Abstract
Despite the high diversity of elasmobranch fishes in the Balearic Islands, knowledge of their biology and population dynamics is still scarce. A recent mark-and-recapture experiment off the Balearic Islands tagged 3738 individuals of 23 shark and batoid species during MEDITS and CANAL bottom trawl scientific surveys from June 2021 to August 2023. Retrieval was reported for the sharks Scyliorhinus canicula and Mustelus mustelus, revealing relatively small home ranges for these species (0.2–38.5 km and 7.8–15.3 km for S. canicula and M. mustelus, respectively). Recapture efficiency was higher from scientific surveys than from commercial catches, highlighting potential challenges in collaboration with fishermen and recapture reports. Density estimates obtained from the MEDITS bottom trawl survey suggest a much larger population for S. canicula than estimates from the mark–recapture data, indicating MEDITS density estimates for this species may be overestimated due to its scavenger behavior perhaps favoring individuals searching for discards aggregated in the fishing grounds. This study emphasizes the importance of monitoring, collaborative efforts, and improved reporting mechanisms to enhance our understanding of elasmobranch populations and provide support for sustainable management of these vulnerable marine species.
Keywords:
batoids; sharks; movements; tag; recapture efficiency; retrieval; population size; vulnerable; western Mediterranean Key Contribution:
A mark–recapture experiment on elasmobranch species in the Balearic Islands provided insights into the ecology and population dynamics of Scyliorhinus canicula and striking differences in population size estimations compared to those based on bottom trawl surveys.
1. Introduction
Elasmobranchs are considered one of the most vulnerable groups of marine species, mainly due to their life history strategy, which includes slow growth, long lifespan, late sexual maturity, and low fecundity. These characteristics result in low reproductive potential and low resilience, making them particularly sensitive to overexploitation and/or environmental change [1,2]. According to the International Union for Conservation of Nature (IUCN), the Mediterranean is considered a key hotspot of extinction risk for elasmobranchs, with more than 60% of the 72 Mediterranean species categorized as threatened, a large part of them under the most imperiled categories of endangered and critically endangered [3]. Recently, the General Fisheries Commission for the Mediterranean (GFCM) adopted recommendation GFCM/42/2018/2 on fisheries management measures for the conservation of sharks and rays and recommendation GFCM/44/2021/16 on additional mitigation measures for the conservation of elasmobranchs in the Mediterranean, including the adoption of measures to reduce their mortality by incidental catch during fishing operations, as well as supporting data collection, monitoring, and research programs on these vulnerable species.
Despite the vulnerability of elasmobranchs, the lack of accurate fisheries landing data and a paucity of biological information has been a drawback for the assessment of their populations [4], and the Mediterranean is not an exception. Mainly caught as a bycatch, elasmobranchs are not frequently the priority of data collection programs, which focus on target species, although in recent years, increasing efforts have been devoted to including them in these programs due to their vulnerability. Hence, the extent of their exploitation is rarely assessed in the main bodies of fisheries advice in the Mediterranean, including the GFCM and the Scientific, Technical and Economic Committee for Fisheries of the European Commission (STECF).
However, some assessments have been conducted based on the analysis of population trends, generally showing declines, such as in the Gulf of Lions [5], the Ligurian Sea [6], the Adriatic Sea [7,8], and the Aegean Sea [9]. However, some signs of stability or even improvement have also been recently detected, such as in the northwestern Mediterranean, where the abundance and biomass of most demersal elasmobranch species suggest stable or even increasing trends [10]. This was particularly remarkable in the Balearic Islands, where although a long-term series (1965–2009) of the whole elasmobranch community lands showed a decreasing trend, data from the last period analyzed revealed differences between the shelf and slope, as some parameters increased significantly over time on the shelf, but did not follow any trend on the slope [11]. This increasing trend continued in the following years at species level, even for the slope, as some of the most abundant species inhabiting the continental shelf, such as Scyliorhinus canicula (Linnaeus, 1758), Raja clavata Linnaeus, 1758, and Raja polystigma Regan, 1923, and the slope, such as Galeus melastomus Rafinesque, 1810, are showing increasing trends in their density and biomass [10].
In the Balearic Islands, the conservation and exploitation status of Raja clavata has been recently assessed using novel molecular techniques and assessment models for data-poor stocks, showing the fishing mortality is already at sustainable levels, although the stock biomass is below that yielding the maximum sustainable yield, but recovering [12]. However, the species shows low genetic diversity, probably due to decades of overexploitation, pointing to poor conservation status.
Several studies have shown that the abundance and diversity of elasmobranchs off the Balearic Islands is among the highest in the western Mediterranean, particularly for batoid species [13,14,15]. According to the IUCN Red List of Threatened Species, up to twelve of the frequent batoid species found in the area are vulnerable species, such as Dasyatis pastinaca (Linnaeus, 1758), and endangered species like the Mediterranean endemic Raja radula Delaroche, 1809, Rostroraja alba (Lacepède, 1803), and Leucoraja circularis (Couch, 1838). For most of the elasmobranch species, there is no information on basic parameters such as population size (except for R. clavata), species spatial ranges, or their migrations [15].
Mark-and-recapture experiments could help to fill these gaps. This type of study provides insight into important characteristics of the biology of the populations studied, such as their size, growth, mobility and migration patterns, degree of philopatry, home ranges, or connectivity with adjacent populations [16]. However, improving the knowledge on these aspects will depend on having enough reported recaptures and the availability of complementary information, such as fishing effort and its spatial distribution and catches [17].
The aim of this work is to increase the knowledge about demersal elasmobranchs populations in the Balearic Islands, particularly regarding their spatial range and migrations, growth, and population size. For that, a short-term data collection was implemented and the first results obtained of the mark-and-recapture experiment are analyzed.
2. Materials and Methods
2.1. Study Area
The Balearic Islands, located in the western Mediterranean, are separated from the Iberian Peninsula by up to 95 nautical miles, with depths ranging from 800 to 1800 m. The continental shelf is narrow, typically 3 km wide, except in southern Mallorca, where it reaches 35 km [18]. The western and southern slopes are gradual with a 6° average inclination, while the northern and eastern slopes are steeper with 16° average inclination, featuring a distinct shelf break. Shelf sediments are primarily biogenic sands and gravels, with sandy–muddy and detrital sediments at the shelf break and slope areas dominated by biogenic muddy sediments [18,19].
The archipelago borders the Balearic sub-basin to the north and the Algerian sub-basin to the south, which present different oceanographic conditions [20]. The Balearic sub-basin is affected by atmospheric forces and colder, saltier Mediterranean waters, while the Algerian sub-basin is influenced by density gradients and warmer, less saline Atlantic waters entering through the Strait of Gibraltar [21].
