Intermittent Detections of ISAV-HPR0 in a Salmon Recirculating Aquaculture System, and Implications for Sampling
Abstract
1. Introduction
2. Materials and Methods
2.1. Water and Fish Sources
2.2. First Detection of ISAV-HPR0 in the Facility
2.3. Molecular Testing
2.4. Exposed Cohort Study
2.5. ISAV-HPR0 Monitoring Program: Spawning
2.6. ISAV-HPR0 Monitoring Program: Full Facility
3. Results
3.1. Initial Detection and Exposed Cohort Study
3.2. ISAV-HPR0 Monitoring Program: Spawning
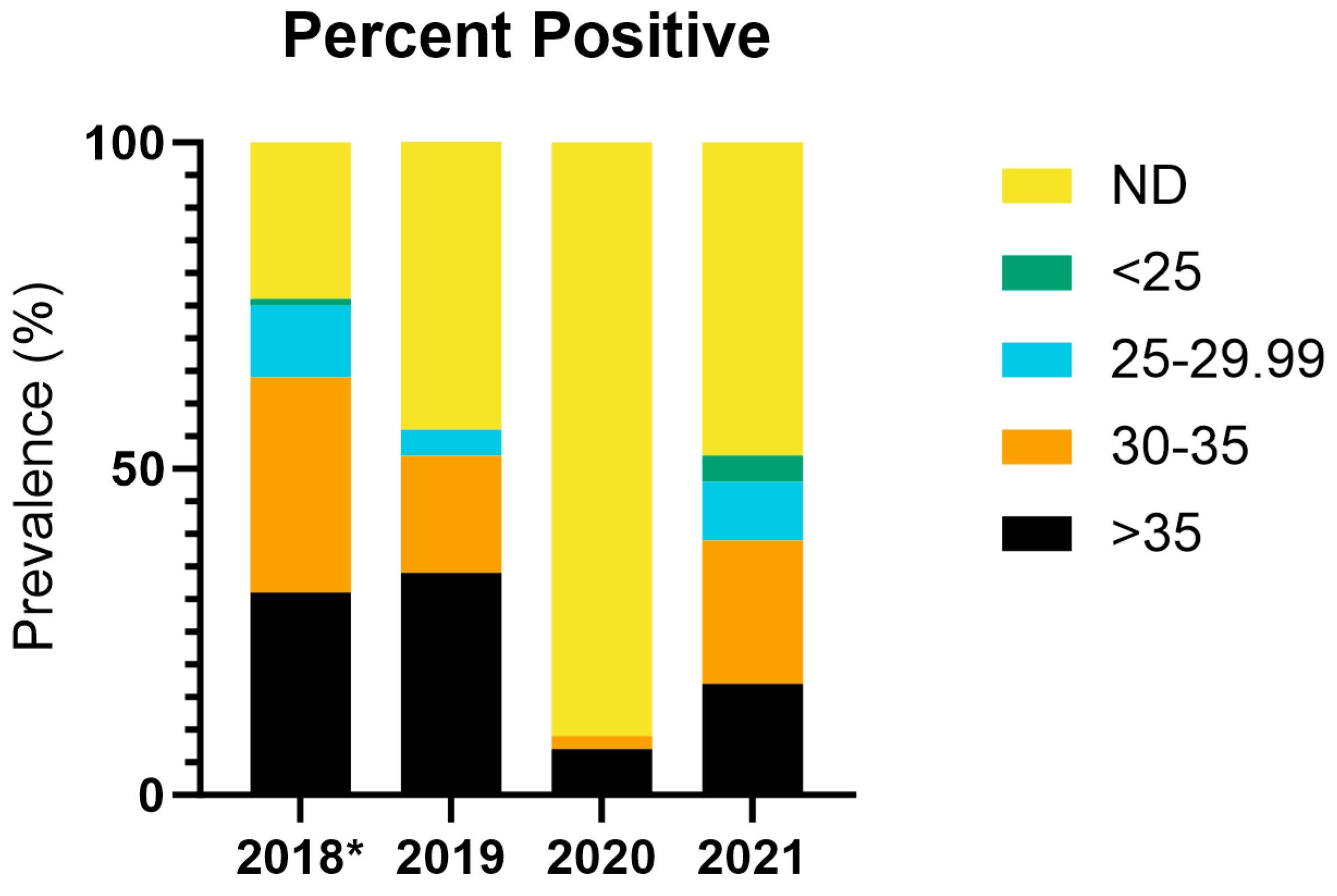
3.3. ISAV-HPR0 Monitoring Program: Full Facility
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorud, K.E.; Djupvik, H.O. Infectious anaemia in Atlantic salmon (Salmo salar L.). Bull. Eur. Assoc. Fish Pathol. 1988, 8, 109–111. [Google Scholar]
- Bouchard, D.; Keleher, W.; Opitz, H.M.; Blake, S.; Edwards, K.C.; Nicholson, B.L. Isolation of infectious salmon anemia virus (ISAV) from Atlantic salmon in New Brunswick, Canada. Dis. Aquat. Organ. 1999, 35, 131–137. [Google Scholar] [CrossRef]
- Bouchard, D.A.; Brockway, K.; Giray, C.; Keleher, W.; Merrill, P.L. First report of Infectious Salmon Anemia (ISA) in the United States. Bull. Eur. Assoc. Fish Pathol. 2001, 21, 86–88. [Google Scholar]
- Christiansen, D.H.; Østergaard, P.S.; Snow, M.; Dale, O.B.; Falk, K. A low-pathogenic variant of infectious salmon anemia virus (ISAV-HPR0) is highly prevalent and causes a non-clinical transient infection in farmed Atlantic salmon (Salmo salar L.) in the Faroe Islands. J. Gen. Virol. 2011, 92, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, F.S.; Garate, O.N.; Johnson, G.; Arriagada, R.; Kibenge, M.J.; Wadowska, D. Isolation and identification of infectious salmon anaemia virus (ISAV) from Coho salmon in Chile. Dis. Aquat. Organ. 2001, 45, 9–18. [Google Scholar] [CrossRef]
- Lovely, J.E.; Dannevig, B.H.; Falk, K.; Hutchin, L.; MacKinnon, A.M.; Melville, K.J.; Rimstad, E.; Griffiths, S.G. First identification of infectious salmon anaemia virus in North America with haemorrhagic kidney syndrome. Dis. Aquat. Organ. 1999, 35, 145–148. [Google Scholar] [CrossRef]
- Mullins, J.E.; Groman, D.; Wadowska, D. Infectious salmon anaemia in salt water Atlantic salmon (Salmo salar L.) in New Brunswick, Canada. Bull. Eur. Assoc. Fish Pathol. 1998, 18, 110–114. [Google Scholar]
- Rodger, H.D.; Turnbull, T.; Muir, F.; Millar, S.; Richards, R.H. Infectious salmon anaemia (ISA) in the United Kingdom. Bull. Eur. Assoc. Fish Pathol. 1998, 18, 115–116. [Google Scholar]
