Structural and Electrochemical Behaviors of ZnO Structure: Effect of Different Zinc Precursor Molarity
Abstract
1. Introduction
2. Results
2.1. Structural Properties
2.2. Morphological Analysis
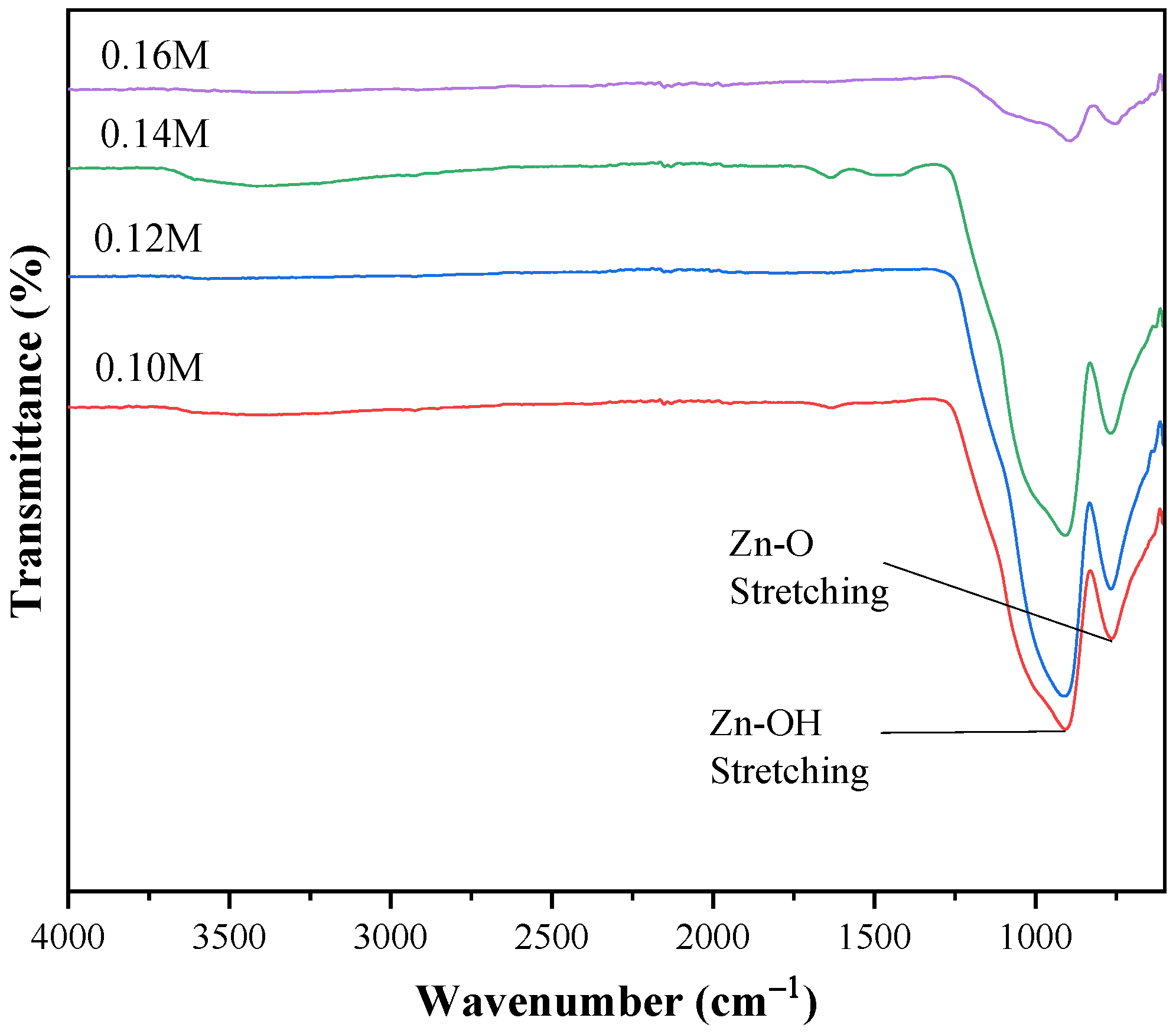
2.3. FTIR Analysis
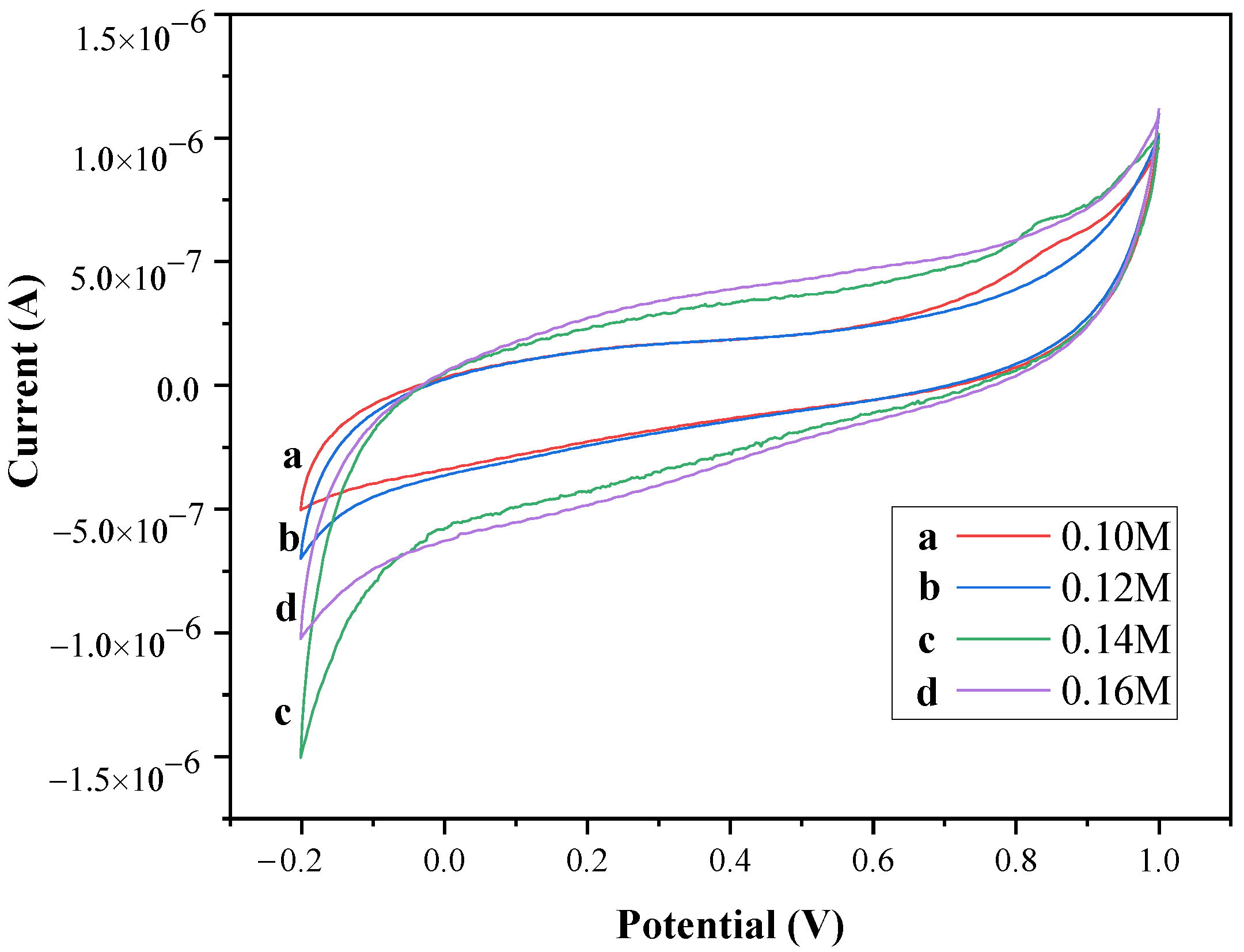
2.4. Cyclic Voltammetry (CV) Measurement
3. Discussion
4. Materials and Methods
4.1. Synthesis of ZnO Structure
4.2. Characterization
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EShaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar]
- Hahn, Y.-B. Zinc oxide nanostructures and their applications. Korean J. Chem. Eng. 2011, 28, 1797–1813. [Google Scholar] [CrossRef]
- Yadav, M.S.; Sinha, A.K.; Singh, M.N. Electrochemical behaviour of ZnO–AC based nanocomposite electrode for supercapacitor. Mater. Res. Express 2018, 5, 085503. [Google Scholar] [CrossRef]
- Coleman, V.A.; Jagadish, C. Basic properties and applications of ZnO. In Zinc Oxide Bulk, Thin Films and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–20. [Google Scholar]
- Abdullah, F.; Abu Bakar, N.; Abu Bakar, M. Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. J. Hazard. Mater. 2021, 424, 127416. [Google Scholar] [PubMed]
- Chu, H.O.; Wang, Q.; Shi, Y.J.; Song, S.G.; Liu, W.G.; Zhou, S.; Gibson, D.; Alajlani, Y.; Li, C. Structural, optical properties and optical modelling of hydrothermal chemical growth derived ZnO nanowires. Trans. Nonferrous Met. Soc. China 2020, 30, 191–199. [Google Scholar] [CrossRef]
