzo [d] thiazol-2-yl) phenyl)-5-chromo-2-methoxybenzamide promotes the expression of p53 and γH2A.X in cancer cells, thereby achieving an inhibitory effect on cancer cell growth [35]. Our preliminary research has shown that the expression of p53 and that of γH2A.X decreases (p < 0.05) after carps are passively administered IgY antibodies against the OmpW and Slp of Pseudomonas fluorescens and challenged with bacteria. The findings indicate that the IgY antibodies of OmpW and Slp reduce apoptosis and DNA damage in carp kidney cells [21]. In this study, after the C. auratus were administered the live or inactivated mouse antisera and challenged with bacteria, the expression of p53 and γH2A.X was decreased (p < 0.05), and the expression of p53 and γH2A.X with the live mouse antiserum was lower than that with the inactivated mouse antiserum (p < 0.05). Therefore, the two mouse antisera could reduce the apoptosis and DNA damage in C. auratus kidney cells induced by bacterial infection, and the immune effects of the live mouse antiserum were better than those of the inactivated mouse antiserum.
Antioxidant and inflammatory responses are important in resisting bacterial infection [36]. To resist bacterial invasion, oxygen is converted into many harmful oxygen free radicals through a series of biotransformations in fish [37,38], which cause oxidative damage to tissues. In addition, the antioxidant system plays a role in scavenging free radicals [39,40]. If the oxidative damage is mild, the activity of antioxidant-related factors decreases. The activity of antioxidant factors (SOD, CAT, and GSH-Px) and the content of MDA indirectly indicate the extent of oxidative tissue damage [41,42]. After animals were administered immunoactive substances and challenged with pathogenic bacteria, the immunoactive substances could inhibit oxidative damage, as manifested by the lower activity of antioxidant factors [18]. The activity of SOD, LYZ, and ACP decreased after IgY administration and bacterial infection in sea cucumbers, which demonstrates that IgY antibodies have an antioxidation effect against bacterial invasion [43]. In addition, after the fish were administered the outer membrane proteins of OmpW, OmpAII, P5, and AHA2685 of A. hydrophila and challenged with bacteria, the activity of the antioxidant factors (SOD, CAT, and GSH-Px) and the content of MDA decreased (p < 0.05), which indicates that these proteins could reduce oxidative damage in fish [20]. After mice with ulcerative colitis were administrated the polysaccharides of Panax quinquefolius, the expression of inflammatory factors (IL-6, IL-8, IL-1β, and TNF-α) was decreased (p < 0.05) in the mouse colon, which indicates that the polysaccharides had anti-inflammatory effects [44]. In previous studies, we found that the levels of inflammatory factors (IL-6, IL-8, TNF-α, and IL-1β) and the activity of antioxidant factors (SOD, MDA, GSH-Px, and CAT) decreased after carps were passively administered IgY antibodies (OmpW, Slp, PF1380, and ExbB) and challenged with bacteria. These results indicated the four IgY antibodies could reduce inflammation and oxidative damage in fish. Furthermore, the four IgY antibodies have antioxidant and anti-inflammatory effects [18,21]. The current study passively administered live or inactivated mouse antisera to C. auratus and challenged them with bacteria. The two mouse antisera could reduce the expression of antioxidant and inflammatory factors (p < 0.05), indicating that live and inactivated mouse antisera have antioxidant and anti-inflammatory effects.