- Rowley, H.M.; Campbell, S.J.; Curran, W.L.; Turnbull, T.; Bryson, D.G. Isolation of infectious salmon anaemia virus (ISAV) from Scottish farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 1999, 22, 483–487. [Google Scholar] [CrossRef]
- Anonymous. ISA hits the Faroes. Fish Farm. Int. 2000, 27, 47. [Google Scholar]
- Evensen, Ø.; Thorud, K.E.; Olsen, Y.A. A morphological study of the gross and light microscopic lesions of infectious anaemia in Atlantic salmon (Salmo salar). Res. Vet. Sci. 1991, 51, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, M.; Lester, K.; Markussen, T.; Falk, K.; Secombes, C.J.; McBeath, A.; Collet, B. Dual Mutation Events in the Haemagglutinin-Esterase and Fusion Protein from an Infectious Salmon Anaemia Virus HPR0 Genotype Promote Viral Fusion and Activation by an Ubiquitous Host Protease. PLoS ONE 2015, 10, e0142020. [Google Scholar] [CrossRef][Green Version]
- Devold, M.; Falk, K.; Dale, B.; Krossoy, B.; Biering, E.; Aspehaug, V.; Nilsen, F.; Nylund, A. Strain variation, based on the hemagglutinin gene, in Norwegian ISA virus isolates collected from 1987 to 2001: Indications of recombination. Dis. Aquat. Org. 2001, 47, 119–128. [Google Scholar] [CrossRef]
- Nylund, A.; Devold, M.; Plarre, H.; Isdal, E.; Aarseth, M. Emergence and maintenance of infectious salmon anaemia virus (ISAV) in Europe: A new hypothesis. Dis. Aquat. Org. 2003, 56, 11–24. [Google Scholar] [CrossRef]
- Cunningham, C.O.; Gregory, A.; Black, J.; Simpson, I.; Raynard, R.S. A novel variant of the infectious salmon anaemia virus (ISAV) haemagglutinin gene suggests mechanisms for virus diversity. Bull. Eur. Assoc. Fish Pathol. 2002, 22, 366–374. [Google Scholar]
- Markussen, T.; Sindre, H.; Jonassen, C.M.; Tengs, T.; Kristoffersen, A.B.; Ramsell, J.; Numanovic, S.; Hjortaas, M.J.; Christiansen, D.H.; Dale, O.B.; et al. Ultra-deep pyrosequencing of partial surface protein genes from infectious Salmon Anaemia virus (ISAV) suggest novel mechanisms involved in transition to virulence. PLoS ONE 2013, 8, e81571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mjaaland, S.; Hungnes, O.; Teig, A.; Dannevig, B.H.; Thorud, K.; Rimstad, E. Polymorphism in the infectious salmon anemia virus hemagglutinin gene: Importance and possible implications for evolution and ecology of infectious salmon anemia disease. Virology 2002, 304, 379–391. [Google Scholar] [CrossRef]
- Cardenas, M.; Galleguillos, C.; Acevedo, K.; Ananias, C.; Alarcon, J.; Michelson, S.; Toledo, J.; Montoya, M.; Meneses, C.; Castro-Nallar, E.; et al. Rapid sequence modification in the highly polymorphic region (HPR) of the hemagglutinin gene of the infectious salmon anaemia virus (ISAV) suggests intra-segmental template switching recombination. J. Fish Dis. 2020, 43, 1483–1496. [Google Scholar] [CrossRef]
