Sex-Linked Changes in Biotransformation of Phenol in Brook Trout (Salvelinus fontinalis) over an Annual Reproductive Cycle
Abstract
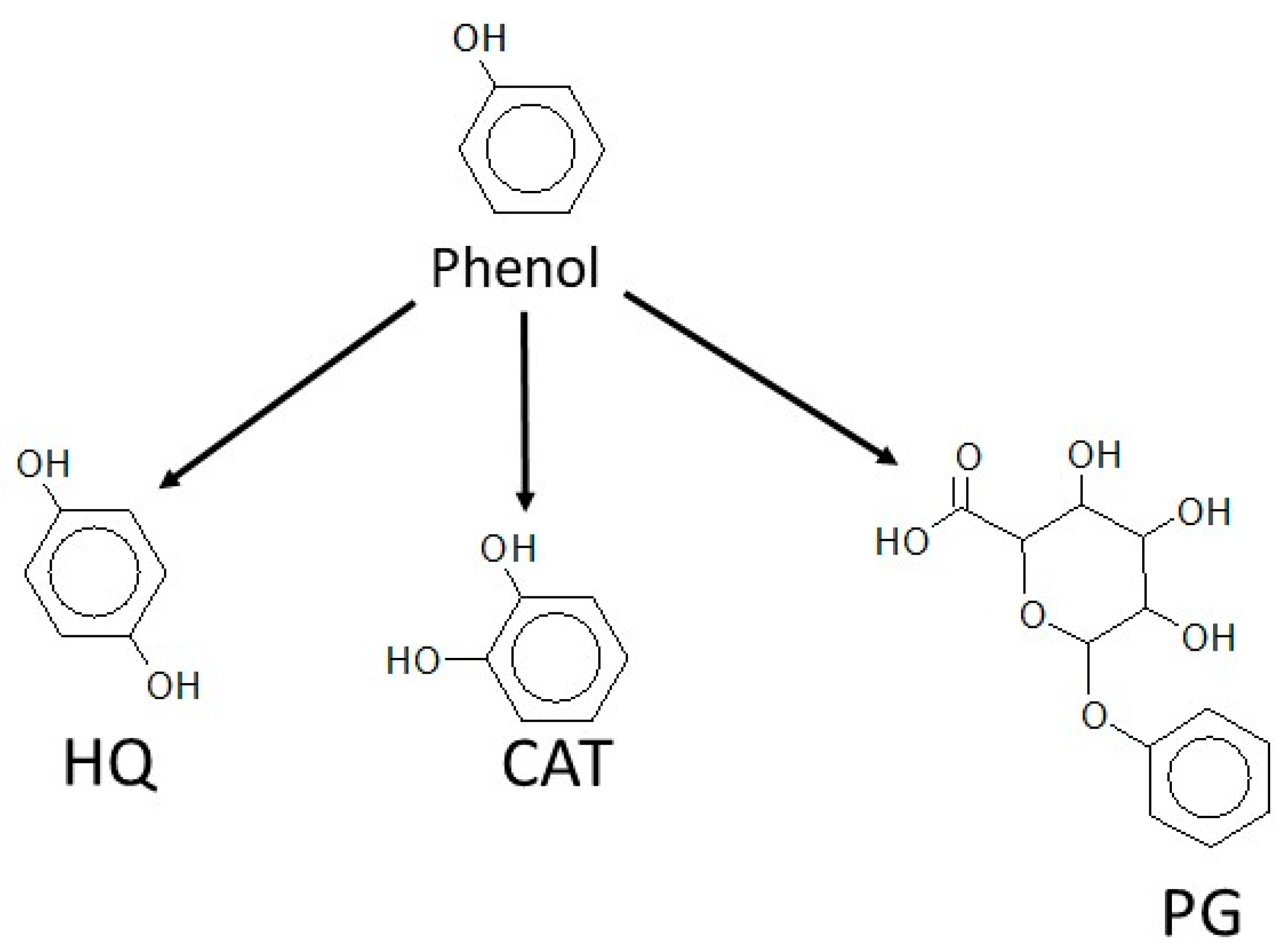
:1. Introduction
2. Methods and Materials
2.1. Chemicals
2.2. Standard and Sample Preparation and Handling
2.3. Animals
2.4. Microsomal Characterization
2.5. Phase I Microsomal Incubations
2.6. Phase II Microsomal Incubations
2.7. Phase I Metabolite Identification and Quantification
2.8. Phase II Metabolite Identification and Quantification
2.9. Data Analysis
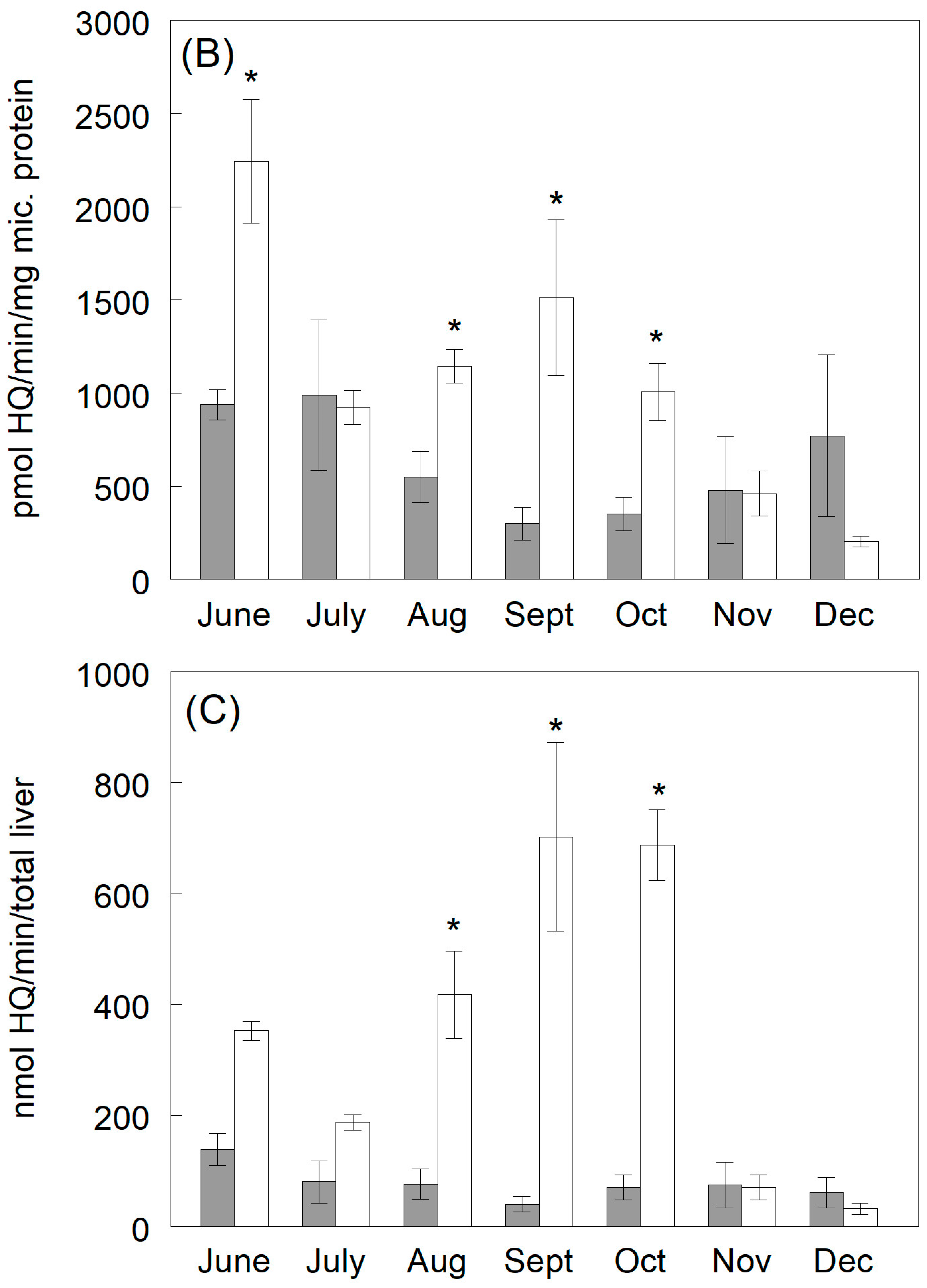
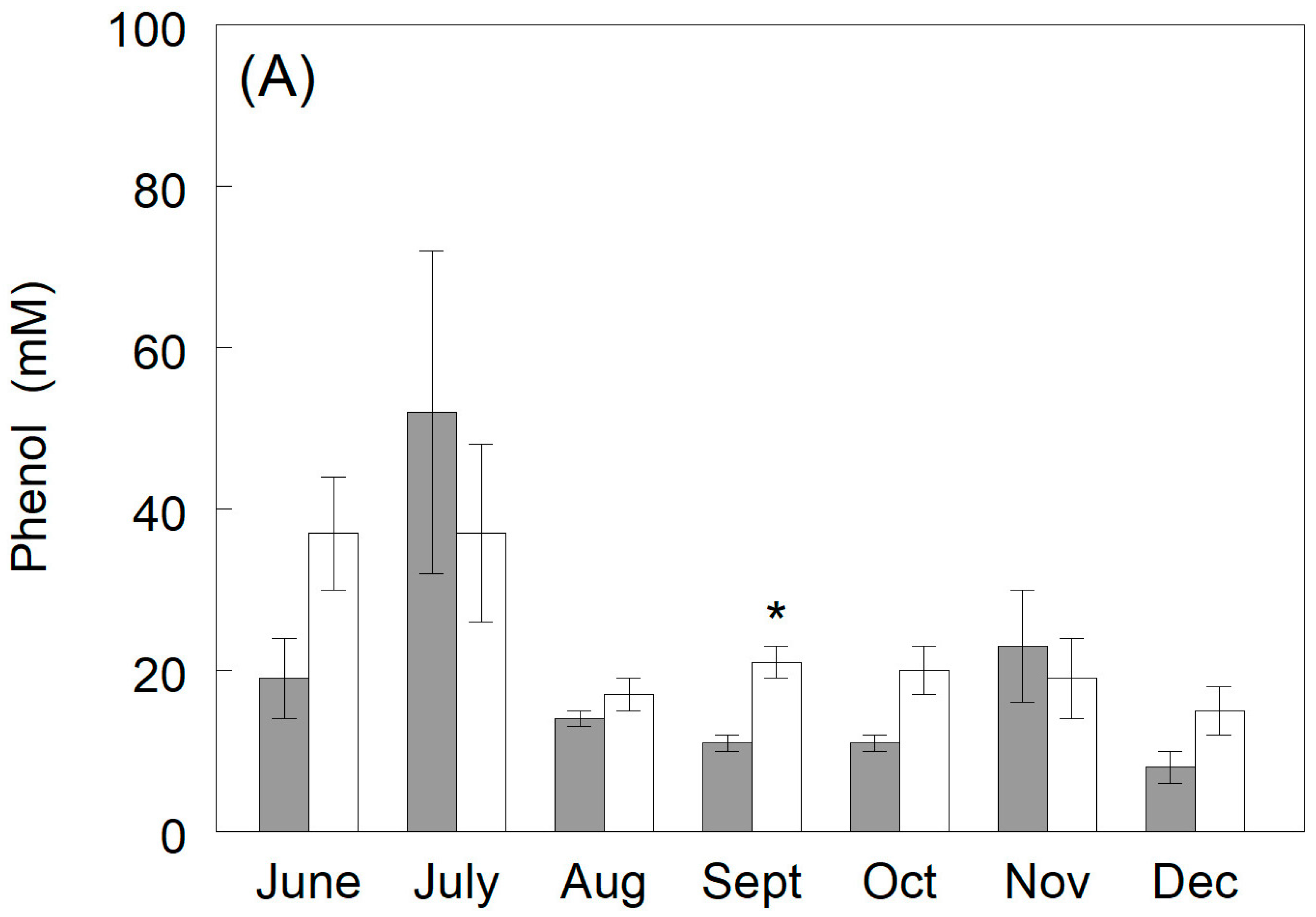
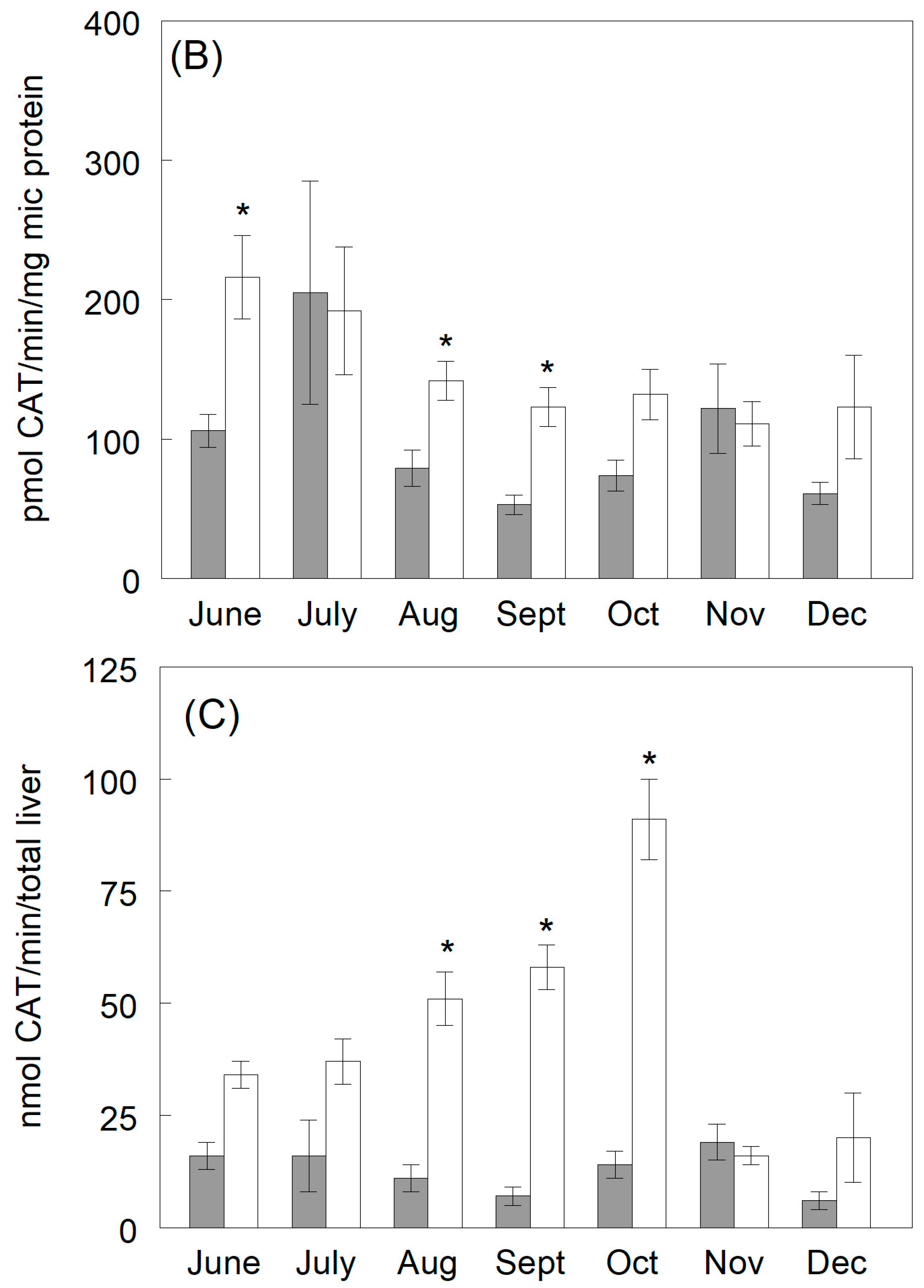
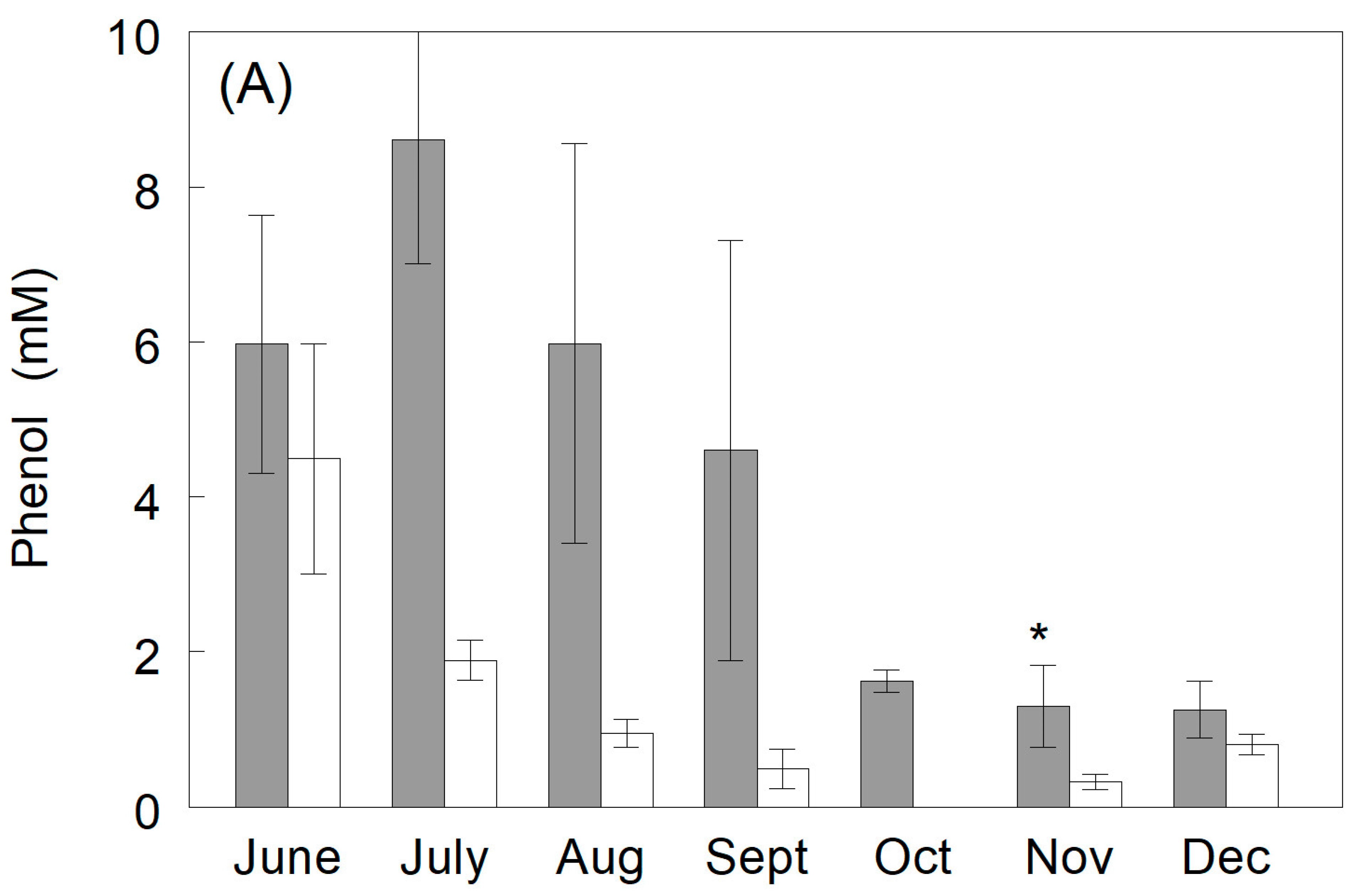
3. Results
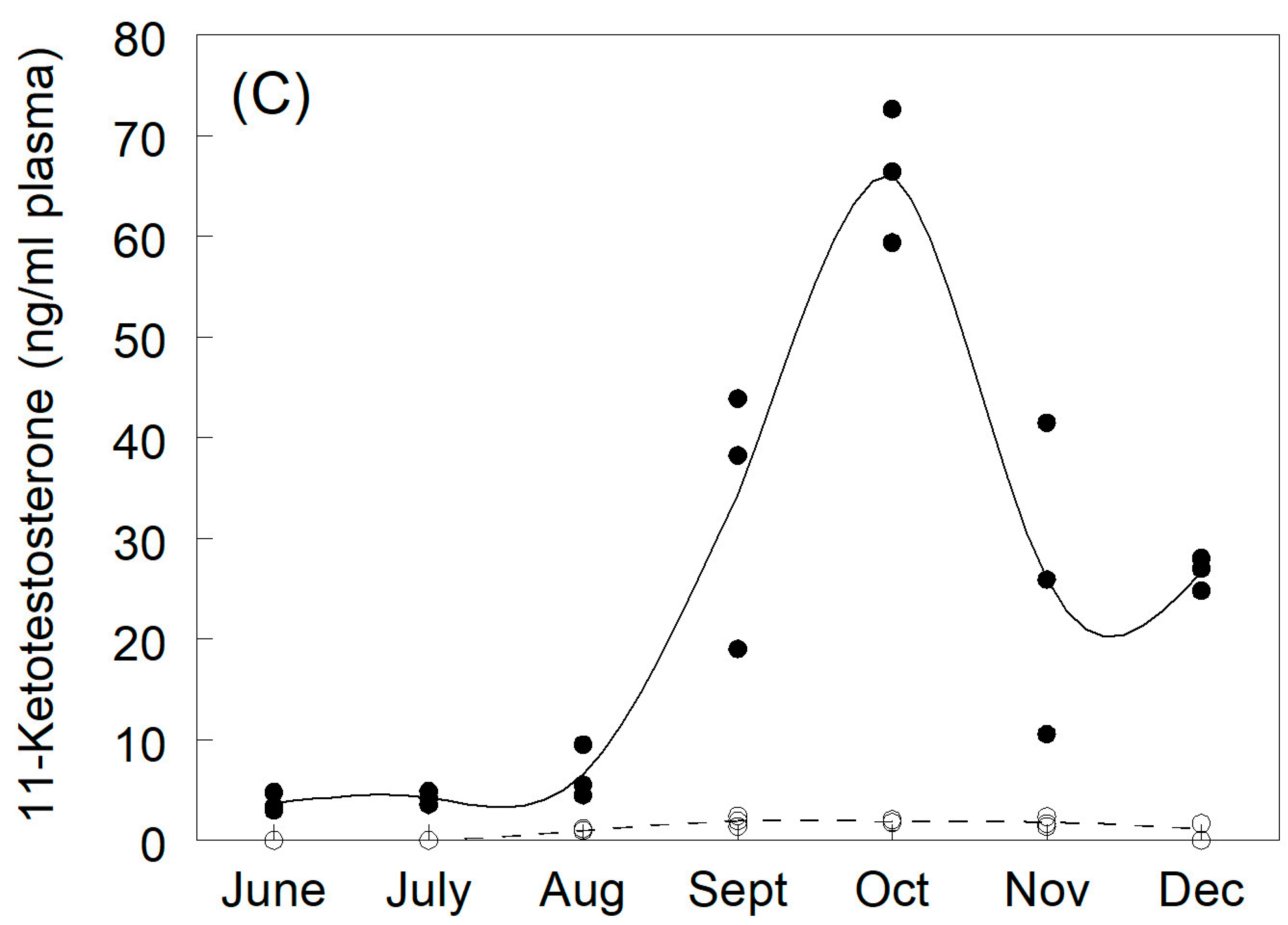
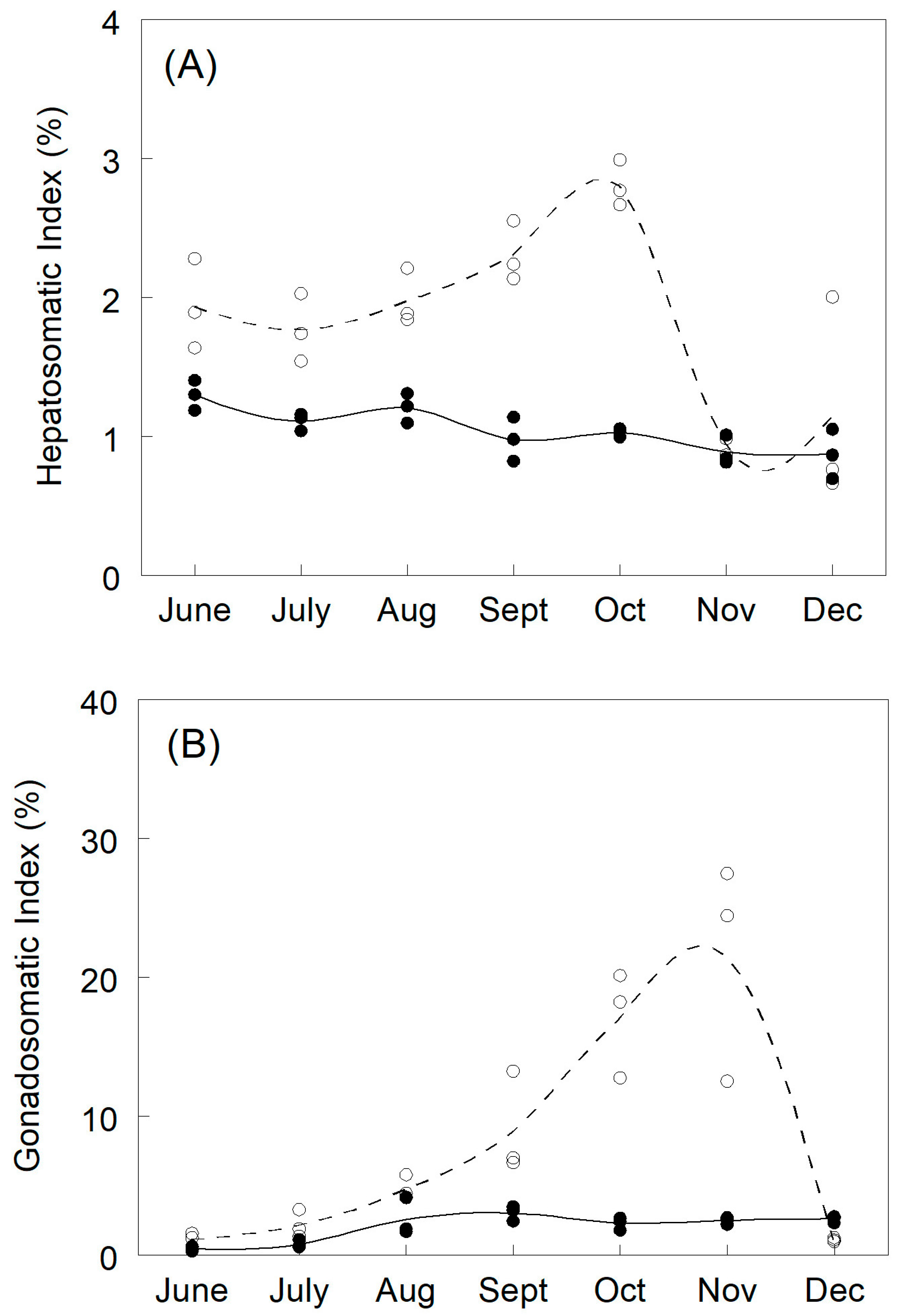
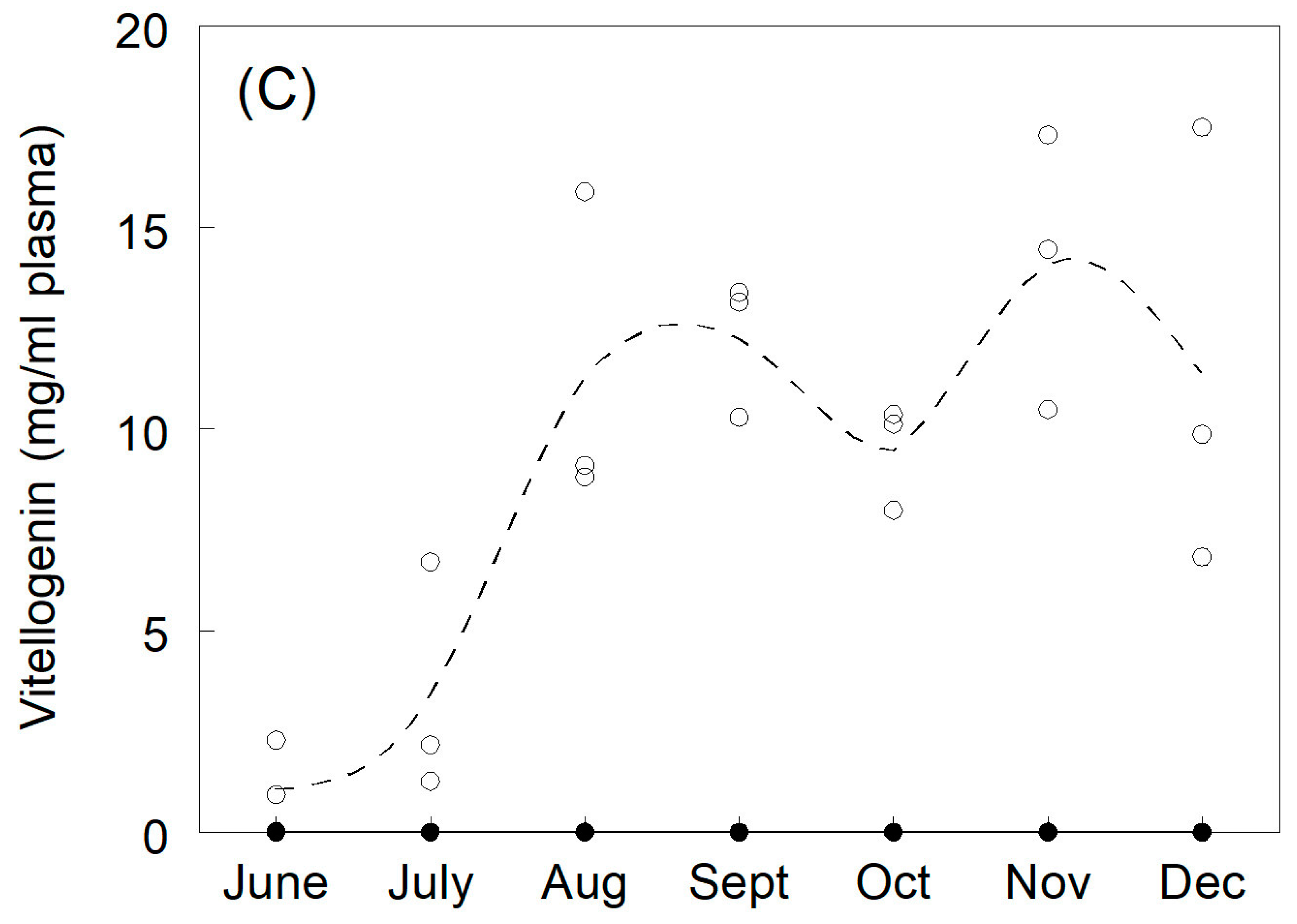
3.1. Plasma Sex Steroids and Vitellogenin
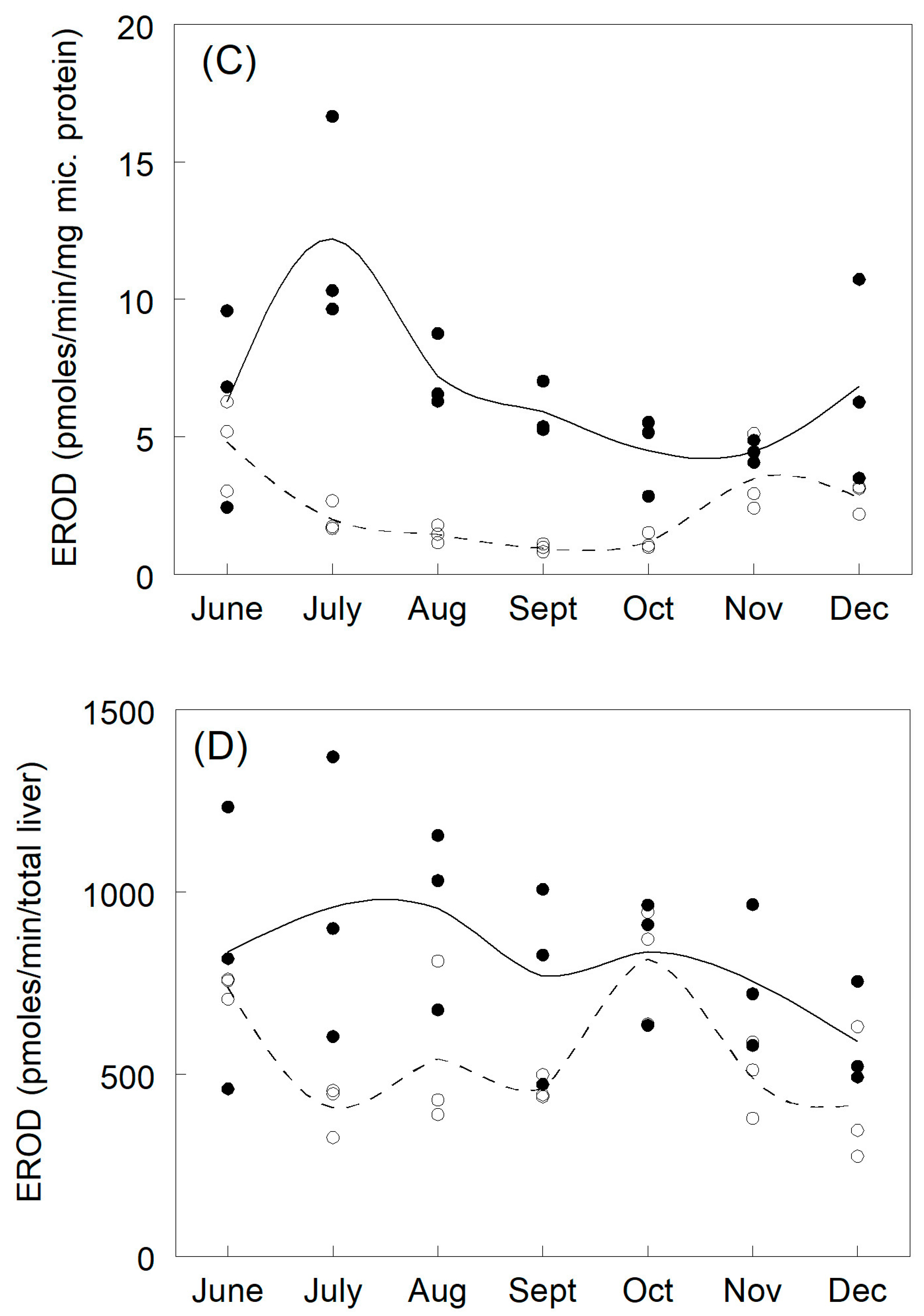
3.2. Microsomal Protein
3.3. Biotransformation Kinetics
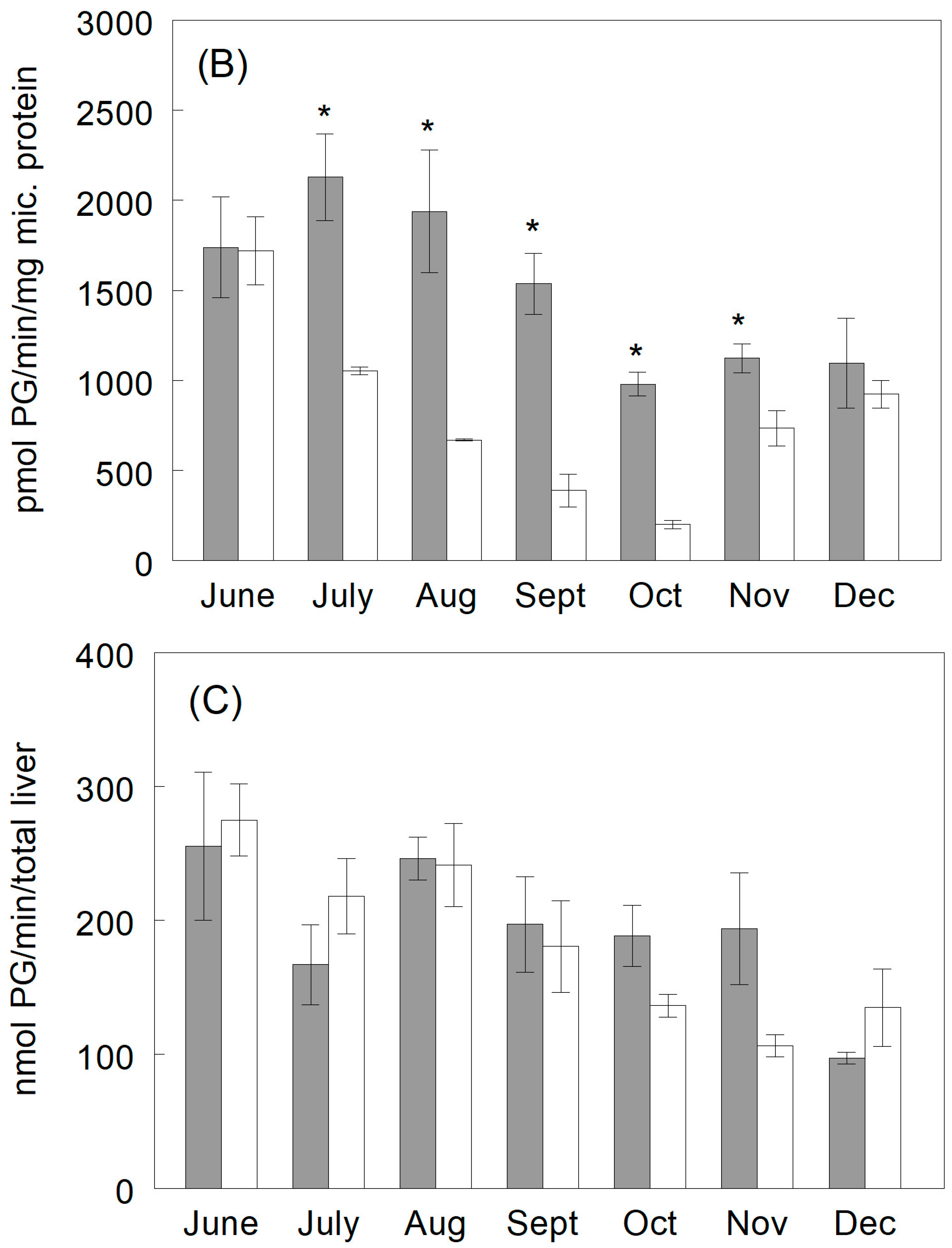
3.3.1. Hydroquinone Production
3.3.2. Catechol Production
3.3.3. Phenylglucuronide Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambers, J.E.; Yarbrough, J.D. Xenobiotic biotransformation systems in fish. Comp. Biochem. Physiol. 1976, 55C, 77–84. [Google Scholar]
- Bend, J.R.; James, M.O. Xenobiotic metabolism in marine and freshwater species. Biochem. Biophys. Perspect. Mar. Biol. 1978, 4, 125–187. [Google Scholar]
- Lech, J.J.; Statham, C.N. Role of glucuronide formation in the selective toxicity of 3-trifluoromethyl-4-nitrophenol (TFM) for the sea lamprey: Comparative aspects of TFM uptake and conjugation in sea lamprey and rainbow trout. Toxicol. Appl. Pharmacol. 1975, 31, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.S.; Kahng, M.W.; Reimschuessel, R.; Nhamburo, P.T.; Lipsky, M.M. UDP-glucuronyltransferase kinetics for 3-trifluoromethyl-4nitrophenol (TFM) in fish. Trans. Am. Fish. Soc. 1994, 123, 217–222. [Google Scholar] [CrossRef]