Bacterial outer membrane proteins are located on the outer layer of cells and have good immunogenicity [45], rendering them valuable for vaccine development [46,47]. Researchers have shown that outer membrane proteins (A, C, K, and F) and their family proteins enhance the ability of animals to resist bacterial infection [48,49,50]. However, there is relatively little research on the immunoprotective ability of the outer membrane proteins of V. fluvialis and the corresponding antibodies. In preliminary research, we found that fish could resist various bacterial infections after the administration of IgY antibodies against the outer membrane proteins (PF1380 and ExbB) of P. fluorescens. This indicates that these IgY antibodies against outer membrane proteins are candidates for multivalent passive vaccines [18]. The present study found that live and inactivated mouse antisera have immunoprotective effects and that it is necessary to explore the types of outer membrane protein antibodies present in the two mouse antisera. We prepared a protein chip technology using 34 purified outer membrane proteins and performed western blotting to detect the types of outer membrane protein antibodies in the live and inactivated mouse antisera. The results showed that the live mouse antiserum held 16 antibodies of outer membrane proteins, while the inactivated mouse antiserum held 9. Moreover, the live V. fluvialis immunity that activated the production of antibodies against outer membrane proteins included inactivated V. fluvialis immunity. These results indirectly indicate that the antigenicity of outer membrane proteins could be modified through formaldehyde treatment in inactivated bacteria. Certain researchers have used the UV-inactivated method on recombinant vesicular stomatitis (rVSV) or enterovirus 70 to prepare vaccines, and found that these vaccines could resist the influenza viruses and enterovirus, respectively [51,52]. In addition, research has compared the stability and immunogenicity of the Coxsackievirus B1 vaccine when subjected to UV irradiation or formalin inactivation, finding that formalin inactivation is better [53]. Thus, it may be worthwhile to assess the possibility of maintaining higher antigenicity using methods such as UV irradiation. Interestingly, we found that the entire OMPs of A. hydrophila and the live or inactivated V. fluvialis antiserum exhibited a cross-binding capacity via a protein chip array, and it will be necessary to reveal which A. hydrophila OMPs are responsible in further research. Thus, A. hydrophila OMPs and V. fluvialis OMPs may have homologous proteins, which is consistent with this study’s results showing that live or inactivated V. fluvialis antisera provide passive immunoprotection to V. fluvialis and A. hydrophila. In addition, the 9 OMP antibodies shared by the live or inactivated V. fluvialis antibodies may enhance the resistance of fish to bacterial infections, and the 7 OMP antibodies contained in the live V. fluvialis antibody may further enhance immune activity. Therefore, live V. fluvialis immunity could induce more antibody responses against outer membrane proteins than inactivated V. fluvialis immunity in mice.
5. Conclusions
This study prepared live and inactivated V. fluvialis mouse antisera and evaluated their immunological abilities through the passive administration of C. auratus and challenge with V. fluvialis or A. hydrophila. The results showed that both the live and inactivated mouse antisera could recognize the bacteria in vitro, effectively clearing the bacterial content in the C. auratus kidney and improving the phagocytic activity of C. auratus leukocytes. Furthermore, the two mouse antisera exhibited significant immune protective rates, promoted anti-inflammatory and antioxidant activities, protected the integrity of visceral organ structures, and reduced the apoptosis and DNA damage among kidney cells. Live V. fluvialis immunity could induce more antibody responses against outer membrane proteins than inactivated V. fluvialis in mice. Moreover, live mouse antiserum better promoted immune activity than inactivated antiserum. Lastly, live mouse antiserum could also be a multivalent passive vaccine candidate for fish.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9080302/s1, Figure S1: Experimental process; Figure S2: The entire OMPs of A. hydrophila recognized with live (A) or inactivated (B) mouse antisera by western blotting. 1–4 represent entire OMPs (0.5 μg) of A. hydrophila; 5, 0.5 μg supernatant of A. hydrophila lysates solution as a positive control; 6, 50 mM Tris-HCl as negative control; Table S1: The information of 34 outer membrane proteins.
Author Contributions
Conceptualization, H.X., P.C. and J.C.; methodology, H.X. and X.H.; investigation, Z.M.; methodology, C.C.; writing—original draft preparation, Y.L. and X.L.; writing—review and editing, X.L.; supervision, X.L.; funding acquisition, Y.L. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Projects of Scientific Research Plan of Colleges and Universities of Anhui Province (2022AH051330), the University Collaborative Innovation Project of Anhui Province (GXXT-2023-077), the Biological and Medical Sciences of Applied Summit Nurturing Disciplines in Anhui Province (Anhui Education Secretary Department [2023]13), the College Student Innovation and Entrepreneurship Project of Anhui Province (S202210371092 and S202310371056), the Outstanding Innovative Research Team for Molecular Enzymology and Detection in Anhui Provincial Universities (2022AH010012), and the University Synergy Innovation Program of Anhui Province (GXXT-2022-067).