- Kulshreshtha, V.; Kibenge, M.; Salonius, K.; Simard, N.; Riveroll, A.; Kibenge, F. Identification of the 3′ and 5′ terminal sequences of the 8 rna genome segments of European and North American genotypes of infectious salmon anemia virus (an orthomyxovirus) and evidence for quasispecies based on the non-coding sequences of transcripts. Virol. J. 2010, 7, 338. [Google Scholar] [CrossRef]
- Christiansen, D.H.; Petersen, P.E.; Dahl, M.M.; Vest, N.; Aamelfot, M.; Kristoffersen, A.B.; Jansen, M.D.; Matejusova, I.; Gallagher, M.D.; Jonsson, G.; et al. No Evidence of the Vertical Transmission of Non-Virulent Infectious Salmon Anaemia Virus (ISAV-HPR0) in Farmed Atlantic Salmon. Viruses 2021, 13, 2428. [Google Scholar] [CrossRef] [PubMed]
- Lyngstad, T.M.; Kristoffersen, A.B.; Hjortaas, M.J.; Devold, M.; Aspehaug, V.; Larssen, R.B.; Jansen, P.A. Low virulent infectious salmon anaemia virus (ISAV-HPR0) is prevalent and geographically structured in Norwegian salmon farming. Dis. Aquat. Org. 2012, 101, 197–206. [Google Scholar] [CrossRef]
- Madhun, A.S.; Maehle, S.; Wennevik, V.; Karlsbakk, E. Prevalence and genotypes of infectious salmon anaemia virus (ISAV) in returning wild Atlantic salmon (Salmo salar L.) in northern Norway. J. Fish Dis. 2019, 42, 1217–1221. [Google Scholar] [CrossRef]
- Christiansen, D.H.; McBeath, A.J.A.; Aamelfot, M.; Matejusova, I.; Fourrier, M.; White, P.; Petersen, P.E.; Falk, K. First field evidence of the evolution from a non-virulent HPR0 to a virulent HPR-deleted infectious salmon anaemia virus. J. Gen. Virol. 2017, 98, 595–606. [Google Scholar] [CrossRef]
- Gustafson, L.; Remmenga, M.; Sandoval Del Valle, O.; Ibarra, R.; Antognoli, M.; Gallardo, A.; Rosenfeld, C.; Doddis, J.; Enriquez Sais, R.; Bell, E.; et al. Area contact networks and the spatio-temporal spread of infectious salmon anemia virus (ISAV) in Chile. Prev. Vet. Med. 2016, 125, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Nylund, A.; Brattespe, J.; Plarre, H.; Kambestad, M.; Karlsen, M. Wild and farmed salmon (Salmo salar) as reservoirs for infectious salmon anaemia virus, and the importance of horizontal- and vertical transmission. PLoS ONE 2019, 14, e0215478. [Google Scholar] [CrossRef]
- Aamelfot, M.; Christiansen, D.H.; Dale, O.B.; McBeath, A.; Benestad, S.L.; Falk, K. Localised Infection of Atlantic Salmon Epithelial Cells by HPR0 Infectious Salmon Anaemia Virus. PLoS ONE 2016, 11, e0151723. [Google Scholar] [CrossRef]