- Haq, A.N.U.; Nadhman, A.; Ullah, I.; Mustafa, G.; Yasinzai, M.; Khan, I. Synthesis approaches of zinc oxide nanoparticles: The dilemma of ecotoxicity. J. Nanomater. 2017, 2017, 8510342. [Google Scholar]
- Perillo, P.M.; Atia, M.N.; Rodríguez, D.F. Effect of the reaction conditions on the formation of the ZnO nanostructures. Physica E Low Dimens. Syst. Nanostruct. 2017, 85, 185–192. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, S.; Wu, X.; Feng, M.; Li, Y.; Han, H.; Li, W. Effect of alkali bases on the synthesis of ZnO quantum dots. Open Chem. 2021, 19, 377–384. [Google Scholar] [CrossRef]
- Jayaraman, V.K.; Hernández-Gordillo, A.; Bizarro, M. Importance of precursor type in fabricating ZnO thin films for photocatalytic applications. Mater. Sci. Semicond. Process. 2018, 75, 36–42. [Google Scholar] [CrossRef]
- Amirthavalli, C.; Manikandan, A.; Prince, A. Effect of zinc precursor ratio on morphology and luminescent properties of ZnO nanoparticles synthesized in CTAB medium. Ceram. Int. 2018, 44, 15290–15297. [Google Scholar] [CrossRef]
- Singh, G.; Shrivastava, S.; Jain, D.; Pandya, S.; Ganesan, V. Effect of Molarity of Precursor Solution on Nanocrystalline Zinc Oxide Thin Films. Defect Diffus. Forum 2009, 293, 99–105. [Google Scholar] [CrossRef]
- Fahimi, Z.; Moradlou, O. Fabrication of ZnO@C foam: A flexible free-standing electrode for energy storage devices. Mater. Des. 2020, 189, 108525. [Google Scholar] [CrossRef]