Institutional Review Board Statement
All animal experimental procedures were conducted in accordance with the guidelines in the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Fuyang Normal University, China (No. 2023–10).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Volpe, E.; Errani, F.; Zamparo, S.; Ciulli, S. Redspotted grouper nervous necrosis virus and the reassortant RGNNV/SJNNV in vitro susceptibility against a commercial peroxy-acid biocide under different conditions of use. Vet. Sci. 2023, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Abioye, O.E.; Osunla, C.A.; Nontongana, N.; Okoh, A.I. Occurrence of virulence determinants in Vibrio cholerae, Vibrio mimicus, Vibrio alginolyticus, and Vibrio parahaemolyticus isolates from important water resources of Eastern Cape, South Africa. BMC Microbiol. 2023, 23, 316. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.A.D.S.; Heo, G.J. Biofilm formation of pathogenic bacteria isolated from aquatic animals. Arch. Microbiol. 2022, 205, 36. [Google Scholar] [CrossRef] [PubMed]
- Ismail, E.T.; El-Son, M.A.M.; El-Gohary, F.A.; Zahran, E. Prevalence, genetic diversity, and antimicrobial susceptibility of Vibrio spp. infected gilthead sea breams from coastal farms at Damietta, Egypt. BMC Vet. Res. 2024, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Muzembo, B.A.; Kitahara, K.; Ohno, A.; Khatiwada, J.; Dutta, S.; Miyoshi, S.I. Vibriosis in South Asia: A systematic review and meta-analysis. Int. J. Infect. Dis. 2024, 141, 106955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, L.; Liu, M.; Liang, W.; Li, Z.; Nan, Z.; Kan, B. Virulence, antibiotic resistance phenotypes and molecular characterisation of Vibrio furnissii isolates from patients with diarrhoea. BMC Infect. Dis. 2024, 24, 412. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Zheng, E.J.; Valeri, J.A.; Donghia, N.M.; Anahtar, M.N.; Omori, S.; Li, A.; Cubillos-Ruiz, A.; Krishnan, A.; Jin, W.; et al. Discovery of a structural class of antibiotics with explainable deep learning. Nature 2024, 626, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Barba, S.; Top, E.M.; Stalder, T. Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era. Nat. Rev. Microbiol. 2024, 22, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, Z. Antibiotic residues, antimicrobial resistance and intervention strategies of foodborne pathogens. Antibiotics 2024, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, Z.G.; Huang, Y.; Jian, J.C.; Tang, J.J. Effects of Chinese herbal medicines on growth performance, intestinal flora, immunity and serum metabolites of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatu ♂). Fish Shellfish Immunol. 2023, 140, 108946. [Google Scholar] [CrossRef] [PubMed]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef]
- Swain, B.; Campodonico, V.A.; Curtiss, R. Recombinant attenuated Edwardsiella piscicida vaccine displaying regulated lysis to confer biological containment and protect catfish against Edwardsiellosis. Vaccines 2023, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Jose Priya, T.A.; Kappalli, S. Modern biotechnological strategies for vaccine development in aquaculture—Prospects and challenges. Vaccine 2022, 40, 5873–5881. [Google Scholar] [CrossRef]
