Carbon Deposition on Hematite (α-Fe2O3) Nanocubes by Annealing in the Air: Morphology Study with Grazing Incidence Small Angle X-ray Scattering (GISAXS)
Abstract
1. Introduction
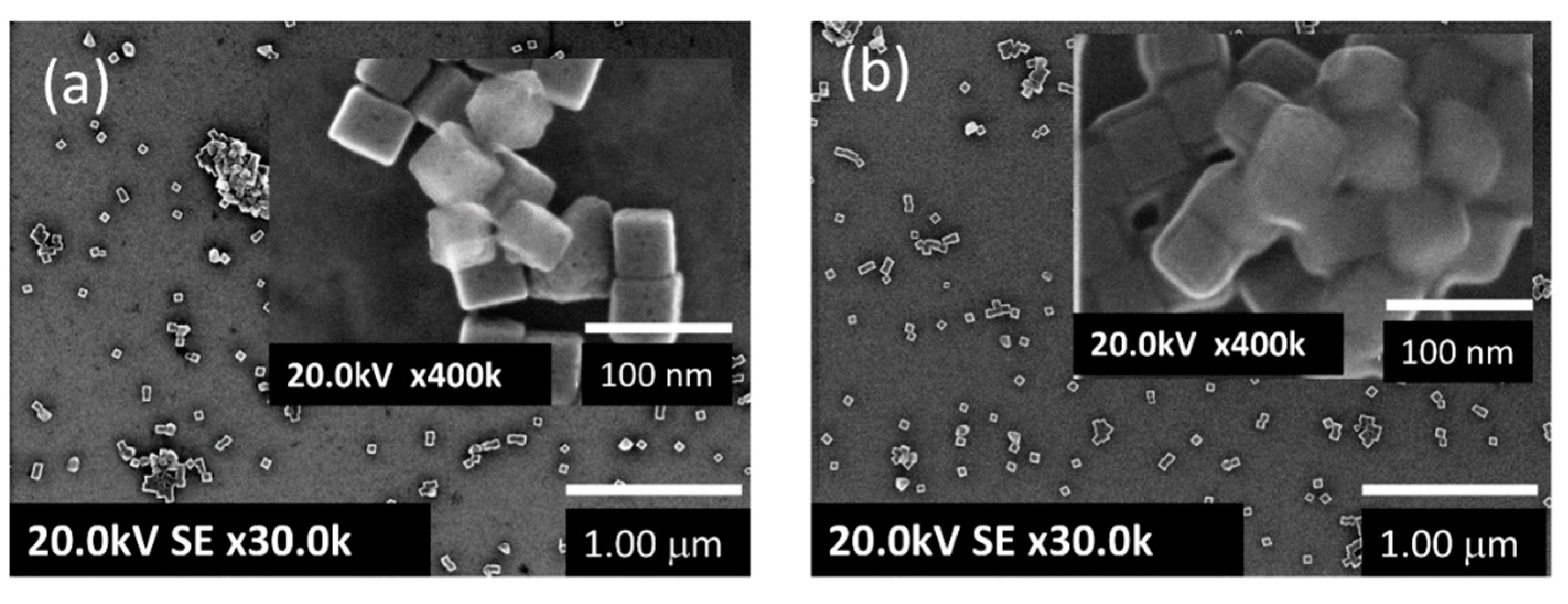
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis α-Fe2O3 Nanocubes
3.2. Fabrication of Submonolayers of α-Fe2O3 Nanocube
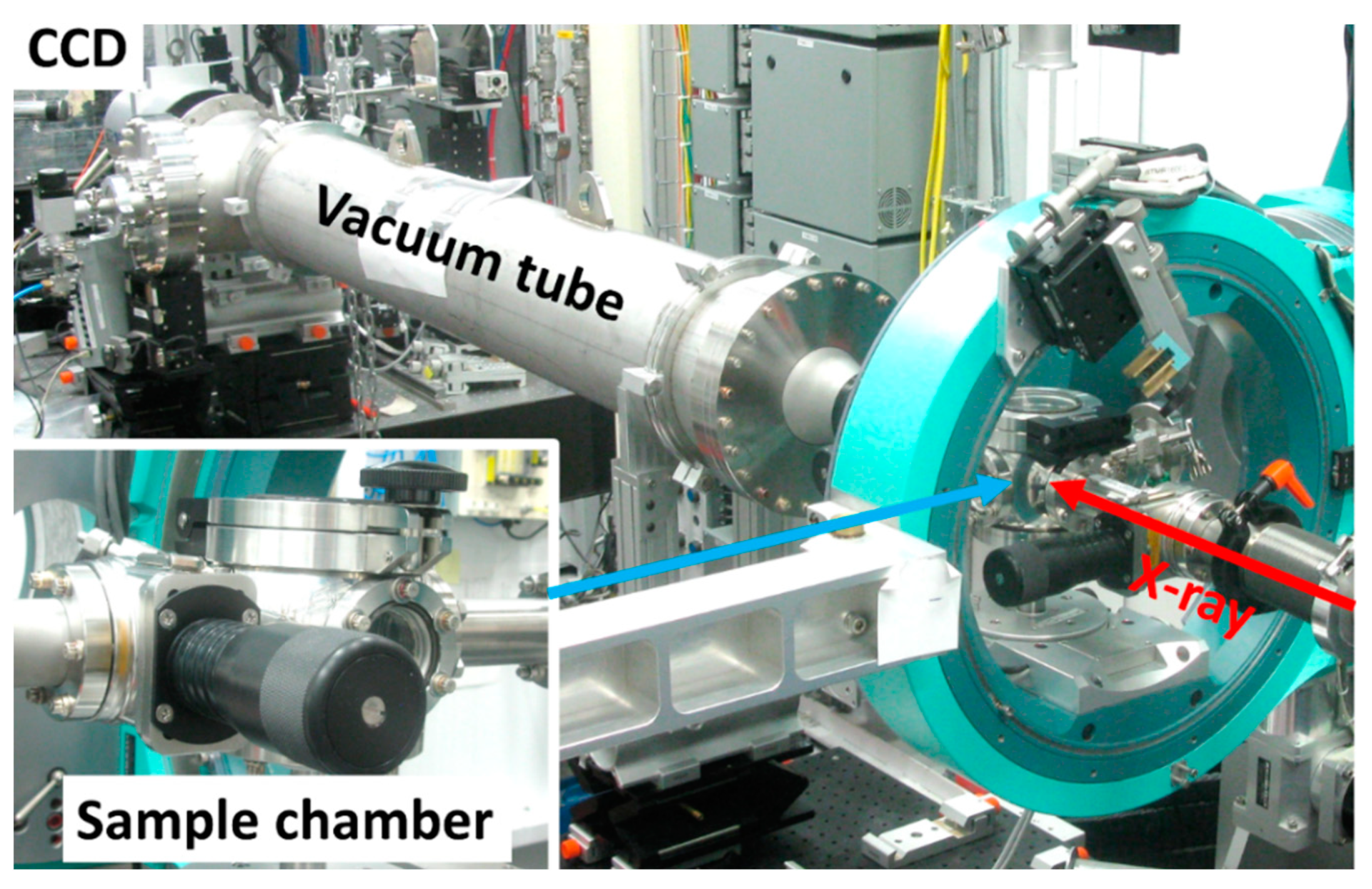
3.3. GISAXS Measurements
3.4. Raman Measurement
Funding
Acknowledgments
Conflicts of Interest
References
- Young, K.M.H.; Klahr, B.M.; Zandi, O.; Hamann, T.W. Photocatalytic water oxidation with hematite electrodes. Catal. Sci. Technol. 2013, 3, 1660–1671. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Bockris, J.O.M. Stacked thin-film photoelectrode using iron oxide. J. Appl. Phys. 1984, 56, 874–876. [Google Scholar] [CrossRef]
- Kim, J.Y.; Youn, D.H.; Kim, J.H.; Kim, H.G.; Lee, J.S. Nanostructure-Preserved Hematite Thin Film for Efficient Solar Water Splitting. ACS Appl. Mater. Interfaces 2015, 7, 14123–14129. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, G.; Zhang, L.; Liang, Y.; Zhang, S.; Zhang, X. Shape and Magnetic Properties of Single-Crystalline Hematite (α-Fe2O3) Nanocrystals. ChemPhysChem 2006, 7, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cheng, Y.; Wang, Y.; Bao, F.; Zhou, L.; Wei, X.; Zhang, Y.; Zheng, Q. Quasicubic α-Fe2O3 Nanoparticles with Excellent Catalytic Performance. J. Phys. Chem. B 2006, 110, 3093–3097. [Google Scholar] [CrossRef]
- Zhifa, P.; Minhua, C.; Jing, Y.; Kunlin, H.; Changwen, H. Controlled synthesis and growth mechanism of hematite nanorhombohedra, nanorods and nanocubes. Nanotechnology 2006, 17, 799–804. [Google Scholar] [CrossRef]
- Cao, H.; Wang, G.; Warner, J.H.; Watt, A.A.R. Amino-acid-assisted synthesis and size-dependent magnetic behaviors of hematite nanocubes. Appl. Phys. Lett. 2008, 92, 013110. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C. Continuous Aspect-Ratio Tuning and Fine Shape Control of Monodisperse α-Fe2O3 Nanocrystals by a Programmed Microwave–Hydrothermal Method. Adv. Funct. Mater. 2008, 18, 880–887. [Google Scholar] [CrossRef]