- Çiçek, O.; Karasüleymanoğlu, M.; Kurnaz, S.; Öztürk, Ö.; Taşçı, A.T. Self-powered visible-UV light photodiodes based on ZnO nanorods-silicon heterojunctions with surface modification and structural enhancement. Optik 2022, 261, 169137. [Google Scholar] [CrossRef]
- Perillo, P.M.; Atia, M.N.; Rodríguez, D.F. Studies on the Growth Control of ZnO Nanostructures synthesized by the Chemical Method. Rev. Mater. 2018, 23. [Google Scholar] [CrossRef]
- Malek, M.F.; Mamat, M.H.; Sahdan, M.Z.; Zahidi, M.M.; Khusaimi, Z.; Mahmood, M.R. Influence of various sol concentrations on stress/strain and properties of ZnO thin films synthesised by sol–gel technique. Thin Solid Films 2013, 527, 102–109. [Google Scholar] [CrossRef]
- Goswami, M.; Adhikary, N.C.; Bhattacharjee, S. Effect of annealing temperatures on the structural and optical properties of zinc oxide nanoparticles prepared by chemical precipitation method. Optik 2018, 158, 1006–1015. [Google Scholar] [CrossRef]
- Mary, A.; Arumugam, S. Structural and optical studies of molarity based ZnO thin films. Nano Hybrids Compos. 2017, 17, 140–148. [Google Scholar] [CrossRef]
- Amakali, T.; Daniel, L.S.; Uahengo, V.; Dzade, N.Y.; de Leeuw, N.H. Structural and optical properties of ZnO thin films prepared by molecular precursor and sol–gel methods. Crystals 2020, 10, 132. [Google Scholar] [CrossRef]