These sub-basins are connected by channels between 100 and 800 m deep facilitating water exchange between them [22]. The region’s pronounced oligotrophy, due to low rainfall and the karstic islands’ terrain, results in high water transparency and deeper algal growth [23,24]. Red algae beds, particularly rhodoliths and Peyssonnelia beds, dominate the continental shelf up to 85 m deep [25,26]. In terms of fisheries, the Balearic Islands have a historically low number of bottom trawl fishing boats compared to the Iberian Peninsula, leading to healthier demersal resources and ecosystems, reflected in the population structure of commercial species and higher abundance and diversity of elasmobranch assemblages [27]. The bottom trawl fleet operates during daytime (from 5:00 a.m. to 5:00 p.m.) between 50 and 800 m depth, exerting the greatest effort in the middle slope, followed by the shelf [28]. The artisanal fleet also operates in the archipelago, primarily on the shelf, and is the only commercial fishery operating at depths shallower than 50 m [29]. Purse seine fishing is scarce in the area and has low impact on the tagged species, i.e., elasmobranchs, as well as recreational fishing [29].
2.2. Mark-and-Recapture Experiment
Tagging of elasmobranchs was carried out during two groups of bottom trawl scientific surveys that took place around the Balearic Islands between June 2021 and August 2023: (1) the MEDITS survey (International Bottom Trawl Survey in the Mediterranean), whose main objective is the evaluation of ecosystems and demersal resources [30] around the Balearic Islands (both eastern —Mallorca and Menorca— and western —Pitiüses—); and (2) the CANAL surveys, which aimed to evaluate the effect of protection on shelf resources in the Menorca Channel and adjacent areas. In these surveys, experimental bottom trawl gear (GOC–73) with a codend mesh size of 18 mm (stretched mesh) was used to sample demersal communities, covering depths between 50 and 800 m during the MEDITS surveys with a depth-stratified random sampling scheme, and between 50 and 85 m during the CANAL surveys. Hauls are carried out during the daytime, with an effective duration between 20 and 60 min, depending on the bathymetric stratum, at 2.5–3 knots of mean speed. Horizontal and vertical openings of the net during the haul (18–22 and 2.5–3.2 m, respectively) are recorded using SCANMAR or MARPORT systems.
As soon as elasmobranch specimens arrived on board, they were immediately placed in a recovery tank with continuous seawater flow to keep them alive until they were tagged and released. Tagging was carried out using external spaghetti-type tags consisting of a striking color plastic strand attached to a T-type or dart-type anchor. Both types were printed with an individual identification number and a contact phone number. The tags were inserted into the middle-outer part of a pectoral fin in the case of batoids and into the base of the first or second dorsal fin in the case of sharks. To do so, we used a tagging gun for the T-type tags and a stainless-steel needle in the case of dart-type tags. The information recorded for each individual was depth and geographical position of the capture, and after tagging and just before the return of the individual to the sea, the identification number, date, species, total length and weight, sex and maturity (if possible, from external characteristics), and the geographical position of the release site.
In order to achieve the maximum reporting of recaptures, either recoveries (recaptured individuals that are not returned to the sea) or recaptures (individuals released alive once again after recapture), an information campaign for the fishermen in the Balearic Islands was carried out. To do so, informative in-person meetings with the fishermen guilds of the different ports in the Balearic Islands were conducted, including a meeting with the president of the Balearic Islands Fishermen Associations and an oral presentation at the XIV Scientific Forum on Spanish fisheries in the Mediterranean Sea [31]. During these meetings and the forum, posters, explanatory documents, and sampling sheets with information about the experiment were handed out (e.g., target species, objective, and study area) together with the instructions of the information that should be collected of each individual in the event of recapture, which were: date, geographical coordinates, depth, identification number, and total length (if possible, also the weight and/or a photograph) of the recaptured specimen. In order to encourage participation and reporting, a reward (T-shirt and a fishing knife) was offered to those who reported recaptures.
2.3. Recapture Efficiency: Scientific Surveys vs. Commercial Fleet
The recapture efficiency was measured in terms of recaptures per unit of fishing effort, which was measured in terms of effective fishing hours (i.e., the period of time during which the gear is in touch with the floor and effectively fishing). This was only done for the bottom trawl activity because (i) it is the only type of gear used in the scientific surveys where tagging was conducted, (ii) recaptures and recoveries of S. canicula from the commercial fleet were only recorded by bottom trawl vessels, and (iii) it is the fishing activity that most overlaps with this species’ bathymetric distribution and that has the highest catch rates in the study area. To do so, we calculated the fishing effort exerted in the bathymetric range of each elasmobranch species that was tagged and recaptured/recovered, i.e., S. canicula, both from scientific surveys and commercial fishing activity.
In the case of scientific surveys, the fishing effort was calculated by adding all the effective fishing hours carried out within the bathymetric range of S. canicula (45–420 m depth, [14]) during the surveys after the tagging experiment had begun, which were all performed in 2022 and 2023.
For the commercial fishing activity, we estimated the number of effective fishing hours on the bathymetric range of S. canicula from the satellite-based Vessel Monitoring System (VMS) from July 2021 to August 2023 (i.e., data of the beginning of the tagging experiment and the last survey conducted, respectively). The VMS data consist of registers of geographical position, date, time, and instant velocity of each vessel, approximately every two hours. A total of 125,000 VMS signals from 2022 and 2023 were analyzed for the present study, as an estimation of the effort exerted after the marking of specimens started. Although tagging began in mid-2021, the overall effort exerted throughout the entire experiment spanned two years, with a notable increase in the number of marked individuals available for recapture by the end of the experiment compared to the beginning. Additionally, there were no recaptures during the 2021 surveys, as only a single tagging survey was conducted that year. Consequently, it was considered more accurate to compare the effort exerted by the fleet using data from the same period, specifically from 2022 and 2023, as only these years were used to calculate recapture efficiency in surveys. These factors altogether justify why the recapture efficiency for the fleet was calculated based on data from 2022 and 2023. In order to take into account only signals emitted when the fleet was fishing, we considered those signals emitted when the boat was in the fishing grounds (as geographically delimited in [28]) and sailing at a velocity within the range used by trawlers during fishing off the Balearic Islands between 2 and 3.6 knots. Then, each signal was assigned to a depth stratum using a geographic information system (specifically, ArcGIS) and the European Marine Observation and Data Network (EMODnet) bathymetry for the Balearic Islands, in order to calculate the fishing effort in terms of fishing days in the bathymetric range of S. canicula (45–420 m depth, [14]). In the Balearic Islands, bottom trawl vessels are allowed to be at sea for a maximum of 12 h per day (daily fishing trips). In a single fishing trip, it is common practice to exploit different depth strata targeting different resources. One of the most common mixed strategies in fishing trips is a first haul on the continental shelf (<100 m depth) targeting coastal species such as striped red mullet, squid, and octopus, followed by one in the middle slope (>500 m depth) targeting red shrimp [27]. Other fishing strategies include the deep shelf and shelf break (100–200 m) and the upper slope (200–500 m), where the bottom trawl fleet mainly targets European hake, and deep rose shrimp and Norway lobster, respectively [32]. Hence, in the case of a boat emitting signals from two or three of those depth strata, only half or a third of the fishing day was considered in each stratum, respectively. The final recount of effective commercial fishing hours consisted in multiplying the fishing days in the S. canicula bathymetric range by eight hours (typically the average fishing hours during a fishing trip, calculated from data obtained by observers on board).
