Correlated Disorder in Myelinated Axons Orientational Geometry and Structure
Abstract
:1. Introduction
2. Results and Discussion
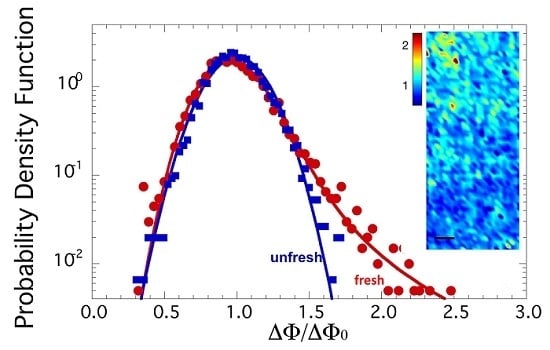
2.1. Axon Orientation Fluctuations
2.2. Mapping of Axon Orientation Fluctuations
- (i)
- stability index of 1.79 ± 0.02 (<2);
- (ii)
- scale parameter: γ of 0.141 ± 0.005.
- (a)
- stability index of 2.00;
- (b)
- scale parameter: γ of 0.131 ± 0.005.
3. Materials and Methods
3.1. Samples Preparation
3.2. Experimental and Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quarles, R.H.; Mcklin, W.B.; Morell, P. Myelin Formation, Structure and Biochemistry; Lippincott-Raven Publishing: Philadelphia, PA, USA, 2006; pp. 51–71. [Google Scholar]
- Zalc, B. The acquisition of myelin: An evolutionary perspective. Brain Res. 2016, 1641, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Avila, R.L.; Inouye, H.; Baek, R.C.; Yin, X.; Trapp, B.D.; Feltri, M.L.; Wrabetz, L.; Kirschner, D.A. Structure and stability of internodal myelin in mouse models of hereditary neuropathy. J. Neuropathol. Exp. Neurol. 2005, 64, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cerullo, J.; Dawli, T.; Priest, C.; Haddadin, Z.; Kim, A.; Inouye, H.; Suffoletto, B.P.; Avila, R.L.; Lees, J.P.B.; et al. Peripheral myelin of Xenopus Laevis: Role of electrostatic and hydrophobic interactions in membrane compaction. J. Struct. Biol. 2008, 162, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Hawk, R.; Avila, R.L.; Inouye, H.; Kirschner, D.A. Internodal myelination during development quantitated using x-ray diffraction. J. Struct. Biol. 2009, 168, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Finean, J.B. Electron microscope and X-ray diffraction studies of the effects of dehydration on the structure of nerve myelin. J. Cell Biol. 1960, 8, 13–29. [Google Scholar] [CrossRef]
- Möbius, W.; Nave, K.A.; Werner, H.B. Electron microscopy of myelin: Structure preservation by high-pressure freezing. Brain Res. 2016, 1641, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Knoll, W.; Peters, J.; Kursula, P.; Gerelli, Y.; Ollivier, J.; Demé, B.; Telling, M.; Kemner, E.; Natali, F. Structural and dynamical properties of reconstituted myelin sheaths in the presence of myelin proteins MBP and p2 studied by neutron scattering. Soft Matter 2014, 10, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Essential Biomaterials Science; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Turner, P.; Nottale, L. The physical principles underpinning self-organization in plants. Prog. Biophys. Mol. Biol. 2017, 123, 48–73. [Google Scholar] [CrossRef] [PubMed]
- Sitharam, M. Modeling Autonomous Supramolecular Assembly; Springer: Berlin/Heidelberg, Germany, 2014; pp. 197–216. [Google Scholar]
- Vattay, G.; Salahub, D.; Csabai, I.; Nassimi, A.; Kaufmann, S.A. Quantum criticality at the origin of life. J. Phys. Conf. Ser. 2015, 626, 012023. [Google Scholar] [CrossRef]
- Honerkamp-Smith, A.R.; Veatch, S.L.; Keller, S.L. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Tauber, U.C. Scale Invariance in non-Equilibrium Systems, Chapter Part II; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Poccia, N.; Campi, G.; Ricci, A.; Caporale, A.S.; Di Cola, E.; Hawkins, T.A.; Bianconi, A. Changes of statistical structural fluctuations unveils an early compacted degraded stage of PNS myelin. Sci. Rep. 2014, 4, 5430. [Google Scholar] [CrossRef] [PubMed]
- Campi, G.; Fratini, M.; Bukreeva, I.; Ciasca, G.; Burghammer, M.; Brun, F.; Tromba, G.; Mastrogiacomo, M.; Cedola, A. Imaging collagen packing dynamics during mineralization of engineered bone tissue. Acta Biomater. 2015, 23, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Campi, G.; Di Gioacchino, M.; Poccia, N.; Ricci, A.; Burghammer, M.; Bianconi, A. Intrinsic dynamical fluctuations of PNS myelin ultrastructure. arXiv, 2017; arXiv:1705.09730. [Google Scholar]