- Mohamed, R.; Mamat, M.H.; Ismail, A.S.; Malek, M.F.; Zoolfakar, A.S.; Khusaimi, Z.; Suriani, A.B.; Ahmad, M.K.; Rusop, M. Hierarchically assembled tin-doped zinc oxide nanorods using low-temperature immersion route for low temperature ethanol sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 16292–16305. [Google Scholar] [CrossRef]
- Mahajan, C.; Takwale, M. Precursor molarity dependent growth rate, microstructural, optical and electrical properties of spray pyrolytically deposited transparent conducting ZnO thin films. Micro Nanostructures 2021, 163, 107131. [Google Scholar] [CrossRef]
- Rayathulhan, R.; Sodipo, B.K.; Aziz, A.A. Nucleation and growth of zinc oxide nanorods directly on metal wire by sonochemical method. Ultrason. Sonochem. 2017, 35, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Soylu, M.; Coskun, M. Controlling the properties of ZnO thin films by varying precursor concentration. J. Alloys Compd. 2018, 741, 957–968. [Google Scholar] [CrossRef]
- Mohamed, R.; Mamat, M.H.; Malek, M.F.; Ismail, A.S.; Rafaie, H.A.; Rusop, M. Controllable synthesis of Sn:ZnO/SnO2 nanorods: pH-dependent growth for an ethanol gas sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 15394–15406. [Google Scholar] [CrossRef]
- Bui, V.K.; Kumar, M.K.; Alinaghibeigi, M.; Moolayadukkam, S.; Eskandarinejad, S.; Mahmoudi, S.; Mirzamohammadi, S.; Rezaei-khamseh, M. A review on zinc oxide composites for energy storage applications: Solar cells, batteries, and supercapacitors. J. Compos. Compd. 2021, 3, 182–193. [Google Scholar]