Finally, we used the effective fishing hours from both scientific surveys and commercial fishing activity to calculate the recapture efficiency related to effort as the number of recaptures/recoveries per effective fishing hour in each case.
2.4. Population Size: Mark and Recapture vs. MEDITS Density Data
We applied the Lincoln–Petersen formula [33,34] to obtain the population size of S. canicula from survey data:
where N is population size, M is the number of marked individuals in the first event, S is the number of captured individuals in the second event, and R is the number of recaptured marked individuals from the first event in the second event.
This approach can only consider two mark–recapture events. Thus, all the surveys performed in 2022 were considered the first event (tagging, because more specimens were tagged and more surveys were performed than in 2021), and the 2023 surveys the second event (retrieves). Since recaptures occurred only in the surveys around Mallorca and Menorca, only this area was considered to calculate the population size. This calculation was only considered valid for the size range covered during the tagging phase. Therefore, once the population size of this size range had been calculated, in order to estimate the size of the whole population, we added the percentage of individuals in the untagged length range in relation to the tagged length range according to the size distributions obtained from the MEDITS surveys during the years of the mark-and-recapture experiment (see Results section).
The population of S. canicula in Mallorca and Menorca was also estimated using MEDITS data. To do so, we calculated the mean standardized density (individuals/km2) by MEDITS depth strata [30]. The density in each haul was calculated using the distance covered by the gear through the bottom (measured using a GPS) and the horizontal opening of the net (measured using an acoustic system—MARPORT®). Then, the average standardized density was multiplied by the area (km2) of every depth stratum. The size distribution was obtained using the same procedure by size interval.
3. Results
3.1. Mark-and-Recapture Experiment
Since the start of the mark-and-recapture experiment in June 2021, we have tagged a total of 3738 individuals of 23 species of sharks and batoids (Table 1). The tagging was conducted during seven scientific surveys until August 2023: five MEDITS (three around Mallorca and Menorca (eastern Balearic Islands) and two around Pitiüses (western Balearic Islands)) and two CANAL in the Menorca Channel (Figure 1). The most tagged shark and batoid species were Scyliorhinus canicula and Raja clavata, with 2192 and 727 marked individuals, respectively (Table 1).

Table 1.
Number of tagged individuals per species and survey and in total (MEDITS_M, MEDITS_P and CANAL are the scientific surveys carried out in Mallorca and Menorca, Pitiüses, and the Menorca channel, respectively), and their total length (TL, in mm) range.
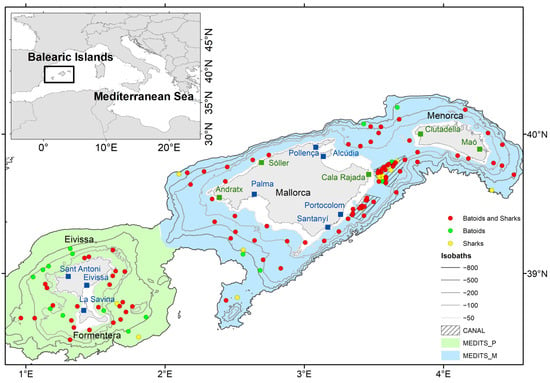
Figure 1.
Map of the Balearic Islands representing all the sampling stations performed during the CANAL and MEDITS surveys, where (i) only batoids were marked (green circles), (ii) only sharks were marked (yellow circles), and (iii) both batoids and sharks were marked (red circles). The green area shows the sampled area during MEDITS surveys in Pitiüses (MEDITS_P), the blue area shows the sampled area during MEDITS surveys in Mallorca and Menorca (MEDITS_M) and the striped area shows the sampled area during CANAL surveys. It is also shown the harbors contacted to inform on the mark-and-recapture experiment during in-person meetings (blue squares) and through email or the Balearic Islands Fishermen Association (green squares).
Recaptures have only been reported for two species of sharks: S. canicula, with six recaptures (three provided by fishermen and three obtained during scientific surveys) and eleven recoveries (six provided by fishermen and five obtained during scientific surveys); and Mustelus mustelus (Linnaeus, 1758), with two recoveries, both provided by two small-scale fishing boats, one using trammel net and the other using bottom longline, both based in a port located at the east of Mallorca and mainly operating in the Menorca Channel (Table 2 and Table 3; Figure 2). In the case of S. canicula, of the nine individuals recaptured by fishermen (all of them from bottom trawlers), six were reported from only two vessels: four by a vessel from Formentera and two from a vessel in Menorca. The three remaining recaptures were reported by three vessels in Mallorca. No recaptures of any batoid species have been reported so far, either in surveys or by the commercial fleet.

Table 2.
Recaptures and recoveries of Scyliorhinus canicula. Dist M-R: distance between mark and recapture (km). Days M-R: days at sea between mark and recapture. TL M-R: difference in total length (TL, mm) between mark and recapture. S.E.: standard error.

Table 3.
Recaptures and recoveries of Mustelus mustelus. Tagging TL: total length at tagging (mm). Dist M-R: distance between mark and recapture (km). Days M-R: days at sea between mark and recapture. TL M-R: difference in total length (TL, mm) between mark and recapture. S.E.: standard error.
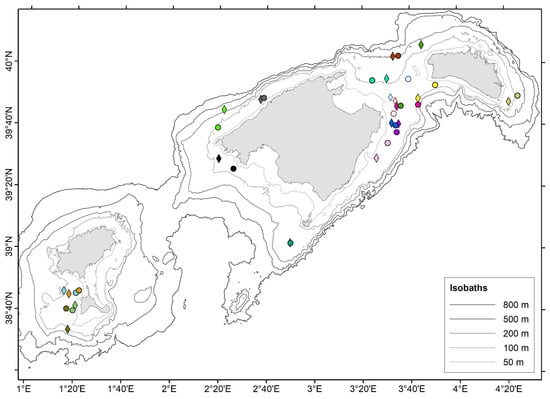
Figure 2.