- Fu, X.; Bei, F.; Wang, X.; Yang, X.; Lu, L. Surface-enhanced Raman scattering of 4-mercaptopyridine on sub-monolayers of α-Fe2O3 nanocrystals (sphere, spindle, cube). J. Raman Specrosc. 2009, 40, 1290–1295. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Chen, J.; Liu, J.; Wu, H.; Zhan, H.; Liang, C.; Wu, M. Continuous Shape- and Spectroscopy-Tuning of Hematite Nanocrystals. Inorg. Chem. 2010, 49, 8411–8420. [Google Scholar] [CrossRef]
- Lu, F.; Wu, Q.; Yang, X.; Chen, L.; Cai, J.; Liang, C.; Wu, M.; Shen, P. Comparative investigation of the performances of hematite nanoplates and nanograins in lithium-ion batteries. PCCP 2013, 15, 9768–9774. [Google Scholar] [CrossRef]
- Ouyang, J.; Pei, J.; Kuang, Q.; Xie, Z.; Zheng, L. Supersaturation-Controlled Shape Evolution of α-Fe2O3 Nanocrystals and Their Facet-Dependent Catalytic and Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 12505–12514. [Google Scholar] [CrossRef]
- Abdul Rashid, N.M.; Haw, C.; Chiu, W.; Khanis, N.H.; Rohaizad, A.; Khiew, P.; Abdul Rahman, S. Structural- and optical-properties analysis of single crystalline hematite (α-Fe2O3) nanocubes prepared by one-pot hydrothermal approach. CrystEngComm 2016, 18, 4720–4732. [Google Scholar] [CrossRef]
- Kim, C.-Y. Atomic structure of hematite (α-Fe2O3) nanocube surface; synchrotron X-ray diffraction study. Nano-Struct. Nano-Objects 2020, 23, 100497. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Zhang, X.; Rosso, K.M.; Zhang, L. Facet-dependent contaminant removal properties of hematite nanocrystals and their environmental implications. Environ. Sci. Nano 2018, 5, 1790–1806. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Cui, Z.; Wang, S.; Cao, M. Facet effect of α-Fe2O3 crystals on photocatalytic performance in the photo-Fenton reaction. RSC Adv. 2014, 4, 34387–34394. [Google Scholar] [CrossRef]
- Morin, F.J. Electrical Properties of a-Fe2O3 and a-Fe2O3 Containing Titanium. Phys. Rev. 1951, 83, 1005–1010. [Google Scholar] [CrossRef]
- Sivula, K.; Zboril, R.; Le Formal, F.; Robert, R.; Weidenkaff, A.; Tucek, J.; Frydrych, J.; Grätzel, M. Photoelectrochemical Water Splitting with Mesoporous Hematite Prepared by a Solution-Based Colloidal Approach. J. Am. Chem. Soc. 2010, 132, 7436–7444. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water Oxidation at Hematite Photoelectrodes: The Role of Surface States. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef]
- Le Formal, F.; Tétreault, N.; Cornuz, M.; Moehl, T.; Grätzel, M.; Sivula, K. Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem. Sci. 2011, 2, 737–743. [Google Scholar] [CrossRef]