- Aljameel, A.I.; Darsin, M. Single Crystalline Zinc Oxide Nanorods Grown by R-F Sputtering Technique Onto P-Si Substrate for Sensing Applications. Mat. Sci. Res. India 2022, 19, 36–43. [Google Scholar] [CrossRef]
- Dhanalakshmi, A.; Natarajan, B.; Ramadas, V.; Palanimurugan, A.; Thanikaikarasan, S. Structural, morphological, optical and antibacterial activity of rod-shaped zinc oxide and manganese-doped zinc oxide nanoparticles. Pramana 2016, 87, 57. [Google Scholar] [CrossRef]
- Chaari, M.; Matoussi, A.; Fakhfakh, Z. Structural and Dielectric Properties of Sintering Zinc Oxide Bulk Ceramic. Mater. Sci. Appl. 2011, 2, 764–769. [Google Scholar] [CrossRef]
- Abdulrahman, A.F.; Ahmed, S.M.; Ahmed, N.M.; Almessiere, M.A. Enhancement of ZnO Nanorods Properties Using Modified Chemical Bath Deposition Method: Effect of Precursor Concentration. Crystals 2020, 10, 386. [Google Scholar] [CrossRef]
- Aksoy, S.; Caglar, Y.; Ilican, S.; Caglar, M. Effect of deposition temperature on the crystalline structure and surface morphology of ZnO films deposited on p-Si. In Proceedings of the European Conference of Chemical Engineering, and European Conference of Civil Engineering, and European Conference of Mechanical Engineering, and European Conference on Control, Tenerife, Spain, 30 November–2 December 2010; pp. 227–231. [Google Scholar]
- Kammel, R.S.; Sabry, R.S. Effects of the aspect ratio of ZnO nanorods on the performance of piezoelectric nanogenerators. J. Sci. Adv. Mater. Dev. 2019, 4, 420–424. [Google Scholar] [CrossRef]
