A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO
Abstract
:1. Introduction
2. Computational Method
3. Results and Discussion
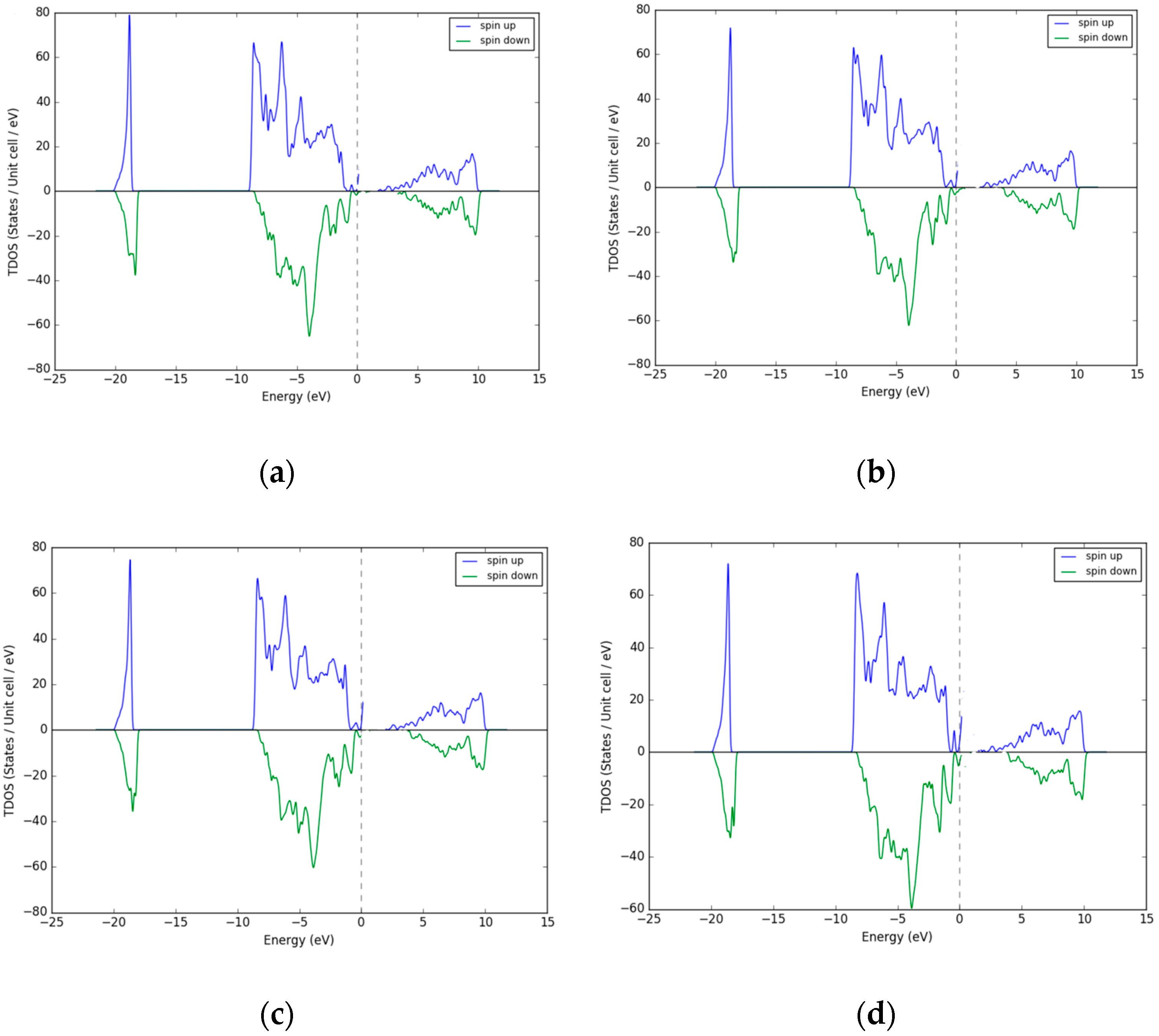
3.1. Density of States (DOS)–Excess Ni Occupying Interstitial Sites inNiO
3.2. Density of States (DOS)—Excess O Occupying Interstitial Sites in NiO
3.3. Formation energies of oxides of Pd and Pt
3.4. Density of States (DOS) - Pd as Dopant Occupying Interstitial Sites inNiO
3.5. Density of States (DOS)—Pt as Dopant Occupying Interstitial Sites inNiO
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide:An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Mott, N.F.; Peierls, R. Discussion on the paper by de Boer and Verwey. Proc. Phys. Soc. 1937, 49, 72–73. [Google Scholar] [CrossRef]
- Davoli, I.; Marcelli, A.; Bianconi, A.; Tomellini, M.; Fanfoni, M. Multi-electron configurations in the X-ray absorption near-edge structure of NiO at the oxygen K threshold. Phys. Rev. B 1986, 33, 2979–2982. [Google Scholar] [CrossRef]
- Tomellini, M.; Gozzi, D.; Bianconi, A.; Davoli, I. Local structure of nickel oxide grown at high temperatures in ceramic electrolyte cells. J. Chem. Soc. Faraday Trans. 1987, 83, 289–298. [Google Scholar] [CrossRef]
- Bengone, O.; Alouani, M.; Blöchl, P.; Hugel, J. Implementation of the projector augmented-wave LDA+U method: Application to the electronic structure of NiO. Phys. Rev. B 2000, 62, 16392–16401. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.M.; Ferrero, M.; Pisani, C. An ab Initio Periodic Study of NiO Supported at the Pd(100) Surface. Part 2: The Nonstoichiometric Ni3O4 Phase. J. Phys. Chem. B 2006, 110, 7918–7927. [Google Scholar] [CrossRef]
- Yi, J.B.; Ding, J.; Feng, Y.P.; Peng, G.W.; Chow, G.M.; Kawazoe, Y.; Liu, B.H.; Yin, J.H.; Thongmee, S. Size-dependent magnetism and spin-glass behavior of amorphous NiO bulk, clusters, and nanocrystals: Experiments and first-principles calculations. Phys. Rev. B 2007, 76, 224402. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Yu, N.; Yu, W.-Y.; Tang, B.-Y. Stability and magnetism of vacancy in NiO: A GGA+U study. Eur. Phys. J. B 2008, 64, 153–158. [Google Scholar] [CrossRef]
- Park, S.; Ahn, H.-S.; Lee, C.-K.; Kim, H.; Jin, H.; Lee, H.-S.; Seo, S.; Yu, J.; Han, S. Interaction and ordering of vacancy defects in NiO. Phys. Rev. B 2008, 77, 134103. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Kim, J.Y.; Pérez, M. Experimental and Theoretical Studies on the Reaction of H2 with NiO: Role of O Vacancies and Mechanism for Oxide Reduction. J. Am. Chem. Soc. 2001, 124, 346–354. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pisani, C.; Cinquini, F.; Giordano, L.; Pacchioni, G. Cationic and anionic vacancies on the NiO(100) surface: DFT+U and hybrid functional density functional theory calculations. J. Chem. Phys. 2007, 127, 174711. [Google Scholar] [CrossRef] [PubMed]