Map of the Balearic Islands showing the locations of release (circles) and recapture (diamonds) of Scyliorhinus canicula (filled symbols) and Mustelus mustelus (empty symbols). Every different color represents an individual.
In general, the geographic positions of release and recapture were close for both recaptured species: 9 and 11 km on average for S. canicula and M. mustelus, respectively (Figure 2). The minimum and maximum distances recorded between release and recapture positions were 0.2 and 38 km and 7.8 and 15.5 km, for S. canicula and M. mustelus, respectively (Table 2 and Table 3).
For S. canicula, the mean size of recaptured individuals was 372 mm total length (TL) at tagging, with the smallest and largest specimens measuring 240 and 433 mm at tagging, respectively (Table 2). In the case of M. mustelus, the mean size of recaptured individuals was 753 mm TL at tagging, with similar lengths at tagging for both recaptured specimens (Table 3). The mean recorded growth between tagging and recapture was 28 mm TL during 298 days at sea for S. canicula (Table 2) and 336 mm during 370 days at sea for M. mustelus (Table 3). Standardizing the growth to a year, the mean growth of the recaptured individuals of S. canicula and M. mustelus was 34 and 331 mm/year, respectively.
3.2. Recapture Efficiency: Scientific Surveys vs. Commercial Fleet
The total effective commercial fishing hours after the beginning of the mark-and-recapture experiment in the S. canicula depth range (50–420 m) account for up to 50,193 (see Table S1 for the effective fishing hours separated by depth strata). In the case of scientific surveys, the total number of fishing hours in the 50–420 m depth range after the beginning of the mark-and-recapture experiment is almost three orders of magnitude lower, with only 88 effective fishing hours (Table 4 and Table S1).

Table 4.
Recapture efficiency for Scyliorhinus canicula from surveys and commercial fleet. Rate of recapture is calculated as recaptures divided by number of effective fishing hours performed in the species’ bathymetric range after the tagging began.
In the Pitiüses Islands (Eivissa and Formentera), where only one scientific survey had taken place after the beginning of the mark-and-recapture experiment, no individual was recovered during the scientific survey, whereas four recaptures were reported by the commercial fishing fleet, yielding a recapture efficiency of 0.00035 recaptures/fishing hour. In contrast, in Mallorca and Menorca, where four scientific surveys had already taken place after the beginning of the mark-and-recapture experiment, the recapture efficiency from scientific surveys was three orders of magnitude higher than from the commercial fleet (0.114 vs. 0.00013 recaptures/fishing hour, respectively). In general, considering both the Pitiüses and Mallorca–Menorca, the recapture efficiency is still almost three orders of magnitude higher from scientific surveys than from the commercial fleet (0.091 vs. 0.00013 recaptures/fishing hour, respectively) (Table 4).
3.3. Population Size: Mark and Recapture vs. MEDITS Density Data
The population size of S. canicula in the Mallorca and Menorca area, calculated by applying the Lincoln–Petersen formula to the recaptures obtained from scientific surveys, was 1.08 million individuals belonging to the tagged length range. The size distribution of S. canicula from MEDITS surveys shows the untagged population fraction represents 2.96% of the tagged fraction, which is equivalent to 32,610 individuals, making a total estimated population of 1.12 million individuals (Figure 3).
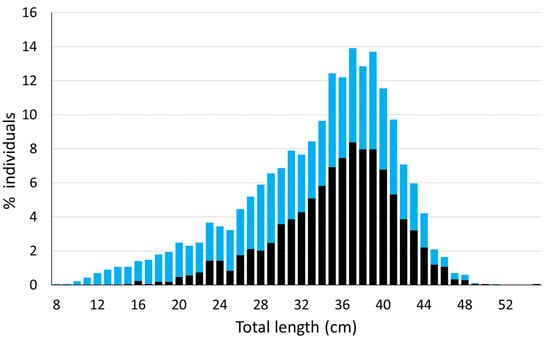
Figure 3.
Size frequency distribution of the captured individuals of Scyliorhinus canicula from the MEDITS survey in Mallorca and Menorca in 2023 (blue) and the tagged individuals of this species throughout the experiment (black).
The estimated population size using the standardized density and the surface of each MEDITS depth stratum yields a total number of 10.99 million of individuals, an order of magnitude higher than the estimation from the mark-and-recapture experiment.
4. Discussion
Mark-and-recapture experiments usually entail a challenge because their success relies on several factors that are difficult to control, such as tagging-related mortality, tag loss and tag detection, and reporting rates [35]. In our experiment, we obtained recaptures for shark species, whereas no recapture was obtained for any batoid species. More specifically, shark recaptures were reported for only two out of the seven shark species that were tagged: S. canicula and M. mustelus.
In general, the release and recapture points of the retrieved individuals are close to each other for both species. This suggests that these specimens have remained in the same area without large movements, suggesting a relatively small home range. The same behavior has been observed for S. canicula in the Cantabrian Sea in a mark-and-recapture experiment in which individuals were also recaptured generally in areas close to the tagging point at an average distance of 30 km, although some individuals were recaptured at more than 200 km, indicating that individuals of this species are capable of much greater movements [36]. In the Balearic Islands, the home range seems even smaller than in the Cantabrian Sea, pointing to a possible effect of isolation in the archipelago. This isolation could be favored by the minimum depth between the Mallorca–Menorca and Eivissa–Formentera platforms (>600 m depth), between Mallorca and Menorca and the Iberian Peninsula (>800–2000 m depth) [18], and the preferred bathymetric range of the S. canicula population inhabiting the Balearic Islands: 45–420 m [14,15]. Another explanation for the smaller distances observed might be the shorter time at liberty, although [36] concluded that there is no relationship between these two parameters for this species. In the case of M. mustelus, a species mainly inhabiting the continental shelf bottoms off the Balearic Islands [15], other studies in South Africa have shown that it has a high degree of philopatry, with most recaptures occurring at distances of less than 50 km from the location of release [37].
The mean growth recorded for the two species differs by approximately 30 cm TL. This difference is due to the different maximum sizes between both species, which are reported to be 1745 mm for M. mustelus and 1000 mm in the case of S. canicula [38]. The recaptures of S. canicula mainly consisted of mature individuals (with a mean size of 36 cm TL at tagging) with sizes closer to the maximum size detected off the Balearic Islands, where individuals usually do not exceed 55 cm TL [15]. On the other hand, M. mustelus individuals were juveniles with 73 and 77 cm TL, respectively. It should be noted that M. mustelus measures around 30 cm TL at birth and that the length at first maturity for males and females is 96–97 and 108–117 cm TL, respectively, with adults that can exceed 160 cm TL in the Mediterranean [39,40,41].