- James, M.O.; Bend, J.R. Polycyclic aromatic hydrocarbon induction of cytochrome P450 dependent mixed-function oxidases of marine fish. Toxicol. Appl. Pharmacol. 1980, 54, 117–133. [Google Scholar] [CrossRef]
- Kleinow, K.M.; Melancon, M.J.; Lech, J.J. Biotransformation and induction: Implications for toxicity, bioaccumulation and monitoring of environmental xenobiotics in fish. Environ. Health Perspect. 1987, 71, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Haasch, M.L.; Wejksnora, P.J.; Stegeman, J.J.; Lech, J.J. Cloned rainbow trout liver P1 450 complimentary DNA as a potential environmental monitor. Toxicol. Appl. Pharmacol. 1989, 98, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Dewaide, J.H.; Henderson, P.T. Seasonal variation of hepatic drug metabolism in the roach, Leuciscus rutilus L. Comp. Biochem. Physiol. 1970, 32, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Forlin, L. Effects of Clophen A50, 3-methylcholanthrene, pregnenolone-16 alpha-carbonitrile and phenobarbital on the hepatic microsomal cytochrome P450 monooxygenase system in rainbow trout, Salmo gairdneri, of different age and sex. Toxicol. Appl. Pharmacol. 1980, 54, 420–430. [Google Scholar] [CrossRef]
- Hansson, T.; Rafter, J.; Gustafsson, J.A. Effects of some common inducers on the hepatic microsomal metabolism of androstenedione in rainbow trout with special reference to cytochrome P450 dependent enzymes. Biochem. Pharmacol. 1980, 29, 582–587. [Google Scholar] [CrossRef]
- Stegeman, J.J.; Chevion, M. Sex differences in cytochrome P450 and mixed-function oxygenase activity in gonadally mature trout. Biochem. Pharmacol. 1980, 29, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Koivusaari, U.; Pesonen, M.; Hanninen, O. Polysubstrate monooxygenase activity and sex hormones in pre- and post-spawning rainbow trout (Salmo gairdneri). Aquat. Toxicol. 1984, 5, 67–76. [Google Scholar] [CrossRef]