- Manders, J.R.; Tsang, S.-W.; Hartel, M.J.; Lai, T.-H.; Chen, S.; Amb, C.M.; Reynolds, J.R.; So, F. Solution-Processed Nickel Oxide Hole Transport Layers in High Efficiency Polymer Photovoltaic Cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, T.Y.; Sun, T.H. Microstructures, electrical and optical properties of non-stoichiometric p-type nickel oxide films by radio frequency reactive sputtering. Surf. Coat. Technol. 2010, 205, S236–S240. [Google Scholar] [CrossRef]
- Sato, H.; Minami, T.; Takata, S.; Yamada, T. Transparent conducting p-type NiO thin films prepared by magnetron sputtering. Thin Solid Films 1993, 236, 27–31. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lu, Y.-M.; Hwang, W.-S. Characterization of sputtered NiO thin films. Surf. Coat. Technol. 2005, 198, 138–142. [Google Scholar] [CrossRef]
- Reddy, Y.A.K. Influence of Growth Temperature on the Properties of DC Reactive Magnetron Sputtered NiO Thin Films. Int. J. Curr. Eng. Technol. 2013, 2, 351–357. [Google Scholar] [CrossRef]
- Subramanian, B.; Ibrahim, M.M.; Murali, K.R.; Vidhya, V.S.; Sanjeeviraja, C.; Jayachandran, M. Structural, optoelectronic and electrochemical properties of nickel oxide films. J. Mater. Sci. Mater. Electron. 2009, 20, 953–957. [Google Scholar] [CrossRef]
- Agrawal, A.; Habibi, H.R.; Agrawal, R.K.; Cronin, J.P.; Roberts, D.M.; Caron-Popowich, R.; Lampert, C.M. Effect of deposition pressure on the microstructure and electrochromic properties of electron-beam-evaporated nickel oxide films. Thin Solid Films 1992, 221, 239–253. [Google Scholar] [CrossRef]
- Yeh, W.; Matsumura, M. Chemical Vapor Deposition of Nickel Oxide Films from Bis-π-Cyclopentadienyl-Nickel. Jpn. J. Appl. Phys. 1997, 36, 6884–6887. [Google Scholar] [CrossRef]
- Raut, B.T.; Pawar, S.G.; Chougule, M.A.; Sen, S.; Patil, V.B. New process for synthesis of nickel oxide thin films and their characterization. J. Alloys Compd. 2011, 509, 9065–9070. [Google Scholar] [CrossRef]
- Guo, W.; Hui, K.N.; Hui, K.S. High conductivity nickel oxide thin films by a facile sol–gel method. Mater. Lett. 2013, 92, 291–295. [Google Scholar] [CrossRef]
- Tanaka, M.; Mukai, M.; Fujimori, Y.; Kondoh, M.; Tasaka, Y.; Baba, H.; Usami, S. Transition metal oxide films prepared by pulsed laser deposition for atomic beam detection. Thin Solid Films 1996, 281, 453–456. [Google Scholar] [CrossRef]
- Reguig, B.A.; Khelil, A.; Cattin, L.; Morsli, M.; Bernède, J.C. Properties of NiO thin films deposited by intermittent spray pyrolysis process. Appl. Surf. Sci. 2007, 253, 4330–4334. [Google Scholar] [CrossRef]
- Kang, J.-K.; Rhee, S.-W. Chemical vapor deposition of nickel oxide films from Ni(C5H5)2/O2. Thin Solid Films 2001, 391, 57–61. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, W.; Yan, X.; Feng, B. Studies on electrochromic properties of nickel oxide thin films prepared by reactive sputtering. J. Alloys Compd. 2008, 462, 356–361. [Google Scholar] [CrossRef]