Recapture efficiency was null for the rest of the tagged species. The lack of recaptures of batoid species could be due to several factors related to tagging or fishing gear. As for tagging, there are currently few mark-and-recapture studies of skates and rays, especially in the Mediterranean, that allow conclusions on this. Only one similar study has been carried out on individuals of Raja clavata in the southern Adriatic, using spaghetti-type tags as well and achieving recaptures from bottom longlines, but none from bottom trawling [42], so it could be hypothesized that the dragging and friction that occurs in bottom trawling could somehow remove the tags from the individuals. A study carried out in northern European seas [16] obtained 50 years of mark–recapture tagging data on skates using a total of 10 different tag types. These authors observed that return rates of different tag types varied, with most recaptures obtained from Petersen discs (21%) and much less from dart and spaghetti tags (1.2% and 2.56%, respectively). Therefore, in future tagging experiments, the use of Petersen discs to tag batoid species would probably be more appropriate to increase the probability of obtaining recaptures.
In any case, the use of these spaghetti tags seems to be more appropriate for sharks. In this sense, the recapture efficiency from bottom trawl scientific surveys was quite high for S. canicula around Mallorca and Menorca (~1.1 recaptures every 10 fishing hours), where several scientific surveys had been performed after tagging had begun. However, the recapture efficiency in the same area was very low when calculated from the commercial bottom trawl fleet (~1.3 recaptures every 10,000 fishing hours). The differences in recapture efficiency between scientific surveys and the commercial fleet point to two different factors that may be hampering reporting of recaptures by the commercial fleet. One is the work burden to sort catches on board and the need for speed to do so, which might hinder the attention needed to detect the presence of tags. However, tags are highly visible, and moreover, we must consider that six out of nine recaptures were reported by just two vessels (one reporting four recaptures), whereas the remaining three recaptures were reported by three different vessels of a total of thirty-one bottom trawl vessels in the Balearic Islands. This points to a second possibility, which is the lack of willingness to become involved in collaborations with scientists during the last few years, due to the presumption of a negative impact of these collaborations in their activity. The onset of the regime applied following the multiannual plan for the fisheries exploiting demersal stocks in the western Mediterranean Sea (Regulation (EU) 2019/1022 of the European Parliament and of the Council of 20 June 2019; EU-MAP) could have exacerbated this situation. This regime has included so far a reduction of more than 30% in fishing days in relation to the period 2015–2017. Moreover, the application of the EU-MAP in the Balearic Islands entails the same effort for reduction than in adjacent areas, despite the state of the demersal resources and habitats in the archipelago showing a remarkably better state of conservation, basically due to the relatively low historical bottom trawl fishing effort [27]. This situation may jeopardize collaboration, as it could be seen as a futile effort that is not profitable for fishermen.
According to the MEDITS survey data from 2023, S. canicula, with an average of 1186 individuals/km2 (averaged accounting for the whole surveyed area in Mallorca and Menorca between 50 and 800 m depth), ranks first among the most abundant demersal fish species exploited in Mallorca and Menorca, such as the teleost fishes Chelidonichthys cuculus (Linnaeus, 1758), Chelidonichthys lastoviza (Bonnaterre, 1788), Merluccius merluccius (Linnaeus, 1758), Mullus surmuletus Linnaeus, 1758, Serranus cabrilla (Linnaeus, 1758), and Trachinus draco Linnaeus, 1758 (856, 514, 503, 352, 533 and 200 individuals/km2, respectively) [43]. The importance of this species is not exclusive to the Balearic Islands, although it is one of the areas in the Mediterranean where its importance is higher. In [44], the authors analyzed the demersal fish assemblages of a large part of the Mediterranean by the geographical sub-area (GSA) established by the General Fisheries Commission for the Mediterranean, and found that S. canicula is among the most important components of the continental shelf and shelf break/upper slope communities in 11 out of the 15 GSAs analyzed. Other authors [45], studying the same GSAs, found S. canicula was the most abundant elasmobranch species of the continental shelf, with maximum values in GSA 8 (Ligurian Sea), followed by GSA 5 (Balearic Islands), both well above the rest of the GSAs and both being among the GSAs subjected to the least bottom trawl fishing activity levels [46].
The high abundance of S. canicula relative to teleost fishes in GSA 5 is striking given the main biological traits of elasmobranch species, including slow growth, late attainment of sexual maturity, long lifespans, and low fecundity, a set of characteristics that makes them more vulnerable to fishing impacts than most teleost species [47,48]. S. canicula is not an exception. In the Mediterranean Sea, its maximum total length is usually between 55 and 65 cm. It can reach an age of up to 12–13 years, and its size at first maturity is around 40–45 cm TL when individuals are 6–7 years old [10,49]. It also presents low fecundity, producing 40–190 egg capsules per year depending on the area ([50] and references therein). The expected vulnerability of S. canicula may be compensated by the fact that (i) most of the smaller individuals captured in the bottom trawl fishery of the western Mediterranean are discarded and returned to the sea [51], and (ii) the high survival-to-catch rates that they present, being able to recover even after relatively prolonged periods of air exposure (up to 70–90% survival after 1 h air exposure [52,53]). The concurrence of these two aspects should be able to increase the resilience of this species to fishing impacts, as these small individuals released would survive and reach maturity.
However, the abundances estimated from MEDITS survey data in Mallorca and Menorca are much higher than the abundances calculated from the results of the mark-and-recapture experiment. Although the scarcity of recaptures reported by the commercial fleet prevented the use of any model to estimate population size using this data, the simplest Lincoln–Petersen model [33,34] could be applied to the retrieval of S. canicula obtained from surveys in Mallorca and Menorca. The results of this model yield an estimation of the population size 10 times smaller than that calculated from density estimations obtained during the MEDITS survey in 2023 (1.1 vs. 11 million of individuals, respectively). Mark-and-recapture experiments in the Cantabrian Sea show S. canicula has a relatively small home range, in general making short movements [36], in agreement with the present results. This behavior, along with scavenging habits with well-known feeding on discards from fishing operations (e.g., [54,55]), may contribute to the concentration of the population of S. canicula in the fishing grounds, hence increasing their density in these areas, which are the sampling objective of MEDITS surveys. Hence, there is also the possibility that the population of S. canicula is overestimated when calculated from MEDITS densities, actually being quite smaller as indicated from present results and more in agreement with its biological traits.
5. Conclusions
A mark-and-recapture experiment was carried out, tagging 3738 individuals of 23 species of sharks and batoids and revealing insights into the growth and home ranges of the sharks Scyliorhinus canicula and Mustelus mustelus. Both species appear to move relatively short distances and hence have a small home range. For S. canicula, the species with more recaptures, the density estimates from bottom trawl surveys suggest a much larger population compared to the mark–recapture results. We hypothesize that the scavenging behavior of this species may lead to overestimations in surveys targeting bottom trawl fishing grounds due to the species’ propensity to aggregate in fishing areas where discards are abundant.