- Aamelfot, M.; Dale, O.B.; Falk, K. Infectious salmon anaemia—Pathogenesis and tropism. J. Fish Dis. 2014, 37, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Aamelfot, M.; Dale, O.B.; McBeath, A.; Falk, K. Host tropism of infectious salmon anaemia virus in marine and freshwater fish species. J. Fish Dis. 2015, 38, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Aamelfot, M.; Dale, O.B.; Weli, S.C.; Koppang, E.O.; Falk, K. Expression of the Infectious Salmon Anemia Virus Receptor on Atlantic Salmon Endothelial Cells Correlates with the Cell Tropism of the Virus. J. Virol. 2012, 86, 10571–10578. [Google Scholar] [CrossRef]
- Fosse, J.H.; Aamelfot, M.; Sønstevold, T.; Weli, S.C.; Vendramin, N.; Petersen, P.E.; Solhaug, A.; Amundsen, M.M.; Heffernan, I.A.; Cuenca, A.; et al. Salmon Erythrocytes Sequester Active Virus Particles in Infectious Salmon Anaemia. Viruses 2022, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Maine, S.o. Importation of Live Marine Organisms. Chapter 24. Available online: https://www.maine.gov/dmr/rules-enforcement/regulations-rules (accessed on 1 February 2022).
- Wolters, W.; Masters, A.; Vinci, B.; Summerfelt, S. Design, loading, and water quality in recirculating systems for Atlantic Salmon (Salmo salar) at the USDA ARS National Cold Water Marine Aquaculture Center (Franklin, Maine). Aquacult. Eng. 2009, 41, 60–70. [Google Scholar] [CrossRef]
- Gustafson, L.L.; Creekmore, L.H.; Snekvik, K.R.; Ferguson, J.A.; Warg, J.V.; Blair, M.; Meyers, T.R.; Stewart, B.; Warheit, K.I.; Kerwin, J.; et al. A systematic surveillance programme for infectious salmon anaemia virus supports its absence in the Pacific Northwest of the United States. J. Fish Dis. 2018, 41, 337–346. [Google Scholar] [CrossRef]
- Snow, M.; McKay, P.; McBeath, A.J.; Black, J.; Doig, F.; Kerr, R.; Cunningham, C.O.; Nylund, A.; Devold, M. Development, application and validation of a Taqman real-time RT-PCR assay for the detection of infectious salmon anaemia virus (ISAV) in Atlantic salmon (Salmo salar). Dev. Biol. 2006, 126, 133–145; discussion 325–326. [Google Scholar]
- Vike, S.; Oelckers, K.; Duesund, H.; Erga, S.R.; Gonzalez, J.; Hamre, B.; Frette, O.; Nylund, A. Infectious salmon anemia (ISA) virus: Infectivity in seawater under different physical conditions. J. Aquat. Anim. Health 2014, 26, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Test Selection and Sampling Requirements for Aquatic Animal Imports. 2021. Available online: https://inspection.canada.ca/animal-health/aquatic-animals/imports/test-selection-and-sampling-requirements/eng/1548710570282/1548710570578 (accessed on 1 February 2022).