- Stehly, G.R.; Hayton, W.L. Disposition of pentachlorophenol in rainbow trout (Salmo gairdneri): Effect of inhibition of metabolism. Aquat. Toxicol. 1989, 14, 131–148. [Google Scholar] [CrossRef]
- McKim, J.M., Jr.; McKim, J.M., Sr.; Naumann, S.; Hammermeister, D.E.; Hoffman, A.D.; Klaassen, C.D. In vivo microdialysis sampling of phenol and phenyl glucuronide in the blood of unanesthetized rainbow trout: Implications for toxicokinetic studies. Fundam. Appl. Toxicol. 1993, 20, 190–198. [Google Scholar] [CrossRef] [PubMed]
- McKim, J.M.; Kolanczyk, R.C.; Lien, G.J.; Hoffman, A.D. Dynamics of renal excretion of phenol and major metabolites in the rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 1999, 45, 265–277. [Google Scholar] [CrossRef]
- Cravedi, J.P.; Lafuente, A.; Baradat, M.; Hillenweck, A.; Perdu-Durand, E. Biotransformation of pentachlorophenol, aniline and biphenyl in isolated rainbow trout (Oncorhynchus mykiss) hepatocytes: Comparison with in vivo metabolism. Xenobiotica 1999, 29, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; George, S.G.; Burchell, B. Glucuronidation in fish. Aquat. Toxicol. 1991, 20, 35–56. [Google Scholar] [CrossRef]
- Stegeman, J.J.; Binder, R.L.; Orren, A. Hepatic and extrahepatic microsomal electron transport components and mixed function oxygenase in the marine fish Stentomus versicolor. Biochem. Pharmacol. 1979, 28, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Andersson, T. Subcellular localization and properties of cytochrome P-450 and UDP-glucuronosyltransferase in the rainbow trout kidney. Biochem. Pharmacol. 1987, 36, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom-Seppa, P.; Koivusaari, U.; Hanninen, O. Extrahepatic metabolism in north european freshwater fish. Comp. Biochem. Physiol. 1981, 69C, 259–263. [Google Scholar] [CrossRef]