- Nayan, M.B.; Jagadish, K.; Abhilash, M.R.; Namratha, K.; Srikantaswamy, S. Comparative Study on the Effects of Surface Area, Conduction Band and Valence Band Positions on the Photocatalytic Activity of ZnO-MxOy Heterostructures. J. Water Resour. Prot. 2019, 11, 35. [Google Scholar] [CrossRef]
- Alves, T.; Kolodziej, C.; Burda, C.; Franco, A., Jr. Effect of particle shape and size on the morphology and optical properties of zinc oxide synthesized by the polyol method. Mater. Des. 2018, 146, 125–133. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural characterisation of ZnO particles obtained by the emulsion precipitation method. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Xue, J.; Yang, Q.; Guan, R.; Shen, Q.; Liu, X.; Jia, H.; Li, Q. High-performance ordered porous Polypyrrole/ZnO films with improved specific capacitance for supercapacitors. Mater. Chem. Phys. 2020, 256, 123591. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hu, Z.-A.; Chang, Y.-Q.; Wang, H.-W.; Zhang, Z.-Y.; Yang, Y.-Y.; Wu, H.-Y. Zinc Oxide/Reduced Graphene Oxide Composites and Electrochemical Capacitance Enhanced by Homogeneous Incorporation of Reduced Graphene Oxide Sheets in Zinc Oxide Matrix. J. Phys. Chem. C 2011, 115, 2563–2571. [Google Scholar] [CrossRef]
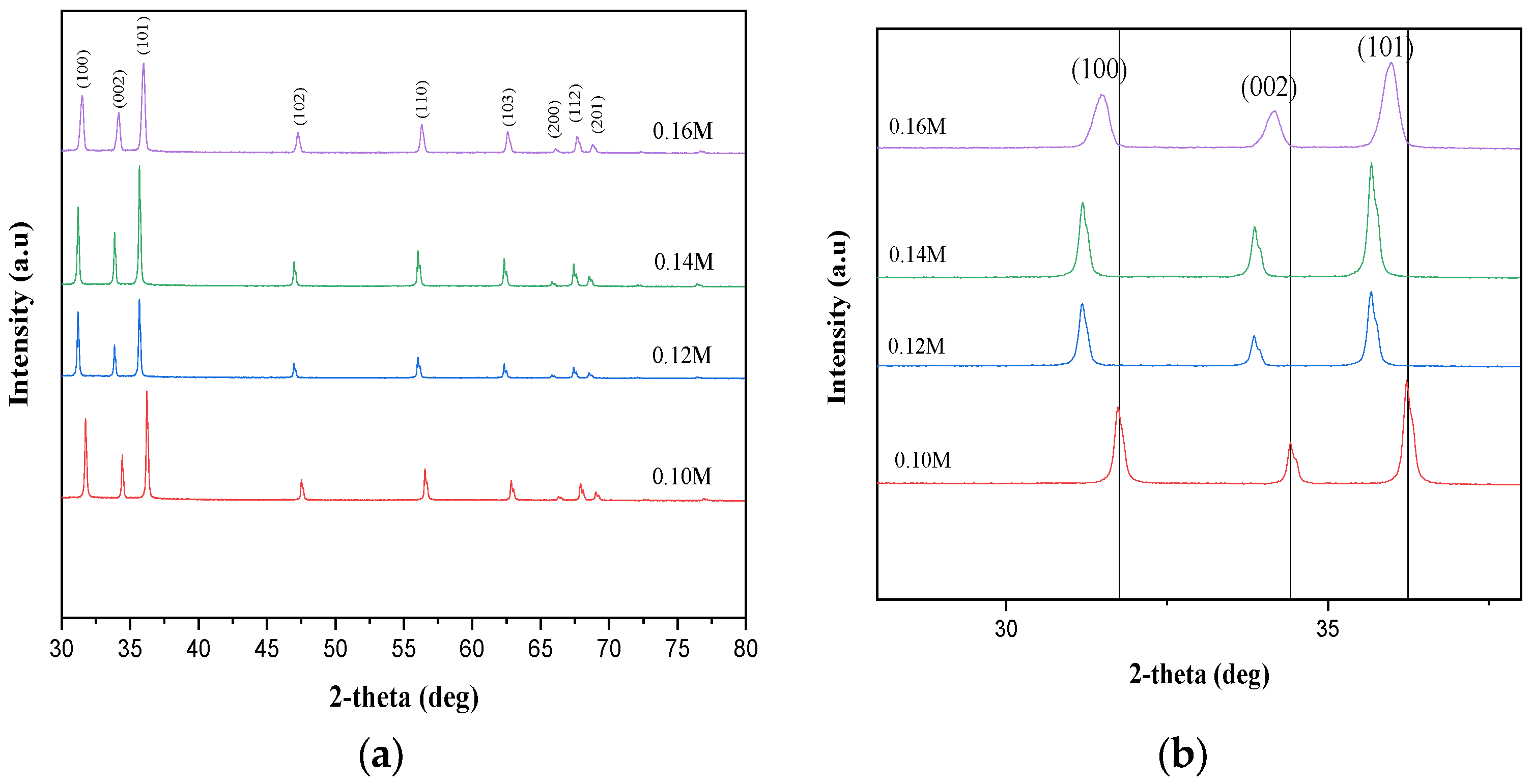

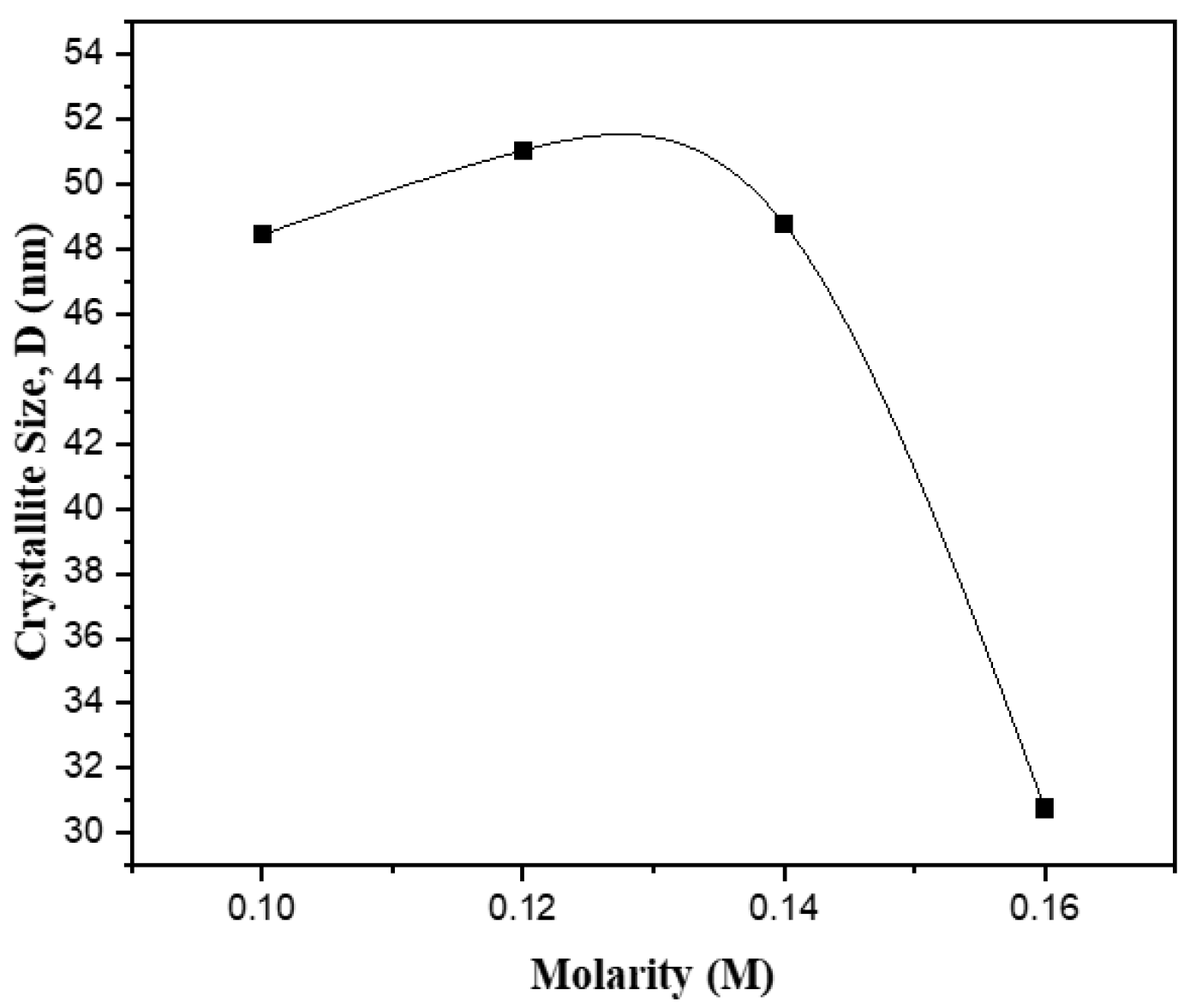