- Le Formal, F.; Sivula, K.; Grätzel, M. The Transient Photocurrent and Photovoltage Behavior of a Hematite Photoanode under Working Conditions and the Influence of Surface Treatments. J. Phys. Chem. C 2012, 116, 26707–26720. [Google Scholar] [CrossRef]
- Deng, J.; Lv, X.; Gao, J.; Pu, A.; Li, M.; Sun, X.; Zhong, J. Facile synthesis of carbon-coated hematite nanostructures for solar water splitting. Energy Environ. Sci. 2013, 6, 1965–1970. [Google Scholar] [CrossRef]
- Hu, X.; Han, S.; Zhu, Y. Facet-controlled synthesis of polyhedral hematite/carbon composites with enhanced photoactivity. Appl. Surf. Sci. 2018, 443, 227–235. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, H.; Li, J.; Wu, F.; Wu, T.; Wang, R.; Liu, D.; Pan, M.; Xie, Z.; Qu, D. Self-assembly synthesis of a unique stable cocoon-like hematite @C nanoparticle and its application in lithium ion batteries. J. Colloid Interface Sci. 2017, 495, 157–167. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H. Facile fire treatment of nanostructured hematite with an enhanced photoelectrochemical water splitting performance. J. Mater. Chem. A 2016, 4, 14974–14977. [Google Scholar] [CrossRef]
- Levine, J.R.; Cohen, J.B.; Chung, Y.W.; Georgopoulos, P. Grazing-incidence small-angle X-ray scattering: New tool for studying thin film growth. J. Appl. Crystallogr. 1989, 22, 528–532. [Google Scholar] [CrossRef]
- Rauscher, M.; Salditt, T.; Spohn, H. Small-angle x-ray scattering under grazing incidence: The cross section in the distorted-wave Born approximation. Phys. Rev. B 1995, 52, 16855–16863. [Google Scholar] [CrossRef]
- Pospelov, G.; Van Herck, W.; Burle, J.; Carmona Loaiza, J.M.; Durniak, C.; Fisher, J.M.; Ganeva, M.; Yurov, D.; Wuttke, J. BornAgain: Software for simulating and fitting grazing-incidence small-angle scattering. J. Appl. Crystallogr. 2020, 53, 262–276. [Google Scholar] [CrossRef]
- Guo, H.; Xu, H.; Barnard, A.S. Can hematite nanoparticles be an environmental indicator? Energy Environ. Sci. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Riedel, T.; Schaub, G.; Jun, K.-W.; Lee, K.-W. Kinetics of CO2 Hydrogenation on a K-Promoted Fe Catalyst. Ind. Eng. Chem. Res. 2001, 40, 1355–1363. [Google Scholar] [CrossRef]