- Dučić, T.; Quintes, S.; Nave, K.A.; Susini, J.; Rak, M.; Tucoulou, R.; Alevra, M.; Guttmann, P.; Salditt, T. Structure and composition of myelinated axons: A multimodal synchrotron spectro-microscopy study. J. Struct. Biol. 2011, 173, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Inouye, H.; Liu, J.; Makowski, L.; Palmisano, M.; Burghammer, M.; Riekel, C.; Kirschner, D.A. Myelin organization in the nodal, paranodal, and juxtaparanodal regions revealed by scanning X-Ray microdiffraction. PLoS ONE 2014, 9, e100592. [Google Scholar] [CrossRef] [PubMed]
- Inouye, H.; Kuo, F.H.; Denninger, A.R.; Weinhausen, B.; Burghammer, M.; Kirschner, D.A. Myelin structure in unfixed, single nerve fibers: Scanning X-ray microdiffraction with a beam size of 200 nm. J. Struct. Biol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Hémonnot, C.Y.J.; Reinhardt, J.; Saldanha, O.; Patommel, J.; Graceffa, R.; Weinhausen, B.; Burghammer, M.; Schroer, C.G.; Köster, S. X-rays reveal the internal structure of keratin bundles in whole cells. ACS Nano 2016, 10, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Campi, G.; Bianconi, A.; Poccia, N.; Bianconi, G.; Barba, L.; Arrighetti, G.; Innocenti, D.; Karpinski, J.; Zhigadlo, N.D.; Kazakov, S.M.; et al. Inhomogeneity of charge-density-wave order and quenched disorder in a high-Tc superconductor. Nature 2015, 525, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Albert, R. Scale-free networks in cell biology. J. Cell Sci. 2005, 118, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, G.; Marsili, M. Emergence of large cliques in random scale-free networks. EPL Europhys. Lett. 2006, 74, 740–746. [Google Scholar] [CrossRef]
- Egu’iluz, V.M.; Chialvo, D.R.; Cecchi, G.A.; Baliki, M.; Apkarian, A.V. Scale-Free brain functional networks. Phys. Rev. Lett. 2005, 94, 018102. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, P.; Goldin, M.; Picardo, M.A.; Jorquera, I.; Cattani, A.; Bianconi, G.; Represa, A.; Ben-Ari, Y.; Cossart, R. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 2009, 326, 1419–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altamura, M.; Elvevag, B.; Campi, G.; De Salvia, M.; Marasco, D.; Ricci, A.; Bellomo, A. Toward scale-free like behavior under increasing cognitive load. Complexity 2012, 18, 38–43. [Google Scholar] [CrossRef]
- Fofack, H.; Nolan, J.P. Tail behavior, modes and other characteristics of stable distributions. Extremes 1999, 2, 39–58. [Google Scholar] [CrossRef]
- Chechkin, A.V.; Gonchar, V.Y.; Klafter, J.; Metzler, R. Fundamentals of Lévy Flight Processes. Adv. Chem. Phys. 2006, 133B, 439–496. [Google Scholar]
- Oświȩcimka, P.; Kwapień, J.; Drożdż, S. Wavelet versus detrended fluctuation analysis of multifractal structures. Phys. Rev. E 2006, 74, 016103. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, P.; Bertolotti, J.; Wiersma, D.S. A Lévy flight for light. Nature 2008, 453, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Humphries, N.E.; Queiroz, N.; Dyer, J.R.M.; Pade, N.G.; Musyl, M.K.; Schaefer, K.M.; Fuller, D.W.; Brunnscheweiler, J.M.; Doyle, T.K.; et al. Environmental context explains Lévy and Brownian movement patterns of marine predators. Nature 2010, 465, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, B.; Valentinčič, A.; Horvatić, D.; Stanley, H.E. Asymmetric Lévy flight in financial ratios. Proc. Natl. Acad. Sci. USA 2011, 108, 17883–17888. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, W. A survey on computing Levy stable distributions and a new MATLAB toolbox. Signal Proc. 2013, 93, 242–251. [Google Scholar] [CrossRef]
- Poccia, N.; Ansuini, A.; Bianconi, A. Far from equilibrium percolation, stochastic and shape resonances in the physics of life. Int. J. Mol. Sci. 2011, 12, 6810–6833. [Google Scholar] [CrossRef] [PubMed]
- Mora, T.; Bialek, W. Are biological systems poised at criticality? J. Stat. Phys. 2011, 144, 268–302. [Google Scholar] [CrossRef]