| Different Molarity of Zn Precursor (M) | Ratio a/c | APF (%) | % | ||||
|---|---|---|---|---|---|---|---|
| a(100) | c(002) | ||||||
| 0.10 M | 3.2522 | 5.2052 | 1.6005 | 75 | −0.015 | 0.35 | 4.26 |
| 0.12 M | 3.3075 | 5.2889 | 1.5990 | 75 | 1.59 | −36.1 | 3.84 |
| 0.14 M | 3.3075 | 5.2889 | 1.5990 | 75 | 1.59 | −36.1 | 4.20 |
| 0.16 M | 3.2779 | 5.2404 | 1.5987 | 75 | 0.66 | −14.1 | 10.56 |
| Different Molarity of Zn Precursor (M) | Area (10−7 AV) | Cs (Fg−1) |
|---|---|---|
| 0.10 | 4.00 | 2.11 |
| 0.12 | 3.85 | 2.03 |
| 0.14 | 6.70 | 3.54 |
| 0.16 | 7.33 | 3.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, R.; Anuar, M.S.A. Structural and Electrochemical Behaviors of ZnO Structure: Effect of Different Zinc Precursor Molarity. Condens. Matter 2022, 7, 71. https://doi.org/10.3390/condmat7040071
Mohamed R, Anuar MSA. Structural and Electrochemical Behaviors of ZnO Structure: Effect of Different Zinc Precursor Molarity. Condensed Matter. 2022; 7(4):71. https://doi.org/10.3390/condmat7040071
Chicago/Turabian StyleMohamed, Ruziana, and Muhammad Syakir Azri Anuar. 2022. "Structural and Electrochemical Behaviors of ZnO Structure: Effect of Different Zinc Precursor Molarity" Condensed Matter 7, no. 4: 71. https://doi.org/10.3390/condmat7040071
APA StyleMohamed, R., & Anuar, M. S. A. (2022). Structural and Electrochemical Behaviors of ZnO Structure: Effect of Different Zinc Precursor Molarity. Condensed Matter, 7(4), 71. https://doi.org/10.3390/condmat7040071