- Johansson, L.-H.; Timmerhaus, G.; Afanasyev, S.; Jørgensen, S.M.; Krasnov, A. Smoltification and seawater transfer of Atlantic salmon (Salmo salar L.) is associated with systemic repression of the immune transcriptome. Fish Shellfish. Immunol. 2016, 58, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Raynard, R.S.; Snow, M.; Bruno, D.W. Experimental infection models and susceptibility of Atlantic salmon Salmo salar to a Scottish isolate of infectious salmon anaemia virus. Dis. Aquat. Org. 2001, 47, 169–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Charu, V.; Zeger, S.; Gog, J.; Bjornstad, O.N.; Kissler, S.; Simonsen, L.; Grenfell, B.T.; Viboud, C. Human mobility and the spatial transmission of influenza in the United States. PLoS Comput. Biol. 2017, 13, e1005382. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Bjornstad, O.N.; Smith, D.L.; Simonsen, L.; Miller, M.A.; Grenfell, B.T. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science 2006, 312, 447–451. [Google Scholar] [CrossRef]
- Nylund, A.; Plarre, H.; Karlsen, M.; Fridell, F.; Ottem, K.F.; Bratland, A.; Saether, P.A. Transmission of infectious salmon anaemia virus (ISAV) in farmed populations of Atlantic salmon (Salmo salar). Arch. Virol. 2007, 152, 151–179. [Google Scholar] [CrossRef] [PubMed]
- Plarre, H.; Nylund, A.; Karlsen, M.; Brevik, O.; Saether, P.A.; Vike, S. Evolution of infectious salmon anaemia virus (ISA virus). Arch. Virol. 2012, 157, 2309–2326. [Google Scholar] [CrossRef]
- Skotheim, S.A. Co-infection with Norwegian Salmonid Alphavirus (NSAV) and Infectious Pancreatic Necrosis Virus (IPNV) in Chinook Salmon Embryo Cells (CHSE-214). Master Thesis, University of Bergen, Bergen, Norway, 2009. [Google Scholar]
- Marshall, S.H.; Ramírez, R.; Labra, A.; Carmona, M.; Muñoz, C. Bona Fide Evidence for Natural Vertical Transmission of Infectious Salmon Anemia Virus in Freshwater Brood Stocks of Farmed Atlantic Salmon (Salmo salar) in Southern Chile. J. Virol. 2014, 88, 6012–6018. [Google Scholar] [CrossRef]
- Vike, S.; Nylund, S.; Nylund, A. ISA virus in Chile: Evidence of vertical transmission. Arch. Virol. 2009, 154, 1–8. [Google Scholar] [CrossRef]
- Melville, K.J.; Griffiths, S.G. Absence of vertical transmission of infectious salmon anemia virus (ISAV) from individually infected Atlantic salmon Salmo salar. Dis. Aquat. Org. 1999, 38, 231–234. [Google Scholar] [CrossRef]
- Sommerset, I.; Wiik-Nielsen, J.; Moldal, T.; Oliveira, V.H.S.; Svendsen, J.C.; Haukaas, A.; Brun, E. Norwegian Fish Health Report 2023; Norwegian Veterinary Institute: Oslo, Norway, 2024. [Google Scholar]

| Target | Primer ID | Sequence | Reference |