- Schmieder, P.K.; Tapper, M.A.; Kolanczyk, R.C.; Hammermeister, D.E.; Sheedy, B.R.; Denny, J.S. Discriminating redox cycling and arylation pathways of reactive chemical toxicity in trout hepatocytes. Toxicol. Sci. 2003, 72, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, R.C.; Schmieder, P.K. Rate and capacity of hepatic microsomal ring-hydroxylation of phenol to hydroquinone and catechol in rainbow trout (Oncorhynchus mykiss). Toxicology 2002, 176, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, R.C.; Tapper, M.A.; Sheedy, B.R.; Serrano, J.A. In vitro metabolism of imidacloprid and acetamiprid in rainbow trout and rat. Xenobiotica 2020, 50, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, R.C.; Denny, J.S.; Sheedy, B.R.; Olson, V.V.; Serrano, J.A.; Tapper, M.A. Increased endocrine activity of xenobiotic chemicals as mediated by metabolic activation. Environ. Toxicol. Chem. 2023, 42, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Tapper, M.A.; Serrano, J.A.; Schmieder, P.K.; Hammermeister, D.E.; Kolanczyk, R.C. Metabolism of Diazinon in rainbow trout liver slices. Appl. Vitr. Toxicol. 2018, 4, 13–23. [Google Scholar] [CrossRef]
- Serrano, J.A.; Kolanczyk, R.C.; Tapper, M.A.; Lahren, T.; Dongari, N.; Hammermeister, D.E.; Kosian, P.A.; Schmieder, P.K.; Sheedy, B.R.; Challis, K.; et al. Characterization and analysis of estrogenic cyclic phenone metabolites produced in vitro by rainbow trout liver slices using GC-MS, LC-MS and LC-TOF-MS. J. Chromatogr. B 2019, 1126–1127, 121717. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, R.C.; Solem, L.E.; McKim, J.M.; Schmieder, P.K. A comparative study of the Phase I and II Biotransformation of phenol in hepatic microsomes from three species of salmonidae: Hydroquinone, catechol and phenylglucuronide formation. Fishes 2024, 9, 284. [Google Scholar] [CrossRef]
- McKim, J.M.; Nichols, J.W. Use of physiologically-based toxicokinetic models in a mechanistic approach to aquatic toxicology. In Aquatic Toxicology: Molecular, Biochemical, and Cellular Perspectives; Malins, D.C., Ostrander, G.K., Eds.; CRC/Lewis Publishers: Boca Raton, FL, USA, 1994; Chapter 11; pp. 469–519. [Google Scholar]