- Temple, P.A.; Hathaway, C.E. Multiphonon Raman Spectrum of Silicon. Phys. Rev. B 1973, 7, 3685–3697. [Google Scholar] [CrossRef]
- Hyatt, H.A.; Cherlow, J.M.; Fenner, W.R.; Porto, S.P.S. Cross section for the Raman effect in molecular nitrogen gas. J. Opt. Soc. Am. 1973, 63, 1604–1606. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Marshall, C.P.; Marshall, A.O. Hematite and carbonaceous materials in geological samples: A cautionary tale. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 80, 133–137. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Jorio, A. Raman Spectroscopy in Graphene-Based Systems: Prototypes for Nanoscience and Nanometrology. ISRN Nanotechnol. 2012, 2012, 234216. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Yamaguchi, Y.; Kinoshita, H.; Ishii, K. In Situ Observation of Reduction Behavior of Hematite with Solid Carbon and Crystallographic Orientation between Hematite and Magnetite. ISIJ Int. 2007, 47, 226–233. [Google Scholar] [CrossRef][Green Version]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Andreazza, P.; Khelfane, H.; Lyon, O.; Andreazza-Vignolle, C.; Ramos, A.Y.; Samah, M. Trends in anomalous small-angle X-ray scattering in grazing incidence for supported nanoalloyed and core-shell metallic nanoparticles. Eur. Phys. J. Spec. Top. 2012, 208, 231–244. [Google Scholar] [CrossRef]




© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-Y. Carbon Deposition on Hematite (α-Fe2O3) Nanocubes by Annealing in the Air: Morphology Study with Grazing Incidence Small Angle X-ray Scattering (GISAXS). Condens. Matter 2020, 5, 54. https://doi.org/10.3390/condmat5030054
Kim C-Y. Carbon Deposition on Hematite (α-Fe2O3) Nanocubes by Annealing in the Air: Morphology Study with Grazing Incidence Small Angle X-ray Scattering (GISAXS). Condensed Matter. 2020; 5(3):54. https://doi.org/10.3390/condmat5030054
Chicago/Turabian StyleKim, Chang-Yong. 2020. "Carbon Deposition on Hematite (α-Fe2O3) Nanocubes by Annealing in the Air: Morphology Study with Grazing Incidence Small Angle X-ray Scattering (GISAXS)" Condensed Matter 5, no. 3: 54. https://doi.org/10.3390/condmat5030054
APA StyleKim, C.-Y. (2020). Carbon Deposition on Hematite (α-Fe2O3) Nanocubes by Annealing in the Air: Morphology Study with Grazing Incidence Small Angle X-ray Scattering (GISAXS). Condensed Matter, 5(3), 54. https://doi.org/10.3390/condmat5030054