- Tharmalingam, T.; Han, X.; Wozniak, A.; Saward, L. Polyclonal hyper immunoglobulin: A proven treatment and prophylaxis platform for passive immunization to address existing and emerging diseases. Hum. Vaccin. Immunother. 2022, 18, 1886560. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Darling, T.L.; Desai, P.; Liang, C.Y.; Dmitriev, I.P.; Soudani, N.; Bricker, T.; Kashentseva, E.A.; Harastani, H.; Raju, S.; et al. Mucosal vaccine-induced cross reactive CD8 (+) T cells protect against SARS-CoV-2 XBB.1.5 respiratory tract infection. Nat. Immunol. 2024, 25, 537–551. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K.; Tan, K.Y.; Pruksaphon, K.; Klinpayom, C.; Gutiérrez, J.M.; Quraishi, N.H.; Tan, C.H. A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms. Sci. Rep. 2020, 10, 11261. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Soontara, C.; Kerddee, P.; Nguyen, D.H.; Senapin, S.; Costa, J.Z.; Del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; et al. Immunization of Nile Tilapia (Oreochromis niloticus) broodstock with Tilapia lake virus (TiLV) inactivated vaccines elicits protective antibody and passive maternal antibody transfer. Vaccines 2022, 10, 167. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, H.H.; Chao, J.; Jian, S.J.; Wu, X.Q.; Lu, J.; Wang, J.; Chen, C.L.; Liu, Y. Polyvalent passive vaccine candidates from egg yolk antibodies (IgY) of important outer membrane proteins (PF1380 and ExbB) of Pseudomonas fluorescens in fish. Fish Shellfish Immunol. 2023, 143, 109211. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Ye, J.Z.; Han, Y.; Zeng, L.; Zhang, J.Y.; Li, H. Identification of polyvalent protective immunogens from outer membrane proteins in Vibrio parahaemolyticus to protect fish against bacterial infection. Fish Shellfish Immunol. 2016, 54, 204–210. [Google Scholar] [CrossRef]
- Liu, X.; Rong, N.; Sun, W.; Jian, S.J.; Chao, J.; Chen, C.; Chen, R.; Ding, R.; Chen, C.; Liu, Y.; et al. The identification of polyvalent protective immunogens and immune abilities from the outer membrane proteins of Aeromonas hydrophila in fish. Fish Shellfish Immunol. 2022, 128, 101–112. [Google Scholar] [PubMed]
- Liu, X.; Chao, J.; Xiao, H.H.; Chen, J.; Cui, P.; Wu, X.Q.; Lu, J.; Wang, J.; Chen, C.L.; Zhang, X.Y.; et al. Identification of polyvalent passive vaccine candidates from egg yolk antibodies (IgY) of important outer membrane proteins of Aeromonas hydrophila in fish. Aquacult. Rep. 2024, 35, 102002. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Marrujo Lopez, F.I.; Aguilar-Rendon, K.G.; Guzmán, R.H. Pathogenic bacteria prevalence in cultured Nile tilapia in Southwest Mexico: A real-time PCR analysis. J. Fish Dis. 2024, 47, e13921. [Google Scholar] [CrossRef] [PubMed]
- Yaparatne, S.; Morón-López, J.; Bouchard, D.; Garcia-Segura, S.; Apul, O.G. Nanobubble applications in aquaculture industry for improving harvest yield, wastewater treatment, and disease control. Sci. Total Environ. 2024, 931, 172687. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, M.Z.; Peng, B.; Wu, H.K.; Xu, C.X.; Xiong, X.P.; Wang, C.; Wang, S.Y.; Peng, X.X. Immunoproteomic identification of polyvalent vaccine candidates from Vibrio parahaemolyticus outer membrane proteins. J. Proteome Res. 2010, 9, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Kumar, A.; Board, N.L.; Kim, J.; Dowdell, K.; Zhang, S.; Lei, Y.; Hostal, A.; Krogmann, T.; Wang, Y.; et al. Epstein-Barr virus gp42 antibodies reveal sites of vulnerability for receptor binding and fusion to B cells. Immunity 2024, 57, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, J.; Jin, L.; Li, X.; Zhen, Y.; Xue, H.; You, J.; Xu, Y. Passive protection of shrimp against white spot syndrome virus (WSSV) using specific antibody from egg yolk of chickens immunized with inactivated virus or a WSSV-DNA vaccine. Fish Shellfish Immunol. 2008, 25, 604–610. [Google Scholar] [CrossRef]