Scyliorhinus canicula is an important component of the structure of the Balearic Islands ecosystem. Thus, knowledge of its population size is essential for understanding its ecological role in this ecosystem and to improve the knowledge of its population dynamics and web structure in the archipelago. The results provided here, along with expectable future recaptures, may contribute to improving estimations of the population of S. canicula off the Balearic Islands, as well as other valuable information, such as the species’ growth and spatial distribution.
Supplementary Materials
The following supporting information can be downloaded at. https://www.mdpi.com/article/10.3390/fishes9080315/s1. Table S1: Comparison of hours of effective fishing between the commercial bottom trawlers and the surveys during the study period in Scyliorhinus canicula bathymetric range.
Author Contributions
Conceptualization, F.F.-P., P.S.-Z., F.O. and S.R.-A.; methodology, F.F.-P., P.S.-Z., F.O. and S.R.-A.; validation, F.O., S.R.-A. and C.R.-C.; investigation, F.F.-P., P.S.-Z., F.O., S.R.-A. and N.P.; data curation, F.F.-P. and P.S.-Z.; writing—original draft preparation, F.F.-P., F.O. and P.S.-Z.; writing—review and editing, F.F.-P., P.S.-Z., F.O., S.R.-A., C.R.-C., M.T.F., N.P. and B.G.; visualization, F.F.-P., P.S.-Z. and M.T.F.; supervision, F.O. and S.R.-A.; project administration, F.O. and B.G.; funding acquisition, F.O. and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
The MEDITS surveys and the observers on-board programme are co-funded by the European Union through the European Maritime and Fisheries Fund (EMFF), within the National Programme of collection, management and use of data in the fisheries sector and support for scientific advice regarding the Common Fisheries Policy. The CANAL surveys are co-funded by the projects MARFISH (A multidisciplinary approach to assess the response of fishery resources to protection, funded by Conselleria de Fons Europeus, Universitat i Cultura del Govern de les Illes Balears: PDR2020/69), and SOSMED (Improving the knowledge for the sustainability of demersal fisheries in the Western Mediterranean; funded by Next Generation European funds (Recovery, Transformation and Resilience Plan), with an agreement between the Spanish Ministry of Agriculture, Fisheries and Food and CSIC by means of the IEO). This research was partly funded by the European Union’s Horizon 2020 Research and Innovation Program (H2020-BG-10-2020-2), grant number No. 101000302—EcoScope (Ecocentric management for sustainable fisheries and healthy marine ecosystems). F.F-P. was supported by the Ministry of European Funds, University and Culture of the Balearic Islands through the grants for the training of research personnel 2021.
Institutional Review Board Statement
The sampling scheme followed a standardized protocol (MEDITS Handbook. MEDITS Working Group, ninth version; 2017; p. 106) approved by international authorities (EU/DG Mare, FAO/GFCM). If a live specimen of a rare species or a species subject to conservation measures were caught, it was quickly sampled (4–5 min) and returned to the sea unharmed, giving it a chance of survival, following recommendation GFCM/36/2012/3 (http://www.gfcmonline.org/decisions/ accessed on 8 August 2024) on fisheries management measures for conservation of sharks and rays in the GFCM area.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from the fisheries sector and bottom trawl surveys and are available from the authors with the permission of the providers.
Acknowledgments
The authors would like to thank the fishermen that reported, recovered, and recaptured specimens. We also wish to thank all the participants in the MEDITS and CANAL surveys, as well as the crew of R/V Miguel Oliver, R/V Ángeles Alvariño and R/V Ramón Margalef.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Frisk, M.; Miller, T.J.; Dulvy, N.K. Life Histories and Vulnerability to Exploitation of Elasmobranchs: Inferences from Elasticity, Perturbation and Phylogenetic Analyses. Artic. J. Northwest Atl. Fish. Sci. 2005, 35, 27–45. [Google Scholar] [CrossRef]
- Last, P.R. Rays of the World; White, W.T., De Carvalho, M.R., Séret, B., Stehmann, M.F., Naylor, G., Eds.; CSIRO: Melbourne, Australia, 2016. [Google Scholar]
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H.L. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea [Brochure]; IUCN: Malaga, Spain, 2016. [Google Scholar]
- Ellis, J.R.; Cruz-Martinez, A.; Rackham, B.D.; Rogers, S.I. The Distribution of Chondrichthyan Fishes around the British Isles and Implications for Conservation. J. Northw. Atl. Fish. Sci. 2005, 35, 195–213. [Google Scholar] [CrossRef]
- Aldebert, Y. Demersal Resources of the Gulf of Lions (NW Mediterranean). Vie Milieu/Life Environ. 1997, 47, 275–284. [Google Scholar]
- Ligas, A.; Osio, G.C.; Sartor, P.; Sbrana, M.; De Ranieri, S. Long-Term Trajectory of Some Elasmobranch Species off the Tuscany Coasts (NW Mediterranean) from 50 Years of Catch Data. Sci. Mar. 2013, 77, 119–127. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and Ecosystem Consequences of Shark Declines in the Ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Barausse, A.; Correale, V.; Curkovic, A.; Finotto, L.; Riginella, E.; Visentin, E.; Mazzoldi, C. The Role of Fisheries and the Environment in Driving the Decline of Elasmobranchs in the Northern Adriatic Sea. ICES J. Mar. Sci. 2014, 71, 1593–1603. [Google Scholar] [CrossRef]