- Nichols, J.W.; Schultz, I.R.; Fitzsimmons, P.N.I. A review of methods, and strategies for incorporating intrinsic clearance estimates into chemical kinetic models. Aquat. Toxicol. 2006, 78, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Fitzsimmons, P.N.; Burkhard, L.P. In vitro-in vivo extrapolation of quantitative hepatic biotransformation data for fish. II. Modeled effects on chemical bioaccumulation. Environ. Toxicol. Chem. 2007, 26, 1304–1319. [Google Scholar] [CrossRef]
- Nichols, J.W.; Fitzsimmons, P.N.; Hoffman, A.D.; Wong, K. In vitro-in vivo extrapolation of hepatic biotransformation data for fish. III. An in-depth case study with pyrene. Environ. Toxicol. Chem. 2023, 42, 1501–1515. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yao, L.; Zhang, Y.Y.; Chen, C.E.; Zhao, J.L.; Ying, G.G. Enhanced prediction of internal concentrations of phenolic endocrine disrupting chemicals and their metabolites in fish by a physiologically based toxicokinetic incorporating metabolism (PBTK-MT) model. Environ. Pollut. 2022, 314, 120290. [Google Scholar] [CrossRef]
- Mit, C.; Bado-Nilles, A.; Daniele, G.; Giroud, B.; Vulliet, E.; Beaudouin, R. The toxicokinetics of bisphenol A and its metabolites in fish elucidated by a PBTK model. Aquat. Toxicol. 2022, 247, 106174. [Google Scholar] [CrossRef] [PubMed]
- Hoar, W.S.; Randall, D.J.; Donaldson, E.M. Fish Physiology; Part A Endocrine Tissues and Hormones; Academic Press Inc., Harcourt Brace Jovanovich Publishers: New York, NY, USA, 1983; Volume IX. [Google Scholar]
- Mommsen, T.P.; Walsh, P.J. Vitellogenesis and oocyte assembly. In Fish Physiology; Hoar, W.S., Randall, R.J., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 11A, pp. 347–405. [Google Scholar]
- Buhler, D.R.; Wang-Buhler, J.L. Rainbow trout cytochrome P450s: Purification, molecular aspects, metabolic activity, induction and role in environmental monitoring. Comp. Biochem. Physiol. Part C 1998, 121, 107–137. [Google Scholar] [CrossRef]