- Peng, B.; Lin, X.P.; Wang, S.N.; Yang, M.J.; Peng, X.X.; Li, H. Polyvalent protective immunogens identified from outer membrane proteins of Vibrio parahaemolyticus and their induced innate immune response. Fish Shellfish Immunol. 2018, 72, 104–110. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Hu, X.; Zhou, Y.; Yang, Z.; Hou, J.; Liu, F.; Liu, Q.; Mabrouk, I.; Yu, J.; et al. Dermal FOXO3 activity in response to Wnt/β-catenin signaling is required for feather follicle development of goose embryos (Anser cygnoides). Poult. Sci. 2024, 103, 103424. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Chen, Y.; Xu, X.; Wu, R.; He, F.; Zhao, Q.; Sun, Q.; Yi, C.; Wu, J.; Najafov, A.; et al. Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer’s disease mouse model. Nat. Commun. 2020, 11, 5731. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, H.; Sun, L.; Shi, K.; Chen, Y.; Ren, X.; Ge, Y.; Jiang, D.; Liu, X.; Knoll, W.; et al. Rapid, label-free histopathological diagnosis of liver cancer based on Raman spectroscopy and deep learning. Nat. Commun. 2023, 14, 48. [Google Scholar] [CrossRef]
- Liang, X.; Liang, J.; Cao, J.; Liu, S.; Wang, Q.; Ning, Y.; Liang, Z.; Zheng, J.; Zhang, Z.; Luo, J.; et al. Oral immunizations with Bacillus subtilis spores displaying VP19 protein provide protection against Singapore grouper iridovirus (SGIV) infection in grouper. Fish Shellfish Immunol. 2023, 138, 108860. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Britt, R.D.J.; Manlove, L.J.; Wicher, S.A.; Roesler, A.; Ravix, J.; Teske, J.; Thompson, M.A.; Sieck, G.C.; Kirkland, J.L.; et al. Hyperoxia-induced cellular senescence in fetal airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Ortiz, G.; Duarte, L.F.; Fernández, C.; Hernández-Armengol, R.; Palacios, P.A.; Prado, Y.; Andrade, C.A.; Rodriguez-Guilarte, L.; Kalergis, A.M.; et al. Contribution of viral and bacterial infections to senescence and immunosenescence. Front. Cell Infect. Microbiol. 2023, 13, 1229098. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Hassan, M.; Tacke, F.; Engelmann, C. Delineating the heterogeneity of senescence induced-functional alterations in hepatocytes. Cell Mol. Life Sci. 2024, 81, 200. [Google Scholar] [CrossRef] [PubMed]
- Suresh Babu, V.; Kizhakeyil, A.; Dudeja, G.; Chaurasia, S.S.; Barathi, V.A.; Heymans, S.; Verma, N.K.; Lakshminarayanan, R.; Ghosh, A. Selective induction of intrinsic apoptosis in retinoblastoma cells by novel cationic antimicrobial dodecapeptides. Pharmaceutics 2022, 14, 2507. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.T.; Schuch, V.; Hossack, D.J.; Chakraborty, R.; Johnson, E.L. Melatonin: The placental antioxidant and anti-inflammatory. Front. Immunol. 2024, 15, 1339304. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Y.; Liu, C.; Ding, J.; Gao, X.M.; Wang, J.Q.; Zhang, Y.B.; Du, C.; Hou, C.C.; Zhu, J.Q.; Lou, B.; et al. Scavenging reactive oxygen species is a potential strategy to protect Larimichthys crocea against environmental hypoxia by mitigating oxidative stress. Zool. Res. 2021, 42, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-inflammatory, antioxidant, and neuroprotective effects of polyphenols-polyphenols as an element of diet therapy in depressive disorders. Int. J. Mol. Sci. 2023, 24, 2258. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar]