- Maravelias, C.D.; Tserpes, G.; Pantazi, M.; Peristeraki, P. Habitat Selection and Temporal Abundance Fluctuations of Demersal Cartilaginous Species in the Aegean Sea (Eastern Mediterranean). PLoS ONE 2012, 7, e35474. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Amaro, S.; Ordines, F.; Esteban, A.; García, C.; Guijarro, B.; Salmerón, F.; Terrasa, B.; Massutí, E. The Diversity of Recent Trends for Chondrichthyans in the Mediterranean Reflects Fishing Exploitation and a Potential Evolutionary Pressure towards Early Maturation. Sci. Rep. 2020, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, B.; Quetglas, A.; Moranta, J.; Ordines, F.; Valls, M.; González, N.; Massutí, E. Inter- and Intra-Annual Trends and Status Indicators of Nektobenthic Elasmobranchs off the Balearic Islands (Northwestern Mediterranean). Sci. Mar. 2012, 76, 87–96. [Google Scholar] [CrossRef]
- Ferragut-Perello, F.; Ramírez-Amaro, S.; Tsikliras, A.C.; Petit-Marty, N.; Dimarchopoulou, D.; Massutí, E.; Serrat, A.; Ordines, F. Exploitation and Conservation Status of the Thornback Ray (Raja clavata) in the Balearic Islands (Western Mediterranean). Fishes 2023, 8, 117. [Google Scholar] [CrossRef]
- Massutí, E.; Moranta, J. Demersal Assemblages and Depth Distribution of Elasmobranchs from the Continental Shelf and Slope off the Balearic Islands (Western Mediterranean). ICES J. Mar. Sci. 2003, 60, 753–766. [Google Scholar] [CrossRef]
- Ordines, F.; Massutí, E.; Moranta, J.; Quetglas, A.; Guijarro, B.; Fliti, K. Balearic Islands vs Algeria: Two Nearby Western Mediterranean Elasmobranch Assemblages with Different Oceanographic Scenarios and Fishing Histories. Sci. Mar. 2011, 75, 707–717. [Google Scholar] [CrossRef]
- Ramírez-Amaro, S.; Ordines, F.; Terrasa, B.; Esteban, A.; García, C.; Guijarro, B.; Massutí, E. Demersal Chondrichthyans in the Western Mediterranean: Assemblages and Biological Parameters of Their Main Species. Mar. Freshw. Res. 2015, 67, 636–652. [Google Scholar] [CrossRef]
- Bird, C.; Burt, G.J.; Hampton, N.; McCully Phillips, S.R.; Ellis, J.R. Fifty Years of Tagging Skates (Rajidae): Using Mark-Recapture Data to Evaluate Stock Units. J. Mar. Biol. Assoc. U. K. 2020, 100, 121–131. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, C.; Gil, J.; Canoura, J.; Sobrino, I.; Piñeiro-Álvarez, C.G.; Rodríguez-Fernández, L.; Camiñas-Hernández, J.A.; Valeiras, J.; Goñi, R.; Díaz, V.D.; et al. Estudios de Marcado y Recaptura de Especies Marinas; Instituto Español de Oceanografía, Ministerio de Ciencia e Innovación: Madrid, Spain, 2008; p. 263. [Google Scholar]
- Acosta, J.; Canals, M.; López-Martínez, J.; Muñoz, A.; Herranz, P.; Urgeles, R.; Palomo, C.; Casamor, J.L. The Balearic Promontory Geomorphology (Western Mediterranean): Morphostructure and Active Processes. Geomorphology 2002, 49, 177–204. [Google Scholar] [CrossRef]
- Canals, M.; Ballesteros, E. Production of Carbonate Particles by Phytobenthic Communities on the Mallorca-Menorca Shelf, Northwestern Mediterranean Sea. Deep-Sea Res. II 1997, 44, 611–629. [Google Scholar] [CrossRef]
- Lehucher, P.; Beautier, L.; Chartier, M.; Martel, F.; Mortier, L.; Brehmer, P.; Millot, C.; Alberola, C.; Benzhora, M.; Taupier-Letage, I.; et al. Progress from 1989 to 1992 in Understanding the Circulation of the Western Mediterranean-Sea. Oceanol. Acta 1995, 18, 255–271. [Google Scholar]
- Pinot, J.-M.; López-Jurado, J.L.; Riera, M. The CANALES Experiment (1996–1998). Interannual, Seasonal, and Mesoscale Variability of the Circulation in the Balearic Channels. Prog. Oceanogr. 2002, 55, 335–370. [Google Scholar] [CrossRef]
- Arnone, R.A.; Wiesenburg, D.A.; Saunders, K.D. The Origin and Characteristics of the Algerian Current. J. Geophys. Res. 1990, 95, 1587–1598. [Google Scholar] [CrossRef]
- Ballesteros, E. Els Fons Rocosos Profunds Amb Osmundaria volubilis (Linné) R. E. Norris a Les Balears. Bolletí Soc. D’història Nat. Balear. 1992, 35, 33–50. [Google Scholar]
- Ballesteros, E. The Deep-Water Peyssonnelia Beds from the Balearic Islands (Western Mediterranean). Mar. Ecol. 1994, 15, 233–253. [Google Scholar] [CrossRef]
- Joher, S.; Ballesteros, E.; Cebrian, E.; Sánchez, N.; Rodríguez-Prieto, C. Deep-Water Macroalgal-Dominated Coastal Detritic Assemblages on the Continental Shelf off Mallorca and Menorca (Balearic Islands, Western Mediterranean). Bot. Mar. 2012, 55, 485–497. [Google Scholar] [CrossRef]
- Joher, S.; Ballesteros, E.; Rodríguez-Prieto, C. Contribution to the Study of Deep Coastal Detritic Bottoms: The Algal Communities of the Continental Shelf off the Balearic Islands, Western Mediterranean. Mediterr. Mar. Sci. 2015, 16, 573–590. [Google Scholar] [CrossRef]
- Quetglas, A.; Guijarro, B.; Ordines, F.; Massutí, E. Stock Boundaries for Fisheries Assessment and Management in the Mediterranean: The Balearic Islands as a Case Study. Sci. Mar. 2012, 76, 17–28. [Google Scholar] [CrossRef]
- Farriols, M.T.; Ordines, F.; Somerfield, P.J.; Pasqual, C.; Hidalgo, M.; Guijarro, B.; Massutí, E. Bottom Trawl Impacts on Mediterranean Demersal Fish Diversity: Not so Obvious or Are We Too Late? Cont. Shelf Res. 2017, 137, 84–102. [Google Scholar] [CrossRef]
- Sánchez-Zulueta, P.; Valls, M.; Guijarro, B.; Ángeles Torres, M.; Ángeles Zapata, M.; Coll, M.; Corrales, X.; Andonegi, E.; Díaz-Valdés, M.; Massutí, E.; et al. Trophic Structure and Fishing Impacts on an Oligotrophic Ecosystem in the Western Mediterranean: The Balearic Islands. Front. Mar. Sci. 2023, 10, 1166674. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS Trawl Survey Specifications in an Ecosystem Approach to Fishery Management. Sci. Mar. 2019, 83, 9–20. [Google Scholar] [CrossRef]
- Sánchez Lizaso, J.L. (Ed.) XIV Reunión del Foro Científico Sobre la Pesca Española en el Mediterráneo; Universitat d’Alacant: San Vicent del Raspeig, Spain, 2023; p. 212. [Google Scholar]