- Koivusaari, U.; Harri, M.; Hanninen, O. Seasonal variation of hepatic biotransformation in female and male rainbow trout (Salmo gairdneri). Comp. Biochem. Physiol. 1981, 70C, 149–157. [Google Scholar] [CrossRef]
- Forlin, L.; Haux, C. Sex differences in hepatic cytochrome P450 monooxygenase activities in rainbow trout during and annual reproductive cycle. J. Endocrinol. 1990, 124, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hansson, T. Androgenic regulation of hepatic metabolism of 4-androstene-3,17-dione in the rainbow trout, Salmo gairdneri. J. Endocrinol. 1982, 92, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, R.; Schmieder, P.; Bradbury, S.; Spizzo, T. Biotransformation of 4-methoxyphenol in rainbow trout (Oncorhynchus mykiss) hepatic microsomes. Aquat. Toxicol. 1999, 45, 47–61. [Google Scholar] [CrossRef]
- Jensen, K.M.; Korte, J.J.; Kahl, M.D.; Pasha, M.S.; Ankley, G.T. Aspects of basic reproductive biology and endocrinology in the fathead minnow (Pimephales promelas). Comp. Biochem. Physiol. 2001, 128C, 127–141. [Google Scholar] [CrossRef]
- Schmieder, P.K.; Tapper, M.A.; Denny, J.S.; Kolanczyk, R.C.; Sheedy, B.R.; Henry, T.R.; Veith, G.D. Use of trout liver slices to enhance mechanistic interpretation of estrogen receptor binding for cost-effective prioritization of chemicals within large inventories. Environ. Sci. Technol. 2004, 38, 6333–6342. [Google Scholar] [CrossRef]
- Forlin, L.; Andersson, T. Storage conditions of rainbow trout liver cytochrome P-450 and conjugating enzymes. Comp. Biochem. Physiol. 1985, 80B, 569–572. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Estabrook, R.; Peterson, W.J.; Baron, J.; Hildebrandt, A.G. The spectrophotometric measurement of turbid suspensions of cytochromes associated with drug metabolism. Methods Pharmacol. 1972, 2, 303–350. [Google Scholar]