- Brückner, W.; Kaltofen, R.; Thomas, J.; Hecker, M.; Uhlemann, M.; Oswald, S.; Elefant, D.; Schneider, C.M. Stress development in sputtered NiO thin films during heat treatment. J. Appl. Phys. 2003, 94, 4853. [Google Scholar] [CrossRef]
- Kuzmin, A.; Purans, J.; Rodionov, A. X-ray absorption spectroscopy study of the Ni K edge in magnetron-sputtered nickel oxide thin films. J. Phys. Condens. Matter 1997, 9, 6979–6993. [Google Scholar] [CrossRef]
- Itapu, S.; Khan, K.; Georgiev, D.G. Effect of UV Laser Irradiation on the properties of NiO films and ZnO/NiO Heterostructures. MRS Adv. 2016, 1, 293–298. [Google Scholar] [CrossRef]
- Itapu, S.; Georgiev, D.G.; Uprety, P.; Podraza, N.J. Modification of reactively sputtered NiOx thin films by pulsed UV laser irradiation. Phys. Status Solidi 2017, 214, 1600414. [Google Scholar] [CrossRef]
- He, J.H.; Yuan, S.L.; Yin, Y.S.; Tian, Z.M.; Li, P.; Wang, Y.Q.; Liu, K.L.; Wang, C.H. Exchange bias and the origin of room-temperature ferromagnetism in Fe-doped NiO bulk samples. J. Appl. Phys. 2008, 103, 23906. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Wang, J.; Yang, P.; Song, D.; Liu, J. Porous lanthanum doped NiO microspheres for supercapacitor application. J. Electroanal. Chem. 2012, 682, 37–44. [Google Scholar] [CrossRef]
- Li, J.-C.; Hou, X.-Y.; Cao, Q. Effect of Cu doping on the resistive switching of NiO thin films. J. Appl. Phys. 2014, 115, 164507. [Google Scholar] [CrossRef]
- Dutta, T.; Gupta, P.; Gupta, A.; Narayan, J. Effect of Li doping in NiO thin films on its transparent and conducting properties and its application in heteroepitaxial p-n junctions. J. Appl. Phys. 2010, 108, 83715. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Jiang, D.; Shi, J. Design of a meso-structured Pd/NiO catalyst for highly efficient low temperature CO oxidation under ambient conditions. RSC Adv. 2015, 5, 40352–40357. [Google Scholar] [CrossRef]
- Sambi, M.; Sensolo, R.; Rizzi, G.A.; Petukhov, M.; Granozzi, G. Growth of NiO ultrathin films on Pd(100) by post-oxidation of Ni films: The effect of pre-adsorbed oxygen. Surf. Sci. 2003, 537, 36–54. [Google Scholar] [CrossRef]
- Zou, X.; Rui, Z.; Ji, H. Core–Shell NiO@PdO Nanoparticles Supported on Alumina as an Advanced Catalyst for Methane Oxidation. ACS Catal. 2017, 7, 1615–1625. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Cheng, B.; Yu, J.; Jiang, C. Flexible nickel foam decorated with Pt/NiO nanoflakes with oxygen vacancies for enhanced catalytic formaldehyde oxidation at room temperature. Environ. Sci. Nano 2017, 4, 2215–2224. [Google Scholar] [CrossRef]
- Nie, L.; Meng, A.; Teng, F.; Cheng, B. Hierarchically macro-mesoporous flowerlike Pt/NiO composite microspheres for efficient formaldehyde oxidation at room temperature. RSC Adv. 2015, 5, 83997–84003. [Google Scholar] [CrossRef]