|---|---|---|---|
| ISAV | Seg8-F | 5′ CTACACAGCAGGATGCAGATGT 3′ | [34] |
| Segment 8 | Seg8-R | 5′ CAGGATGCCGGAAGTCGAT 3′ | |
| Seg8-P | 5′ (6-FAM) CATCGTCGCTGCAGTTC (MGB-NFG) 3′ | ||
| European | 872 EU | 5′ GCT GCT TCG TGT GAA TAT GAC 3′ | Warg |
| Segment 6 | 1118EU | 5′ TTC CAA CCT GCT AGG AAC 3′ | Unpub. |
| Fixative | Tissue | 15 Feb | 12 Apr | 26 May | 29 Jun | 19 Jul | 14 Aug |
|---|---|---|---|---|---|---|---|
| RNA Later | Blood | 10 | 5 | 5 | 5 | 5 | 5 |
| Gill | 10 | 5 | 5 | 5 | 5 | 5 | |
| Heart | 10 | 5 | 5 | 5 | 5 | 5 | |
| Kidney | 10 | 5 | 5 | 5 | 5 | 5 | |
| Liver | 5 | 5 | 5 | 5 | 5 | ||
| Spleen | 5 | 5 | 5 | 5 | 5 | ||
| Frozen | Gill Arch | 10 | 5 | 5 | 5 | 5 | 38 |
| Heart | 5 | 5 | 5 | 5 | 5 | ||
| Kidney | 5 | 5 | 5 | 5 | 5 | ||
| Liver | 5 | 5 | 5 | 5 | 5 | ||
| Spleen | 5 | 5 | 5 | 5 | 5 |
| 2018 | 2019 | 2020 | 2021 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YC | Nov. | Dec. | Apr. | May | Jul. | Sep. | Nov. | Dec. | May | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | Apr. | May-early | May-late | Jun. | Jul. | Aug. | Sep. | Nov. |
| 14–15 | 174/133/B | 47/10/B | ||||||||||||||||||||||||
| 15–16 | 133/75/B | 4/1/B | ||||||||||||||||||||||||
| 16–17 | 30/0/G | 30/0/B | 180/0/B | 218/23/B | 33/0/B | |||||||||||||||||||||
| 17–18 | 30/0/S | 60/0/S | 90/0/B | 90/0/B | 308/151/B | |||||||||||||||||||||
| 18–19 | 30/0/P | 30/0/P | 15/0/S | 60/0/G | 120/1/G | |||||||||||||||||||||
| 19–20 | 60/0/P–S | 120/72/S | 120/0/S | 115/0/S 115/0/B2 | ||||||||||||||||||||||
| 20–21 | 180/0/P | |||||||||||||||||||||||||
| Water | 1 | 2 | 3 | 4 | 5 | 3 | 2 | 2 | 2 | 4 | 4 | 4 | 1 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrak, M.; Warg, J.; Gustafson, L.; Peterson, B.C. Intermittent Detections of ISAV-HPR0 in a Salmon Recirculating Aquaculture System, and Implications for Sampling. Fishes 2024, 9, 325. https://doi.org/10.3390/fishes9080325
Pietrak M, Warg J, Gustafson L, Peterson BC. Intermittent Detections of ISAV-HPR0 in a Salmon Recirculating Aquaculture System, and Implications for Sampling. Fishes. 2024; 9(8):325. https://doi.org/10.3390/fishes9080325
Chicago/Turabian StylePietrak, Michael, Janet Warg, Lori Gustafson, and Brian C. Peterson. 2024. "Intermittent Detections of ISAV-HPR0 in a Salmon Recirculating Aquaculture System, and Implications for Sampling" Fishes 9, no. 8: 325. https://doi.org/10.3390/fishes9080325
APA StylePietrak, M., Warg, J., Gustafson, L., & Peterson, B. C. (2024). Intermittent Detections of ISAV-HPR0 in a Salmon Recirculating Aquaculture System, and Implications for Sampling. Fishes, 9(8), 325. https://doi.org/10.3390/fishes9080325