- Pohl, R.J.; Fouts, J.R. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal. Biochem. 1980, 107, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.R.; Chowdhury, N.R.; Arias, I.M. Bilirubin conjugation in the spiny dogfish, Squalus acanthias, the small skate, Raja erinacea and the winter flounder, Pseudopleuronectes americanas. Comp. Biochem. Physiol. 1980, 66B, 523–528. [Google Scholar] [CrossRef]
- Sakai, T.; Kawatsu, H.; Gotoh, O. Intracellular distribution and enzymatic properties of bilirubin UDP glucuronyl transferase in carp, eel and yellow tail. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 679–682. [Google Scholar]
- Sturm, A.; Hodson, P.V.; Carey, J.H.; Hansen, P.D. Hepatic UDP-glucuronyltransferase in rainbow trout (Oncorhynchus mykiss) and preliminary assessment of response to pulp mill cooking liquor. Bull. Environ. Contam. Toxicol. 1999, 62, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom-Seppa, P.; Hanninen, O. Sampling and storage conditions of rainbow trout liver affects monooxygenase and conjugation enzymes. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1988, 89, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Sahawata, T.; Neal, R.A. Biotransformation of phenol to hydroquinone and catechol by rat liver microsomes. Mol. Pharmacol. 1983, 23, 453–460. [Google Scholar]
- Hibbs, B.; Wilbur, S.; George, J. Toxicokinetics. In Toxicological Profile for Benzene (Update); ATSDR (Agency for Toxic Substances and Disease Registry): Atlanta, GA, USA, 1997; p. 129. [Google Scholar]
- Scott, A.P.; Liley, N.R. Dynamics of excretion of 17a,20B-dihydroxy-4-pregnen-3-one 20-sulfate, and of the glucuronides of testosterone and 17B-oestradiol, by urine of reproductively mature male and female rainbow trout (Oncorhynchus mykiss). J. Fish Biol. 1994, 44, 117–129. [Google Scholar]
- Pajor, A.M.; Stegeman, J.J.; Thomas, P.; Woodin, B.R. Feminization of the hepatic microsomal cytochrome P-450 system in brook trout by estradiol, testosterone and pituitary factors. J. Exp. Zool. 1990, 253, 51–60. [Google Scholar] [CrossRef]