- Palmer, M.; Quetglas, A.; Guijarro, B.; Moranta, J.; Ordines, F.; Massutí, E. Performance of Artificial Neural Networks and Discriminant Analysis in Predicting Fishing Tactics from Multispecific Fisheries. Can. J. Fish. Aquat. Sci. 2009, 66, 224–237. [Google Scholar] [CrossRef]
- Petersen, C.G.J. The Yearly Immigration of Young Plaice into the Limfjord from the German Sea. Rep. Dan. Biol. Stn. 1896, 6, 1–48. [Google Scholar]
- Lincoln, F.C. Calculating Waterfowl Abundance on the Basis of Banding Returns; U.S. Department of Agriculture: Washington, DC, USA, 1930; p. 118. [Google Scholar]
- Björnsson, B.; Karlsson, H.; Thorsteinsson, V.; Solmundsson, J. Should All Fish in Mark–Recapture Experiments Be Double-Tagged? Lessons Learned from Tagging Coastal Cod (Gadus morhua). ICES J. Mar. Sci. 2011, 68, 603–610. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, C.; Sánchez, F.; Fernández, A.; Olaso, I. Is the Lesser Spotted Dogfish (Scyliorhinus canicula) Population from the Cantabrian Sea a Unique Stock? Fish. Res. 2004, 69, 57–71. [Google Scholar] [CrossRef]
- Klein, J.D.; Asbury, T.A.; da Silva, C.; Hull, K.L.; Dicken, M.L.; Gennari, E.; Maduna, S.N.; Bester-van der Merwe, A.E. Site Fidelity and Shallow Genetic Structure in the Common Smooth-Hound Shark Mustelus mustelus Confirmed by Tag-Recapture and Genetic Data. J. Fish. Biol. 2022, 100, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, S. Annotated Checklist of the Living Sharks, Batoids and Chimaeras (Chondrichthyes) of the World, with a Focus on Biogeographical Diversity. J. Fish. Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef] [PubMed]
- Capapé, C. Observations Sur La Sexualité, La Reproduction et La Fécondité de 8 Sélaciens Pleurotrèmes, Vivipares Placentaires Des Côtes Tunisiennes. Arch. Inst. Pasteur Tunis. 1974, 51, 329–344. [Google Scholar]
- Morte, S.; Redon, M.J.; Sanz-Brau, A. Feeding Habits of Juvenile Mustelus mustelus (Carcharhiniformes, Triakidae) in the Western Mediterranean. Cah. Biol. Mar. 1997, 38, 103–107. [Google Scholar]
- Saïdi, B.; Bradaï, M.N.; Bouaïn, A. Reproductive Biology of the Smooth-Hound Shark Mustelus mustelus (L.) in the Gulf of Gabès (South-Central Mediterranean Sea). J. Fish. Biol. 2008, 72, 1343–1354. [Google Scholar] [CrossRef]
- Carbonara, P.; Bellodi, A.; Palmisano, M.; Mulas, A.; Porcu, C.; Zupa, W.; Donnaloia, M.; Carlucci, R.; Sion, L.; Follesa, M.C. Growth and Age Validation of the Thornback Ray (Raja clavata Linnaeus, 1758) in the South Adriatic Sea (Central Mediterranean). Front. Mar. Sci. 2020, 7, 586094. [Google Scholar] [CrossRef]
- Farré, M.; Joher, S.; Farriols, M.T.; Ferragut-Perello, F.; Pasini, N.; Ramírez-Amaro, S.; Valls, M.; Guijarro, B.; Massutí, E.; Ordines, F. Informe Campaña MEDITS_ES_2023 (GSA 5E, Eastern Balearic Islands), Unpublished Work. 2023.
- Farriols, M.T.; Ordines, F.; Carbonara, P.; Casciaro, L.; Di Lorenzo, M.; Esteban, A.; Follesa, C.; García-Ruiz, C.; Isajlovic, I.; Jadaud, A.; et al. Spatio-Temporal Trends in Diversity of Demersal Fish Assemblages in the Mediterranean. Sci. Mar. 2019, 83, 189–206. [Google Scholar] [CrossRef]
- Follesa, M.C.; Marongiu, M.F.; Zupa, W.; Bellodi, A.; Cau, A.; Cannas, R.; Colloca, F.; Djurovic, M.; Isajlovic, I.; Jadaud, A.; et al. Spatial Variability of Chondrichthyes in the Northern Mediterranean. Sci. Mar. 2019, 83, 81–100. [Google Scholar] [CrossRef]
- Ferrà, C.; Tassetti, A.N.; Grati, F.; Pellini, G.; Polidori, P.; Scarcella, G.; Fabi, G. Mapping Change in Bottom Trawling Activity in the Mediterranean Sea through AIS Data. Mar. Policy 2018, 94, 275–281. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The Effects of Fishing on Sharks, Rays, and Chimaeras (Chondrichthyans), and the Implications for Marine Ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Graham, K.J.; Andrew, N.L.; Hodgson, K.E. Changes in Relative Abundance of Sharks and Rays on Australian South East Fishery Trawl Grounds after Twenty Years of Fishing. Mar. Freshw. Res. 2001, 52, 549–561. [Google Scholar] [CrossRef]
- Moreira, I.; Figueiredo, I.; Farias, I.; Lagarto, N.; Maia, C.; Robalo, J.; Moura, T. Growth and Maturity of the Lesser-spotted Dogfish Scyliorhinus canicula (Linnaeus, 1758) in the Southern Portuguese Continental Coast. J. Fish Biol. 2022, 100, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Capapé, C.; Vergne, Y.; Reynaud, C.; Guélorget, O.; Quignard, J.P. Maturity, Fecundity and Occurrence of the Small Spotted Catshark Scyliorhinus canicula (Chondrichthyes: Scyliorhinidae) off the Languedocian Coast (Southern France, North-Western Mediterranean). Vie Milieu/Life Environ. 2008, 58, 47–55. [Google Scholar]
- Carbonell, A.; Alemany, F.; Merella, P.; Quetglas, A.; Román, E. The By-Catch of Sharks in the Western Mediterranean (Balearic Islands) Trawl Fishery. Fish. Res. 2003, 61, 7–18. [Google Scholar] [CrossRef]
- Revill, A.S.; Dulvy, N.K.; Holst, R. The Survival of Discarded Lesser-Spotted Dogfish (Scyliorhinus canicula) in the Western English Channel Beam Trawl Fishery. Fish. Res. 2005, 71, 121–124. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; Ruiz-Jarabo, I.; Fuentes, J.; Mancera, J.M.; Sobrino, I. Survival Rates and Physiological Recovery Responses in the Lesser-Spotted Catshark (Scyliorhinus canicula) after Bottom-Trawling. Comp. Biochem. Physiol. Part. A 2019, 233, 1–9. [Google Scholar] [CrossRef]
- Olaso, I.; Velasco, F.; Pérez, N. Importance of Discarded Blue Whiting (Micromesistius Poutassou) in the Diet of Lesser Spotted Dogfish (Scyliorhinus canicula) in the Cantabrian Sea. ICES J. Mar. Sci. 1998, 55, 331–341. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Spencer, B.E. Fish Scavenging Behaviour in Recently Trawled Areas. Mar. Ecol. Prog. Ser. 1994, 112, 41–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