- Dongjie, S.; Rajendran, R.S.; Xia, Q.; She, G.; Tu, P.; Zhang, Y.; Liu, K. Neuroprotective effects of Tongtian oral liquid, a traditional Chinese medicine in the Parkinson’s disease-induced zebrafish model. Biomed. Pharmacother. 2022, 148, 112706. [Google Scholar] [CrossRef] [PubMed]
- Naraki, K.; Rezaee, R.; Karimi, G. A review on the protective effects of naringenin against natural and chemical toxic agents. Phytother. Res. 2021, 35, 4075–4091. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, Y.P.; He, L.Y.; Zhang, M.X.; Wang, L.L.; Li, Z.; Li, X.Y. Immunomodulatory effects of chicken egg yolk antibodies (IgY) against experimental Shewanella marisflavi AP629 infections in sea cucumbers (Apostichopus japonicus). Fish Shellfish Immunol. 2019, 84, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.D.; Chen, K.C.; Li, S.S.; Zhang, Y.T.; Li, Z.M.; Liu, S.; Sun, Y.S. Panax quinquefolius polysaccharides ameliorate ulcerative colitis in mice induced by dextran sulfate sodium. Front. Immunol. 2023, 14, 1161625. [Google Scholar] [CrossRef] [PubMed]
- Janssens, A.; Nguyen, V.S.; Cecil, A.J.; Van der Verren, S.E.; Timmerman, E.; Deghelt, M.; Pak, A.J.; Collet, J.F.; Impens, F.; Remaut, H. SlyB encapsulates outer membrane proteins in stress-induced lipid nanodomains. Nature 2024, 626, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.; Rodrigues, S.C.; Duarte, F.V.; Costa, P.M.D.; Costa, P.M.D. The role of outer membrane proteins in UPEC antimicrobial resistance: A systematic review. Membranes 2022, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Salgado, L.; Souto, S.; Olveira, J.G.; Bandín, I. A potential Nervous Necrosis Virus (NNV) live vaccine for sole obtained by genomic modification. Animals 2024, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Meena, J.K.; Sharma, M.; Dixit, A. Recombinant outer membrane protein C of Aeromonas hydrophila elicits mixed immune response and generates agglutinating antibodies. Immunol. Res. 2016, 64, 1087–1099. [Google Scholar] [CrossRef]
- Tang, X.; Wang, H.; Liu, F.; Sheng, X.; Xing, J.; Zhan, W. Outer membrane protein A: An immunogenic protein induces highly protective efficacy against Vibrio ichthyoenteri. Microb. Pathog. 2017, 113, 152–159. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Hu, D.; Dou, Y.; Xiong, L.; Wang, X.; Hu, J.; Xing, S.Z.; Li, W.; Cai, J.P.; Jin, M.; et al. Identification and evaluation of recombinant outer membrane proteins as vaccine candidates against Klebsiella pneumoniae. Front. Immunol. 2021, 12, 730116. [Google Scholar] [CrossRef] [PubMed]
- Olukitibi, T.A.; Ao, Z.; Azizi, H.; Ouyang, M.J.; Omole, T.; McKinnon, L.; Kobasa, D.; Coombs, K.; Kobinger, G.; Yao, X. UV-inactivated rVSV-M2e-based influenza vaccine protected against the H1N1 influenza challenge. Front. Biosci. 2024, 29, 195. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Duggan, C.; Texada, D.E.; Reden, T.B.; Kooragayala, L.M.; Langford, M.P. Immunogenicity of enterovirus 70 capsid protein VP1 and its non-overlapping N-and C-terminal fragments. Antiviral Res. 2005, 66, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Hankaniemi, M.M.; Stone, V.M.; Sioofy-Khojine, A.B.; Heinimäki, S.; Marjomäki, V.; Hyöty, H.; Blazevic, V.; Laitinen, O.H.; Flodström-Tullberg, M.; Hytönen, V.P. A comparative study of the effect of UV and formalin inactivation on the stability and immunogenicity of a Coxsackievirus B1 vaccine. Vaccine 2019, 37, 5962–5971. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).