- Qi, L.; Cheng, B.; Ho, W.; Liu, G.; Yu, J. Hierarchical Pt/NiO Hollow Microspheres with Enhanced Catalytic Performance. ChemNanoMat 2015, 1, 58–67. [Google Scholar] [CrossRef]
- Walker, M.; Parkinson, C.R.; Draxler, M.; Brown, M.G.; Mcconville, C.F. Initial growth of platinum on oxygen-covered Ni(1 1 0) surfaces. Surf. Sci. 2006, 600, 3327–3336. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Av, G.K.; Furthmiiller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter. 1994, 6, 8245–8257. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Hahn, T. (Ed.) International Tables for Crystallography Volume A: Space-Group Symmetry, 5th ed.; Springer: Amsterdam, The Netherlands, 2002; p. 688. [Google Scholar]
- Bruska, M.K.; Czekaj, I.; Delley, B.; Mantzaras, J.; Wokaun, A. Electronic structure and oxygen vacancies in PdO and ZnO: Validation of DFT models. Phys. Chem. Chem. Phys. 2011, 13, 15947–15954. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T. Theoretical investigations on the potential-induced formation of Pt-oxide surfaces. J. Electroanal. Anal. Chem. 2007, 607, 158–166. [Google Scholar] [CrossRef] [Green Version]





| % Excess Ni | 3 | 12 | 18 | 25 |
| Bandgap (eV) | 3.8 ± 0.003 | 3.83 ± 0.005 | 3.85 ± 0.006 | 3.86 ± 0.008 |
| % Excess O | 3 | 12 | 18 | 25 |
| Bandgap (eV) | 3.80 ± 0.02 | 3.85 ± 0.03 | 3.90 ± 0.03 | 4.00 ± 0.05 |
| Types of Defects | O-Rich Condition Eform(eV) | O-Poor Condition Eform(eV) |
|---|---|---|
| PdNi | −0.33 | 1.54 |
| PtNi | −0.05 | 2.18 |
| IPd | 2.55 | 2.55 |
| IPt | 3.18 | 3.18 |
| % Pd doping | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 25 |
| Bandgap (eV) | 3.80 ± 0.02 | 3.75 ± 0.08 | 3.50 ± 0.06 | 3.20 ± 0.04 | 2.95 ± 0.03 | 2.80 ± 0.04 | 2.70 ± 0.02 | 2.50 ± 0.02 |
| % Pt doping | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 25 |
| Bandgap (eV) | 2.50 ± 0.02 | 2.40 ± 0.04 | 2.40 ± 0.04 | 2.25 ± 0.05 | 2.15 ± 0.06 | 2.10 ± 0.03 | 2.0 ± 0.01 | 2.0 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itapu, S.; Borra, V.; Mossayebi, F. A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO. Condens. Matter 2018, 3, 46. https://doi.org/10.3390/condmat3040046
Itapu S, Borra V, Mossayebi F. A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO. Condensed Matter. 2018; 3(4):46. https://doi.org/10.3390/condmat3040046
Chicago/Turabian StyleItapu, Srikanth, Vamsi Borra, and Faramarz Mossayebi. 2018. "A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO" Condensed Matter 3, no. 4: 46. https://doi.org/10.3390/condmat3040046
APA StyleItapu, S., Borra, V., & Mossayebi, F. (2018). A Computational Study on the Variation of Bandgap Due to Native Defects in Non-Stoichiometric NiO and Pd, Pt Doping in Stoichiometric NiO. Condensed Matter, 3(4), 46. https://doi.org/10.3390/condmat3040046