- Goldfeld, N.; Woese, C. Life is Physics: Evolution of a collective phenomenon out of equilibrium. Annu. Rev. Condens. Matter Phys. 2011, 2, 375. [Google Scholar] [CrossRef]
- Ciasca, G.; Sassun, T.E.; Minelli, E.; Antonelli, M.; Papi, M.; Santoro, A.; Giangaspero, M.; Delfini, R.; De Spirito, M. Nano-mechanical signature of brain tumours. Nanoscale 2016, 8, 19629–19643. [Google Scholar] [CrossRef] [PubMed]
- Ciasca, G.; Papi, M.; Di Claudio, S.; Chiarpotto, M.; Palmieri, V.; Maulucci, G.; Nocca, G.; Rossi, C.; De Spirito, M. Mapping viscoelastic properties of healthy and pathological red blood cells at the nanoscale level. Nanoscale 2015, 7, 17030–17037. [Google Scholar] [CrossRef] [PubMed]
- Piccioli, A.; Donati, F.; Di Giacomo, G.; Ziranu, A.; Careri, S.; Spinelli, M.S.; Giannini, S.; Giannicola, G.; Perisano, C.; Maccauro, G. Infective complications in tumour endoprostheses implanted after pathological fracture of the limbs. Injury 2016, 47, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Poccia, N.; Ricci, A.; Innocenti, D.; Bianconi, A. A possible mechanism for evading temperature quantum decoherence in living matter by Feshbach resonance. Int. J. Mol. Sci. 2009, 10, 2084–2106. [Google Scholar] [CrossRef] [PubMed]
- Lambreva, M.; Russo, D.; Polticelli, F.; Scognamiglio, V.; Antonacci, A.; Zobnina, V.; Campi, G.; Rea, G. Structure/Function/dynamics of photosystem II plastoquinone binding sites. Curr. Protein Pept. Sci. 2014, 15, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, A.; Ciasca, G.; Tenenbaum, A.; Battisti, A.; Campi, G. Temperature and solvent dependence of the dynamical landscape of tau protein conformations. J. Biol. Phys. 2012, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Florenzano, F.; Veronica, C.; Ciasca, G.; Ciotti, M.T.; Pittaluga, A.; Olivero, G.; Sinopoli, A.; Filomena, I.; Valentina, L.; Michele, F.M.S.; et al. Extracellular truncated tau causes early presynaptic dysfunction associated with Alzheimer’s disease and other tauopathies. Oncotarget 2017, in press. [Google Scholar] [CrossRef]
- Bianconi, A. On the Fermi liquid coupled with a generalized Wigner polaronic CDW giving high Tc superconductivity. Solid State Commun. 1994, 91, 1–5. [Google Scholar] [CrossRef]
- Campi, G.; Ricci, A.; Poccia, N.; Fratini, M.; Bianconi, A. X-Rays Writing/Reading of charge density waves in the CuO2 plane of a simple cuprate superconductor. Condens. Matter 2017, 2, 26. [Google Scholar] [CrossRef]
- Caivano, R.; Fratini, M.; Poccia, N.; Ricci, A.; Puri, A.; Ren, Z.-A.; Dong, X.-L.; Yang, J.; Lu, W.; Zhao, Z.-X.; et al. Feshbach resonance and mesoscopic phase separation near a quantum critical point in multiband FeAs-based superconductors. Supercond. Sci. Technol. 2009, 22, 014004. [Google Scholar] [CrossRef]
- Campi, G.; Bianconi, A. High-temperature superconductivity in a hyperbolic geometry of complex matter from nanoscale to mesoscopic scale. J. Supercond. Novel Magn. 2016, 29, 627–631. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gioacchino, M.; Campi, G.; Poccia, N.; Bianconi, A. Correlated Disorder in Myelinated Axons Orientational Geometry and Structure. Condens. Matter 2017, 2, 29. https://doi.org/10.3390/condmat2030029
Di Gioacchino M, Campi G, Poccia N, Bianconi A. Correlated Disorder in Myelinated Axons Orientational Geometry and Structure. Condensed Matter. 2017; 2(3):29. https://doi.org/10.3390/condmat2030029
Chicago/Turabian StyleDi Gioacchino, Michael, Gaetano Campi, Nicola Poccia, and Antonio Bianconi. 2017. "Correlated Disorder in Myelinated Axons Orientational Geometry and Structure" Condensed Matter 2, no. 3: 29. https://doi.org/10.3390/condmat2030029
APA StyleDi Gioacchino, M., Campi, G., Poccia, N., & Bianconi, A. (2017). Correlated Disorder in Myelinated Axons Orientational Geometry and Structure. Condensed Matter, 2(3), 29. https://doi.org/10.3390/condmat2030029