- Williams, D.E.; Buhler, D.R. Purification of cytochrome P448 from B-naphthoflavone-treated rainbow trout. Biochim. Biophys. Acta 1982, 717, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Buhler, D.R. Benzo[a]pyrene-hydroxylase catalyzed by purified isozymes of cytochrome P-450 from beta-naphthoflavone-fed rainbow trout. Biochem. Pharmacol. 1984, 33, 3743–3753. [Google Scholar] [CrossRef] [PubMed]
- Klotz, A.V.; Stegeman, J.J.; Walsh, C. An arylhydrocarbon hydroxylating hepatic cytochrome P450 from the marine fish, Stenotumus chrysops. Arch. Biochem. Biophys. 1983, 227, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Goksoyr, A. Purification of hepatic microsomal cytochrome P450 from B-naphthoflavone-treated Atlantic cod (Gadus morhua), a marine teleost fish. Biochim. Biophys. Acta 1985, 850, 409–417. [Google Scholar] [CrossRef]
- Stegeman, J.J.; Kloepper-Sams, X.X. Cytochrome P450 isoenzymes and monooxygenase activity in aquatic animals. Environ. Health Perspect. 1987, 71, 87–95. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolanczyk, R.C.; Solem, L.E.; Tapper, M.A.; Hoffman, A.D.; Sheedy, B.R.; Schmieder, P.K.; McKim, J.M., III. Sex-Linked Changes in Biotransformation of Phenol in Brook Trout (Salvelinus fontinalis) over an Annual Reproductive Cycle. Fishes 2024, 9, 311. https://doi.org/10.3390/fishes9080311
Kolanczyk RC, Solem LE, Tapper MA, Hoffman AD, Sheedy BR, Schmieder PK, McKim JM III. Sex-Linked Changes in Biotransformation of Phenol in Brook Trout (Salvelinus fontinalis) over an Annual Reproductive Cycle. Fishes. 2024; 9(8):311. https://doi.org/10.3390/fishes9080311
Chicago/Turabian StyleKolanczyk, Richard C., Laura E. Solem, Mark A. Tapper, Alex D. Hoffman, Barbara R. Sheedy, Patricia K. Schmieder, and James M. McKim, III. 2024. "Sex-Linked Changes in Biotransformation of Phenol in Brook Trout (Salvelinus fontinalis) over an Annual Reproductive Cycle" Fishes 9, no. 8: 311. https://doi.org/10.3390/fishes9080311





